Abstract
Pinworm infection (oxyuriasis) is a common problem in rodent colonies. Facility-wide prophylactic treatment of all mice with a diet containing therapeutic levels of fenbendazole for several weeks is often used to control pinworm outbreaks. We examined the effect of feeding a therapeutic diet containing 150 ppm fenbendazole on the growth of EMT6 mouse mammary tumors implanted into BALB/c Rw mice. Mice were randomized to receive either a fenbendazole-containing or control diet for 1 wk before tumor cells were injected intradermally in the flanks and throughout tumor growth. Tumor growth was monitored by serial measurements of tumor diameters from the time tumors became palpable until they reached 1000 mm3. The medicated diet did not alter tumor growth, invasion, or metastasis. When tumors reached volumes of approximately 100 mm3, some were irradiated locally with 10 Gy of X-rays. Irradiation significantly delayed tumor growth; fenbendazole did not alter the radiation-induced growth delay. However, cell culture studies showed that fenbendazole concentrations not far above those expected in the tissues of mice on this diet altered the growth of the tumor cells in culture. Recent data from other laboratories also have demonstrated effects of fenbendazole that could complicate experiments. Care should therefore be exercised in deciding whether chow containing fenbendazole should be administered to mouse colonies being used in cancer research.
Mouse pinworms (Syphacia obvelata and Aspiculuris tetraptera) are a common problem even in well-run rodent colonies. Infections with pinworms can be asymptomatic, but heavy infections can produce rectal prolapse, intussusception, enteritis, fecal impaction, diarrhea, hematologic changes, and poor weight gain.12,20,22 Pinworms can be difficult to eradicate. The eggs are light and sticky and therefore are disseminated readily through transfer from contaminated hands, clothing, surfaces, instruments, or other fomites. The eggs require incubation at room temperature for 6 to 7 d to become infective and can survive for weeks outside the host. Their resistance to common disinfectants allows them to survive many routine decontamination procedures. Moreover, diagnostic tests for pinworms often yield false-negative results in low-level infections, and treating only those mice proven to be infected can leave undetected pockets of infected animals that can serve as a source of recurrent infections within the facility. For this reason, facility-wide prophylactic treatments of all mice with a medicated diet for several weeks combined with rigorous measures for both decontamination and prevention of reinfection are often used to control or eliminate pinworm outbreaks.
Fenbendezole, [5-(phenylthio)-1H-benzimidazol-2-yl]carbamic acid methyl ester, is widely used in the medicated foods used to treat pinworms and various parasitic infections in humans, livestock, companion animals, and laboratory animals.15,20,22 The drug acts primarily by binding to tubulin, thereby disrupting the tubulin–microtubule equilibrium.5,16 The differential effects of fenbendazole across species and its utility as an antiparasitic agent largely reflect differences in the structures of tubulin in mammalian cells and lower organisms; these differences lead to increased binding of the drug to tubulin and therefore to greater inhibition of tubulin polymerization, in lower organisms.5,16 Oral fenbendazole is not well absorbed from the intestine, likely reflecting the limited aqueous solubility of the drug, but the ability of transporter proteins (including certain anticancer drug resistance proteins) to prevent transport across the intestinal wall also contributes to poor absorption.6,15,18 In rodents more than 90% of the drug is excreted in the feces unchanged;15 in the bloodstream, the primary metabolite is oxfenbendazole; small amounts of fenbendazole sulfone and sulfoxide also are seen in plasma.15,21 Fenbendazole has been shown repeatedly to lack significant toxicities to mice and rats during regimens that are effective in the eradication of pinworms.12,15,20,22,37
A general assumption relevant to use of these treatment regimens is that food containing fenbendazole at therapeutic concentrations will not affect the outcomes of the experiments being performed in research facilities. However, some recent publications have suggested that this assumption is not always true. A fenbendazole-containing diet had no significant effects on several behavioral tests, but it did impede for motor function, as measured by rotarod tests.8 Fenbendazole treatment did not alter many standard measures of immune function in mice in one study.4 However, fenbendazole decreased function in B lymphocytes in another study;17 this reduction in immune function was greater in aged mice and was reversed when treated mice subsequently were fed the control food for 2 to 4 wk. Reports that fenbendazole-containing diets produced strain-specific changes in the onset and severity of autoimmune enchephalomyelitis23 and allergic airways inflammation2 but did not alter the incidence and onset of type 1 diabetes in nonobese diabetic mice7 show that the immunologic effects of fenbendazole can compromise experiments and that the effects of the drug depend on the mouse strain and experimental model used.
