Abstract
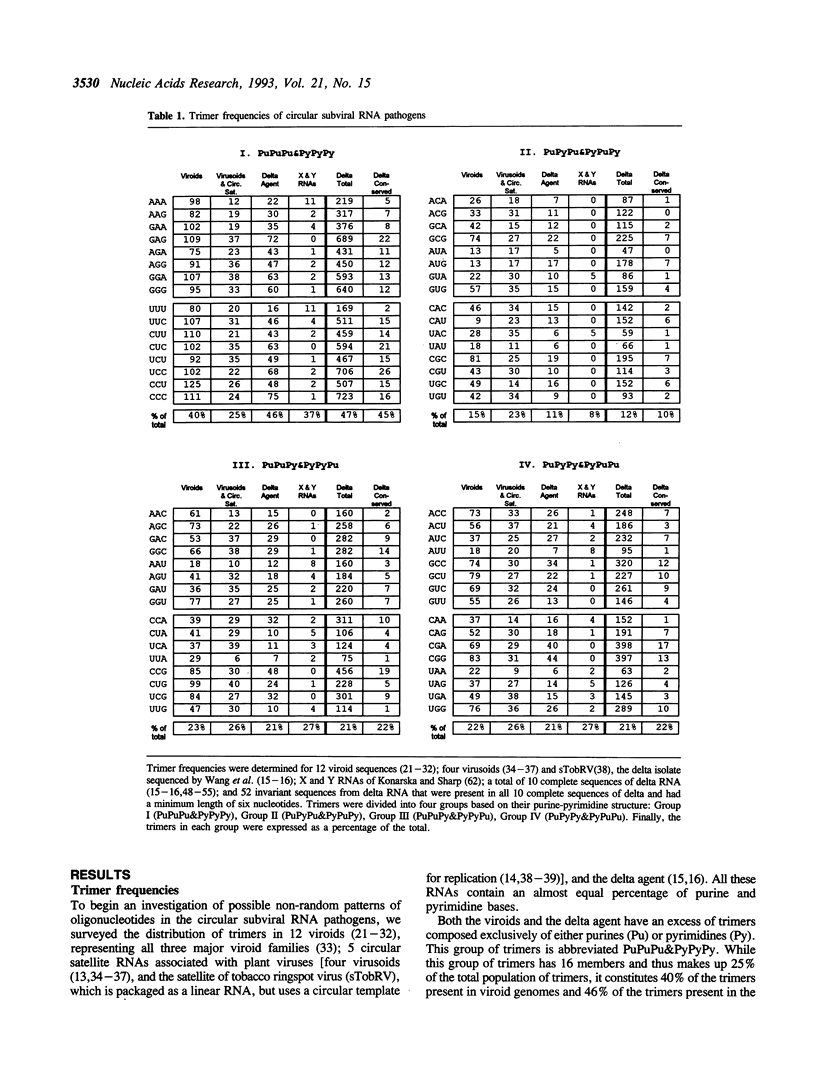
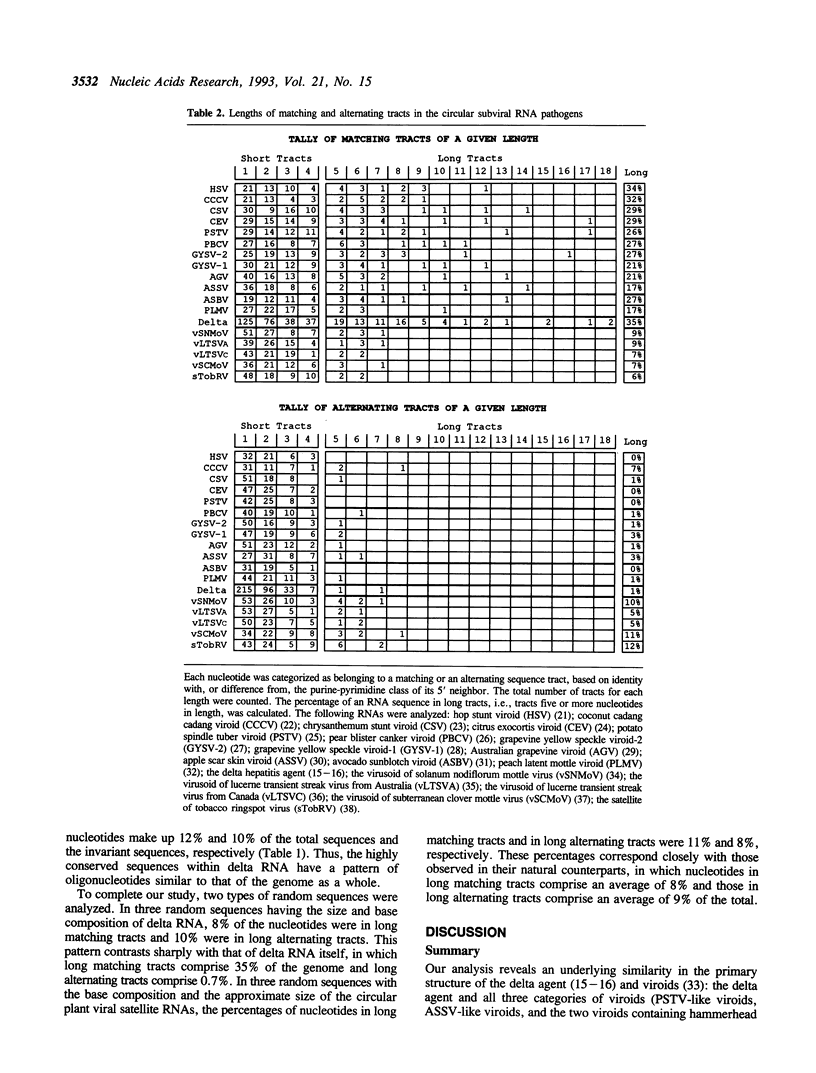
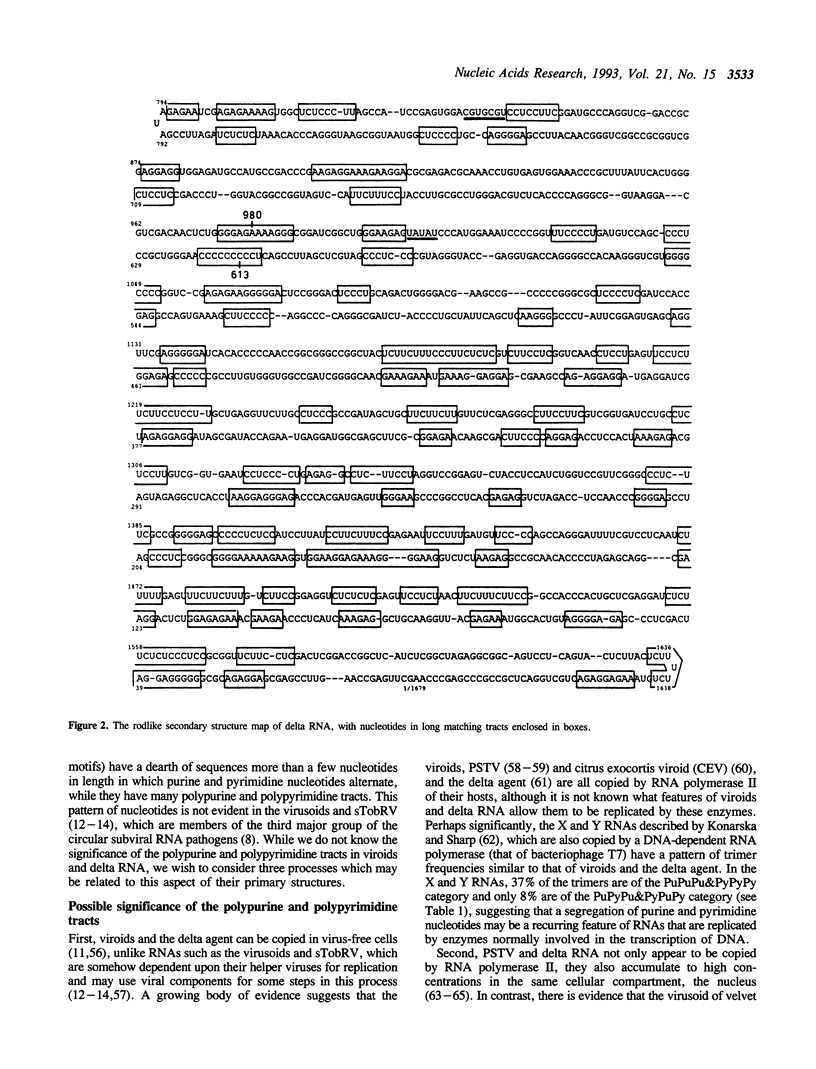
To seek patterns of nucleotide usage in the three types of circular subviral RNA pathogens, trimer frequencies and nearest-neighbor biases were studied in 12 plant viroid sequences; five sequences of circular plant viral satellite RNAs; and the sequence of RNA from the human hepatitis delta agent. The viroids and RNA of the delta agent contain tracts of polypurines and polypyrimidines which make up substantial portions of their genomes. Such tracts are not common in the virusoids or in the satellite RNA of tobacco ringspot virus. Viroids, the delta hepatitis agent, and the circular satellite RNAs of certain plant viruses have several features in common: all have circular genomic RNA and replicate through an RNA to RNA rolling circle replication cycle. However, virusoids and related satellite RNAs are directly or indirectly dependent on their helper viruses for replication, while the delta agent and viroids are not. The difference in the pattern of nucleotide usage between the plant viral satellite RNAs on the one hand, and viroids and delta RNA on the other, may relate to this difference in replication strategy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrai I., Scapoli C., Barale R., Volinia S. Oligonucleotide correlations between infector and host genomes hint at evolutionary relationships. Nucleic Acids Res. 1990 May 25;18(10):3021–3025. doi: 10.1093/nar/18.10.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch A. D., Benenfeld B. J., Baroudy B. M., Wells F. V., Gerin J. L., Robertson H. D. An ultraviolet-sensitive RNA structural element in a viroid-like domain of the hepatitis delta virus. Science. 1989 Feb 3;243(4891):649–652. doi: 10.1126/science.2492676. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. Efficient trans cleavage and a common structural motif for the ribozymes of the human hepatitis delta agent. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10163–10167. doi: 10.1073/pnas.88.22.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Bergmann K. F., Brown T. L., Gerin J. L. Structural requirements for RNA editing in hepatitis delta virus: evidence for a uridine-to-cytidine editing mechanism. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7149–7153. doi: 10.1073/pnas.89.15.