Abstract
Background and Purpose
To test whether changes in plasma tissue factor pathway inhibitor (TFPI) levels or activated protein C resistance (normalized APC resistance ratio, nAPCsr) modify the increased risk of ischemic stroke due to postmenopausal hormone therapy (PHT).
Methods
Nested case-control study of 455 cases of ischemic stroke and 565 matched controls in the Women’s Health Initiative trials of PHT.
Results
Baseline free TFPI was associated with ischemic stroke risk, OR (95% CI) per SD increase = 1.17 (1.01, 1.37, p=0.039, but baseline nAPCsr was not, OR per SD increase = 0.89 (0.75, 1.05), p=0.15. Baseline TFPI levels and nAPCsr did not modify the effect of PHT on ischemic stroke. Treatment-induced mean changes of -28% in free TFPI and +65% in nAPCsr did not change the risk of ischemic stroke (interaction p = 0.452 and 0.971 respectively). In subgroup analyses baseline nAPCsr was inversely associated with lacunar strokes, OR per SD increase = 0.74 (0.57, 0.96), p=0.025, and baseline free TFPI interacted with treatment to increase large vessel atherosclerotic strokes, p=0.008.
Conclusions
Pro-coagulant changes in TFPI or nAPCsr do not modify the increased ischemic stroke risk due to PHT.
Keywords: Cerebrovascular Accident, Estrogen, Hemostasis, Menopause, Randomized Controlled Trials
INTRODUCTION
The Women’s Health Initiative (WHI) trials of showed an increased risk of ischemic stroke for postmenopausal hormone therapy (PHT) compared to placebo.1,2 Baseline levels of several hemostatic markers and genotypic Factor V Leiden (FVL) status did not identify women at increased risk of ischemic stroke on PHT in the WHI trials.3 PHT increased D-dimer and plasmin-antiplasmin levels and decreased fibrinogen and PAI-1, and change in D-dimer interacted with PHT to increase risk of stroke.3 The fact that PHT increased the risk of venous thrombo-embolism (VTE) in WHI, especially in subjects with FVL is consistent with a possible role for hemostatic factors in explaining the increased risk of stroke also.4,5
Oral PHT increases markers of activated coagulation, reduces coagulation inhibitors, and induces an acquired resistance to the natural anticoagulant activated protein C (APC).6,7,8,9 Reduction in tissue factor pathway inhibitor (TFPI) and protein S are thought to be important mechanisms underlying the activation of coagulation and acquired protein C resistance associated with oral contraceptives (OCs), and both have been implicated in VTE.10 Cross-sectional studies suggest that low levels of TFPI or increased APC resistance may play a role in childhood ischemic stroke, but their roles in adult strokes are unclear.11,12,13 Higher TFPI levels in subjects with subclinical carotid and coronary atherosclerosis and with increased arterial stiffness may reflect endothelial dysfunction.14,15, 16
Here we report the first prospective study of the associations of acquired APC resistance and of TFPI levels with stroke, and we examine whether PHT-induced changes in these factors are associated with stroke risk in the WHI trials. We also examine associations with major subgroups of stroke.
METHODS
Details of the design, recruitment, randomization, data collection, intervention, and outcomes ascertainment procedures in the WHI PHT trials, including CONSORT diagrams, have been published previously.3,17,18
Study population and interventions
The WHI hormone trials enrolled 27 347 postmenopausal women aged 50-79 years from 1993 to 1998 at 40 US clinical centers based on hysterectomy status: 16 608 without hysterectomy in a trial of CEE+MPA; 10 739 with hysterectomy in a trial of CEE alone. Blood specimens were collected at baseline and the one-year visit. The study was approved by the human subjects review committee at each participating institution, and all participants provided written informed consent.
Participants were randomly assigned to take a single daily tablet containing a placebo or active medication: women without hysterectomy took 0.626 mg CEE plus 2.5 mg MPA (Prempro), and women with hysterectomy took 0.625 mg CEE (Premarin). Study drugs and placebo were supplied by Wyeth-Ayerst, St. Davids, PA. The planned end-date of the trials was March 31, 2005 for a total follow up of 8.4 years; however, CEE+MPA trial medications were stopped on July 7, 2002 and CEE was stopped on March 1, 2004 after mean follow-up periods of 5.6 and 7.1 years, respectively.1,2
Follow-up and outcome ascertainment
Stroke outcomes were identified by semi-annual questionnaires followed by review of medical records and classification by centrally adjudicated by stroke-trained neurologists blinded to treatment assignment. This report is based on 565 centrally adjudicated strokes with measurements of APC resistance or TFPI at baseline and controls matched 1:1 on age, race, randomization date, hysterectomy status, and self-reported prevalent stroke or transient ischemic attack at baseline. Strokes were classified into ischemic (N=455), hemorrhagic (N=82), other (N=4), cause of death only (N=21), and missing (N=3).). Ischemic strokes were subclassified according to the Trial of Org 10172 in Acute Stroke Therapy (TOAST).19 The major identifiable subgroups were large artery atherosclerosis N=43, cardioembolism N=66, and small vessel occlusion (lacune) N=117. The remaining 229 ischemic strokes had insufficient information to make a determination or had 2 or more potential etiologies.