A fenbendazole-containing diet was reported to inhibit the growth of a human lymphoma xenograft in SCID mice and to produce significant increases in total WBC and neutrophil counts, but only when given with high-dose vitamins. 9 It is unclear whether the changes in the growth of these xenografts reflected a direct effect of the drug on the tumor cells or stimulation of an immune response that inhibited the growth of the human tumors, which can survive xenotransplantation only in severely immunodeficient hosts. Other data suggest that fenbendazole can act as an antitumor agent. A presentation at the 2010 meeting of the American Association of Cancer Research3 reported that high doses of fenbendazole, albendazole, and mebendazole inhibited the growth of paclitaxel-resistant tumors. Because fenbendazole acts by inhibiting microtubule formation5,16 and several widely used anticancer drugs produce their antineoplastic effects by disrupting either microtubule formation (vincristine, vinblastine)36 or microtubule depolymerization (paclitaxel, docetaxel),36 fenbendazole could have significant antitumor effects when given in sufficiently intensive regimens.
Several studies raised concerns about the potential for interactions between fenbendazole and radiation in solid tumors. The effect of the fenbendazole on the rotarod test8 is reminiscent of that seen with the nitroimidazoles (including metronidazole and misonidazole). Nitroimidazoles were shown to be hypoxic cell radiosensitizers and hypoxic cytotoxins in preclinical studies using solid rodent tumors and have been used clinically in combination with radiotherapy.19,25-27 Our past studies showed that these nitroimidazoles were extremely effective in sensitizing EMT6 tumors to radiation when given in high concentrations.24-27,33 Several modified benzimidazoles were devised and synthesized10 as potential hypoxic cell radiosensitizers and proved to be effective in laboratory studies. Moreover, several series of substituted bis-benzimidazoles have been developed that bind in the minor groove of DNA at specific DNA sequences, inhibit DNA helicase activity, and thereby inhibit cell proliferation;32,34 some of these bis-benzimidazoles undergo bioreductive activation in hypoxic conditions, which could produce selective antitumor activity through preferential activation to the cytotoxic species in the hypoxic cells of solid tumors.25-27 Many benzimidazoles also have effects on glucose uptake and carbohydrate metabolism,6,15,37 which conceivably could produce cytotoxic and antitumor effects under appropriate circumstances, including the conditions of hypoxia and low pH that occur within solid tumors.
Ongoing research in our laboratories uses assays of the growth and metastatic spread of tumors in rats and mice and concomitant rodent health assessment to evaluate the effects of new regimens for treating solid tumors with radiation, anticancer drugs, and combined modality regimens. Many of our studies examine novel compounds developed specifically to attack cells that have become severely hypoxic because of the adverse microenvironments that occur naturally within solid tumors.24-27,33 Because of this research focus and in light of the data on the effects of benzimidazoles described earlier, when the clinical veterinarians in our university recommended that all mouse colonies in our facility be placed on a diet containing 150 ppm fenbendazole for 6 wk, we were concerned that this treatment might confound our experiments. The studies described here were performed to examine the effects on our primary experimental tumor model system of the standard commercial fenbendazole-containing chow being used to treat mice and to confirm or allay these concerns.
Materials and Methods
Mice and tumors.
These experiments were performed by using EMT6 mouse mammary tumors growing in BALB/cRw mice. This model system has been in use in our laboratories since 1975 to study the response of tumors in vivo to X-rays and to a wide variety of anticancer agents.24-31,33 EMT6 is an anaplastic, cloned, cell-culture-adapted tumor cell line derived from a mouse mammary tumor; the origin, characteristics, growth pattern, cell proliferation kinetics, and metastatic profile of this line have been described in our past publications.24,28 The tumor is negative for both estrogen and progesterone receptors, and its growth is similar in male and female mice; its status regarding human epidermal growth factor receptor 2 (HER2) has not been determined. The tumor cells, tumors, and mice and their response to standard anticancer therapeutic agents, are well characterized.24-31,33 Protocols for the experiments performed here were reviewed and approved in advance by the Yale IACUC. The experiments were performed in compliance with these protocols, Yale policies, and the principles outlined in the Guide for the Care and Use of Laboratory Animals.13
These experiments used adult female BALB/cRw mice (age, 2.5 to 3 mo) that had been bred and reared in our production colony. These mice were housed under barrier conditions in ‘full-service’ individually ventilated microisolation caging, with autoclaved cage, food, water bottles, and bedding and are serviced in a clean-air hood or change station. These SPF mice are screened routinely (Veterinary Preventive Medicine Group, Section of Comparative Medicine, Yale University) by serology for ectromelia virus, lymphocytic choriomeningitis virus, minute virus of mice, mouse hepatitis virus, mouse parvovirus, murine rotavirus, Sendai virus, pneumonia virus of mice, and Mycoplasma pulmonis; by examination of the pelage for ectoparasites and the cecum and proximal colon for endoparasites; and by microbiologic culture of the nasopharynx and cecum for pathogenic bacteria. Mice were housed on a 12:12-h light:dark cycle and had access to water and to either standard autoclaved rodent chow or radiation-sterilized fenbendazole-containing chow ad libitum. Tumor measurements and experimental manipulations were performed in a biologic safety cabinet within the animal room. No evidence of pinworm infection was found in examinations of sentinel mice from either the experimental colony or production colony or in necropsies of approximately 35 mice from the experimental colony that had been sent to veterinary clinical services for testing just before this experiment began. These mice were being euthanized at the conclusion of an experiment and had been housed on the same rack as the mice assigned to the experiment reported here. There therefore was no evidence of pinworm infection in our colonies; rather, our colonies were treated because of pinworm infections detected in other rooms in the same animal facility.