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M., Hsieh S. Y., Taylor J. The antigen of hepatitis delta virus: examination of in vitro RNA-binding specificity. J Virol. 1991 Aug;65(8):4057–4062. doi: 10.1128/jvi.65.8.4057-4062.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y. C., Chang M. F., Gust I., Lai M. M. Sequence conservation and divergence of hepatitis delta virus RNA. Virology. 1990 Oct;178(2):384–392. doi: 10.1016/0042-6822(90)90335-o. [DOI] [PubMed] [Google Scholar]
- Chao Y. C., Lee C. M., Tang H. S., Govindarajan S., Lai M. M. Molecular cloning and characterization of an isolate of hepatitis delta virus from Taiwan. Hepatology. 1991 Feb;13(2):345–352. [PubMed] [Google Scholar]
- Chen P. J., Kalpana G., Goldberg J., Mason W., Werner B., Gerin J., Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C., Haseloff J., Symons R. H. Structure, self-cleavage, and replication of two viroid-like satellite RNAs (virusoids) of subterranean clover mottle virus. Virology. 1990 Jul;177(1):216–224. doi: 10.1016/0042-6822(90)90475-7. [DOI] [PubMed] [Google Scholar]
- Elena S. F., Dopazo J., Flores R., Diener T. O., Moya A. Phylogeny of viroids, viroidlike satellite RNAs, and the viroidlike domain of hepatitis delta virus RNA. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5631–5634. doi: 10.1073/pnas.88.13.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores R., Semancik J. S. Properties of a cell-free system for synthesis of citrus exocortis viroid. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6285–6288. doi: 10.1073/pnas.79.20.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francki R. I. Plant virus satellites. Annu Rev Microbiol. 1985;39:151–174. doi: 10.1146/annurev.mi.39.100185.001055. [DOI] [PubMed] [Google Scholar]
- Gartenberg M. R., Crothers D. M. Synthetic DNA bending sequences increase the rate of in vitro transcription initiation at the Escherichia coli lac promoter. J Mol Biol. 1991 May 20;219(2):217–230. doi: 10.1016/0022-2836(91)90563-l. [DOI] [PubMed] [Google Scholar]
- Glenn J. S., Taylor J. M., White J. M. In vitro-synthesized hepatitis delta virus RNA initiates genome replication in cultured cells. J Virol. 1990 Jun;64(6):3104–3107. doi: 10.1128/jvi.64.6.3104-3107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans E. J., Baroudy B. M., Negro F., Ponzetto A., Purcell R. H., Gerin J. L. Evidence for replication of hepatitis delta virus RNA in hepatocyte nuclei after in vivo infection. Virology. 1988 Nov;167(1):274–278. doi: 10.1016/0042-6822(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence-directed curvature of DNA. Annu Rev Biochem. 1990;59:755–781. doi: 10.1146/annurev.bi.59.070190.003543. [DOI] [PubMed] [Google Scholar]
