Abstract
Background and Purpose
Pyruvate is known to be cytoprotective through antioxidant and anti-inflammatory mechanisms. We tested the hypothesis that pyruvate protects brain against ischemia-reperfusion injury by inducing endogenous erythropoietin (EPO) expression.
Methods
Pyruvate’s protective effect was evaluated in C6 glioma cells and HT22 neuronal cells subjected to transient oxygen glucose deprivation (OGD). Cell viability (calcein AM assay) and expression of HIF-1α, EPO, Akt and Erk (immunoblot) and EPO receptor (RT-PCR) were analyzed. Transient focal cerebral ischemia in rats was induced by 2h middle cerebral artery occlusion followed by 24h reperfusion. Pyruvate or saline was infused from 60 min occlusion until 30 min reperfusion. Lesion volume and DNA fragmentation were assessed by 2,3,5-triphenyltetrazolium staining and TUNEL assay, respectively. Immunoblots were conducted to determine cerebral EPO contents.
Results
Pyruvate increased cell viability, HIF-1α, EPO, and Akt phosphorylation. siRNA suppression of HIF-1α and EPO abolished pyruvate-induced cytoprotection. In the rat stroke model, pyruvate reduced lesion volume by 84% and DNA fragmentation by 77% vs. controls; increased EPO content paralleled these cerebroprotective actions of pyruvate.
Conclusions
Pyruvate activation of the HIF-1α-EPO signaling cascade in neurons and glia could protect brain from ischemia-reperfusion injury.
Introduction
Despite decades of research and promising results from animal studies, effective treatments to protect brain from ischemic stroke have proven elusive. Erythropoietin (EPO) has been found to be neuroprotective in stroke models.1 However, because the blood-brain barrier (BBB) limits EPO entry into the brain parenchyma,2 high doses are required which can produce untoward side effects including hypercoagulability and polycythemia in clinical practice.3,4 Strategies to lower the EPO dosages necessary to protect ischemic brain merit investigation.
Preclinical studies have shown that mammalian brain can synthesize substantial amounts of EPO.5,6 Astrocytes6 and neurons7 are the major sources of EPO in brain. Moreover, EPO’s membrane receptors (EPOR) are expressed in brain.8 Therefore, alternative approaches that induce endogenous EPO production in brain might obviate the detrimental side effects of EPO.
Pyruvate has proven to be protective in cerebropathological conditions,9,10 but its mechanisms remain unclear. We demonstrated that pyruvate-enriched cardioplegia induced EPO mRNA and protein expression in swine myocardium undergoing cardiopulmonary bypass.11 Moreover, monocarboxylate transporters in the BBB12 and plasma membranes13 efficiently deliver circulating pyruvate to the brain parenchyma.
Erythropoietin is the first identified target gene induced by hypoxia inducible factor-1 (HIF-1), a key transcription factor for cellular responses to hypoxia.14 Pyruvate stabilizes the subunit of HIF-1, HIF-1α, by inhibiting its proteosomal degradation.15 Accordingly, pyruvate-enriched cardioplegia increased myocardial HIF-1α.11
This study tested the hypotheses that pyruvate induces EPO signaling, and interference with EPO’s signaling pathways abrogates pyruvate-induced protection in neuronal and glial cells, thereby implicating EPO as a pivotal mediator of pyruvate cerebroprotection. Additionally, we determined whether exogenous pyruvate can protect the rat brain from ischemic stroke, and whether this protection parallels EPO induction within the brain.
Materials and Methods
Hypoxia-reoxygenation of neuronal and glial cells
Glia and neurons are the major cell types in brain; therefore, delineating cytoprotective signaling cascades in these cells can provide crucial information regarding pyruvate’s protective mechanisms against ischemic stroke. Mouse neuronal HT22 cells were a gift from Dr. David Schubert, Salk Institute, San Diego, CA, and GFAP-positive rat C6 glioma cells were obtained from ATCC (Rockville, MD). For hypoxia/reoxygenation (H/R), cells were switched to glucose- and fetal bovine serum (FBS)-free DMEM containing 0, 5 or 10 mM pyruvate, and incubated in a hypoxic atmosphere (0.5% O2) for 12h followed by 24h of reoxygenation and renewed D-glucose (11 mM) availability.