In vivo protocol.
In the experiment reported here, mice were randomized either to continue receiving the standard diet being used in the experimental colony or to receive the fenbendazole-containing diet prescribed for mice throughout the facility. Within these groups, cages of mice were randomized to be untreated controls or to receive 10 Gy of radiation localized to the tumor. Eight mice were assigned to each of the 4 experimental groups.
Both diets came from Harlan Teklad (Madison, WI). Mice on the control diet received Global 2018S (autoclavable), which was added to the cages before autoclaving; cages, bedding, and food were autoclaved as an intact unit. Mice on the medicated diet received TD.01432 Irradiated; the sterile food (sterilized by irradiation by the vendor) was added to the sterile caging in the clean washroom after the bedding units had been autoclaved. The TD.01432 medicated diet is composed of the Global 2018 diet with 150 ppm fenbendazole added. Both diets contain 18.6% protein and 6.2% fat and have the same content of other macronutrients, minerals, amino acids, and fatty acids. The initial content of heat-labile vitamins is higher in the 2018S diet than in the TD.01432 diet; because autoclaving reduces the content of these vitamins whereas irradiation does not, the vitamin contents in the 2 sterilized foods should be similar. The complete compositions and nutrient profiles of the 2 diets are available on the vendor website (http://www.harlan.com).
At 1 wk after mice were placed on the specified diets, 2 × 105 EMT6 tumor cells, harvested from exponentially-growing cell cultures and suspended in sterile pyrogen-free cell culture medium, were injected intradermally in a volume of 0.05 mL into the shaved right flank. This position allows the tumors to grow without impeding normal movement and without invading the body wall, to be measured easily, and to be retracted slightly from the body of the mouse for localized tumor irradiation. Tumors were measured 3 times weekly from the time they became palpable,14,24,28 by using vernier calipers to measure the 3 diameters of the tumor: the length (the longest diameter), width (the diameter perpendicular to the length), and height. Tumor volumes were calculated by using the formula for a hemiellipsoid (volume = 0.5236 × length × width × height), the geometric form best approximating their shape.
When the tumors reached an average calculated volume of 100 mm3, mice to be irradiated were anesthetized by intramuscular injection of 100 mg/kg ketamine and 10 mg/kg xylazine. They then were positioned on an irradiation platform, with their tumors retracted slightly from the body to be fully within the radiation port, and the tumors were irradiated with 10 Gy of 250-kV X-rays (Stabilipan, Siemans, Malvern, PA) delivered at 12.5 mA, with 2-mm Al filtration and a dose rate of 6.4 Gy/min. The bodies and limbs of the mice were shielded with lead, so that the dose to critical normal tissues such as the bone marrow, intestines, and lungs was less than 5% of the tumor dose; this low dose of radiation does not cause significant injury.11 After irradiation, mice were observed until they were sternally recumbent and returned to their cages. Because the X-ray machine is located in a shielded room adjacent to the animal housing room and is used only with animals from this colony, irradiation could be performed without compromising the microbiology or pinworm status of the mice. Tumors continued to be measured 3 times weekly for the duration of the study. At the times of tumor measurement, mice also were weighed and examined for appearance (for example, fur condition, appearance of eyes) and behavior (for example, changes in grooming, spontaneous movement, or response to handling; breathing rate and pattern) to detect any signs of toxicity from the food, radiation treatment, or growing tumors. As specified in our IACUC protocol, mice were euthanized when the appearance (for example, failure to groom, evidence of infection of the tumor), behavior (for example, tumor interfering with gait or movement, lethargy, failure to respond normally to handling, difficulty breathing), or weight (weight loss of 20%) exceeded the specified toxicity criteria. In the absence of such toxicities, each mouse was euthanized when its tumor reached a volume of 1000 mm3.