Biomarker Analyses
Blood samples were collected into tubes containing 1.8% sodium citrate and centrifuged within 2 hours at 1300xg for 10 minutes at 4° C and stored at -70°C. Assays were run in duplicate in single batches including cases and controls and 10% blind duplicates within 8 years of collection. The endogenous thrombin potential-based activated protein C resistance test (ETP-based APC resistance test) was performed at the Department of Biochemistry at the University of Maastricht, the Netherlands, as described earlier.20 The test result is expressed as the ratio of thrombin generation without and with added APC, normalized against pooled normal plasma (nAPC-sr). In this assay higher nAPCsr values indicate increasing APC resistance. Total and free TFPI antigen were assayed using the same batch of Asserochrom ELISA (Stago) kits for each, and TFPI activity was measured in citrated plasma by an in-house chromogenic substrate activity assay at the Department of Hematology, Oslo University Hospital, Oslo.21,22 Inter-and intra-assay coefficients of variation were 3-5% and 1-3% for all assays. In 150 blind duplicates from the current study the intra-class correlations for nAPCsr and TFPI (total, free, and activity) were 0.73, 0.83, 0.44, and 0.84 respectively.
Statistical Analyses
The primary analysis tested the hypothesis that PHT-induced increases in APC resistance and reductions in free TFPI levels increase the risk of ischemic stroke. Cases of ischemic stroke (N=455) and all controls (N=565) were used to study associations of baseline values with ischemic stroke; associations of change in TFPI values were examined in 317 cases occurring after year 1 and in 465 controls while change in nAPCsr values were examined in 220 cases and 330 controls with both baseline and year 1 values available. Lacunar and non-lacunar ischemic stroke, hemorrhagic stroke, and total stroke were examined in secondary analyses. We combined the trial data since their effects on stroke were similar.1,2 Sensitivity analyses excluded women not adherent to their study medication 6 months before their stroke.
Biomarkers were examined on a continuous (linear) scale, after log-transformation for biomarkers that had skewed distributions. We used markers linearly to assess significance but reported odds ratios (OR) and 95% confidence intervals (CI) per standard deviation (SD) increase. Thus, there was not one-to-one correspondence between p-values below 0.05 and CIs for OR not containing 1. For the interaction of change in biomarkers levels from baseline to year 1, we computed p-values from logistic coefficients for change as a continuous variable but showed ORs by tertiles of change. We also examined whether changes in individual biomarkers were intermediates in the pathway of HT effects on stroke by comparing regression models with and without terms for biomarker change covariates.
We tested for nominal significance at p<0.05 without adjustment for multiple testing. Statistical analyses were performed on SAS statistical software (version 9; SAS Institute Inc, Cary, North Carolina).
RESULTS
In this case-control dataset PHT compared to placebo yielded overall multivariable-adjusted ORs (95% CI) for total, ischemic, and hemorrhagic stroke of 1.60 (1.23, 2.09), 1.81(1.36, 2.40), and 0.87 (0.52, 1.46) respectively. Cases of ischemic stroke were more likely to be smokers, non-drinkers, inactive, diabetic, hypertensive, have left ventricular hypertrophy, or a history of prior cardiovascular disease, higher body mass index, waist-to-hip ratio, systolic and diastolic blood pressure compared to controls (Table 1). Due to matching factors race/ethnicity and age did not differ between cases and controls. In unadjusted analyses baseline free TFPI levels were significantly higher in ischemic stroke cases than controls (p=0.002) but total TFPI, TFPI activity, and nAPCsr did not differ between cases and controls. In multivariable-adjusted models baseline free TFPI was positively associated with ischemic stroke, OR per SD increase = 1.17 (1.01, 1.37, p=0.037, but baseline nAPCsr was not associated with ischemic stroke, OR per SD increase = 0.89 (0.75, 1.05), p=0.15 (Table 2). Baseline TFPI and nAPCsr did not modify the effect of hormone therapy on stroke risk (Table 3).
Table 1.
