Abstract
Here we show that chronic application of low concentrations (0.01–0.05 U/ml) or a single application of 1–5 U/ml acetylcholinesterase (AChE) promotes the extension of neuronal processes, synapse formation, and α-amino-3-hydroxy-5-methylisoxazolepropionate receptor (AMPAR) surface expression in both embryonic and postnatal hippocampal cultures. The total number of AMPARs was unchanged but the proportion of receptors that were surface-expressed, predominantly at synapses, was approximately doubled following AChE treatment. Blockade of the peripheral anionic site of endogenous AChE in the cultures dramatically reduced neurite outgrowth but did not alter the appearance of synaptic markers SV2a and PSD95. These results indicate that AChE is necessary for normal dendrite and axon formation in hippocampal neurones and suggest that it may also play a role in excitatory synapse development, plasticity, and remodelling.
Introduction
Acetylcholinesterase (AChE, EC 3.1.1.7) is the enzyme that inactivates the neurotransmitter acetylcholine in the synaptic cleft of cholinergic synapses and neuromuscular junctions. In addition to this function, a growing body of evidence demonstrates that this protein has a number of other noncholinergic, synaptic, and extrasynaptic roles during neural development (Soreq and Seidman, 2001). For example, dorsal root ganglion (DRG) neurones show a transient peak of AChE expression during periods of axonal outgrowth prior to synaptogenesis (Bigbee et al., 2000). Furthermore, in the developing brain AChE expression coincides with axon tract formation even in noncholinergic brain regions (Anderson and Key, 1999).
Dendritic outgrowth, synapse formation, and synaptic plasticity in hippocampal neurones are of particular interest because these processes are widely accepted to provide the molecular and anatomical basis of some types of memory. For example, long-term potentiation (LTP) and long-term depression (LTD) are extensively studied models for memory formation and storage (Bear and Abraham, 1996; Bliss and Collingridge, 1993). Furthermore, it is becoming increasingly clear with advances in real-time imaging techniques that neuronal processes and especially the presynaptic terminals and postsynaptic dendritic spines of excitatory synapses are highly dynamic, activity-dependent structures (for reviews see Matus and Shepherd, 2000; Sorra and Harris, 2000).
α-Amino-3-hydroxy-5-methylisoxazolepropionate receptor (AMPARs) mediate most excitatory synaptic transmission in the mammalian CNS. Developmental and activity-dependent regulation of the insertion and removal of AMPARs at the postsynaptic membrane provides a mechanism for altering synaptic efficacy. There has been considerable progress toward identifying intracellular molecules that traffic and anchor AMPARs at the synapse (for reviews see Braithwaite et al., 2000; Scannevin and Huganir, 2000; Sheng, 2001). Much less is known, however, about extra-cellular factors that facilitate the formation of excitatory central synapses and initiate recruitment of functional receptors.
It has been proposed that the dendritic spines of glutamatergic synapses are initially formed in response to NMDAR activation and that synapses are subsequently stabilised by the expression of functional AMPARs (Fischer et al., 2000). Interestingly, Narp, a secreted immediate-early gene product regulated by synaptic activity in brain, increases the number of excitatory but not inhibitory synapses in cultured spinal neurones. Narp forms large surface clusters that coaggregate AMPAR subunits (O’Brien et al., 1999). We have demonstrated previously that AChE potently increases the number of AMPARs in rat brain homogenates (Olivera et al., 1999), that the effect is direct and not dependent on membrane lipids, and that this action is developmentally and regionally regulated (Olivera et al., 2001). Since AChE has previously been reported to promote hippocampal LTP (Appleyard, 1995), here we extend these previous studies to report the actions of AChE on neuronal growth, synapse formation, and AMPAR recruitment to synapses in cultured hippocampal neurones.
Results and discussion
AChE causes neurite extension in embryonic hippocampal neurones in culture
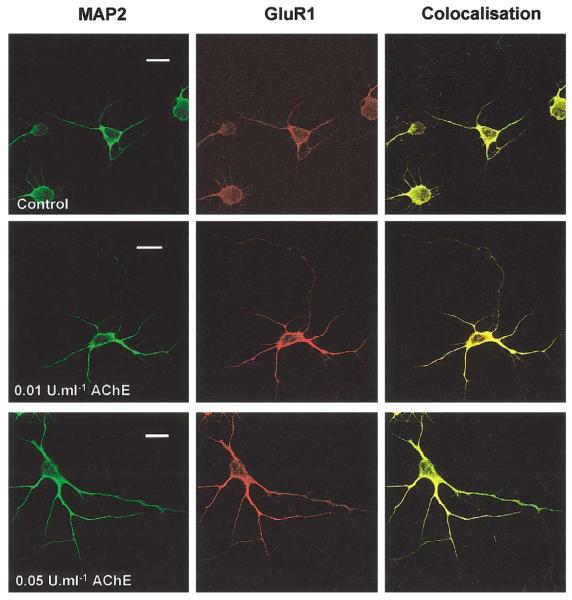
We investigated a range of AChE concentrations on both embryonic and postnatal hippocampal neurones at different ages in culture and using different application protocols. Exposure of 1 day in vitro (DIV) cultured neurones to 0.01–1 U/ml AChE once a day for 5 days evoked a dramatic increase in the number and length of dendrites visualized with the dendritic marker MAP2 and the AMPAR subunit GluR1 (Fig. 1). We quantified the changes induced by AChE treatment in dendritic outgrowth and structural organisation using a modified Sholl analysis (Sholl, 1953). In the original protocol concentric circles of increasing diameter are superimposed on camera lucida images of neurones and the number of intersections between dendrites and circles is counted. Here we analysed digitised transmission images of neurones directly from the confocal images. Concentric circles were drawn around the soma of control and AChE-treated cells at 20, 40, 60, 80, and 100 μm. In both groups, the number of intersections was higher in the inner circles and dropped markedly in the outer ones. In the outermost circle (100 μm), dendritic intersections were only seen in AChE-treated neurones. Dendritic intersections of treated cells were significantly increased (P < 0.001) compared to that in control cells at 20, 40, and 80 μm circles. No intersections were found in control cells after 80 μm. The effects of chronic application of AChE or BW284c51 to inhibit endogenous AChE in cultured hippocampal neurones are shown in Table 1.