After euthanasia, each mouse was necropsied to assess local infiltration and metastatic spread by the tumor. The lungs (the most common site of metastases for this tumor line) were removed, fixed in Bouin solution, washed in 95% ethanol, and stored in ethanol until metastases were counted. Any tumor nodules on the surface of the lungs were counted under a dissecting microscope. Because identification and counting of these microscopic metastases can be subjective, the vials containing the samples were coded, and the tumors were evaluated by a blinded observer.
Cell culture studies.
Cell culture studies were performed by using the same tumor cell line as for the in vivo studies. In these studies, 5 × 104 EMT6 cells, harvested from exponentially-growing monolayer cultures and grown as detailed previously,24,29,30 were plated into culture dishes containing Waymouth medium (Gibco, Grand Island, NY) supplemented with 15% serum (FetalPlex, Invitrogen, Carlsbad, CA) and antibiotics. Cells were incubated at 37°C in a humid atmosphere of 95% air, 5% CO2 for 4 h, to allow them to attach and begin growing. Graded doses of fenbendazole (Sigma, St Louis, MO), dissolved in DMSO (Sigma), and diluted in Waymouth medium, were then added to the culture medium. The growth of treated and control cultures then was followed throughout the next week. Each day, 2 dishes from each group were selected at random, the medium was removed, and the cells were washed with 0.05% trypsin in balanced salt solution and trypsinized for 10 min to release the cells from the plate surface. The trypsin then was inactivated by the addition of an equal volume of medium containing serum, and the cells were suspended into a single-cell suspension by gentle pipetting. The cells were counted (Coulter Counter, Beckman Coulter, Brea, CA), and the total number of cells per dish was calculated. Data from 2 experiments are shown.
Results
Experiments with tumors in vivo.
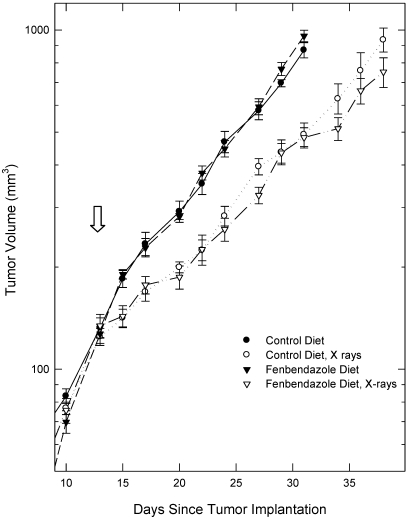
One week after the mice were randomized and began receiving either the fenbendazole-containing or control diets, they were injected with tumor cells. Tumors were measured 3 times weekly from the time they became palpable. The growth curves for tumors in mice receiving the fenbendazole-containing and control diets were indistinguishable throughout the growth of the tumors to the predetermined maximal volume of 1000 mm3 (Figure 1).
Figure 1.
Growth of EMT6 tumors in mice fed the fenbendazole-containing or control diets, unirradiated mice, and mice receiving 10 Gy of radiation delivered locally to the tumors on day 14 (arrow). Data are given as mean ± SEM (n = 8 per group initially).
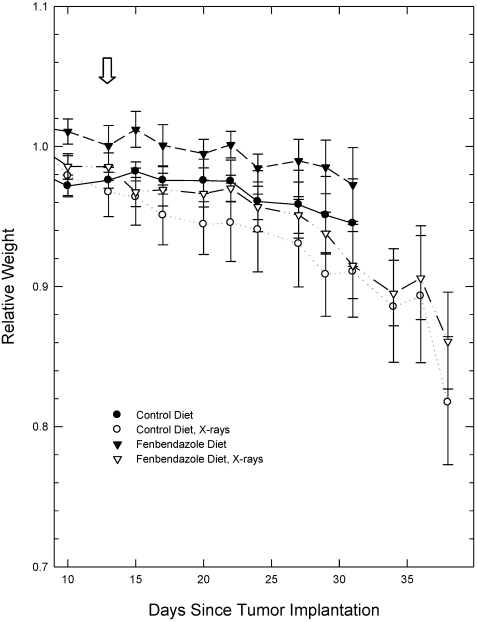
The appearance and behavior of the mice were monitored throughout the course of the experiment. The mice showed no changes in the appearance over this period (other than tumor development) and had no behavioral changes indicative of toxicities or stress. Mice were weighed on each day that the tumors were measured. Because of differences in the initial weights of the individual mice, the weight of each mouse during the course of the experiment was normalized to its weight on the first measurement to allow better visualization of changes in weight during tumor growth. The changes in the relative weights of the mice on the 2 diets over the course of the tumor volume measurements were not significantly different (Figure 2). In addition, ANOVA of the measured weights of the mice at the initial measurement and during the growth of the tumors did not show significant differences between the weights of the mice in the different groups.