Baseline characteristics of women in the nested case control study of ischemic stroke
| Estrogen Trial | Estrogen + Progestin Trial | P-Value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (N=297) | Ischemic Stroke (N=239) | Control (N=268) | Ischemic Stroke (N=216) | ||||||
| Race/ethnicity | 0.22 | ||||||||
| White | 229 | 77.1 | 176 | 73.6 | 232 | 86.6 | 180 | 83.3 | |
| Black | 45 | 15.2 | 49 | 20.5 | 18 | 6.7 | 19 | 8.8 | |
| Other/Unspecified | 23 | 7.7 | 14 | 5.9 | 18 | 6.7 | 17 | 7.9 | |
|
| |||||||||
| Smoking status | 0.007 | ||||||||
| Never | 151 | 51.5 | 116 | 49.4 | 152 | 57.6 | 96 | 44.7 | |
| Past | 119 | 40.6 | 93 | 39.6 | 91 | 34.5 | 87 | 40.5 | |
| Current | 23 | 7.8 | 26 | 11.1 | 21 | 8.0 | 32 | 14.9 | |
|
| |||||||||
| Alcohol use | 0.003 | ||||||||
| Non drinker | 134 | 45.3 | 150 | 62.8 | 115 | 43.2 | 100 | 46.5 | |
| ≤ 1 drink/day | 120 | 40.5 | 68 | 28.5 | 113 | 42.5 | 91 | 42.3 | |
| >1 drink/day | 42 | 14.2 | 21 | 8.8 | 38 | 14.3 | 24 | 11.2 | |
|
| |||||||||
| Total expenditure from physical activity (MET-hrs/wk) | 0.01 | ||||||||
| Inactive (0) | 43 | 16.3 | 61 | 28.2 | 43 | 18.0 | 42 | 22.0 | |
| <5 | 69 | 26.1 | 59 | 27.3 | 56 | 23.4 | 44 | 23.0 | |
| 5-<12 | 62 | 23.5 | 34 | 15.7 | 48 | 20.1 | 47 | 24.6 | |
| ≥12 | 90 | 34.1 | 62 | 28.7 | 92 | 38.5 | 58 | 30.4 | |
|
| |||||||||
| Treated diabetes | 21 | 7.1 | 35 | 14.6 | 7 | 2.6 | 25 | 11.6 | <0.001 |
|
| |||||||||
| History of hypertension | <0.001 | ||||||||
| Never hypertensive | 149 | 56.4 | 61 | 29.3 | 159 | 66.3 | 98 | 52.7 | |
| Untreated hypertensive | 23 | 8.7 | 36 | 17.3 | 19 | 7.9 | 23 | 12.4 | |
| Treated hypertensive | 92 | 34.8 | 111 | 53.4 | 62 | 25.8 | 65 | 34.9 | |
|
| |||||||||
| Treated for high cholesterol | 50 | 19.2 | 36 | 17.1 | 34 | 14.5 | 38 | 20.7 | 0.49 |
|
| |||||||||
| Left ventricular hypertrophy on ECG | 22 | 7.6 | 36 | 15.1 | 11 | 4.2 | 19 | 8.9 | <0.001 |
|
| |||||||||
| Aspirin use | 79 | 26.6 | 62 | 25.9 | 60 | 22.4 | 51 | 23.6 | 0.96 |
|
| |||||||||
| Statin use | 27 | 9.1 | 17 | 7.1 | 15 | 5.6 | 18 | 8.3 | 0.86 |
|
| |||||||||
| History of CVD† | 47 | 16.3 | 64 | 27.6 | 34 | 12.8 | 31 | 14.8 | 0.005 |
| Estrogen Trial | Estrogen + Progestin Trial | P-Value* | |||||||||||
| Control | Ischemic Stroke | Control | Ischemic Stroke | ||||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | ||
|
| |||||||||||||
| Age at screening | 297 | 67.3 | 6.5 | 239 | 67.3 | 6.4 | 268 | 67.4 | 6.7 | 216 | 67.6 | 6.6 | 0.73 |
|
| |||||||||||||
| Body-mass index (kg/m2) | 297 | 28.8 | 5.8 | 238 | 30.1 | 5.5 | 266 | 28.0 | 5.6 | 213 | 28.2 | 5.0 | 0.02 |
|
| |||||||||||||
| Waist/hip ratio | 295 | 0.8 | 0.1 | 237 | 0.9 | 0.1 | 266 | 0.8 | 0.1 | 216 | 0.8 | 0.1 | <0.001 |
|
| |||||||||||||
| Systolic BP (mm Hg) | 297 | 132.8 | 17.0 | 239 | 139.8 | 18.9 | 268 | 129.2 | 16.5 | 216 | 137.0 | 18.7 | <0.001 |
|
| |||||||||||||
| Diastolic BP (mm Hg) | 297 | 76.3 | 9.3 | 239 | 77.8 | 9.2 | 268 | 74.4 | 9.0 | 216 | 76.4 | 10.3 | 0.002 |
|
| |||||||||||||
| Total TFPI (ng/mL) | 288 | 86.2 | 19.2 | 224 | 87.8 | 20.3 | 250 | 87.7 | 20.1 | 184 | 89.0 | 21.0 | 0.35 |
|
| |||||||||||||
| Free TFPI (ng/mL) | 288 | 18.6 | 12.3 | 224 | 21.2 | 12.8 | 250 | 18.1 | 11.0 | 184 | 19.0 | 11.6 | 0.002 |
|
| |||||||||||||
| TFPI Activity (%) | 288 | 111.0 | 24.4 | 223 | 110.2 | 27.7 | 250 | 114.1 | 26.0 | 184 | 117.4 | 28.7 | 0.86 |
|
| |||||||||||||
