Abstract
Background & Aims
Non-alcoholic steatohepatitis (NASH) is characterized by steatosis and inflammation, which can further progress into fibrosis and cirrhosis. Recently, we demonstrated that combined deletion of the two main scavenger receptors, CD36 and macrophage scavenger receptor 1 (MSR1), which are important for modified cholesterol-rich lipoprotein uptake, reduced NASH. The individual contributions of these receptors to NASH and the intracellular mechanisms by which they contribute to inflammation have not been established. We hypothesize that CD36 and MSR1 contribute independently to the onset of inflammation in NASH, by affecting intracellular cholesterol distribution inside Kupffer cells (KCs).
Methods & Results
Ldlr−/− mice were transplanted with wild-type (Wt), Cd36−/− or Msr1−/− bone marrow and fed a Western diet for 3months. Cd36−/−- and Msr1−/−- transplanted (tp) mice showed a similar reduction in hepatic inflammation compared to Wt-tp mice. While the total amount of cholesterol inside KCs was similar in all groups, KCs of Cd36−/−- and Msr1−/−-tp mice showed increased cytoplasmic cholesterol accumulation, while Wt-tp mice showed increased lysosomal cholesterol accumulation.
Conclusion
CD36 and MSR1 contribute similarly and independently to the progression of inflammation in NASH. One possible explanation for the inflammatory response related to expression of these receptors could be abnormal cholesterol trafficking in KCs. These data provide a new basis for prevention and treatment of NASH.
Introduction
Non-alcoholic steatohepatitis (NASH) is increasingly recognized as a major health burden in developed countries, with a prevalence of 2–3% in the general population and up to 37% of the severely obese population of Western countries [1]. It is characterized by hepatic lipid accumulation (steatosis) combined with inflammation. While steatosis itself is generally considered benign, the presence of inflammation can lead to progression of NASH, resulting in liver fibrosis, cirrhosis and eventually liver failure and hepatocellular carcinoma [2]. The inflammatory response in the liver represents a key step in NASH development as it contributes to further liver damage by cirrhosis and hepatocellular carcinoma.
We have previously shown that hyperlipidemic mice fed a Western diet show an early onset of hepatic inflammation, which is associated with bloated Kupffer cells (KCs). These KCs resemble the foam cells of atherosclerotic lesions [3]. The current view favours a model where scavenger receptors (SR) mediate uptake of oxidized lipoproteins by macrophages, leading to foam cell formation and initiation of the inflammatory response. In line with this, we have already shown that the haematopoietic deletion of scavenger receptors Cd36 and macrophage scavenger receptor 1 (Msr1) in low density lipoprotein (LDL) receptor-deficient (Ldlr−/−) mice attenuates hepatic inflammation [4]. However, while hepatic inflammation, lipid oxidation and liver fibrosis were significantly reduced, these mice showed no difference in foamy KCs. Similarly, it has also been shown that deletion of CD36, MSR1 or both do not completely abrogate foam cell formation in vitro or in vivo. In terms of atherosclerosis, there is controversy as to whether there are reduced lesions in apoE/Cd36/Msr1 triple knock-out mice, but there is consensus that absence of these receptors reduced lesion complexity and inflammation [5], [6]. Thus, in contrast to the current view, these observations suggest that scavenger receptors modulate inflammation without altering total lipid accumulation in macrophages. Likewise, there is a growing appreciation of the numerous similar but independent functions of each one of these scavenger receptors in addition to lipoprotein uptake, including their role in inflammatory signal transduction and tissue homeostasis [7]. Currently, the individual contributions of these receptors to the onset of inflammation and the intracellular mechanisms by which they contribute to inflammation have not yet been established. Based on their different functions in inflammatory signal transduction, we hypothesized that CD36 and MSR1 contribute independently to the onset of hepatic inflammation, by affecting intracellular cholesterol distribution inside KCs.
