Abstract
In Spring 2008, 175 adult female Xenopus laevis were exposed to construction-related vibrations that caused overt water rippling in the frog tanks. The 3 affected tanks were custom-built static, 300-gal ‘pond-style’ tanks that sat on the floor of the housing room. The water in the tank developed visible ripples as a result of the vibrations transmitted through the floor during jack-hammering in an adjacent room that was approximately 10 ft away. All frogs in the tanks displayed buoyancy problems, excessive air gulping, and skin sloughing; ultimately 7 frogs died. In addition, these 7 animals were bloated, and 5 of these 7 had regurgitated and everted their stomach and distal esophagus into the oral cavity, resulting in airway obstruction and death. Gross pathologic findings included regurgitation and eversion of the stomach of the distal portion of the esophagus into the oral cavity, obstruction of the airway, and lung overinflation. No significant histologic lesions were observed. Construction vibrations transmitted through the water appeared to have disrupted the mechanoreceptive function of the lateral line system, resulting in overstimulation of the noxious feeding response, regurgitation, and eversion of the stomach and distal esophagus into the oral cavity and subsequent suffocation due to airway obstruction. After immediate cessation of the jack-hammering and relocation of the remaining frogs, no additional morbidities or mortalities occurred.
Although the detrimental health effects of occupationally generated noise (hearing loss) and vibration (Hand–Arm Vibration Syndrome, degenerative lumbar spine injuries, CNS, digestive and urogenital disturbances) have been well characterized in people,2,9,16,17,21,22 very little is known regarding potential health implications to animals. To date, published studies on animals have focused on laboratory rodents and have described either the effect that occupation-related noise and vibration might have on these animals in terms of health (reduced reproductive performance, increased disease incidence, cardiovascular system changes, immunosuppression, hormonal effects)10,5,19 or noise perception differences between mice and humans.20
There is limited information on the effects of construction noise on aquatic laboratory animal species in general. However, important extrapolations and inferences can be made from examining the reported affects of construction noise and vibrations on research fish, a species that has hair cells with the same morphology and physiology as those in the lateral line of Xenopus.6 For example, repeated exposure to noise from an air-gun caused visible damage to the sensory epithelia of pink snappers (Pagrus auratus).12 In addition, this noise caused histologically evident disruption of the hair cells.12 Furthermore, displacement stimulation of the mechanoreceptors in the skin generated by sound and vibrations caused significant damage to the hair cells of the short lateral line of clupeidae fishes (marine forage fishes such as herring and sardines).4 This damage caused overstimulation of the cupula (a structure that surrounds the hair cell receptors, which are sensitive to hydrodynamic flow in fish) and to the hair cells themselves.4
To our knowledge, no report to date specifically describes detrimental effects of construction noise and vibrations on the fully aquatic amphibian species Xenopus laevis. Here we present the case history and pathology findings in laboratory Xenopus that were exposed to vibration and noise during facility renovations and construction.
Case History
The animals described in this report are part of a colony of sexually mature, adult female South African clawed frogs (Xenopus laevis) between the ages of 2 and 4 y that were used for egg harvesting by research laboratories as outlined in protocols that were approved by Stanford University's IACUC. In Spring 2008, acute mortalities and morbidities affected 7 of 175 (4%) previously healthy adult female Xenopus laevis after exposure to a short period of intermittent noise and vibration. The emission source was a jack hammer (Bosch Model GSH 32, Stuttgart, Germany) that was being used in an adjacent room approximately 10 ft away and resulted in a noise level of 65 to 72 dB (determined retrospectively) and visible water rippling of the frog tanks. Previous jack-hammering during renovations earlier in the year had occurred approximately 20 ft away from Xenopus housing and resulted in no visible changes in the water or health effects on the same animals. The facility is composed of 12-in. thick concrete slab flooring, and each housing room is separated by 12-in. cinderblock walls. Animals were housed in floor-based, plastic bathtub-style static tanks (4 × 6 × 4 ft) filled with 300 L dechloraminated, potable water maintained at 23 to 25 °C (Figure 1). Frogs were fed commercial chow (Xenopus Brittle, NASCO, Madison, WI) 2 to 3 times weekly. The room was maintained on a12:12-h light:dark cycle.
Figure 1.
Flow-through ‘pond’-style housing system for laboratory Xenopus. Image reproduced from reference 7 with permission.