Cell viability
Cell viability was determined with calcein AM assay. C6 glial and HT22 neuronal cells were assigned to control or pyruvate groups of 6 independent trials, each in sextuplicate. Cells were incubated with 1 µM calcein AM at 37°C for 10 min. Fluorescence was measured with excitation/emission at 485/530 nm.
Immunoblot of hypoxia-responsive proteins
Contents of HIF-1α, EPO signaling pathway components, and actin were analyzed by immunoblotting of brain extracts and cell lysates. Protein concentrations were measured by the Bradford assay16 to ensure equal loading of 30 µg/lane. Primary antibodies were mouse monoclonal antibodies against HIF-1α, EPO, Erk/p-Erk, Akt/p-Akt, and actin (Santa Cruz Biotechnology). Goat anti-mouse secondary antibody was obtained from Jackson Immunoresearch (West Grove, PA). Protein bands were quantified (Ultraviolet Products, Upland, CA) and normalized to actin.
Real time RT-PCR measurements of mRNA
Real time RT-PCR was used to assess abundances of EPOR and actin mRNA in HT 22 neuronal cells. Total RNA was isolated using Trizol™ Regent (Invitrogen). cDNA was synthesized from total RNA with Taqman reverse transcriptase (Applied Biosystems, Foster City, CA). EPOR and β-actin cDNA were amplified in a Smart Cycler II (Cepheid, Sunnyvale, CA) by SYBR Green PCR Master Mix (Promega) and an Applied Biosystems 7300 Real-Time PCR System. Two-step real-time PCR was performed (95°C for 15 sec, 60°C for 60 sec extension and detection, 40 cycles) with specific primers for EPOR (forward: 5’-GTAGGGGCCGTCAGATGAGT-3’; reverse: 5’-CCTTTTCCTGCTCTGTTTGG-3’) and β-actin (forward: 5’- TCTTTTCCAGCCTTCCTTCTTG-3’; reverse: 5’-GCACTGTGTTGGCATAGAGGTC-3’). Abundances of amplified genes were assessed by analysis of cycle threshold.
siRNA silencing of HIF-1α
HIF-1α siRNA transfection was performed with siRNA transfection reagent (Santa Cruz Biotechnology) according to the manufacturer’s protocols. HIF-1α siRNA (2 nM) was mixed with transfection reagent added to neuronal cells (n=6/group) and equivalent concentrations of scrambled sequence siRNA were transfected for negative control. Silencing of HIF-1α transcription was confirmed by immunoblot.
Erythropoietin pathway inhibition
To interrogate the involvement of EPO signaling in pyruvate-induced cytoprotection, soluble EPO receptor (sEPOR, Sigma) was applied to HT22 neuronal (n=15/group) and C6 glial (n=10/group) cells pretreated for 6h with sEPOR.17 After H/R, cell viability was determined as described above.
Surgical preparation for rat stroke experiments
Animal experimentation was approved by the Institutional Animal Care and Use Committee of University of North Texas Health Science Center. Male Sprague-Dawley rats (275–300 g; Charles River, Wilmington, MA) were housed under 12h light:dark cycles and consumed food and water ad libitum. Rats were anesthetized with 1.5% isoflurane and temperature was maintained at 37.0 ± 0.5°C with an electric heating pad. Ventilatory frequency, heart rate and SpO2 were monitored. The right jugular vein was cannulated for pyruvate or normal saline infusion.
Pharmacokinetics of infused pyruvate
Circulating pyruvate concentrations during pyruvate infusion and washout were determined colorimetrically18 in a Shimadzu dual wavelength spectrophotometer (337/417 nm, ε=5.65 M−1·cm−1). Plasma (300 µl) was sampled at 10 min intervals before, during, and after 30 min pyruvate infusion (0.05 mmol·kg−1·min−1), flash-frozen in liquid N2, and extracted in 1 vol. 0.6 N HClO4.
Middle cerebral artery occlusion-reperfusion protocol
Transient focal cerebral ischemia was induced by middle cerebral artery occlusion (MCAO),19 in which the left middle cerebral artery was occluded by a 3-0 monofilament suture for 2h, and then reperfused for 24h by withdrawing the suture. Rats were randomly assigned to control (normal saline, n=8) or pyruvate (n=12) treatments. Sodium pyruvate (1M) prepared in sterile water was infused at 0.05 mmol·kg−1·min−1 for 90 min, from 60 min occlusion until 30 min after reperfusion.