| nAPCsr (ratio) | 236 | 3.8 | 2.5 | 181 | 3.6 | 2.4 | 218 | 3.4 | 2.0 | 167 | 3.2 | 2.0 | 0.11 |
MI, PCI, CABG, CHF, angina, stroke, or atrial fibrillation
The p values quantify the marginal association of each baseline characteristic and biomarker with ischemic stroke and are obtained from logistic regression models in combined trials adjusted for treatment assignment (CEE, CEE placebo, CEE+MPA, CEE+MPA placebo) using a 1-df test for association except for the categorical values ethnicity, smoking status, alcohol use, physical activity, and history of hypertension.
Table 2.
Adjusted Ischemic Stroke Risk per Standard Deviation Higher Baseline Tissue Factor Pathway Inhibitor and Activated Protein C Resistance (N=455 ischemic strokes, N=565 controls)
| Odds Ratio per SD Increase (95% Confidence Interval)* | ||||
|---|---|---|---|---|
| Estrogen Trial | Estrogen + Progestin Trial | Combined Trials† | P-Value‡ | |
| Total TFPI, ng/mL | 1.06 (0.86, 1.31) | 1.02 (0.82, 1.28) | 1.08 (0.93, 1.25) | 0.329 |
| Free TFPI, ng/mL | 1.37 (1.11, 1.70) | 0.95 (0.74, 1.21) | 1.17 (1.01, 1.37) | 0.039 |
| TFPI Activity, % | 0.88 (0.71, 1.08) | 1.17 (0.93, 1.47) | 1.04 (0.90, 1.21) | 0.556 |
| nAPCsr, ratio | 0.87 (0.69, 1.10) | 0.86 (0.66, 1.11) | 0.89 (0.75, 1.05) | 0.153 |
Odds ratio for ischemic stroke compared with controls per standard deviation of biomarker (in controls) from logistic regression models adjusted for treatment assignment (CEE, CEE placebo, CEE+MPA, CEE+MPA placebo), interaction with treatment assignment, age, race, BMI, waist-hip ratio, smoking, alcohol use, physical activity, diabetes mellitus, prevalent cardiovascular disease (including atrial fibrillation), systolic and diastolic blood pressure, LVH on ECG, use of antihypertensive medications, aspirin, statins, and ever treated for high cholesterol.
Covariate adjustment for hysterectomy status and as in the preceding footnote.
P values are based on logistic regression models in combined trials using a 1-df test for association. Covariate adjustment as in the preceding footnotes.
Table 3.
Associations of Baseline Tissue Factor Pathway Inhibitor and Activated Protein C Resistance with Ischemic Stroke Risk by Treatment Assignment
| Odds Ratio per SD Increase (95% Confidence Interval)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estrogen Trial | Estrogen + Progestin Trial | ||||||||
| CEE | CEE Placebo | CEE+MPA | CEE+MPA Placebo | P Value for Interaction† | |||||
| N | OR (CI) | N | OR (CI) | N | OR (CI) | N | OR (CI) | ||
| Total TFPI, ng/mL | 264 | 1.16 (0.88, 1.52) | 248 | 1.00 (0.75, 1.33) | 236 | 1.13 (0.84, 1.49) | 198 | 1.01 (0.72, 1.41) | 0.408 |
| Free TFPI, ng/mL | 264 | 1.55 (1.15, 2.08) | 248 | 1.21 (0.91, 1.60) | 236 | 0.81 (0.59, 1.12) | 198 | 1.16 (0.82, 1.63) | 0.861 |
| TFPI Activity, % | 263 | 0.93 (0.71, 1.21) | 248 | 0.91 (0.68, 1.22) | 236 | 1.23 (0.92, 1.65) | 198 | 1.18 (0.87, 1.61) | 0.946 |
| nAPCsr, ratio | 216 | 1.02 (0.74, 1.39) | 201 | 0.79 (0.58, 1.07) | 208 | 0.99 (0.72, 1.37) | 177 | 0.78 (0.55, 1.10) | 0.134 |
From logistic regression models adjusted for age, race, BMI, waist-hip ratio, smoking, alcohol use, physical activity, diabetes mellitus, prevalent cardiovascular disease (including atrial fibrillation), systolic and diastolic blood pressure, LVH on EKG, use of antihypertensive medications, aspirin, statins, and ever treated for high cholesterol.