Figure 2.
Relative weights of mice during tumor growth. Because individual mice varied in weight, weight during the observation period was normalized to the initial weight recorded for an individual mouse to better visualize changes in body weight over time. Arrow indicates 10-Gy irradiation of tumors of irradiated mice (day 14). Data are given as mean ± SEM (n = 8 per group initially). Differences between groups were not statistically significant (P > 0.05; ANOVA).
When each tumor reached a volume of 1000 mm3, or when prespecified toxicity criteria were met, each mouse was euthanized and necropsied to assess local invasion of the tumor and regional metastasis. As anticipated, local invasion of the body wall and lymph node metastases were not detected in any of the mice in this experiment (Table 1). Because the earliest site of distant metastasis in EMT6 tumors generally is the lung, lungs were harvested and fixed, and the metastases on the lungs were counted under a dissecting microscope. Mice on the 2 diets showed no significant differences in the numbers of metastases (Table 1; Mann–Whitney U test, P > 0.05). The spontaneous lung metastases in all mice were microscopic and had not yet produced clinical symptoms in the hosts.
Table 1.
Analyses of tumor growth, invasion, and metastasis
| Treatment | Time (d) to reach 4 times the treatment volume (mean ± SEM) | Evidence of local invasion or lymph node metastases | No. of lung metastases (mean ± SEM) |
| Control diet, no radiation | 12.7 ± 0.8 | none | 29.7 ± 7.2d |
| Fenbendazole diet, no radiation | 12.1 ± 0.6a | none | 29.7 ± 4.3d |
| Control diet, tumor irradiation | 17.5 ± 1.3b | none | 28.6 ± 3.9d |
| Fenbendazole diet, tumor irradiation | 20.4 ± 0.9bc | none | 24.3 ± 9.1d |
The significance of differences between groups was assessed by using Mann–Whitney U tests.
Not significantly different (P = 0.32) from value for tumors in unirradiated mice on the control diet.
Significantly different (P < 0.05) from value for unirradiated tumors.
Not significantly different (P = 0.12) from value for tumors in irradiated mice on the control diet.
None of the differences between groups were statistically significant (P > 0.05 for all comparisons).
Overall, these observations revealed no significant differences in the growth or metastatic pattern of the tumors in mice on the fenbendazole-containing and control diets, and no differences in the health of the hosts on the 2 diets.
As expected, localized irradiation of the tumors with 10 Gy 14 d after inoculation, when tumors were approximately 100 mm3 in volume, produced a statistically significant inhibition of tumor growth (Figure 1, Table 1). All tumors continued to grow, as was expected for this subcurative radiation dose. The growth curves for irradiated tumors in mice receiving the fenbendazole-containing food and the irradiated tumors in mice receiving the control diet were not significantly different (Figure 1, Table 1). The behavior and appearance of the irradiated mice on the treated and control diet were similar also. The weights of mice with unirradiated and irradiated tumors were similar during the time that the mice with unirradiated tumors were alive, but mice with irradiated tumors lived several days longer than did mice with tumors that were not irradiated, and these mice continued to lose weight during that time (Figure 2).
When each irradiated tumor reached a volume of 1000 mm3, the mouse was euthanized and necropsied, and the metastases were counted. The numbers of lung metastases in the mice with irradiated tumors were not significantly different from those in the nonirradiated mice, and the numbers of metastases in irradiated mice on the 2 diets were not significantly different (Table 1; Mann–Whitney U test, P > 0.05). Overall, these data did not reveal any significant effects of fenbendazole on the response of EMT6 tumors to a single, subcurative dose of localized radiation.
Cell culture studies.
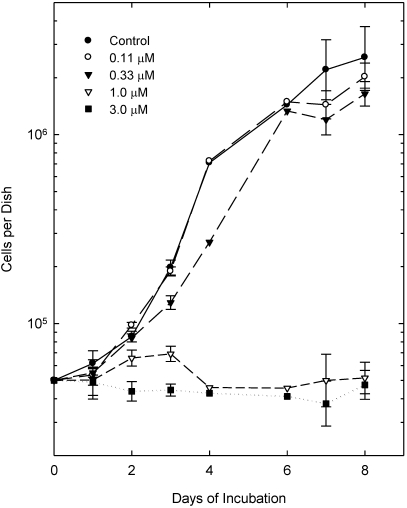
Because fenbendazole at high concentrations reportedly can have cytotoxic effects on tumor cells,3,9 we performed limited cell culture studies to ascertain whether EMT6 cells were sensitive to the effects of this drug given in continuous incubations at doses that might be expected to occur in the tissues of mice fed the therapeutic diet (Figure 3). At concentrations of 0.11 and 0.33 µM, fenbendazole did not produce significant, consistent changes in the growth of EMT6 cells in vitro. However, at doses of 1 and 3 µM, fenbendazole strikingly inhibited the growth of these cells. We also noted changes in the appearance of the cultures at these higher doses: cells in treated cultures were rounder than the control cells and less firmly attached to growth surface.