Under normal conditions, lipoproteins which are endocytosed by KCs will initially be directed to the lysosomes, where they are hydrolyzed by lysosomal enzymes and transferred into the cytoplasm. However, the uptake of oxLDL by macrophages, which is modulated by CD36 and MSR1, is associated with lysosomal trapping in vitro [8]. Several lines of evidence indicate an association between lysosomal cholesterol accumulation and inflammation [9]–[12].
In order to investigate whether a macrophage-specific deletion of each scavenger receptor individually affects hepatic inflammation, Ldlr−/− mice were lethally irradiated and transplanted with bone marrow from Wild type (Wt), Msr1−/− or Cd36−/− mice and fed a high-fat-high-cholesterol (HFC) diet for 3 months. We found that CD36 and MSR1 contribute in a similar manner to the progression of NASH in the presence of high levels of plasma-modified lipoproteins. The effect of these receptors on inflammation is likely modulated via impaired cholesterol trafficking inside KCs and increased lysosomal cholesterol accumulation.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee for Animal Welfare of Maastricht University (Permit Number: 2006-177). The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Mice and diet
Mice were housed under standard conditions and given free access to food and water. Female Ldlr−/− mice were lethally irradiated and transplanted with Wt, Cd36−/− and Msr−/− bone marrow as previously described [4]. After a recovery period of 9 weeks, the mice were given a HFC diet for 3 months (n = 9 Wt-tp mice, n = 8 for both Cd36 −/−-tp and Msr1 −/−-tp mice), containing 21% milk butter, 0.2% cholesterol, 46% carbohydrates and 17% casein. Collection of blood and specimens, biochemical determination of lipids in plasma and liver, liver histology, RNA isolation, cDNA synthesis and qPCR, aminotransferases, oxysterols and auto-antibody titers against IgG and IgM antibodies to CuOx-LDL and MDA-LDL, as well as T15id+ IgM were determined as previously described [4].
Electron microscopy
A detailed overview about the (post)fixation, embedding, cutting and type of electron microscope was described previously [3]. Scoring of electron microscopy (EM) pictures was performed by a trained specialist. Fifty KCs per mouse were analysed and scored. The KCs were divided into three different categories, i.e., primary lysosomes, secondary fatty lysosomes and cytoplasmic fat accumulation and the percentage of each subtype was calculated. Furthermore, the secondary fatty lysosomes were scored from 0 to 3 for their fat content, where a score of 0 indicated not positive and a score of 3 indicated extreme fat accumulation inside the lysosomes of the KCs. The same scoring index was used for the cytoplasmic fat accumulation inside KCs.
Statistical analysis
The data were statistically analysed by performing a one-way ANOVA test with a Bonferroni post-test using GraphPad Prism for comparing Wt-tp, Cd36−/−-tp and Msr−/−-tp mice with each other. The same was done for the human samples where the different groups of patients were compared with each other. The data were expressed as mean ± SEM and considered significant at p<0.05. *, ** and *** indicate p<0.05, 0.01 and 0.001, respectively.
Results
Inflammation is reduced in Cd36−/−-tp and Msr1−/−-tp mice compared to Wt-tp mice
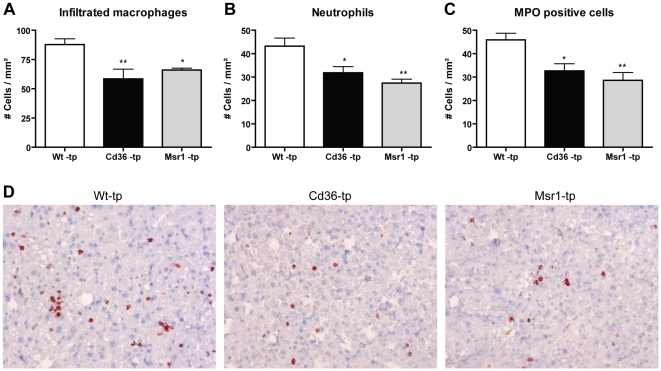
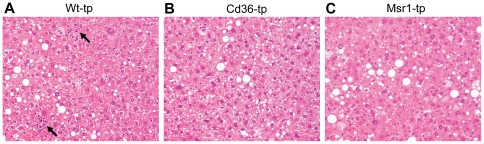
To determine whether both Cd36 and Msr1 are independently involved in diet-induced hepatic inflammation, Ldlr−/− mice were transplanted with Cd36−/− or Msr1−/− bone marrow and fed a HFC diet for 3 months. Immunohistochemistry revealed that infiltrated macrophages and neutrophils were significantly decreased in the livers of mice transplanted with both Cd36−/− and Msr1−/− bone marrow compared to Wt- transplanted (tp) mice (Fig. 1A+B+D). The same decrease was seen after staining with an antibody against myeloperoxidase (MPO), which is an oxidative stress/neutrophil marker (Fig. 1C). These data on inflammation were confirmed by a general HE staining, indicating less hepatic inflammation in Cd36−/−- and Msr1−/−-tp mice (Fig. 2).