P values for the interaction in combined trials of active treatment/placebo X biomarker on ischemic stroke risk based on a 1-df test for biomarkers. Covariate adjustment for hysterectomy status and as in the preceding footnote.
Free TFPI was inversely related to nAPCsr at baseline (linear regression β (SE) -0.84 (0.18), p<0.0001) and at 1 year (β -1.41 (0.19), p<0.0001). TFPI was correlated with lipoproteins but adding lipoprotein covariates to the models did not alter any results. PHT variably decreased TFPI levels and increased nAPCsr compared to no changes in the placebo group, with mean net decreases of −19, -28, and -22% respectively for total, free and TFPI activity and an increase of 65% for nAPCsr (Table 4 and Figure 1). In the subset with known FVL carrier status baseline mean (SD) nAPCsr values were 4.9 (2.2) in the 25 FVL heterozygotes and 7.2 in the single homozygote, compared to a mean of 3.0 (1.9) in non-carriers. Baseline and year 1 nAPCsr exceeded 2.0 in all but one of the heterozygotes; however, their values were overlapped completely by those of known non-carriers.
Table 4.
Change in Tissue Factor Pathway Inhibitor and Activated Protein C Resistance from Baseline to Year 1
| Median (Interquartile Range) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estrogen Trial | Estrogen + Progestin Trial | ||||||||
| CEE | CEE Placebo | CEE+MPA | CEE+MPA Placebo | P Value for Change* | |||||
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | ||
| Total TFPI, ng/mL | 226 | -18.10 (21.70) | 207 | -1.40 (16.60) | 195 | -17.40 (19.00) | 154 | -1.40 (15.40) | <0.001 |
| Free TFPI, ng/mL | 226 | -3.60 (8.00) | 207 | 0.20 (6.90) | 195 | -3.10 (6.40) | 154 | 0.30 (6.10) | <0.001 |
| TFPI Activity, % | 225 | -27.00 (30.00) | 207 | -4.00 (22.00) | 195 | -25.00 (29.00) | 154 | 0.00 (19.00) | <0.001 |
| nAPCsr, ratio | 147 | 1.60 (3.22) | 147 | -0.16 (2.16) | 128 | 1.45 (2.54) | 128 | 0.14 (1.73) | <0.001 |
P values are from a paired t test (per participant) of change in biomarker in combined trials during hormone treatment compared to control adjusted for hysterectomy status, baseline levels of biomarkers, and the same covariates as in Table 3.
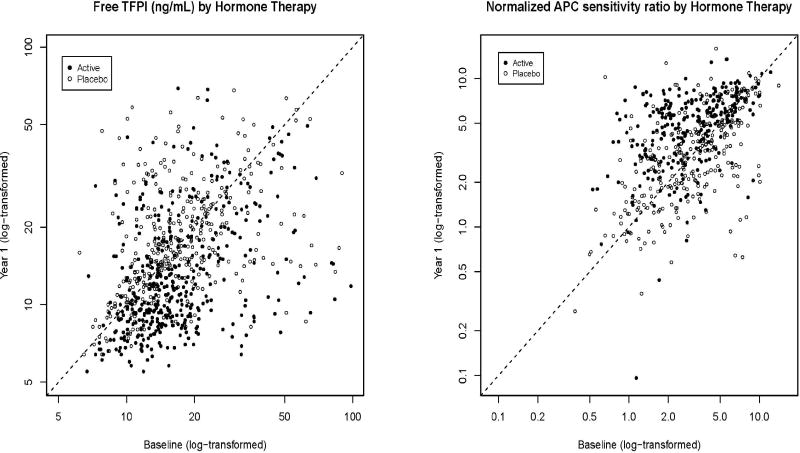
Figure 1.
Free TFPI and normalized APC sensitivity ratio at baseline and year 1 in women randomized to active hormone therapy or placebo therapy (N=782 with repeat measures of TFPI, N=550 of nAPCsr).