Fig. 1.
Chronic exposure to low levels of AChE promotes dendrite extension and arborisation. Embryonic cells were treated every day for 5 days from 1 DIV with 0.01 or 0.05 U/ml AChE and then fixed and immunostained with antibodies against MAP2 and GluR1. Calibration bar, 20 μm.
Table 1.
Sholl analysis of the chronic effects of AChE or BW284c51 treatment on neurite outgrowth in young neurones
| Treatment | Dendrites per cell |
||
|---|---|---|---|
| 1st order (D1) | 2nd order (D2) | 3rd (D3) | |
| Control | 3.0 ± 1 | 0 | 0 |
| AChE 0.05 U/ml | 5.0 ± 1.46 | 3.5 ± 2.1 | 0.5 ± 0.7 |
| BW284c51 5 × 10−6 M | 2.5 ± 0.7 | 0 | 0 |
| BW284c51 10−5 M | 1 ± 0.7 | 0 | 0 |
Note. AChE 0.05 U/ml or BW284c51 (5 × 10−6 and 10−5 M) was added to neurones (starting at 5 DIV) every day for 5 days. The cells were then fixed and permeabilised and immunostained for MAP2 and GluR1. Results were obtained from three separate experiments in each of which 25 neurones were analysed. The data are the mean ± SD.
These results are consistent with previous studies using different types of neurones and experimental paradigms, in that they show that AChE evokes increases in neurite length (Jones et al., 1995; Layer et al., 1993; Small et al., 1995). This is the first report, however, to use rat hippocampal neurones, which are a widely studied model for synaptic plasticity, and to demonstrate an increase in both the number and the length of dendrites as well as in the absolute number of terminal dendritic branches caused by AChE. This latter action is of interest because it suggests that AChE can accelerate the differentiation of neurones and that it could play a role in priming the postsynaptic area to receive afferent connections and promote synaptogenesis. Furthermore, we show that dendritic outgrowth is matched by increased AMPARs throughout the processes.
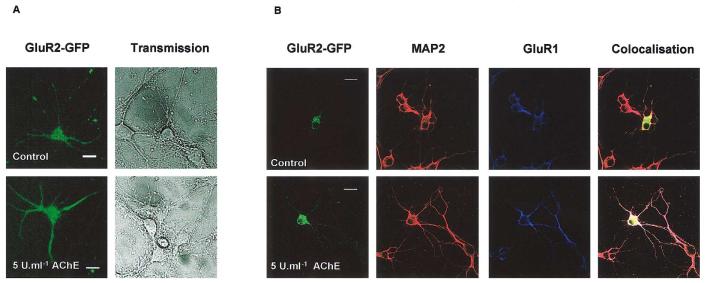
Because AChE increased GluR1 concomitantly with dendrite extension (Fig. 1), we investigated the effects on two different AMPAR subunits. First we infected the neurones with an adenovirus expressing GFP-GluR2 and investigated the effect of a single application of 1–5 U/ml AChE by imaging GFP-GluR2 fluorescence in living cells (Fig. 2A). The use of GFP-tagged GluR2 allows rapid and non-invasive visualisation of receptor distribution in neuronal processes and it is particularly suited for use in very young cells. It does not require additional secondary antibodies and simplifies triple-labelling experiments. In cells infected at, or after, 5 DIV and stained 2–4 days later, there was near complete colocalisation of GluR1, GluR2, and MAP2 in infected cells (Fig. 2B). As expected, AChE treatment increased the length of primary and secondary dendrites compared to control nontreated neurones (2.26 ± 0.7 and 2.21 ± 0.2, respectively, ratio of AChE treated versus control; n = 35) and there was strong expression of GluR2 and GluR1 throughout the dendrites. Interestingly, however, in infected cells younger than 5 DIV the distribution of GFP-GluR2 did not mirror that of MAP2 in the AChE-treated cells (data not shown). We attribute this age-dependent difference to the fact that endogenous GluR2 is poorly expressed in very young neurones and that GluR2-containing AMPARs are not fully functionally expressed until around 10–14 DIV (Cotton and Henley, unpublished observations). Thus, neurones may lack the correct trafficking/targeting proteins to allow the transport of the virally expressed GluR2 to distal dendrites.