- Hammond R., Smith D. R., Diener T. O. Nucleotide sequence and proposed secondary structure of Columnea latent viroid: a natural mosaic of viroid sequences. Nucleic Acids Res. 1989 Dec 11;17(23):10083–10094. doi: 10.1093/nar/17.23.10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harders J., Lukács N., Robert-Nicoud M., Jovin T. M., Riesner D. Imaging of viroids in nuclei from tomato leaf tissue by in situ hybridization and confocal laser scanning microscopy. EMBO J. 1989 Dec 20;8(13):3941–3949. doi: 10.1002/j.1460-2075.1989.tb08577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucleic Acids Res. 1982 Jun 25;10(12):3681–3691. doi: 10.1093/nar/10.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C., Daròs J. A., Elena S. F., Moya A., Flores R. The strands of both polarities of a small circular RNA from carnation self-cleave in vitro through alternative double- and single-hammerhead structures. Nucleic Acids Res. 1992 Dec 11;20(23):6323–6329. doi: 10.1093/nar/20.23.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C., Elena S. F., Moya A., Flores R. Pear blister canker viroid is a member of the apple scar skin subgroup (apscaviroids) and also has sequence homology with viroids from other subgroups. J Gen Virol. 1992 Oct;73(Pt 10):2503–2507. doi: 10.1099/0022-1317-73-10-2503. [DOI] [PubMed] [Google Scholar]
- Hernández C., Flores R. Plus and minus RNAs of peach latent mosaic viroid self-cleave in vitro via hammerhead structures. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3711–3715. doi: 10.1073/pnas.89.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazeki F., Omata M., Ohto M. Complete nucleotide sequence of hepatitis delta virus RNA in Japan. Nucleic Acids Res. 1991 Oct 11;19(19):5439–5439. doi: 10.1093/nar/19.19.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhász A., Hegyi H., Solymosy F. A novel aspect of the information content of viroids. Biochim Biophys Acta. 1988 Sep 7;950(3):455–458. doi: 10.1016/0167-4781(88)90145-5. [DOI] [PubMed] [Google Scholar]