Change in free TFPI did not modify stroke risk (interaction p=0.452, Table 5). Though women in upper and lower tertiles of change in nAPCsr appeared to be at higher risk of ischemic stroke on HT, the interaction was not significant (interaction p=0.971 in linear models, p=0.38 in quadratic models). Models with change in TFPI or nAPCsr added as covariate showed no evidence that change in these biomarkers mediated the effect of PHT on ischemic stroke risk.
Table 5.
Effect of Hormone Therapy on Ischemic Stroke Risk by Change in Tissue Factor Pathway Inhibitor and Activated Protein C Resistance
| First Tertile | Second Tertile | Third Tertile | |||||
|---|---|---|---|---|---|---|---|
| Change Value | OR (95% CI) | Change Value | OR (95% CI) | Change Value | OR (95% CI) | P Value for Interaction* | |
| Total TFPI, ng/mL | < -17.5 | 1.38 (0.65, 2.94) | -17.6 - -2.4 | 2.06 (1.16, 3.64) | >-2.4 | 2.03 (1.08, 3.81) | 0.769 |
| Free TFPI, ng/mL | < -4.1 | 2.10 (1.08, 4.09) | -4.1 - 0.6 | 1.27 (0.72, 2.24) | > 0.6 | 2.62 (1.46. 4.71) | 0.452 |
| TFPI Activity, % | < -25.0 | 1.29 (0.59, 2.80) | -25 - -4 | 2.07 (1.14, 3.76) | > -4.0 | 1.74 (0.93, 3.28) | 0.714 |
| nAPCsr, ratio | < -0.1 | 2.29 (1.08, 4.86) | -0.2 - 1.5 | 1.09 (0.54, 2.22) | > 1.5 | 2.60 (1.14, 5.90) | 0.971 |
P values for interaction in combined trials of active treatment/placebo X change in biomarker adjusting for hysterectomy status, baseline level of biomarkers, and the same covariates as in Table 3, based on 317 cases and 465 controls for TFPI and 220 cases and 330 controls for nAPCsr. Tertile cutpoints for change are derived from controls.
Secondary analyses restricted to adherent participants yielded results similar to those for the overall study. Models comparing the extreme deciles of TFPI or nAPCsr with values below or above the median, respectively, did not yield significant associations with ischemic stroke for baseline values or for change at 1 year. In other secondary analyses the findings for total stroke were similar in direction and strength to those for ischemic stroke, while hemorrhagic stroke showed no evidence of association with TFPI or nAPCsr. Subtypes of stroke by TOAST criteria indicated that lacunar strokes were inversely associated with baseline nAPCsr, OR 0.74 (0.57, 0.96), p= 0.025; however, nAPCsr did not interact with treatment on ischemic stroke. Higher baseline levels of free TFPI were not associated with large artery atherosclerotic strokes, but interacted with treatment to increase risk (p=0.008).
DISCUSSION
Even though PHT induced substantial increases in nAPCsr and decreases in TFPI, these changes were not related to the increased risks of ischemic stroke observed in the WHI trials. The mechanisms underlying the increased stroke risks on postmenopausal hormone therapy remain elusive, though hemostatic mechanisms remain the most likely culprits with increases in D-dimer implicated in prior analyses of a subset of WHI data.3 Without knowledge of a mechanism it is difficult to design strategies to prevent this complication of oral PHT therapy. Transdermal estradiol does not appear to share the pro-thrombotic potential of oral estrogens with respect to venous thrombosis or stroke, but randomized trial evidence is lacking.23,24
The relationships of nAPCsr and TFPI to ischemic stroke risk are complex. Contrary to our hypothesis that reduced anti-coagulant function would increase risk of ischemic stroke, in this study baseline free TFPI levels were higher in cases compared to controls while no significant differences were observed for nAPCsr. The findings for TFPI are consistent with previous studies of carotid atherosclerosis, arterial stiffness, and ischemic heart disease.14,15,16, 25 Previous case-control studies did not find convincing evidence that FVL is associated with stroke.26
In the subset of lacunar strokes nAPCsr values were significantly lower in stroke cases than in controls. Lacunar strokes result from pathology of the small perforating arteries supplying the deep subcortical areas of the brain. Previous work has suggested that lacunar arteriopathy may differ from occlusive atherothombosis of the large arteries responsible for other ischemic strokes, and that the risk factor profile of lacunar stroke may differ from that of non-lacunar stroke.27 Higher free TFPI levels increased the risk of large vessel atherosclerotic stroke due to hormone therapy. However, these subgroup findings may have occurred by chance due to the multiple testing performed.