Fig. 2.
A single addition of AChE evokes dendritic growth. The effects of short-term, high-concentration AChE exposure on living neurones using virally expressed GluR2-GFP. Neurones were infected at 5 DIV and treated with a single concentration of 5 U/ml AChE at the same time. After 3 days, cells were imaged alive (A) or fixed and immunostained with antibodies to GluR2, MAP2, and GluR1 (B). AChE treatment increased the length of primary and secondary dendrites (Ratios for dendrite length of treated/control cells were 2.26 ± 0.7 and 2.21 ± 0.2, respectively, n = 35). There was strong expression of GluR2 and GluR1 in dendrites. Calibration marker, 20 μm.
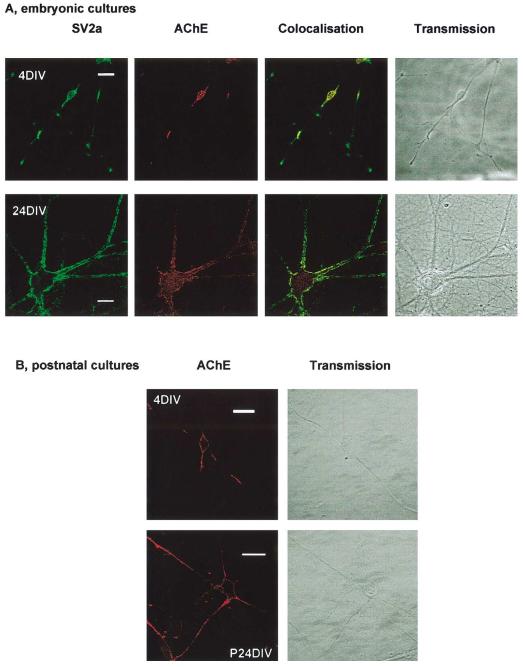
Hippocampal neurones express cytosolic and membrane-associated AChE
To test if cultured hippocampal neurones express endogenous AChE embryonic cells were immunostained using an anti-AChE antibody. As shown in Fig. 3A, from early time points (4 DIV) the cells expressed both cytosolic and surface AChE. Furthermore, in older 24 DIV neurones there was marked colocalisation of AChE and the presynaptic marker SV2a. Strong surface expression of AChE was also observed in 5 DIV nonpermeabilised postnatal neurones (Fig. 3B).
Fig. 3.
Expression and localisation of AChE in hippocampal cultures. Endogenous expression of AChE was determined in fixed, permeabilised embryonic neurones with anti-AChE antibody and compared to the synaptic marker SV2a. In very young cells (4DIV), there was a high AChE expression in the soma and neuronal processes. In older neurones, there was extensive colocalisation, suggesting that AChE remained in synapses even after synapse formation. (B) Surface staining of AChE in postnatal cells.
Inhibition of the peripheral anionic binding site of endogenous AChE potently inhibits and reverses dendrite outgrowth
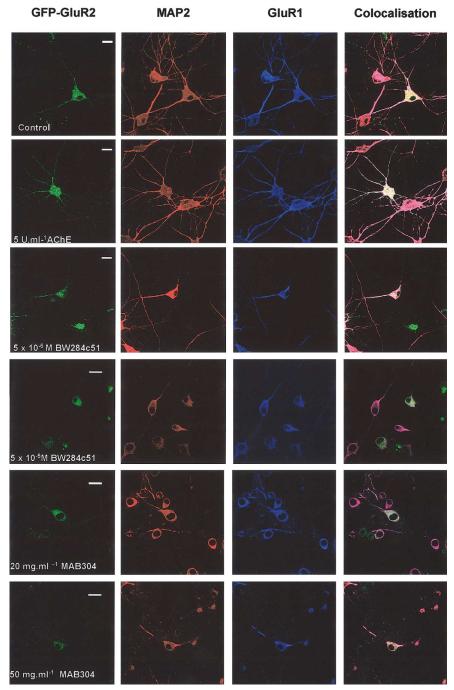
Previous reports have used retinal explants (Layer et al., 1993) or primary DRG cultures (Sharma and Bigbee, 1998) to study the actions of AChE. To determine if endogenous AChE is a factor in neurite development in cultured hippocampal neurones we investigated the effects of two well-characterised inhibitors of AChE action, namely BW284c51 and MAB304. BW284c51 binds primarily to the peripheral anionic site of AChE (Taylor and Radic, 1994). Embryonic cultured hippocampal neurones (5 DIV) were treated with a single application of 10−7, 10−6, 5 × 10−6, 10−5, or 10−4 M BW284c51. Two days later the cells were fixed, imaged for GFP-GluR2, and stained with anti-MAP2 and anti-GluR1 antibodies. At the lowest BW284c51 concentration used dendrite outgrowth was markedly retarded and at the higher concentrations it was completely inhibited and reversed with significant levels of cell death (Table 1 and Fig. 4).
Fig. 4.