- Keese P., Symons R. H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4582–4586. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow A. M., Rezaian M. A. A scheme for viroid classification. Intervirology. 1989;30(4):194–201. doi: 10.1159/000150093. [DOI] [PubMed] [Google Scholar]
- Koltunow A. M., Rezaian M. A. Grapevine viroid 1B, a new member of the apple scar skin viroid group contains the left terminal region of tomato planta macho viroid. Virology. 1989 Jun;170(2):575–578. doi: 10.1016/0042-6822(89)90450-9. [DOI] [PubMed] [Google Scholar]
- Koltunow A. M., Rezaian M. A. Grapevine yellow speckle viroid: structural features of a new viroid group. Nucleic Acids Res. 1988 Feb 11;16(3):849–864. doi: 10.1093/nar/16.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Structure of RNAs replicated by the DNA-dependent T7 RNA polymerase. Cell. 1990 Nov 2;63(3):609–618. doi: 10.1016/0092-8674(90)90456-o. [DOI] [PubMed] [Google Scholar]
- Kos T., Molijn A., van Doorn L. J., van Belkum A., Dubbeld M., Schellekens H. Hepatitis delta virus cDNA sequence from an acutely HBV-infected chimpanzee: sequence conservation in experimental animals. J Med Virol. 1991 Aug;34(4):268–279. doi: 10.1002/jmv.1890340412. [DOI] [PubMed] [Google Scholar]
- Kostura M., Mathews M. B. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol Cell Biol. 1989 Apr;9(4):1576–1586. doi: 10.1128/mcb.9.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. Y., Goldberg J., Coates L., Mason W., Gerin J., Taylor J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J Virol. 1988 Jun;62(6):1855–1861. doi: 10.1128/jvi.62.6.1855-1861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. Y., Sharmeen L., Dinter-Gottlieb G., Taylor J. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988 Dec;62(12):4439–4444. doi: 10.1128/jvi.62.12.4439-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman D. K., Tavantzis S. M. RNA progeny of an infectious two-base deletion cDNA mutant of potato spindle tuber viroid (PSTV) acquire two nucleotides in Planta. Virology. 1992 Apr;187(2):565–572. doi: 10.1016/0042-6822(92)90459-3. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Bih F. Y., Chao Y. C., Govindarajan S., Lai M. M. Evolution of hepatitis delta virus RNA during chronic infection. Virology. 1992 May;188(1):265–273. doi: 10.1016/0042-6822(92)90756-f. [DOI] [PubMed] [Google Scholar]