The 5.7% prevalence of FVL ascertained by genotype is similar to that reported in the literature9 but nAPCsr did not clearly discriminate FVL carriers from non-carriers. This may be due to high overall nAPCsr values in this study compared to values reported in the literature, making the window for discrimination smaller. The high values are likely caused by the fact that APCsr determined with the ETP-based APC resistance test is normalized by dividing through the APCsr measured in normal pooled plasma.20 Differences in pre-analytical variables such as venipuncture, citrate concentration, centrifugation, plasma handling, and storage of the WHI plasma and the normal pooled plasma used in the laboratory may result in high sensitivity of pooled plasma for APC compared to WHI plasma.
Strengths of the current study include its setting in the context of a randomized controlled clinical trial, which allows for an unbiased assessment of treatment effects on hemostatic factors and stroke. Limitations include the relatively small number of strokes overall and the large number of strokes of undetermined etiology. Only large interactions of treatment with biomarkers could have been detected; however the primary analysis of interaction by change in biomarker offered no hint of potential effect modification. Another limitation is the variability in the laboratory measurements, especially for free TFPI. Clinical trial participants may differ from the general population and the results may not be generalizable.
We conclude that changes nAPCsr and TFPI in response to PHT do not modify or mediate the increased ischemic stroke risk due to hormone therapy. Higher baseline free TFPI was associated with overall risk of ischemic stroke and with increased risk of large vessel atherosclerotic strokes but the direction of these associations was the opposite of what one might have expected from studies of venous thrombo-embolism. Future studies are needed to confirm the apparent protective association of nAPCsr with lacunar stroke and to explore the biology of this association.
Acknowledgments
Sources of Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The study drugs were provided by Wyeth Research (St. Davids, Pa). The National Institutes of Health had input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. Wyeth did not participate in any aspect of the aforementioned.
Footnotes
Disclosures: None.
Contributor Information
Jacques E Rossouw, National Heart, Lung, and Blood Institute, Bethesda, MD.
Karen C Johnson, University of Tennessee Health Science Center, Memphis, TN kjohnson@uthsc.edu.
Mary Pettinger, Fred Hutchinson Cancer Research Center, Seattle, WA mpetting@whi.org.
Mary Cushman, University of Vermont, Burlington, VT Mary.Cushman@uvm.edu.
Per Morten Sandset, Oslo University Hospital, Oslo, Norway p.m.sandset@medisin.uio.no.
Lewis Kuller, University of Pittsburgh, Pittsburgh, PA kullerl@edc.pitt.edu.
Frits Rosendaal, Leiden University Medical Center, Leiden, The Netherlands F.R.Rosendaal@lumc.nl.
Jan Rosing, Maastricht University, Maastricht, The Netherlands j.rosing@bioch.unimaas.nl.
Sylvia Wasserthal-Smoller, Albert Einstein College of Medicine, New York, NY sylvia.smoller@einstein.yu.edu.
Lisa W Martin, George Washington University, Washington, DC lwmartin@mfa.gwu.edu.
JoAnn E Manson, Brigham and Women’s Hospital, Boston, MA jmanson@rics.bwh.harvard.edu.
Kamakshi Lakshminarayan, University of Minnesota, Minneapolis, MN laksh004@umn.edu.
Jose G Merino, National Institute of Neurological Disorders and Stroke, Bethesda, MD j.merino@mac.com.
John Lynch, National Institute of Neurological Disorders and Stroke, Bethesda, MD lynchj@ninds.nih.gov.