At 24h post-reperfusion, brains were harvested, divided into slices at 3, 5, 7, 9, 11, 13, and 15 mm posterior to the olfactory bulb, stained with 2% 2,3,5-triphenyltetrazolium chloride, and planimetrically analyzed (Image-Pro Plus 4.1, Media Cybernetics, Silver Spring, MD) to determine lesion volume. In another 18 rats (9 control, 9 pyruvate), the cerebral hemispheres were harvested at 24h reperfusion, and the cortex and subcortex were separated, snap frozen in liquid nitrogen, and extracted in buffer containing phosphatase/protease inhibitor cocktail. Proteins were analyzed by immunoblot as described above.
TUNEL detection of DNA fragmentation
Paraffin-embedded brain sections (8 µm) were stained for TUNEL-positive cells with Dead END Fluorescent kit (Promega, Madison, WI) according to the manufacturer’s instructions, and then counterstained with DAPI nuclear stain. The fluorescent signal was visualized and photographed on a Zeiss fluorescence microscope. TUNEL-positive cells were counted in 20× microscope fields from a single section for each animal using a CAST-Grid system (Nikon, Inc). Seven microscope fields were selected within frontoparietal cortices and subcortex as previously described.20
Statistical Analysis
Data are expressed as mean values ± SEM. Single comparisons between 2 groups employed Student’s t-test. Multiple comparison between-hypoxia groups and to normoxic control were accomplished by two-way ANOVA combined with Tukey’s multiple comparison test to identify statistically significant differences. Plasma pyruvate concentrations before and after pyruvate infusion were compared by single-factor ANOVA followed by Tukey’s multiple comparison test. P values < 0.05 were taken to indicate statistically significant effects.
Results
Cytoprotection by pyruvate against hypoxia-reoxygenation
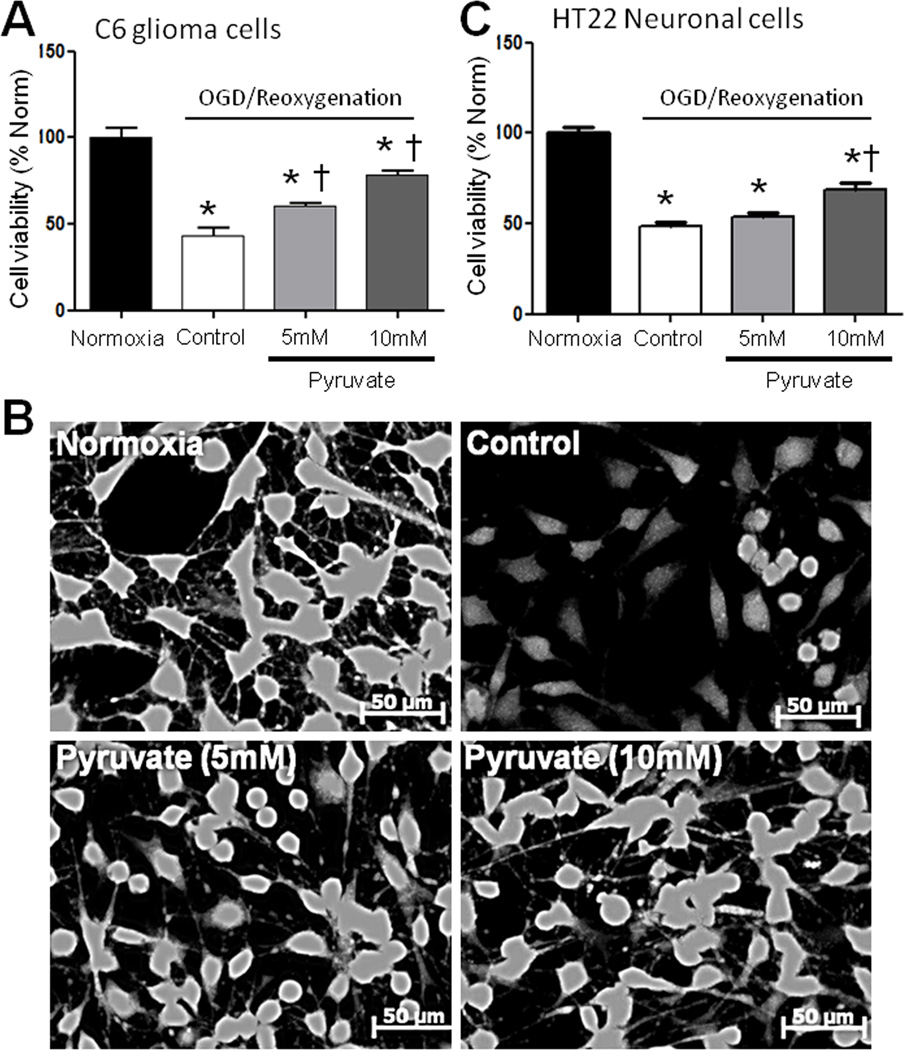
Pyruvate-induced cytoprotection was tested in C6 glioma and HT22 neuronal cell lines subjected to H/R. Twelve hours of hypoxia and 24h reoxygenation killed over 50% of C6 cells (Figure 1A). Five and 10 mM pyruvate increased viability of these cells by 39% and 81%, respectively (Figure 1A, B). A similar response was detected in HT22 cells: 10 mM pyruvate increased viability by 40% vs. control (Figure 1C).