Effects of BW284c51 and MAB304 in infected neurones. Embryonic cells (DIV) were infected with GFP-GluR2 and a single application of either 5 U/ml AChE, 10−7–10−5 M BW284c51, or 20–100 μg/ml MAB304. After 2 days, cells were fixed and immunostained. BW284c51 and MAB304 evoked a concentration-dependent reduction in the number and length of dendrites. High concentrations of either BW284c51 or MAB304 caused significant cell death. Calibration bar, 20 μm
MAB304 is an AChE-scavenging monoclonal antibody raised against human erythrocyte AChE. It recognises the peripheral anionic site and does not inhibit the catalytic activity of AChE. Consistent with previous reports in other systems (Bigbee et al., 2000; Sharma and Bigbee, 1998), incubation of embryonic hippocampal neurones with 20 or 50 μg/ml MAB304 for 2 days effectively prevented neurite extension and at higher concentrations caused cell death (Fig. 4). We also tested the selective inhibitors of the catalytic site of AChE ethopropazine (10−5–10−3 M) and eserine (10−4–5×10−3 M; see Taylor and Radic, 1994). Neither compound caused any significant changes in cell morphology at these concentrations (data not shown). Thus, our experimental evidence indicates that only the peripheral site of AChE is important for the regulation of neurite outgrowth. Furthermore, results from the pharmacological inhibition of endogenous AChE indicate that AChE is required for normal development of hippocampal neurones in culture.
AChE enhances synapse formation and AMPAR surface expression
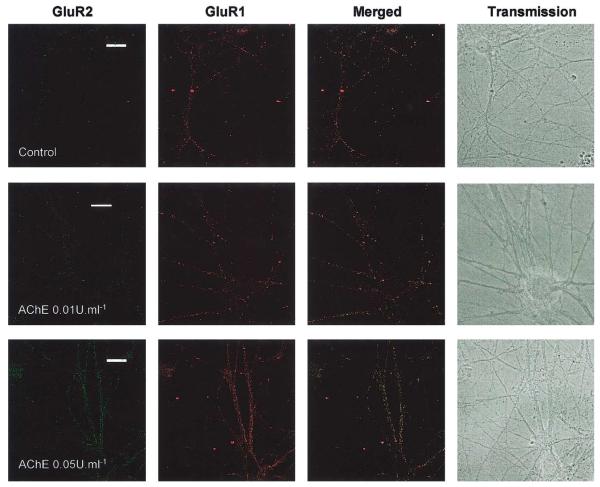
We next investigated the action of AChE on AMPAR surface expression at synapses. For these experiments we used postnatal cultures because AMPAR surface expression has been particularly well-characterised in these cells (Noel et al., 1999; Pickard et al., 2000). Chronic application of AChE (0.05 U/ml) evoked a significant increase in the surface expression of AMPAR subunits (Fig. 5). Quantification of the data from these experiments, expressed as GluR1, GluR2, or NR1 surface puncta per 100 μm of dendrite, is shown in Table 2. There were significant AChE-evoked increases in the number of surface-expressed GluR1 or GluR2 puncta but no change in NR1 surface expression. Since NR1 is present in all NMDARs these data suggest that AChE does not regulate NMDAR surface expression. In parallel experiments we permeabilised cells from the same cultures to show that AChE treatment did not alter the total expression (surface plus intradendritic puncta) of AMPARs (Table 2).
Fig. 5.
AChE enhances surface expression of AMPARs. Postnatal neurones from 5 DIV were treated with 0.01 U/ml AChE or 10−5 M BW284c51 for 5 days. Surface-expressed receptors were visualised using N-terminal-directed antibodies. Calibration bar, 20 μm.
Table 2.
Chronic AChE affects AMPAR surface expression but not total numbers of AMPARs
| GluR2 |
GluR1 |
NR1 surface | |||
|---|---|---|---|---|---|
| Surface | Total | Surface | Total | ||
| Control | 27 ± 6 | 65 ± 12 | 36 ± 6 | 76 ± 11 | 46 ± 8 |
| 0.01 U/ml AChE | 42 ± 8 | 63 ± 9 | 52 ± 8 | 83 ± 10 | 51 ± 12 |
| 0.05 U/ml AChE | 60 ± 4 | 72 ± 9* | 62 ± 4 | 80 ± 9 | 47 ± 6 |
Note. Hippocampal neurones were daily treated with 0.01 and 0.05 U/ml AChE from 3 to 8 DIV and then fixed, permeabilised, and double-stained for either GluR2 and GluR1 or GluR2 and NR1. The results are expressed as the number of immunoreactive puncta per 100 μm of dendrite length in control and treated cells. AChE evoked a significant increase in the surface expression of AMPAR subunits but did not affect the number of surface-expressed NR1 clusters. Cells from the same batch of cultures that were fixed and permeabilised prior to incubation with antibodies showed that AChE did not affect the total expression of GluR2 or GluR1. The data are the mean ± SD.