Figure 3.
Effect of fenbendazole on the growth of EMT6 tumor cells in cell culture. Cells were plated at 5 × 104 cells per dish on day 1 and fenbendazole was added to the culture medium for the treated groups 4 h after plating to produce concentrations of 0.11, 0.33, 1.0 or 3.0 μM, which were left in the culture medium for the duration of the 2 experiments. Points show the total number of cells per culture and are the means of 2 or 4 measurements; SEM are shown for values with 4 observations.
The limited data available on the pharmacokinetics of fenbendazole in rodents6,21,37 suggest that the experimental diet used in our tumor studies produce maximal tissue levels of approximately 0.1 µM or lower. Therefore, our cell culture data support the in vivo data by suggesting that chronic exposure to the drug in concentrations expected in tissues of mice fed a fenbendazole-containing diet does not alter the growth of EMT6 tumor cells. However, the in vitro data also provide evidence that higher drug concentrations do have significant effects on the growth of these tumor cells.
Discussion
Our experiments tested for 2 possible effects of fenbendazole: alteration of tumor growth, through a cytotoxic effect of the drug on cells within these solid tumors, and alteration of tumor radiosensitivity. We saw no evidence that either effect occurred when mice were placed on a standard fenbendazole-containing diet appropriate for the treatment of pinworms in rodent colonies. In this sense, the data from our tumor growth study support the notion that this intervention can be used safely in mice being used in experimental cancer therapy studies.
However, our cell culture data show that fenbendazole inhibits the growth of EMT6 tumor cells in vitro when given continuously at doses not far above those expected in the tissues of rodents fed this diet. EMT6 cells are very proficient in the repair of damage induced by radiation and by many anticancer drugs24,26,27,30 and are relatively resistant to radiation and many anticancer drugs, including docetaxel,29 which binds to and stabilizes microtubules, thereby disrupting progression through the cell cycle. These cells are also resistant to injury from hypoxia and low pH.24-27,33 It is therefore possible that tumor cell lines that are more sensitive to the cytotoxic effects of either tubulin-directed agents or the metabolic disruption induced by benzimidazoles might be more sensitive to fenbendazole than are EMT6 tumors. In this regard, it is notable that experiments demonstrating that fenbendazole inhibited the growth of a human leukemia in SCID mice9 used a model system with notable sensitivity in 2 respects. First, leukemia cells (like most cells of hematopoietic origin) are less able to repair damage and more likely to undergo apoptosis in response to injury from radiation, drugs or metabolic disruption than are cells of other lineages.11,36 Second, the cells in the cited study9 were xenografted into SCID mice, which are used as hosts for xenografts because they have an mutation that produces defective V(D)J joining, producing deficits in T and B cell immunity that prevent the mice from rejecting grafts of foreign tissues.1 However, this same mutation also prevents the repair of DNA double-strand breaks produced by radiation, anticancer drugs, and other agents and increases the radiation sensitivity of the bone marrow cells in SCID mice by a factor of 3 over that of the cells in normal BALB/c mice.1 Both the tumors and the hosts used in the earlier studies9 therefore would be expected to be unusually sensitive to the effects of agents that damaged DNA directly or indirectly through effects on metabolic processes or tubulin.
The effects of fenbendazole on tubulin might synergize with or antagonize the effects of anticancer drugs with mechanisms of action that involve stabilization or disruption of microtubules (for example, paclitaxel, docetaxel, vincristine, vinblastine, colchicine and podophyllotoxin);36 such synergism has been reported for the closely related benzimidazole flubendazole.35 The possibility of such interactions would not be anticipated by the many cancer researchers who are unaware that the mechanism of action of this antihelminth involves altered tubulin stability. Furthermore, it is possible that fenbendazole would have effects that varied within the different microenvironments within solid tumors. If this effect occurred, the interactions of fenbendazole with anticancer drugs having activity that is influenced by these tumor microenvironments25-27 could be complex. The effects of the benzimidazoles on glucose uptake and metabolism6,15,16,37 raise the possibility of interactions between fenbendazole and drugs that are activated in hypoxia or drugs that alter energy metabolism. Therefore, significant interactions between fenbendazole and anticancer drugs having several different mechanisms of action are quite possible.