Figure 1. Viability of glial and neuronal cell lines during reoxygenation ± pyruvate.
A) 5 and 10 mM pyruvate dose-dependently protected post-hypoxic C6 glioma cells vs. control. B) Representative calcein AM images under each treatment in C6 glioma cells. C) Ten but not 5 mM pyruvate preserved post-hypoxic HT22 neuronal cells. *P<0.05 vs. Normoxia; †P<0.05 vs. Control.
Pyruvate-induced EPO signaling
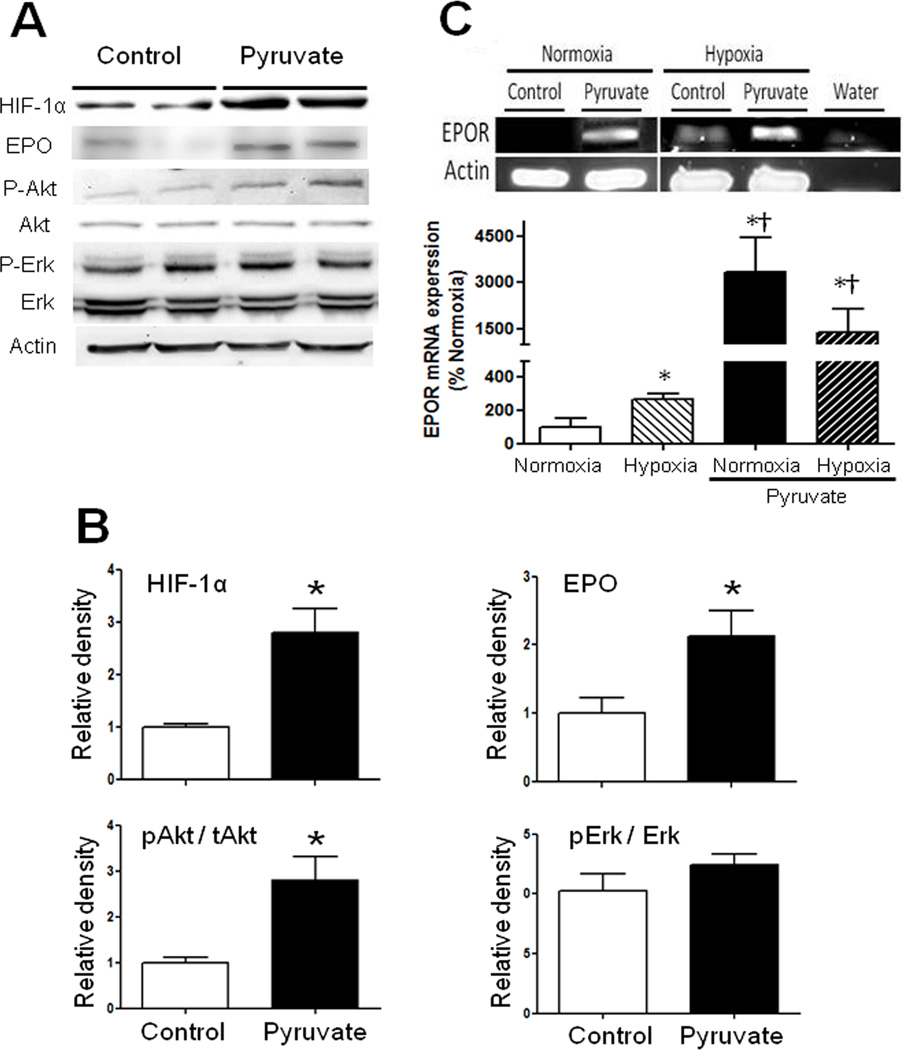
Components of the EPO signaling cascade were examined in HT22 neuronal and C6 glioma cells to determine if pyruvate can activate cytoprotective signaling. Relative to control, 10 mM pyruvate increased HIF-1α nearly 3- and 2-fold in HT22 and C6 cells, respectively. Pyruvate increased EPO contents 2-fold in HT22 cells (Figure 2A,B) and 2.5-fold in C6 cells (Figure S1). Because EPOR primary antibody reportedly cross-reacts with heat shock protein,21 EPOR mRNA expression was analyzed. Pyruvate increased EPOR mRNA abundance in HT22 cells about 20-fold, both before and after H/R (Figure 2C). Pyruvate increased Akt phosphorylation 4- and 2.5-fold in HT22 neuronal and C6 glioma cells, respectively (Figure 2A,B; Figure S1), but did not alter Erk-1/2 phosphorylation (Figure 2A,B).