AChE promotes the appearance of synaptic markers
We also compared the number of clusters of GluR2 and the presynaptic marker protein SV2a and measured the degree of colocalisation following 0.01 U/ml or 0.05 U/ml AChE treatment for 5 days. Levels of 0.01 U/ml or 0.05 U/ml AChE caused a significant increase in the number of SV2a but 0.05 U/ml AChE was needed to bring about a significant increase in GluR2 expression (Table 3). Consistent with the increase in AMPAR surface expression, there was a dramatic increase in the number of puncta of both SV2a and the postsynaptic marker protein PSD95 in cells treated with 0.01 U/ml AChE (Table 4). Interestingly, however, and consistent with the differential dose-response data for presynaptic SV2a and postsynaptic GluR2 shown in Table 3, AChE treatment for 5 days evoked a significant increase in the number of SV2a puncta but a significant increase in PSD95 puncta did not occur until after 10 days of AChE treatment. Inhibition of the peripheral anionic site on endogenous AChE by BW284c51 in growing neurones did not decrease the number of SV2a or PSD95 puncta, suggesting that synaptogenic activity of AChE is likely mediated by its catalytic domain (Table 5). Taken together these data suggest that AChE has effects on both pre- and postsynaptic membranes to promote synapse formation but that presynaptic processes are more sensitive to, and occur prior to, AChE-evoked changes in the nascent postsynaptic membrane and recruitment of functional postsynaptic AM-PARs. This modulation by AChE of AMPAR localisation and of postsynaptic membranes may represent a molecular mechanism able to transduce localised neural activity into durable modifications of synaptic and perisynaptic molecular structure.
Table 3.
Chronic AChE increases the synaptic expression of GluR2
| 1 DIV |
3 DIV |
5 DIV |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | AChE 0.01 U/ml |
AChE 0.05 U/ml |
Control | AChE 0.01 U/ml |
AChE 0.05 U/ml |
Control | AChE 0.01 U/ml |
AChE 0.05 U/ml |
|
| SV2a | 23 ± 1 | 42 ± 2 | 38 ± 1 | 23 ± 9 | 33 ± 1 | 38 ± 4 | 40 ± 3 | 55 ± 8 | 66 ± 11 |
| GluR2 | 11 ± 2 | 14 ± 3 | 28 ± 1 | 20 ± 8 | 21 ± 1 | 31 ± 9 | 28 ± 12 | 36 ± 7 | 47 ± 13 |
| Colocalisation | 5 ± 0 | 10 ± 1 | 17 ± 2 | 17 ± 6 | 18 ± 1 | 23 ± 6 | 17 ± 1 | 25 ± 4 | 40 ± 9 |
Note. Postnatal hippocampal neurones from 1, 3, and 5 DIV were treated with 0.01 and 0.05 U/ml AChE every day for 5 days. The number of clusters of the presynaptic marker SV2a, for GluR2 and the extent of colocalisation (puncta immunostained with both antibodies), are shown per 100 μm of dendrite.
Table 4.
AChE-evoked synaptogenesis in cultured hippocampal neurones
| Age of cells at start of AChE treatment | 1 DIV |
3 DIV |
5 DIV |
7 DIV |
||||
|---|---|---|---|---|---|---|---|---|
| Treatment duration (days) | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 |
| SV2a | ||||||||
| Control | 18 ± 5 | 28 ± 7 | 21 ± 4 | 31 ± 3 | 35 ± 5 | 29 ± 7 | 36 ± 6 | 42 ± 6 |
| AChE | 27 ± 5 | 42 ± 6 | 29 ± 6 | 48 ± 7 | 50 ± 6 | 44 ± 8 | 46 ± 5 | 49 ± 6 |
| AChE: control ratio | 1.5 ± 0.2 | 1.5 ± 0.3 | 1.4 ± 0.2 | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.5 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.9 |
| PSD95 | ||||||||
| Control | 6 ± 2 | 12 ± 4 | 18 ± 4 | 13 ± 4 | 26 ± 7 | 31 ± 3 | 34 ± 6 | 26 ± 5 |
| AChE | 5 ± 3 | 29 ± 3 | 28 ± 4 | 29 ± 6 | 37 ± 5 | 45 ± 7 | 48 ± 5 | 39 ± 3 |
| AChE: control ratio | ND* | 2.4 ± 0.9 | 1.6 ± 0.2 | 2.2 ± 0.2 | 1.4 ± 0.3 | 1.5 ± 0.4 | 1.4 ± 0.2 | 1.5 ± 0.4 |
Note. Postnatal hippocampal neurones at different ages in vitro (DIV) were treated with 0.01 U/ml AChE every day for 5 or 10 days. The number of puncta of the pre- and postsynaptic markers SV2a and PSD95 were counted in AChE-treated and control cells in five separate experiments. The data are expressed as immunoreactive puncta per 100 μm dendtrite (mean ± SD) and also as the ratio of puncta (treated/control). P < 0.05 for all comparisons.
ND, not determined because staining of PSD95 was predominantly diffuse.
Table 5.