Figure 1. Parameters of hepatic inflammation.
(A–C) Liver sections were stained for infiltrated macrophages and neutrophils (Mac-1), neutrophils (NIMP) and MPO (D) Representative pictures of Mac-1 staining (×200 magnification) after 3 months of HFC feeding in Wt-tp, Cd36−/−-tp and Msr1−/−-tp mice. *Significantly different from Wt-tp group. * and ** indicate p<0.05 and 0.01, respectively.
Figure 2. General histology of the liver.
HE staining on livers of Wt-tp (A), Cd36 −/−-tp (B) and Msr1 −/−-tp (C) mice indicating steatosis and inflammation (arrows).
The plasma transaminases alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are considered as sensitive indicators of liver damage. Although the ALT levels were equal in all transplanted groups, the AST levels were significantly lower in Cd36−/−-tp and Msr1−/−-tp mice compared to Wt-tp mice. Moreover, the ratio of AST/ALT was lower in Msr1−/−-tp mice compared to Wt-tp mice (Table 1). In general, these data indicate that both Cd36−/−-tp and Msr1−/−-tp mice showed less liver damage.
Table 1. Parameters for lipid related products in liver and plasma.
| Wt-tp mice | Cd36−/−-tp mice | Msr1 −/−-tp mice | |
| Liver | |||
| Triglycerides (µg) | 0.38 (±0.02) | 0.40 (±0.03) | 0.47 (±0.02)* |
| Free fatty acids (µg) | 0.17 (±0.003) | 0.19 (±0.006) | 0.20 (±0.01)* |
| Cholesterol (µg) | 0.08 (±0.0038) | 0.10 (±0.0062) | 0.11 (±0.0071)** |
| Lathosterol (ng/mg liver) | 21.8 (±1.76) | 19.71 (±2.44) | 19.10 (±2.55) |
| Desmosterol (ng/mg liver) | 16.11 (±1.06) | 14.44 (±0.85) | 12.62 (±0.92)* |
| Cholestanol (ng/mg liver) | 93.03 (±8.42) | 97.33 (±6.38) | 126.7 (±11.22)*, # |
| Cholesterol esters (µg/mg liver) | 14.37 (±1.58) | 21.2 (±1.88)* | 20.99 (±1.27)** |
| Free cholesterol (µg/mg liver) | 8.63 (±0.28) | 9.00 (±0.23) | 8.16 (±0.36) |
| Plasma | |||
| Triglycerides (mM) | 1.49 (±0.16) | 1.29 (±0.14) | 1.27 (±0.18) |
| Free fatty acids (mM) | 0.58 (±0.04) | 0.59 (±0.06) | 0.45 (±0.03) |
| Cholesterol (mg/dl) | 1052 (±113.1) | 1210 (±37.76) | 1097 (±69.45) |
| Lathosterol (mg/dl) | 0.31 (±0.03) | 0.24 (±0.04) | 0.17 (±0.03)** |
| Desmosterol (mg/dl) | 0.77 (±0.06) | 0.77 (±0.08) | 0.52 (±0.06)*, # |
| Cholestanol (mg/dl) | 3.03 (±0.31) | 4.34 (±0.13)** | 4.60 (±0.14)** |
| 7α-OH cholesterol (ng/ml) | 1.50 (±0.13) | 1.02 (±0.13)* | 0.92 (±0.03)** |
| 24-OH cholesterol (ng/ml) | 448.8 (±46.85) | 506.5 (±28.77) | 505.6 (±41.62) |
| 27-OH cholesterol (ng/ml) | 504 (±59.81) | 504.8 (±42.63) | 455 (±46.12) |
| MDA-LDL IgM (RLU) | 86348 (±4857) | 89405 (±6189) | 113896 (±2889)***,## |
| CuOx-LDL IgM (RLU) | 31987 (±6331) | 62903 (±11844)* | 102371 (±6341)***,## |
| T15id+ (IgM) (RLU) | 3726 (±395.2) | 9000 (±1815)* | 19079 (±3412)**, # |
| ALT (U/l) | 61 (±9.35) | 58.43 (±3.40) | 65.5 (±3.53) |
| AST (U/l) | 129.5 (±13.77) | 79 (±14.3)* | 84 (±6.52)* |