References
- 1.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 3.Kooperberg C, Cushman M, Hsia J, Robinson JG, Aragaki AK, Lynch JK, et al. Can biomarkers identify women at increased stroke risk? The Women’s Health Initiative Hormone Trials. PLoS Clin Trials. 2007;2:e28. doi: 10.1371/journal.pctr.0020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292:1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 5.Curb JD, Prentice RL, Bray PF, Langer RD, Van Horn L, Barnabei VM, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med. 2006;166:772–780. doi: 10.1001/archinte.166.7.772. [DOI] [PubMed] [Google Scholar]
- 6.Sandset Per Morten, Hoibraaten E, Eilertsen AL, Dahm A. Mechanisms of thrombosis related to hormone therapy. Thrombosis Research. 2009;123(Suppl. E):S70–S73. doi: 10.1016/S0049-3848(09)70015-5. [DOI] [PubMed] [Google Scholar]
- 7.Hoibraaten E, Qvigstad, Andersen TO, Mowinckel M-C, Sandset PM. The effects of hormone replacement therapy (HRT) on hemostatic variables in women with previous venous thromboembolism-results from a randomized, double-blind clinical trial. Thromb Haemost. 2001;85:773–781. [PubMed] [Google Scholar]
- 8.Hoibraaten E, Mowinckel M-C, De Ronde H, Bertina RM, Sandset PM. Hormone replacement therapy and acquired resistance to protein C: results of a randomized, double-blind, placebo-controlled trial. British J Haematol. 2001;115:415–420. doi: 10.1046/j.1365-2141.2001.03111.x. [DOI] [PubMed] [Google Scholar]
- 9.Curvers J, Thomassen MCLGD, Nicolaes GAF, Van Oerle R, Hamulyak K, Hemker HC, et al. Acquired APC resistance and oral contraceptives: differences between two functional tests. Br J Haematol. 1999;105:88–94. [PubMed] [Google Scholar]
- 10.Van Vliet HAAM, Bertina RM, Dahm AEA, Rosendaal FR, Rosing J, Sandset PM, et al. Different effects of oral contraceptives containing different progestogens on protein S and tissue factor inhibitor. J Thromb Haemost. 2008;6:346–351. doi: 10.1111/j.1538-7836.2008.02863.x. [DOI] [PubMed] [Google Scholar]
- 11.Duering C, Kosch A, Langer C, Thedieck S, Nowak-Gottl U. Total tissue factor pathway inhibitor is an independent risk factor for pediatric venous thromboembolism and stroke. Thromb Haemost. 2004;92:701–712. doi: 10.1160/TH04-05-0293. [DOI] [PubMed] [Google Scholar]
- 12.Abumiya T, Yamaguchi T, Terasaki T, Kowakawa T, Kario K, Kato H. Decreased plasma tissue factor inhibitor activity in ischemic stroke patients. Thromb Haemost. 1995;74:1050–1054. [PubMed] [Google Scholar]
- 13.Zunker P, Hohenstein C, Plendl H-J, Zeller JA, Caso V, Georgiadis D, et al. Activated protein C resistance and acute ischemic stroke: relation to stroke causation and age. J Neurol. 2001;248:701–704. doi: 10.1007/s004150170117. [DOI] [PubMed] [Google Scholar]
- 14.Novo G, Caplice N, Tantillo R, Bonura F, Simari R, Novo S. TFPI antigen and activity levels in patients with asymptomatic atherosclerosis and target organ acute and chronic complications. Int Angiol. 2005;24:366–371. [PubMed] [Google Scholar]
- 15.Mitchell CT, Kamineni A, Palmas W, Cushman M. Tissue factor inhibitor pathway inhibitor, vascular risk factors and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;207:277–283. doi: 10.1016/j.atherosclerosis.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regnault V, Perret-Guillaume C, Kearney-Schwartz A, Max JP, Labat C, Louis H, et al. Tissue factor pathway inhibitor: a new link among arterial stiffness, pulse pressure, and coagulation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2011;31:1226–1232. doi: 10.1161/ATVBAHA.110.221911. [DOI] [PubMed] [Google Scholar]
- 17.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of Conjugated Equine Estrogen in Postmenopausal Women with Hysterectomy. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 19.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Dahm A, Van Hylckama Vlieg A, Bendz B, Rosendaal F, Bertina RM, et al. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood. 2003;101:4387–4392. doi: 10.1182/blood-2002-10-3188. [DOI] [PubMed] [Google Scholar]
- 21.Ostergaard PB, Beck TC, Orsted H, Svendsen A, Nordfang O, Sandset PM, et al. An enzyme linked immunosorption assay for tissue factor pathway inhibitor. Thromb Res. 1997;87:4447–4459. doi: 10.1016/s0049-3848(97)00161-8. [DOI] [PubMed] [Google Scholar]
- 22.Bendz B, Andersen TO, Sandset PM. A new sensitive chromogenic substrate assay of tissue factor pathway inhibitor type 1. Thromb Res. 2000;97:463–472. doi: 10.1016/s0049-3848(99)00183-8. [DOI] [PubMed] [Google Scholar]
- 23.Canonico M, Fournier A, Carcaillon L, Olie V, Plu-Bureau G, Oger E, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscl Thromb Vasc Biol. 2010;30:136–137. doi: 10.1161/ATVBAHA.109.196022. [DOI] [PubMed] [Google Scholar]
- 24.Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- 25.Falciani M, Gori AM, Fedi S, Chiarugi L, Simonetti I, Dabizzi RP, et al. Elevated tissue factor and tissue factor pathway inhibitor circulating levels in ischaemic heart disease patients. Thromb Haemost. 1998;79:495–499. [PubMed] [Google Scholar]
- 26.Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias and arterial stroke. Can it cause more harm than good? Stroke. 2010;41:2985–2990. doi: 10.1161/STROKEAHA.110.595199. [DOI] [PubMed] [Google Scholar]
- 27.Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, et al. Differing risk factor profiles of ischemic stroke subtypes. Evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–629. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]