In tanks where water rippling occurred, frogs displayed combinations of the following clinical signs: bloating, skin sloughing, respiratory distress as evidenced by increases in respiratory frequency and the number of frogs rising to the water's surface to gulp air, buoyancy problems (including floating on their sides at the water surface [Figure 2] and inability to submerge), and unexpected death. The jack-hammering that had been initiated approximately 30 min earlier was stopped immediately and the frogs were moved to a temporary housing location in another room approximately 25 ft away. In this location, no surface rippling was observed when jack hammering resumed, and, although audible, the noise of jack-hammering was reduced approximately to the level of an ordinary conversation. Water samples were obtained from the tanks with affected frogs and were tested for standard water quality parameters including total gas pressure, pH, temperature, adjusted conductivity, ammonia, nitrite, nitrate, copper, dissolved oxygen, monochloramine, total chlorine and free chlorine. All values were within normal limits and were consistent with earlier water testing results.
Figure 2.
Photograph of an affected X. laevis immediately after exposure to construction-generated noise and vibrations. This frog floated on the water surface and was unable to submerge or maintain an upright position (lateral surface floating). Note: the picture does not depict the frog's original housing.
Five dead frogs and 2 live affected frogs were submitted for necropsy. The 2 morbid frogs were unable to submerge and were found floating on their sides (Figure 1); they subsequently were euthanized with tricaine (MS222) according to AVMA guidelines.1 In 3 of the 5 frogs found dead and in both of the euthanized frogs, gross examination revealed external eversion of the stomach and esophagus such that both were extruding through the oral cavity (Figures 2 and 3). The eversion was severe enough to occlude the airway, resulting in secondary overinflation of the lungs (Figure 4). All other organs and tissues were in normal gross limits in all of the found-dead and euthanized frogs. Microscopic examination was performed on all soft tissues and organs of the 2 euthanized frogs, which in addition revealed multifocal necrosis of pulmonary parenchyma of the overinflated lungs and marked congestion of the everted parts of the stomach and esophagus. The totality of these clinical, gross, and microscopic findings resulted in a final diagnosis of esophageal and gastric eversion–regurgitation secondary to construction-related vibrations.
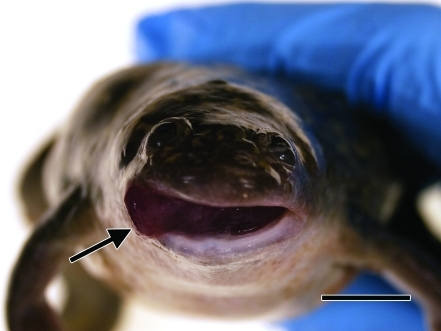
Figure 3.
Photograph of a freshly euthanized X. laevis frog submitted to necropsy immediately after exposure to construction-generated vibrations. Note the external presence of everted esophageal and gastric mucosa through the oral cavity (black arrow). Bar, 1 cm.
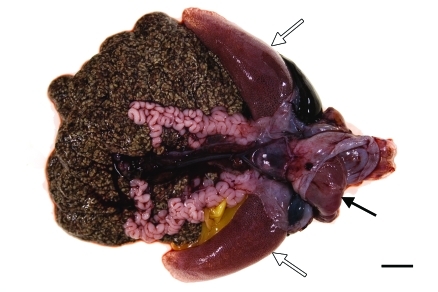
Figure 4.
Gross photograph of an organ pluck from a euthanized X. laevis frog submitted to necropsy immediately after exposure to construction-generated vibrations. Note the presence of everted esophageal and gastric mucosa (black arrow) and overinflated lungs (white arrows). Bar, 1 cm.
Discussion
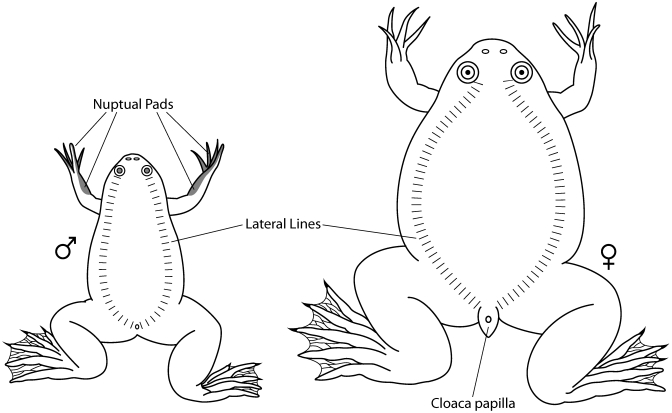
To our knowledge, this report is the first to describe the transmission of construction-related vibrations through water to result in possible activation of the noxious feeding stimulus via the lateral line system of Xenopus and subsequent regurgitation and eversion of the esophagus and stomach into the oral cavity. The lateral line sensory system of Xenopus is comprised of hair cell receptors aggregated into neuromast organs, which are found along the sides of the body and around the head and eyes (Figure 5).18 Xenopus use the lateral line to analyze water waves and obtain information about nearby movement in the water as a means of detecting prey.6 Because they are indiscriminate feeders, Xenopus frogs have evolutionarily developed the unique ability to regurgitate and evert their stomach, expel the contents, and wipe the stomach mucosa free of any accidentally ingested noxious substances. Although studies elucidating the neurophysiology and mechanics of this natural behavior in frogs have not yet been done, chemicals,14,15,23 radiation,13 and mechanical stimulation8 have all been shown to induce this response. In addition, Xenopus frogs have been observed to display this behavior when stressed or stimulated too soon after eating.7
Figure 5.