BW284C5 does not inhibit the appearance of synaptic markers in developing neurones
| Duration of treatment | 3 Days |
7 Days |
10 Days |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Marker | SV2a | PSD95 | Both | SV2a | PSD95 | Both | SV2a | PSD95 | Both |
| Control | 30 ± 5 | 19 ± 6 | 9 ± 3 | 35 ± 8 | 24 ± 4 | 10 ± 6 | 29 ± 7 | 15 ± 4 | 13 ± 2 |
| BW284C5 | 22 ± 4 | 19 ± 6 | 9 ± 1 | 33 ± 7 | 26 ± 4 | 10 ± 2 | 23 ± 3 | 12 ± 3 | 8 ± 2 |
| BW:control ratio | 0.7 ± 0.5 | 1.0 ± 0.6 | 1.0 ± 0.6 | 0.9 ± 0.5 | 1.1 ± 0.3 | 1.0 ± 0.8 | 0.8 ± 0.5 | 0.8 ± 0.6 | 0.6 ± 0.6 |
Note. Postnatal hippocampal neurones (5 DIV) were treated with BW284C5 (10−6 M) every day for 3, 7, or 10 days. The number of puncta of the pre- and postsynaptic markers SV2a and PSD95, respectively, were counted in BW284C5-treated and control cells in five separate experiments. The data are expressed as the number of immunoreactive puncta for SV2a alone, PSD95 alone, and puncta showing colocalised SV2a and PSD95 per 100 μm dendrite (mean ± SD). The ratio of puncta (treated/control) is also shown. BW284C5 had no significant effect on either synaptic marker.
A number of previous reports on the properties of AChE have used neurones from lower vertebrates such as Xenopus and chick as model systems. Based on these and other studies AChE has been reported to have three main functions in the CNS, namely: (1) the hydrolysis of acetylcholine at cholinergic synapses; (2) a synaptogenic property; and (3) the promotion of neurite extension. The latter two functions go some way towards explaining the fact that AChE is commonly expressed in developing neurones irrespective of the neurotransmitters they finally utilise (Layer and Willbold, 1995). The hydrolytic capacity of AChE is essential for the breakdown of acetylcholine and for the promotion of synaptogenesis. However, the neurite growth activity depends on the E6-encoded C-terminal domain of the protein and not on its catalytic activity (Sternfeld et al., 1998). This enzyme activity-independent action of AChE is most likely due to its sequence similarity to cell adhesion molecules such as laminin (Small et al., 1995) and neuroligin (Ichtchenko et al., 1995).
Neuroligins are a protein family of well-characterised cell surface adhesion molecules that are concentrated at synapses (Ichtchenko et al., 1995, 1996; Koenigsberger et al., 1997; Sharma et al., 2001). It has been reported that AChE and neuroligins have overlapping actions on neurite outgrowth in NGF-differentiated PC12 cells, leading to the suggestion that interactions of AChE-like proteins and neurexins are potential mediators of cytoarchitectural changes supporting neuritogenesis (Grifman et al., 1998). Indeed, AChE contains an HNK-like carbohydrate epitope that is considered a marker of adhesion proteins (Krejci et al., 1991) and AChE can attach to the cell membrane in a way that resembles the attachment mechanism of some cell adhesion molecules (Massoulie et al., 1993).
The current view is that the neuritogenic, cell adhesion-like properties of AChE seem not to require any catalytic activity (for a review see Broide et al., 1999). The synaptogenic properties of AChE, however, require catalytic activity of the enzyme, but not membrane association (Sternfeld et al., 1998). Thus, synaptogenic properties of AChE appear distinct from its neuritogenic effects. Our results are in good agreement with this model. Consistent with these previous observations in other cell types, our data using AChE site-selective inhibitors in cultured hippocampal neurones show that the neurite extension actions of AChE are mediated by the peripheral anionic site.
Furthermore, since inhibition of the anionic site does not prevent the appearance of synaptic markers, our data are also consistent with the synaptogenic properties of AChE being mediated by the catalytic site.
In conclusion, here we have demonstrated that AChE has profound effects on the morphology of cultured hippocampal neurones, causing pronounced increases in dendritic outgrowth and branching. AChE treatment promotes an increase in immunoreactivity for synaptic markers and increases surface expression of AMPARs. We show that the cultured neurones express endogenous AChE and that inhibitors of AChE severely retard dendritic growth and increase cell death. Overall, our data indicate that AChE may play a central role in the functional development and plasticity of hippocampal excitatory synapses.
Experimental methods
Hippocampal cell culture
Embryonic cultures were prepared from E18 rat pups and postnatal cultures were from P2–4 rats as previously described (Noel et al., 1999; Ralph et al., 2001).
Infection and AChE treatment of embryonic hippocampal cells
GluR2-GFP adenovirus (4 μl) was added to cultures 5–12 DIV. Infected cells were used 2–4 days later. In some experiments cells were treated with 1–10 U/ml AChE, 10−7–10−4 M 1,5bis-(4-allyldimethylammoniumphenyl)pentan-3-one-bromide (BW284c51, Sigma) or 10–100 μg/ml of AChE-monoclonal antibody MAB304 (Chemicom). Each of the inhibitors was used at concentration ranges calculated to fully inhibit AChE based on their published IC50 values. Compounds were diluted directly in growth medium (50 μl) and added to the medium in the culture dish (2 ml).
AChE, BW284c51, or MAB304 remained with cultures until the day of immunostaining, when all media was replaced by Hepes buffered saline solution (HBS; 25 mM Hepes, 30 mM glucose, 119 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2mM MgCl2, 0.5 μM TTX at a final pH of 7.4). AChE was from electric eel (Type V, Sigma C-2888).