Figure 2. HIF-1α, EPO, pAkt/Akt, and pErk/Erk contents and EPOR mRNA expression in HT22 neuronal cells.
A) 10 mM pyruvate increased HIF-1α and EPO contents in parallel with Akt activation. B) Immunoblot band densities in pyruvate-treated cells were normalized to respective controls (*P<0.05 vs. Control). C) EPOR mRNA expression was evaluated with real time RT-PCR (*P<0.05 vs. Normoxic control; †P<0.05 vs. Hypoxic control).
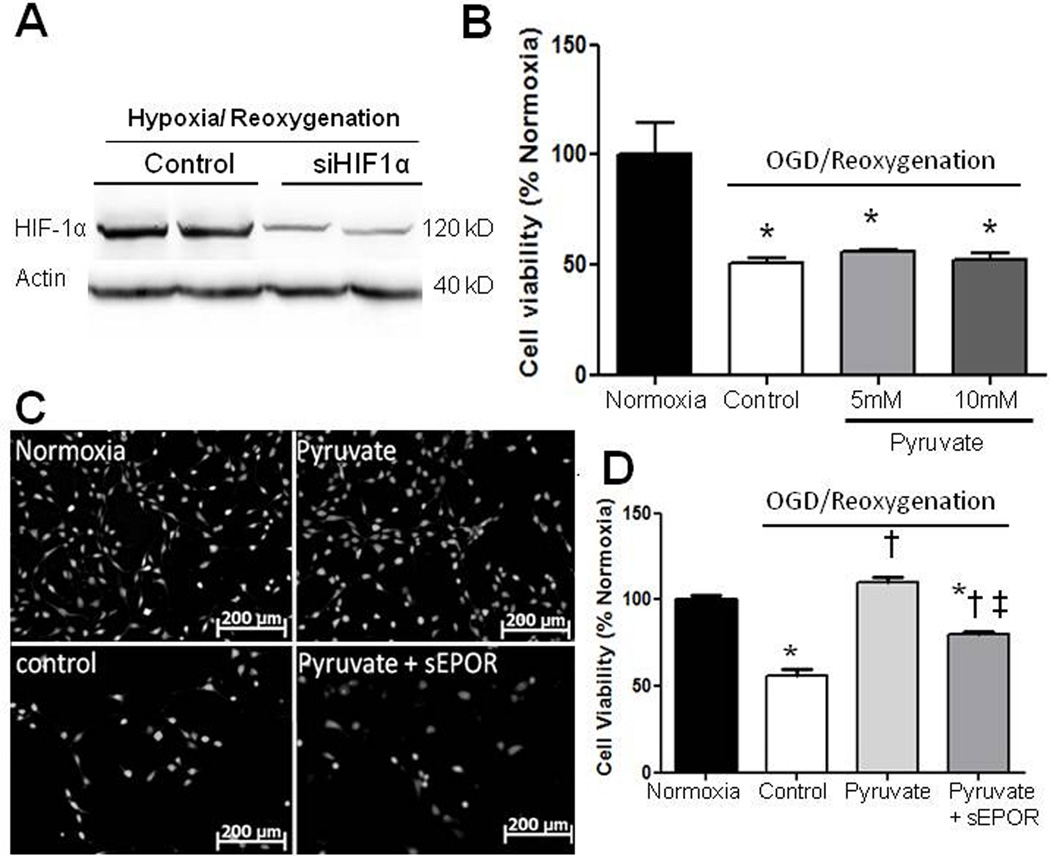
To interrogate the roles of HIF-1α and EPO in pyruvate-induced neuroprotection, HT22 cells were transfected with HIF-1α siRNA, which lowered H/R-induced HIF-1α expression 50% (Figure 3A). HIF-1α siRNA abrogated the cytoprotection of 5 and 10 mM pyruvate (Figure 3B). To further define EPO’s role in protection, HT22 neuronal and C6 glioma cells were incubated with soluble EPOR to bind EPO originating in the cells. This strategy blunted pyruvate-induced protection of HT22 neuronal (Figure 3C,D) and C6 glioma (Figure S2) cells subjected to H/R.