The hatch marks represent a portion of the lateral line system in Xenopus. The lateral line system is composed of rows of hair cells, a portion of which function as mechanoreceptors that respond to water movement and vibrations. Image reproduced from reference 7 with permission.
The stomach regurgitation reported here possibly was due to excessive water vibrations as a result of nearby jack-hammering, resulting in a powerful and prolonged central activation of the noxious feeding stimulus (via overstimulation of the lateral line system). This strong and protracted stimulation associated with construction vibrations may have inhibited the normal stomach retraction mechanism, created a partial airway obstruction, and prevented expulsion of air from the lungs. We also considered an alternate explanation for the stomach regurgitation—exposure to toxic substances—but this cause seemed unlikely, given that frogs had not been fed during the 48 h prior to the problem, were maintained in a secure location, lived in water that was verified to be of appropriate quality based on laboratory testing, and lacked lesions in liver, kidney, or nervous tissues—targets of many toxins. The underlying pathogenesis of the skin sloughing (for example, excessive exfoliated skin in water) remains uncharacterized, but possibilities include traumatic injuries, an underlying dermatopathy, and partial anorexia, because healthy frogs ingest dead skin.7 A leading cause of acute death and buoyancy disturbances in Xenopus—supersaturation of water with gas—was ruled out quickly given that the water total gas pressure was within normal limits and that no gas bubbles (emboli) were visible under the skin of affected frogs. We also suggest that the proposed overstimulation of the lateral line system from jack-hammering would not necessarily manifest in histologic damage to the lateral line, given the brief exposure time, intensity level of the emitted sound and vibration, and unknown sensitivity of Xenopus to various noise and vibration levels.
There are currently no published threshold recommendations for vibration and noise exposure levels for Xenopus.20 Xenopus may be especially susceptible to adverse health effects resulting from construction-associated vibration due to their unique nervous system anatomy and fully aquatic status. Vibration may have adverse health effects on aquatic species even when noise level is still considered to be safe for humans.19 According to the jackhammer manufacturer's operator's manual,11 this device emits a sound pressure level of approximately 95 dB and has vibration emission value to the hand of 19 m/s2. Hearing protection is required by operators and those in close vicinity during use of this tool. The Occupational Health and Safety Administration currently does not have published standards concerning vibration exposure limits in workers, but the American Conference of Governmental Industrial Hygienists has developed threshold limit values for vibration exposure to handheld tools. According to these recommendations, operators of devices that have a vibration emission value to the hand of 12 m/s2 should limit exposure duration to less than 1 h daily.3,12
Given the absence of such recommendations for laboratory animals housed in vivaria and given consequences described here, aquatic species, particularly Xenopus, should be relocated whenever possible, before noise- and vibration-generating construction begins. The affect of noise and vibration on laboratory animals that cannot easily be relocated (aquatics) may be further reduced by the use of noise-dampening and antivibration materials.
Acknowledgment
We thank Janis Atuk-Jones for her technical writing assistance with the manuscript.
