Abstract
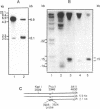
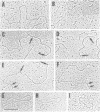
Transposition of Saccharomyces cerevisiae Ty1 retroelements has been shown to involve reverse transcription in intracytoplasmic virus-like particles (Ty-VLPs). Ty DNA present in the particles specified by Ty1-H3 element was found to consist of the full-length genomic DNA as well as incomplete cDNAs mainly of plus polarity. Our results indicate that identical sequences (TGGGTGGTA) are used as primers for the synthesis of plus strand cDNA, generating cDNAs of 0.345 kb (analogous to the retroviral strong-stop plus cDNA) and of 2.1 kb. Electron microscopic analyses of Ty1-VLP DNA revealed two distinct classes, one full-length and the other corresponding to 0.34 kbp molecules, the size of a LTR sequence. The full-length molecules are either completely double-stranded or only partially double- stranded at one end or at both ends. These double-stranded regions are of a length corresponding to those of incomplete plus strands detected by biochemical techniques. Double-stranded circular molecules mainly of a length corresponding to that of two-LTR circles were also detected on electron micrographs. These analyses allowed us to propose a scheme for reverse transcription in Ty particles.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballario P., Filetici P., Junakovic N., Pedone F. Ty1 extrachromosomal circular copies in Saccharomyces cerevisiae. FEBS Lett. 1983 May 8;155(2):225–229. doi: 10.1016/0014-5793(82)80608-x. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Eichinger D., Castrillon D., Fink G. R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988 Apr;8(4):1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Chapman K. B., Byström A. S., Boeke J. D. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3236–3240. doi: 10.1073/pnas.89.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charneau P., Alizon M., Clavel F. A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J Virol. 1992 May;66(5):2814–2820. doi: 10.1128/jvi.66.5.2814-2820.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charneau P., Clavel F. A single-stranded gap in human immunodeficiency virus unintegrated linear DNA defined by a central copy of the polypurine tract. J Virol. 1991 May;65(5):2415–2421. doi: 10.1128/jvi.65.5.2415-2421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibel H., Gafner J., Stotz A., Philippsen P. Characterization of the yeast mobile element Ty1. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):609–617. doi: 10.1101/sqb.1981.045.01.079. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Boeke J. D., Fink G. R. Ty element transposition: reverse transcriptase and virus-like particles. Cell. 1985 Sep;42(2):507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Schulze D. H., Pease L. R., Weiss E. H., Mellor A. L., Flavell R. A., Nathenson S. G. Interaction between Kb and Q4 gene sequences generates the Kbm6 mutation. Mol Cell Biol. 1986 Feb;6(2):645–652. doi: 10.1128/mcb.6.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. J., Chalker D. L., Sandmeyer S. B. Ty3, a yeast retrotransposon associated with tRNA genes, has homology to animal retroviruses. Mol Cell Biol. 1988 Dec;8(12):5245–5256. doi: 10.1128/mcb.8.12.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Hudspeth M. E., Shumard D. S., Tatti K. M., Grossman L. I. Rapid purification of yeast mitochondrial DNA in high yield. Biochim Biophys Acta. 1980 Dec 11;610(2):221–228. doi: 10.1016/0005-2787(80)90003-9. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Fung Y. K., Majors J. E., Bishop J. M., Varmus H. E. Synthesis of plus strands of retroviral DNA in cells infected with avian sarcoma virus and mouse mammary tumor virus. J Virol. 1981 Jan;37(1):127–138. doi: 10.1128/jvi.37.1.127-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Laufer W., Pott U., Ciriacy M. Characterization of products of TY1-mediated reverse transcription in Saccharomyces cerevisiae. Mol Gen Genet. 1991 Apr;226(1-2):145–153. doi: 10.1007/BF00273598. [DOI] [PubMed] [Google Scholar]
- Pochart P., Agoutin B., Fix C., Keith G., Heyman T. A very poorly expressed tRNA(Ser) is highly concentrated together with replication primer initiator tRNA(Met) in the yeast Ty1 virus-like particles. Nucleic Acids Res. 1993 Apr 11;21(7):1517–1521. doi: 10.1093/nar/21.7.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset S., Nocentini S., Revet B., Moustacchi E. Molecular analysis by electron microscopy of the removal of psoralen-photoinduced DNA cross-links in normal and Fanconi's anemia fibroblasts. Cancer Res. 1990 Apr 15;50(8):2443–2448. [PubMed] [Google Scholar]
- Warmington J. R., Waring R. B., Newlon C. S., Indge K. J., Oliver S. G. Nucleotide sequence characterization of Ty 1-17, a class II transposon from yeast. Nucleic Acids Res. 1985 Sep 25;13(18):6679–6693. doi: 10.1093/nar/13.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]