| AST/ALT | 2.25 (±0.38) | 1.52 (±0.15) | 1.28 (±0.07)* |
Significantly different from Wt-tp group. *, ** and *** indicate p<0.05, 0.01 and 0.001, respectively. RLU: Relative light unit.
Significantly difference between Cd36−/−-tp and Msr1−/−-tp mice. # and ## indicate p<0.05 and 0.01 respectively.
The reduced inflammatory response in Cd36−/−-tp and Msr1−/−-tp mice is not correlated with the changes in lipid levels
To determine whether deletion of Cd36 and Msr1 on bone marrow cells affected steatosis, liver lipid levels were analysed in order to investigate the extent of steatosis present in these mice. Table 1 show that liver triglycerides (TG), free fatty acids (FFA) and total cholesterol were all higher in the Msr1 −/−-tp mice compared to Wt-tp mice. The amount of cholesterol esters (CE) in the liver was elevated in both Cd36−/−-tp and Msr1−/−-tp mice compared to Wt-tp mice. Desmosterol, a cholesterol synthesis marker, was decreased in the Msr1 −/−-tp mice compared to Wt-tp mice, whereas the cholesterol absorption marker cholestanol was higher in Msr1 −/−-tp mice compared to Wt-tp and Cd36−/−-tp mice (Table 1). Nevertheless, HE staining (Fig. 2) and Oil red O staining (data not shown) revealed no differences between any of the groups. Thus, the observed differences found in hepatic steatosis between Wt-tp, Cd36−/−-tp and Msr1−/−-tp mice were mild and not consistent.
Table 1 shows that plasma TG and total cholesterol were similar in all transplanted groups. Plasma FFA levels were significantly lower in Msr1−/−-tp mice compared to Wt-tp mice. Although the total amount of cholesterol did not differ between the groups, the cholesterol synthesis markers lathosterol and desmosterol were significantly lower in the plasma of Msr1−/−-tp mice compared to Wt-tp mice. Moreover, the cholesterol absorption marker cholestanol was significantly higher in both Cd36−/−-tp and Msr1−/−-tp mice compared to Wt-tp mice. Plasma levels of 7α-hydroxy (OH) cholesterol were decreased in both Cd36−/−-tp and Msr1−/−-tp mice compared to Wt-tp mice, while the other oxysterols (24-OH and 27-OH cholesterol) were not different from Wt-tp mice. Overall, these plasma lipid data were inconclusive and did not reveal any major differences between Wt-tp, Cd36−/−-tp and Msr1−/−-tp mice.
In order to investigate whether natural antibodies, known to decrease atherogenesis, are affected by deletion of Cd36 and Msr1, the protective immunoglobulin M auto-antibody levels against MDA-LDL, Cu-OxLDL and T15id+ were measured and showed significantly higher levels in Msr1−/−-tp mice compared to Wt-tp mice and Cd36−/−-tp mice. The Cu-OxLDL and T15id+ IgM auto-antibodies were also elevated in Cd36−/−-tp mice compared to Wt-tp mice. Thus, both Cd36−/−-tp and Msr1−/−-tp mice have an increased protective phenotype compared to Wt-tp mice.