Figure 3. Effects of HIF-1α silencing and suppression of EPO signaling on cell viability.
A) HIF-1α siRNA blunted HIF-1α expression in HT22 neuronal cells. B) HIF-1α silencing abrogated pyruvate protection of hypoxic, glucose-deprived cells (*P<0.05 vs. Normoxia). C, D) Interruption of EPO pathway with soluble EPOR dampened pyruvate-induced protection in HT22 cells. *P<0.05 vs. Normoxia; †P< 0.05 vs. Control; ‡P< 0.05 vs. Pyruvate.
Pyruvate cerebroprotection in in vivo stroke model
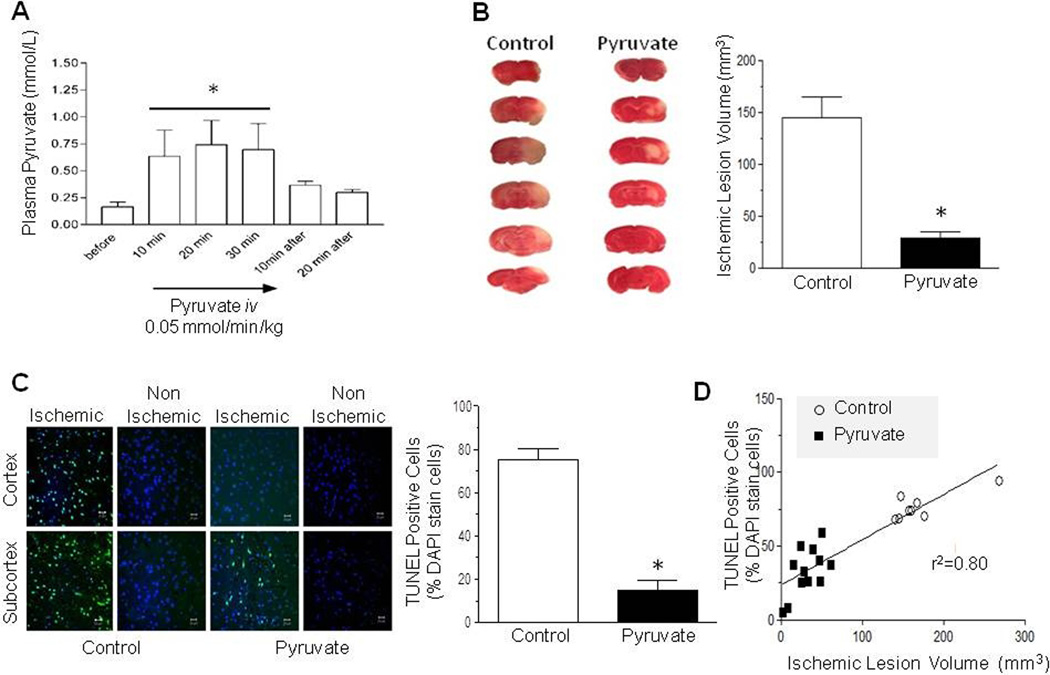
The impact of pyruvate infusion on circulating pyruvate was evaluated. Venous pyruvate concentration rapidly increased at the onset of infusion, stabilized at ~0.7 mM within 10 min, and then subsided after pyruvate infusion was discontinued (Figure 4A). Next, the cerebroprotective effects of pyruvate infusion were examined in rats subjected to 2h occlusion and 24h reperfusion of the MCAO. Pyruvate or normal saline was infused iv for 90 min, from 60 min MCAO until 30 min reperfusion. Pyruvate sharply attenuated lesion volume by 84% vs. control (Figure 4B).