Characteristics of the mice also might influence the effects of fenbendazole on experiments. Mice that have greater drug absorption from the intestine because of hereditary disease phenotypes, certain microbiologic profiles (induced by intercurrent or experimental infections), or concomitant treatment with drugs that alter absorption might take up more of this poorly absorbed drug and therefore have higher blood and tissue levels of fenbendazole and its active metabolites,15,18,21 which in turn could produce greater confounding effects. Tumor–host systems that are especially sensitive to small changes in the host immune system, such as tumor xenografts in immunocompromised animals or tumors that are immunogenic in the rodent strain of origin,31 might also be more sensitive to the subtle immunologic effects of fenbendazole.
Because of these potential problems, we advise caution when fenbendazole-containing diets are used to treat rodent colonies actively used in research on experimental cancer therapy.
Acknowledgments
We thank Jacqueline Mendes for her assistance with the experiments and Gail Cameron for providing detailed information on the foods and procedures used by the Yale ARC. This research was supported by grants P01 CA129186 and CA129186-03S2 from the National Cancer Institute (NCI). Core facilities supported by the Yale Cancer Center and NCI center grant 16359 were used in the performance of the studies.
References
- 1.Biedermann KA, Sung J, Giaccia AJ, Tosto LM, Brown JM. 1991. SCID mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci USA 88:1394–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Y, Zhou J, Webb DC. 2009. Treatment of mice with fenbendazole attenuates allergic airways inflammation and Th2 cytokine production in a model of asthma. Immunol Cell Biol 87:623–629 [DOI] [PubMed] [Google Scholar]
- 3.Chung I, Barrows C, Wilson A, Rummel N, Badaruddin S, Mizokami A, Banyard J, Zetter B. [Internet] 2010. Benzimidazole as novel therapeutic agent for metastatic prostate cancer. [Cited 01 November 2011]. Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=94285e5c-4f3a-49b4-8641-03cf9a804f77&cKey=fa44601a-4893-4f23-9039-6c017cbc23b5&mKey=%7b0591FA3B-AFEF-49D2-8E65-55F41EE8117E%7d
- 4.Cray C, Villar D, Zaias J, Altman NH. 2008. Effects of fenbendazole on routine immune response parameters in BALB/c mice. J Am Assoc Lab Anim Sci 47:32–36 [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson PJ, Gutteridge WE, Gull K. 1984. A comparison of the interaction of antihelminth benzimidazoles with tubulin isolated from mammalian tissue and the parasitic nematode Ascaridia galli. Biochem Pharmacol 33:1069–1074 [DOI] [PubMed] [Google Scholar]
- 6.Düwel D. 1977. Fenbendazole. II. Biological properties and activity. Pesticide Sci 8:550–555 [Google Scholar]
- 7.Franke DDH, Shirwan H. 2006. Prophylactic fenbendazole therapy does not affect the incidence and onset of type 1 diabetes in nonobese diabetic mice. Int Immunol 18:453–458 [DOI] [PubMed] [Google Scholar]
- 8.Gadad BS, Daher JPL, Hutchinson EK, Brayton CF, Dawson TM, Plentnikov MV, Watson J. 2010. Effect of fenbendazole on 3 behavioral tests in male C57BL/6N mice. J Am Assoc Lab Anim Sci 49:821–825 [PMC free article] [PubMed] [Google Scholar]
- 9.Gao P, Dang CV, Watson J. 2008. Unexpected antitumorigenic effect of fenbendazole when combined with supplementary vitamins. J Am Assoc Lab Anim Sci 47:37–40 [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta RP, Larroquette CA, Agrawal KC. 1982. Potential radiosensitizing agents. 5. 2-substituted benzimidazole derivatives. J Med Chem 25:1342–1346 [DOI] [PubMed] [Google Scholar]
- 11.Hall EJ, Giaccia AJ. 2006. Radiobiology for the radiologist, 6th ed. Philadelphia (PA): Lippincott, Williams and Wilkins. [Google Scholar]
- 12.Harkness JE, Wagner JE. 1995. Specific diseases and conditions, p 171–321. In: The biology and medicine of rabbits and rodents, 4th ed. Media (PA): Williams and Wilkins. [Google Scholar]
- 13.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 14.Kallman RF. 1987. Rodent tumors models in experimental cancer therapy. New York (NY): Plenum Press. [Google Scholar]
- 15.Keller WC. Fenbendazole. (WHO Food Additive Series 29). [Cited 30 June 2011]. Available at www.inchem.org/documents/jecfa/jecmono/v29je04.htm.