- Lin J. H., Chang M. F., Baker S. C., Govindarajan S., Lai M. M. Characterization of hepatitis delta antigen: specific binding to hepatitis delta virus RNA. J Virol. 1990 Sep;64(9):4051–4058. doi: 10.1128/jvi.64.9.4051-4058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Chao M., Hsieh S. Y., Sureau C., Nishikura K., Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990 Mar;64(3):1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton T. B., Gowans E. J., McNamara S. P., Burrell C. J. Hepatitis delta antigen is necessary for access of hepatitis delta virus RNA to the cell transcriptional machinery but is not part of the transcriptional complex. Virology. 1991 Sep;184(1):387–390. doi: 10.1016/0042-6822(91)90855-6. [DOI] [PubMed] [Google Scholar]
- Makino S., Chang M. F., Shieh C. K., Kamahora T., Vannier D. M., Govindarajan S., Lai M. M. Molecular cloning and sequencing of a human hepatitis delta (delta) virus RNA. Nature. 1987 Sep 24;329(6137):343–346. doi: 10.1038/329343a0. [DOI] [PubMed] [Google Scholar]
- Merkl R., Kröger M., Rice P., Fritz H. J. Statistical evaluation and biological interpretation of non-random abundance in the E. coli K-12 genome of tetra- and pentanucleotide sequences related to VSP DNA mismatch repair. Nucleic Acids Res. 1992 Apr 11;20(7):1657–1662. doi: 10.1093/nar/20.7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y. Hop stunt viroid: molecular cloning and nucleotide sequence of the complete cDNA copy. Nucleic Acids Res. 1983 Sep 24;11(18):6185–6197. doi: 10.1093/nar/11.18.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta A. T., Been M. D. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature. 1991 Apr 4;350(6317):434–436. doi: 10.1038/350434a0. [DOI] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Puchta H., Luckinger R., Yang X. C., Hadidi A., Sänger H. L. Nucleotide sequence and secondary structure of apple scar skin viroid (ASSVd) from China. Plant Mol Biol. 1990 Jun;14(6):1065–1067. doi: 10.1007/BF00019406. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Rohde W., Sänger H. L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature. 1981 May 28;291(5813):297–301. doi: 10.1038/291297a0. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A. Australian grapevine viroid--evidence for extensive recombination between viroids. Nucleic Acids Res. 1990 Apr 11;18(7):1813–1818. doi: 10.1093/nar/18.7.