Immunocytochemistry
Neurones were washed and then fixed and permeabilised with −20°C methanol for 15–30 s. After 30 min of rehydration cells were incubated overnight with the following antibodies at 4°C in HBS:5% BSA: (i) mouse monoclonal anti-MAP2 (10 μg/ml; Sigma); (ii) rabbit polyclonal anti-GluR1 (10 μg/ml; Oncogene); (iii) mouse monoclonal anti-PSD95 (50 μg/ml; Upstate Biotechnology). After three 10-min washes with ice-cold HBS, cells were incubated with appropriate secondary antibodies, washed, and imaged.
For surface staining, living hippocampal neurones were washed three times at 37°C with HBS and then incubated for 2 h with one or two of the following extracellular epitope-directed antibodies: (i) mouse monoclonal anti-GluR2 (100 μg/ml; Chemicom); (ii) rabbit polyclonal anti-GluR1 (5 μg/ml; Oncogene); or (iii) rabbit polyclonal anti-NR1 (50 μg/ml; Pharmingen). All incubations were performed at room temperature; under these conditions, there is minimal receptor internalisation in our cells (Noel et al., 1999; Pickard et al., 2000). The cells were then fixed and permeabilised with methanol (−20°C, for 1–2 min) and then allowed to rehydrate in HBS at 4°C.
To measure the total number of receptors (surface-expressed plus intracellular receptors) cells were fixed and permeabilised with methanol (−20°C, for 1–2 min), rehydrated, and then incubated with the relevant primary anti-body. Exactly the same protocol was used to visualise intracellular synaptic marker proteins using mouse monoclonal anti-PSD95 (50 μg/ml) and/or rabbit polyclonal anti-SV2a (10 μg/ml; StressGen Biotechnologies). In all cases, the concentrations of primary and secondary antibodies were optimised for our conditions.
Imaging
Images were captured using a Leica TCS-NT confocal laser-scanning microscope. Excitation was at 488 and 568 nm for dual imaging and 488, 568, and 647 nm for triplelabel imaging with FITC/TRITC or FITC/TRITC/Cys5 emission filters. There was no bleed-through between green–red and red–Cys5 channels. For quantitative analysis, confocal images were processed in Adobe Photoshop. Puncta were designated as discrete regions (1–3 μm) where the fluorescence intensity was at least double the intensity of the background. Colocalisation was defined when at least 60% of the pixels of each puncta were superimposed. Background was less than 10% of the intensity of a positive immunostaining.
Measurement of neurite number and length
The number of dendrites was determined from MAP2-positive staining and from a modification of the Sholl method in transmission images. To determine neurite length, line drawings of isolated neurones and their processes were traced from the video monitor display of recorded images. The tip of the growing neurite was defined as the distal leading edge of the phase-dark palm of the growth cone (Sternfeld et al., 1998). The total trajectory of the neurite, including all of its branches, was measured using Scion Image. Software was calibrated using the relationship between pixels and micrometers determined during the acquisition of images, usually 320 pixels/100 μm when zoom was 1.
Statistics
Each experiment was performed at least three times and independent group t tests were used for determining the statistical values.
Acknowledgments
We thank PEDECIBA Biologia, Uruguay, for continued financial support to S.O. and D.R.I. This work was funded by the Wellcome Trust and the MRC. We are grateful to Scott Ralph and Sarah De La Rue for providing the GFP-GluR2 adenovirus and Lisa Pickard and Josh Duckworth for assistance with the immunocytochemistry and imaging.
References
- Anderson RB, Key B. Role of acetylcholinesterase in the development of axon tracts within the embryonic vertebrate brain. Int. J. Dev. Neurosci. 1999;17:787–793. doi: 10.1016/s0736-5748(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Appleyard ME. Acetylcholinesterase induces long-term potentiation in CA1 pyramidal cells by a mechanism dependent on metabotropic glutamate receptors. Neurosci. Lett. 1995;190:25–28. doi: 10.1016/0304-3940(95)11491-e. [DOI] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in the hippocampus. Ann. Rev. Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Bigbee JW, Sharma KV, Chan EL, Bogler O. Evidence for the direct role of acetylcholinesterase in neurite outgrowth in primary dorsal root ganglion neurons. Brain Res. 2000;861:354–362. doi: 10.1016/s0006-8993(00)02046-1. [DOI] [PubMed] [Google Scholar]