No difference in the foamy appearance of Kupffer cells
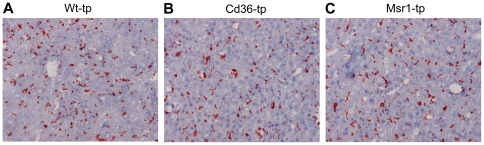
To characterize the KCs, immunohistochemistry for CD68 (macrophage marker) was performed. This staining revealed no differences in the size or presence of KCs between Wt-tp, Cd36−/−-tp and Msr1−/−-tp mice (Fig. 3A–C). Moreover, CD68 mRNA expression showed similar results (data not shown).
Figure 3. Foamy Kupffer cells.
CD68 staining of Kupffer cells in liver of Wt-tp (A), Cd36 −/−-tp (B) and Msr1 −/−-tp (C) mice.
Cd36 −/−-tp and Msr1 −/−-tp mice have decreased lysosomal cholesterol storage inside the KCs compared to Wt-tp mice
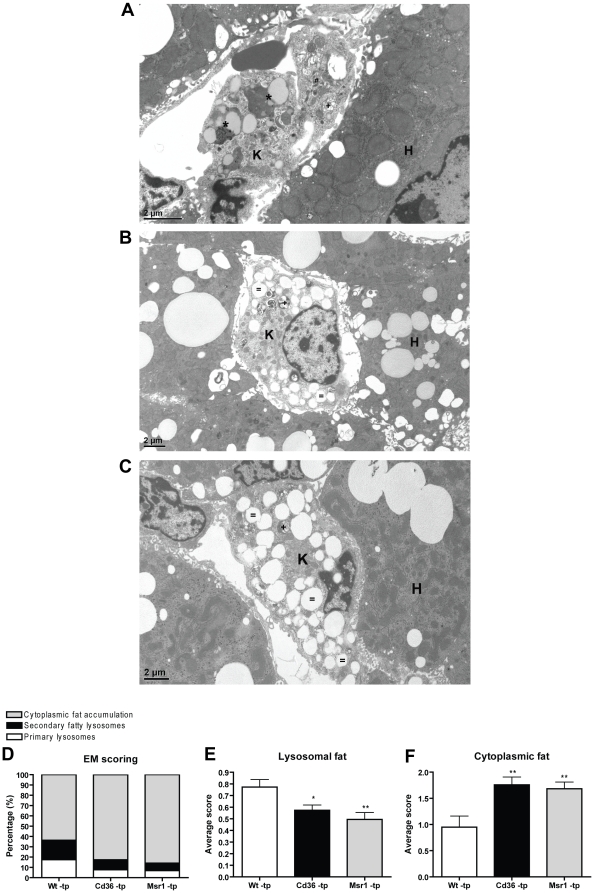
To determine whether the reduced inflammatory response in Cd36−/−-tp and Msr1−/−-tp mice was associated with decreased lysosomal fat accumulation, detailed analyses of the KCs were performed using electron microscopy (EM) (Fig. 4A–C). Interestingly, lipid distribution inside these cells revealed a clear difference between Wt-tp and Cd36 −/− -tp/Msr1 −/− -tp mice (Fig. 4D). Cd36 −/−-tp and Msr1 −/−-tp mice showed decreased lysosomal cholesterol storage inside the KCs compared to Wt-tp mice, whereas the first two had increased cytoplasmic fat storage compared to Wt-tp mice (Fig. 4E+F). Moreover, Wt-tp mice had more primary and secondary lysosomes compared to Cd36 −/−-tp and Msr1 −/−-tp mice.
Figure 4. Electron microscopic views of Kupffer cells (K) after 3 months of a high fat diet.