Figure 4. Pyruvate reduces lesion volume and DNA fragmentation following middle cerebral artery occlusion-reperfusion.
A: Plasma pyruvate concentration plateaued during pyruvate infusion and subsided within 20 min post-infusion (*P<0.05 vs. pre-infusion). Pyruvate treatment during cerebral ischemia-reperfusion sharply lowered brain lesion volume (B) and TUNEL positive cells (C: % of DAPI stained cells) in cortex and subcortex 24 h later (* P<0.05 vs. Control). D) Linear correlation between TUNEL positive cells and lesion volume.
The effect of pyruvate on ischemia/reperfusion-induced DNA fragmentation was examined. In control rats, DNA fragmentation in subcortex and cortex was sharply increased vs. non-ischemic contralateral hemisphere. Pyruvate decreased DNA fragmentation by 77% vs. control (Figure 4C). A significant correlation (r2=0.80) emerged between DNA fragmentation and lesion volume at 24h reperfusion (Figure 4D).
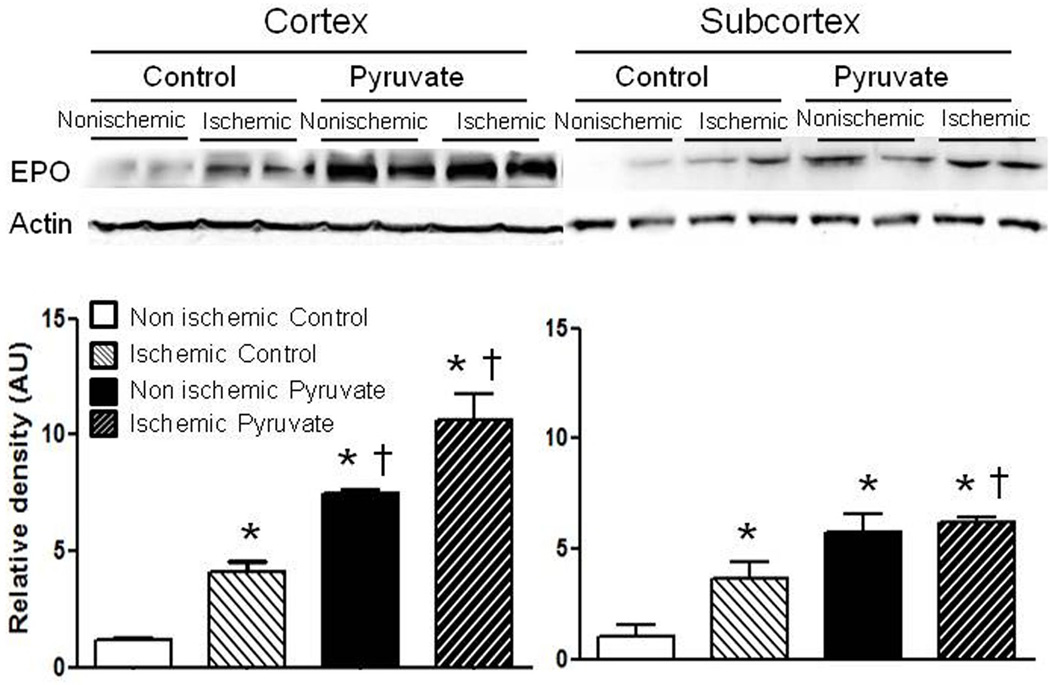
Effect of pyruvate on EPO content in brain after stroke
Erythropoietin contents in ischemic cortex and subcortex were about 4-fold increased vs. contralateral non-ischemic regions (Figure 5). Pyruvate appreciably increased EPO in ischemic and contralateral cortex and subcortex vs. the respective controls. Interestingly, pyruvate did not increase renal EPO content (Figure S3).
Figure 5. Cerebral EPO content after transient cerebral ischemia.
Ischemia and pyruvate augmented EPO contents separately, and more effectively in combination. *P<0.05 vs. non-ischemic control; †P<0.05 vs. ischemic control.
Discussion
This study tested the hypothesis that pyruvate treatment protects the brain from ischemia/reperfusion injury by evoking EPO synthesis and cytoprotective signaling. In vivo and in vitro results demonstrated that pyruvate treatment 1) increased HIF-1α and EPO, 2) activated Akt, 3) enhanced cell viability, 4) decreased lesion volume and 5) suppressed DNA fragmentation.