- 16.Lacey E. 1988. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Parasitol 18:885–936 [DOI] [PubMed] [Google Scholar]
- 17.Landin AM, Frasca D, Zaias J, Van der Put E, Riley RL, Altman NH, Blomberg BB. 2009. Effects of fenbendazole on the murine humoral immune system. J Am Assoc Lab Anim Sci 48:251–257 [PMC free article] [PubMed] [Google Scholar]
- 18.Merino G, Jonker JW, Wagenaar E, Pulido MM, Alvarez AI, Schinkel AH. 2005. Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab Dispos 33:614–618 [DOI] [PubMed] [Google Scholar]
- 19.Overgaard J. 2007. Hypoxic radiosensitization: adored and ignored. J Clin Oncol 25:4066–4074 [DOI] [PubMed] [Google Scholar]
- 20.Percy DH, Barthold SW. 2007. Mice, p 3–95. In: Pathology of laboratory rodents and rabbits, 3rd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 21.Prichard RK, Kelly JD, Bolin TD, Duncombe VM, Fagan MR. 1981. The effect of iron and protein deficiency on plasma levels and parasite uptake of [14C]fenbendazole in rats infected with Nippostrongylus brasiliensis. Aust J Exp Biol Med Sci 59:567–573 [DOI] [PubMed] [Google Scholar]
- 22.Pritchett KR, Johnson NA. 2002. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci 41:36–46 [PubMed] [Google Scholar]
- 23.Ramp AA, Hall C, Orian JM. 2010. Strain-related effects of fenbendazole treatment on murine experimental autoimmune encephalomyelitis. Lab Anim 44:271–273 [DOI] [PubMed] [Google Scholar]
- 24.Rockwell S. 1972. In vivo–in vitro tumor systems: new models for studying the response of tumors to therapy. Lab Anim Sci 27:831–851 [PubMed] [Google Scholar]
- 25.Rockwell S. 1983. Hypoxic cells as targets for cancer chemotherapy, p 157–172. In: Cheng YC, Goz B, Minkoff M. Development of targeted-oriented anticancer drugs. New York (NY): Raven Press. [Google Scholar]
- 26.Rockwell S. 1992. Use of hypoxia-directed drugs in the therapy of solid tumors. Semin Oncol 19:29–40 [PubMed] [Google Scholar]
- 27.Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. 2009. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med 9:442–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwell SC, Kallman RF, Fajardo LF. 1972. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst 49:735–749 [PubMed] [Google Scholar]
- 29.Rockwell S, Liu Y, Higgins SA. 2005. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat 90:233–239 [DOI] [PubMed] [Google Scholar]
- 30.Rockwell S, Liu Y, Seow HA, Ishiguro K, Baumann RP, Penketh PG, Shyam K, Glazer PM, Sartorelli AC. 2011. Preclinical evaluation of laromustine for use in combination with radiation therapy in the treatment of solid tumors. Int J Radiat Biol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockwell S, Rockwell KR. 2008. Mouse models for experimental cancer therapy, p 623–630. In: Conn PM. Source book of models for biomedical research. Totowa (NJ): Humana Press. [Google Scholar]
- 32.Seaton A, Higgins C, Mann J, Baron A, Bailly C, Neidle S, van den Berg H. 2003. Mechanistic and antiproliferative studies of 2 novel, biologically-active bis-benzimidazoles. Eur J Cancer 39:2548–2555 [DOI] [PubMed] [Google Scholar]
- 33.Seow HA, Penketh PG, Shyam K, Rockwell S, Sartorelli AC. 2005. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine: an anticancer agent targeting hypoxic cells. Proc Natl Acad Sci USA 102:9282–9287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soderlind K-J, Gorodetsky B, Singh AK, Bacher NR, Miller GG, Lown JW. 1999. Bis-benzimidazole anticancer agents: targeting human tumor helicases. Anticancer Drug Des 14:19–36 [PubMed] [Google Scholar]
- 35.Spagnuolo PA, Hu J, Hurren R, Wang X, Gronda M, Sukhai MA, Di Meo A, Boss J, Ashall I, Zavareh RB, Fine N, Simpson CD, Sharmeen S, Rottapel R, Schimmer AD. 2010. The antihelmintic flubendazole inhibits microtubule function through a mechanism distinct from vinca alkaloids and displays preclinical activity in leukemia and myeloma. Blood 115:4824–4833 [DOI] [PubMed] [Google Scholar]
- 36.Tannock IF, Hill RP, Bristow RG, Harrington L. 2005. The basic science of oncology, 4th ed. New York (NY): McGraw Hill. [Google Scholar]
- 37.Villar D, Cray C, Zaias J, Altman NH. 2007. Biological effects of fenbendazole in rats and mice: a review. J Am Assoc Lab Anim Sci 46:8–15 [PubMed] [Google Scholar]