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Riesner D., Colpan M., Goodman T. C., Nagel L., Schumacher J., Steger G., Hofmann H. Dynamics and interactions of viroids. J Biomol Struct Dyn. 1983 Dec;1(3):669–688. doi: 10.1080/07391102.1983.10507474. [DOI] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Saldanha J. A., Thomas H. C., Monjardino J. P. Cloning and sequencing of RNA of hepatitis delta virus isolated from human serum. J Gen Virol. 1990 Jul;71(Pt 7):1603–1606. doi: 10.1099/0022-1317-71-7-1603. [DOI] [PubMed] [Google Scholar]
- Schneider I. R. Characteristics of a satellite-like virus of tobacco ringspot virus. Virology. 1971 Jul;45(1):108–122. doi: 10.1016/0042-6822(71)90118-8. [DOI] [PubMed] [Google Scholar]
- Sharmeen L., Kuo M. Y., Dinter-Gottlieb G., Taylor J. Antigenomic RNA of human hepatitis delta virus can undergo self-cleavage. J Virol. 1988 Aug;62(8):2674–2679. doi: 10.1128/jvi.62.8.2674-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Koller T., Diener T. O. Potato spindle tuber viroid. X. Visualization and size determination by electron microscopy. Virology. 1973 Sep;55(1):70–80. doi: 10.1016/s0042-6822(73)81009-8. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe H., Bürk R. R., Crawford L. V., Morrison J. M., Hay J., Keir H. M. An approach to evolutionary relationships of mammalian DNA viruses through analysis of the pattern of nearest neighbor base sequences. Cold Spring Harb Symp Quant Biol. 1966;31:737–748. doi: 10.1101/sqb.1966.031.01.094. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Small catalytic RNAs. Annu Rev Biochem. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Diener T. O. Potato spindle tuber viroid. XIV. Replication in nuclei isolated from infected leaves. Virology. 1975 Mar;64(1):106–114. doi: 10.1016/0042-6822(75)90083-5. [DOI] [PubMed] [Google Scholar]
- Taylor J., Mason W., Summers J., Goldberg J., Aldrich C., Coates L., Gerin J., Gowans E. Replication of human hepatitis delta virus in primary cultures of woodchuck hepatocytes. J Virol. 1987 Sep;61(9):2891–2895. doi: 10.1128/jvi.61.9.2891-2895.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Choo Q. L., Wang K. S., Govindarajan S., Redeker A. G., Gerin J. L., Houghton M. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988 Feb;62(2):594–599. doi: 10.1128/jvi.62.2.594-599.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. N., Lin Y. J., Lin F. P., Makino S., Chang M. F., Lai M. M. Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]