(A) The Kupffer cell of Wt-tp mice shows several fatty lysosomes (*). Primary lysosomes (#) and secondary lysosomes without fat accumulation (+) can be seen as well. Kupffer cells of Cd36 −/− -tp (B) and Msr1 −/− -tp (C) mice show massive cytoplasmic lipid accumulation ( = ). H: hepatocytes. (D) Percentage of Kupffer cells with primary lysosomes, secondary fatty lysosomes and cytoplasmic fat accumulation. (E+F) Arbitrary scoring of secondary fatty lysosomes and cytoplasmic fat accumulation inside Kupffer cells. *Significantly different from Wt-tp group. * and ** indicate p<0.05 and 0.01, respectively.
Discussion
Our study showed for the first time that the uptake of modified lipids in vivo leads to lysosomal cholesterol accumulation in KCs. By specifically inactivating one of the two predominant scavenger receptors on macrophages, we established that the contribution of each one of these two receptors to hepatic inflammation is similar. Internalization of lipids by these receptors leads to increased levels of cholesterol in lysosomes and increased hepatic inflammation.
CD36 and MSR1 contribute in a similar manner to the progression of NASH
Currently, the risk factors that drive hepatic inflammation during the progression of NASH are largely unknown. We previously showed that hyperlipidemic mice are more sensitive to developing early diet-induced NASH [3]. We also demonstrated that the increased sensitivity of these mice towards developing NASH is related to the expression of Msr1 and Cd36 [4].
Scavenger receptors MSR1 and CD36 have been identified as the principal receptors responsible for the uptake of modified lipids and cholesterol into macrophages [13]. MSR1 was found to account for the majority (80%) of the macrophage uptake of acetylated low density lipoproteins (acLDL), but it also binds oxidized LDL (oxLDL), albeit with lower affinity. CD36 binds oxLDL rather than acLDL, and it is involved in oxLDL-induced JNK activation. While the relative affinities of MSR1 and CD36 to modified and oxidized lipids are different, the effects on inflammation in our study were similar. These results are surprising, since it was found that cultured macrophages incubated with oxLDL showed lysosomal cholesterol accumulation while incubation with acLDL did not lead to lysosomal cholesterol accumulation but rather to cytoplasmic cholesterol storage inside macrophages [8]. Thus, it is possible that in contrast to the in vitro data, the affinity of both receptors towards oxLDL in vivo is similar. Another possible explanation is that the markedly increased levels of oxLDL in the plasma of Ldlr−/− mice fed the HFC diet exceeded the maximal amount of cholesterol that can be taken up by KCs and trapped inside their lysosomes. The scavenger receptors CD36 and MSR1 differ from each other not only in their relative affinities to modified and oxidized lipids, but also in their signalling pathways and other important functions triggered by the binding and uptake of modified LDL, such as innate immune responses, cellular adhesion, and the phagocytosis of apoptotic cells [7]. These functions may also be involved in the development of NASH. The similarity in the degree of inflammation between the two models suggests that this inflammation is a consequence of the two receptors sharing similar modified lipid internalization mechanisms, rather than each receptor having its own specialized functions. In line with this hypothesis, previous studies with MSR1 and CD36-knockout mice showed that disruption of each receptor pathway partially inhibited the uptake of modified LDL in macrophages and retarded atherosclerosis progression in a similar manner [14]–[16]. All together, this study provides evidence of a central contribution of both of these receptors to the pathogenesis of NASH. Further investigations are necessary to determine the exact interaction between CD36 and MSR1 upon the internalization of lipids.