References
- 1.American Veterinary Medical Association. [Internet] 2007. AVMA guidelines on euthanasia, 2007 update. [Cited 07 October 2011]. Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf
- 2.Bovenzi M, Hulshof CT. 1999. An updated review of epidemiologic studies on the relationship between exposure to whole-body vibration and low back pain (1986-1997). Int Arch Occup Environ Health 72:351–365 [DOI] [PubMed] [Google Scholar]
- 3.Canadian Centre for Occupational Health and Safety. [Internet] Vibration—measurement, control standards. [Cited June 2011]. Available at: http://www.ccohs.ca/oshanswers/phys_agents/vibration/vibration_measure.html
- 4.Denton EJ, Gray JAB. 1993. Stimulation of the acoustico-lateralis system of clupeid fish by external sources and their own movements. Philos Trans R Soc Lond B Biol Sci 341:113–127 [Google Scholar]
- 5.Diercks AK, Schwab A, Rittgen W, Kruspel A, Heuss E, Schenkel J. 2010. Environmental influences on the production of preimplantation embryos. Theriogenology 73:1238–1243 [DOI] [PubMed] [Google Scholar]
- 6.Elepfandt A. 1996. Sensory perception and the lateral line system in the clawed frog, Xenopus, p 97–120. In: Tinsley HR, Kobel HR. The biology of Xenopus. New York (NY): Oxford University Press. [Google Scholar]
- 7.Green SL. 2009. The laboratory Xenopus sp. In: Suckow MA. Boca Raton (FL): CRC Press. [Google Scholar]
- 8.Hukuhara T, Naitoh T, Kamevama H. 1973. [A peculiar phenomenon, the prolapsus of the esophagus–stomach, observed in the vomiting of the frog (author's transl)]. Nihon Heikatsukin Gakkai Zasshi. 9: 1–8. [Article in Japanese] [Google Scholar]
- 9.Hulshof C, van Zanten BV. 1987. Whole-body vibration and low-back pain. A review of epidemiologic studies. Int Arch Occup Environ Health 59:205–220 [DOI] [PubMed] [Google Scholar]
- 10.Jensen K, Hahn NE, Palme R, Saxton K, Francis DD. 2010. Vacuum-cleaner noise and acute stress responses in female C57BL/6 mice (Mus musculus). J Am Assoc Lab Anim Sci 49:300–306 [PMC free article] [PubMed] [Google Scholar]
- 11.Master Hire. [Internet] GSH 27 Professional [Cited 21 June 2011]. Available at: http://www.masterhire.net.au/uploads/operatormanuals/EJ%204%20Bosch.pdf. [Google Scholar]
- 12.McCauley RD, Fewtrell J, Popper AN. 2003. High-intensity anthropogenic sound damages fish ears. J Acoust Soc Am 113:638–642 [DOI] [PubMed] [Google Scholar]
- 13.Miyachi Y, Koike T, Murol K, Kanao T, Kawamoto T, Yamada T. 2002. Marked depression of radiation-induced emesis in frogs following prior exposure to a brief dose of X-rays. Can J Physiol Pharmacol 80:828–832 [DOI] [PubMed] [Google Scholar]
- 14.Naitoh T, Wassersug RJ, Leslie RA. 1989. The physiology, morphology, and ontogeny of emetic behavior in anuran amphibians. Physiol Zool 62:819–843 [Google Scholar]
- 15.Nigam HC. 1977. Vomiting response in the Indian frogs Rana tigrina (Daud) and Rana cyanophylctis (Boulenger). Curr Sci 46:791–792 [Google Scholar]
- 16.Pelmear PL, Leong D. 2000. Review of occupational standards and guidelines for hand–arm (segmental) vibration syndrome (HAVS). Appl Occup Environ Hyg 15:291–302 [DOI] [PubMed] [Google Scholar]
- 17.Pelmear PL, Taylor W. 1994. Hand–arm vibration syndrome. J Fam Pract 38:180–185 [PubMed] [Google Scholar]
- 18.Pough FH, Janis CM, Heiser JB. 2009. Living in water, p 84–87. In: Pough FH, Janis CM, Heiser JB. Vertebrate life. San Francisco (CA): Pearson Education / Benjamin Cummings. [Google Scholar]
- 19.Raff H, Bruder ED, Cullinan WE, Ziegler DR, Cohen EP. 2011. Effect of animal facility construction on basal hypothalamic–pituitary–adrenal and renin–aldosterone activity in the rat. Endocrinology 152:1218–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds RP, Kinard WL, Degraff JJ, Leverage N, Norton JN. 2010. Noise in a laboratory animal facility from the human and mouse perspectives. J Am Assoc Lab Anim Sci 49:592–597 [PMC free article] [PubMed] [Google Scholar]
- 21.Rozema R. [Internet]. Acoustic and vibration levels in research animal facilities - what is required? [Cited 21 June 2011]. Available at: http://www.alnmag.com/article/acoustic-and-vibration-levels-research-animal-facilities-what-required.
- 22.Seidel H. 1993. Selected health risks caused by long-term, whole-body vibration. Am J Ind Med 23:589–604 [DOI] [PubMed] [Google Scholar]
- 23.Seidel H, Heide R. 1986. Long-term effects of whole-body vibration: a critical survey of the literature. Int Arch Occup Environ Health 58:1–26 [DOI] [PubMed] [Google Scholar]
- 24.Tokita K, Iwasaki S, Yui F. 1953. On the opening motion of mouth and vomiting of frogs after the digitalis preparations and on their mechanism. Folia Pharmacol Japan 49:138–142 [Google Scholar]