- Bliss T, Collingridge G. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Meyer G, Henley JM. Interactions between AMPA receptors and intracellular proteins. Neuropharmacology. 2000;39:919–930. doi: 10.1016/s0028-3908(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Broide RS, Grifman M, Loewenstein A, Grisaru D, Timberg R, Stone J, Shani M, Patrick JW, Soreq H. Manipulations of AChE gene expression suggest non-catalytic involvement of acetylcholinesterase in the functioning of mammalian photoreceptors but not in retinal degeneration. Brain Res. Mol. Brain Res. 1999;71:137–148. doi: 10.1016/s0169-328x(99)00169-2. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nature Neurosci. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- Grifman M, Galyam N, Seidman S, Soreq H. Functional redundancy of acetylcholinesterase and neuroligin in mammalian neuritogenesis. Proc. Natl. Acad. Sci. USA. 1998;95:13935–13940. doi: 10.1073/pnas.95.23.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Südhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J. Biol. Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Jones SA, Holmes C, Budd TC, Greenfield SA. The effect of acetylcholinesterase on outgrowth of dopaminergic neurons in organotypic slice culture of rat mid-brain. Cell Tissue Res. 1995;279:323–330. doi: 10.1007/BF00318488. [DOI] [PubMed] [Google Scholar]
- Koenigsberger C, Chiappa S, Brimijoin S. Neurite differentiation is modulated in neuroblastoma cells engineered for acetylcholinesterase expression. J. Neurochem. 1997;69:1389–1397. doi: 10.1046/j.1471-4159.1997.69041389.x. [DOI] [PubMed] [Google Scholar]
- Krejci E, Duval N, Chatonnet A, Vincens P, Massoulie J. Cholinesterase-like domains in enzymes and structural proteins: functional and evolutionary relationships and identification of a catalytically essential aspartic acid. Proc. Natl. Acad. Sci. USA. 1991;88:6647–6651. doi: 10.1073/pnas.88.15.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer PG, Weikert T, Alber R. Cholinesterases regulate neurite growth of chick nerve cells in vitro by means of a non-enzymatic mechanism. Cell Tissue Res. 1993;273:219–226. doi: 10.1007/BF00312823. [DOI] [PubMed] [Google Scholar]
- Layer PG, Willbold E. Novel functions of cholinesterases in development, physiology and disease. Prog. Histochem. Cytochem. 1995;29:1–93. doi: 10.1016/s0079-6336(11)80046-x. [DOI] [PubMed] [Google Scholar]
- Massoulie J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993;41:31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- Matus A, Shepherd GM. The millennium of the dendrite? Neuron. 2000;27:431–434. doi: 10.1016/s0896-6273(00)00054-4. [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Olivera S, Rodriguez-Ithurralde D, Henley JM. Acetylcholinesterase potentiates [3H]fluorowillardiine and [3H]AMPA binding to rat cortical membranes. Neuropharmacology. 1999;38:505–512. doi: 10.1016/s0028-3908(98)00214-7. [DOI] [PubMed] [Google Scholar]
- Olivera S, Rodriguez-Ithurralde D, Henley JM. Regional localisation and developmental profile of acetylcholinesterase-evoked increases in [3H] 5-fluorowillardiine binding to AMPA receptors in rat brain. Brit. J. Pharmacol. 2001;133:1055–1062. doi: 10.1038/sj.bjp.0704167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard L, Noel J, Henley JM, Collingridge GL, Molnar E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J. Neurosci. 2000;20:7922–7931. doi: 10.1523/JNEUROSCI.20-21-07922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph GS, Bienemann A, Ma J, Tan HK, Noel J, Henley JM, Uney JB. Disruption of the glur2-nsf interaction protects primary hippocampal neurons from ischemic stress. Mol. Cell Neurosci. 2001;17:662–670. doi: 10.1006/mcne.2000.0959. [DOI] [PubMed] [Google Scholar]
- Scannevin RH, Huganir RL. Postsynaptic organization and regulation of excitatory synapses. Nature Rev. Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- Sharma KV, Bigbee JW. Acetylcholinesterase antibody treatment results in neurite detachment and reduced outgrowth from cultured neurons: further evidence for a cell adhesive role for neuronal acetylcholinesterase. J. Neurosci. Res. 1998;53:454–464. doi: 10.1002/(SICI)1097-4547(19980815)53:4<454::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Sharma KV, Koenigsberger C, Brimijoin S, Bigbee JW. Direct evidence for an adhesive function in the noncholinergic role of acetylcholinesterase in neurite outgrowth. J. Neurosci. Res. 2001;63:165–175. doi: 10.1002/1097-4547(20010115)63:2<165::AID-JNR1008>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sheng M. Molecular organization of the postsynaptic specialization. Proc. Natl. Acad. Sci. USA. 2001;98:7058–7061. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurones of the visual and motor cortices of the cat. J. Anat. 1953;87:387–407. [PMC free article] [PubMed] [Google Scholar]
- Small DH, Reed G, Whitefield B, Nurcombe V. Cholinergic regulation of neurite outgrowth from isolated chick sympathetic neurons in culture. J. Neurosci. 1995;15:144–151. doi: 10.1523/JNEUROSCI.15-01-00144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H, Seidman S. Acetylcholinesterase—new roles for an old actor. Nature Rev. Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sternfeld M, Ming G, Song H, Sela K, Timberg R, Poo M, Soreq H. Acetylcholinesterase enhances neurite growth and synapse development through alternative contributions of its hydrolytic capacity, core protein, and variable C termini. J. Neurosci. 1998;18:1240–1249. doi: 10.1523/JNEUROSCI.18-04-01240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Radic Z. The cholinesterases: from genes to proteins. Annu. Rev. Pharmacol. Toxicol. 1994;34:281–320. doi: 10.1146/annurev.pa.34.040194.001433. [DOI] [PubMed] [Google Scholar]