Hepatic inflammation is correlated with increased lysosomal cholesterol accumulation
Interestingly, it was shown that lysosomal cholesterol accumulation in vitro was associated with increased pH inside lysosomes [17], which can lead to partial lysosomal enzyme inactivation and an inflammatory response. Similarly, it was shown that in foam cells of advanced atherosclerotic plaques, cholesterol is not transferred into the cytoplasm but rather accumulates in the lysosomes of the macrophages [18]. Several lines of evidence further indicate an association between lysosomal cholesterol accumulation and inflammation: first, lysosomal acid lipase (LAL) is the essential enzyme for the hydrolysis of triglycerides and cholesteryl esters in lysosomes. Similar to our observations in hyperlipidemic mice, a deficiency of this enzyme was found to lead to lysosomal cholesterol accumulation and inflammation [12]. Moreover, exogenous LAL administration in Ldlr−/− mice led to a significant reduction in hepatic inflammation and lipid accumulation. Furthermore, the macrophage-specific expression of human LAL was found to correct inflammation in Lal−/− mice [12]. Likewise, patients with mutations in Niemann-Pick type C (NPC) 1 and 2, proteins which facilitate the movement of cholesterol from the lysosomes to the cytoplasmic compartment, commonly have a very poorly functioning liver, and about 10% of these patients dies from liver failure [19], [20]. Second, lysosomes have been assigned a central role in many processes involving tissue injury and inflammation [21]. Likewise, increased lysosomal cholesterol accumulation contributes to disturbed autophagy, a process that has recently emerged as an important regulatory pathway of the innate immune response [22]. Finally, it was recently demonstrated that oxLDL has the potential to damage lysosomal membranes and thereby prime cells for inflammation [23].
Taken altogether, our novel observations point towards the role of scavenger receptors during hepatic inflammation, resulting in abnormal cholesterol trafficking in KCs. These data provide a new basis for prevention and treatment of NASH.
Acknowledgments
We are grateful to Hans Duimel and Marie-Hélène Lenders (Maastricht University) for their excellent technical help.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Veni grant: 916.76.070 (2006/00496/MW) www.nwo.nl and Maag Lever Darm Stichting (MLDS) (WO 08-16) www.mlds.nl. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 2.Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 4.Bieghs V, Wouters K, van Gorp PJ, Gijbels MJ, de Winther MP, et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology. 2010;138:2477–2486, 2486 e2471–2473. doi: 10.1053/j.gastro.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerome WG, Yancey PG. The role of microscopy in understanding atherosclerotic lysosomal lipid metabolism. Microsc Microanal. 2003;9:54–67. doi: 10.1017/S1431927603030010. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro LJ. Regulation of lysosomal enzyme secretion: role in inflammation. Agents Actions. 1974;4:241–258. doi: 10.1007/BF01965227. [DOI] [PubMed] [Google Scholar]
- 10.Liao G, Cheung S, Galeano J, Ji AX, Qin Q, et al. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1−/− mouse brain. Brain Res. 2009;1270:140–151. doi: 10.1016/j.brainres.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tessitore A, Pirozzi M, Auricchio A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics. 2009;2:4. doi: 10.1186/1755-8417-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan C, Lian X, Li Y, Dai Y, White A, et al. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal−/− mice. Am J Pathol. 2006;169:916–926. doi: 10.2353/ajpath.2006.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennings M, Meurs I, Ye D, Out R, Hoekstra M, et al. Regulation of cholesterol homeostasis in macrophages and consequences for atherosclerotic lesion development. FEBS Lett. 2006;580:5588–5596. doi: 10.1016/j.febslet.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 17.Cox BE, Griffin EE, Ullery JC, Jerome WG. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J Lipid Res. 2007;48:1012–1021. doi: 10.1194/jlr.M600390-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Griffin EE, Ullery JC, Cox BE, Jerome WG. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J Lipid Res. 2005;46:2052–2060. doi: 10.1194/jlr.M500059-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Kelly DA, Portmann B, Mowat AP, Sherlock S, Lake BD. Niemann-Pick disease type C: diagnosis and outcome in children, with particular reference to liver disease. J Pediatr. 1993;123:242–247. doi: 10.1016/s0022-3476(05)81695-6. [DOI] [PubMed] [Google Scholar]
- 20.Vanier MT, Millat G. Niemann-Pick disease type C. Clin Genet. 2003;64:269–281. doi: 10.1034/j.1399-0004.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 21.Weissmann G. The role of lysosomes in inflammation and disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]
- 22.Saitoh T, Akira S. Regulation of innate immune responses by autophagy-related proteins. J Cell Biol. 2010;189:925–935. doi: 10.1083/jcb.201002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 466:652. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]