A pivotal transcription factor, HIF-1 mediates cellular adaptations to hypoxia.22,23 Transcriptional activity of HIF-1 is controlled by regulating cellular content of its α subunit. In the presence of α-ketoglutarate, Fe2+ and normal intracellular O2 concentrations, prolyl residues in HIF-1α are hydroxylated by prolyl hydroxylase, leading to proteosomal degradation and, thus, preventing assembly of HIF-1 heterodimers.22,23 Pyruvate inhibits prolyl hydroxylase by competing with α-ketoglutarate for access to the enzyme’s catalytic domain.15 Recently, we demonstrated pyruvate-enriched cardioplegia increased HIF-1α, EPO and EPOR contents, and suppressed post-bypass inflammation and edema in myocardium of pigs maintained on cardiopulmonary bypass.11 In the brain, astrocytes6 and neurons7 can synthesize EPO,24 EPOR are present in the brain parenchyma,8 and pyruvate readily traverses the BBB.12 Since preclinical studies have shown exogenous EPO to be cerebroprotective, pyruvate induction of endogenous cerebral EPO production could be powerfully cerebroprotective. In accordance with this hypothesis, pyruvate afforded significant protection in the rat stroke model, manifest as marked reductions in ischemic lesion volume and DNA fragmentation. Moreover, pyruvate sharply increased EPO contents in both ischemic and non-ischemic brain.
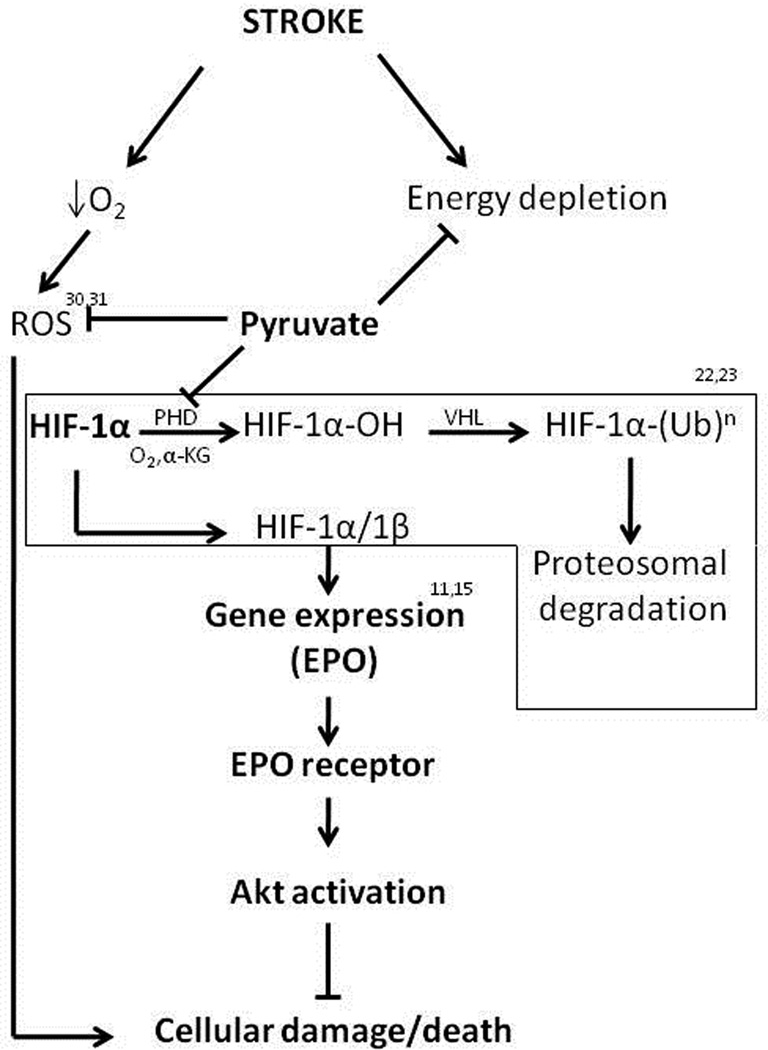
Two strategies were employed to interrogate the cytoprotective actions of the pyruvate-induced HIF-1/EPO axis. First, transfection of cultured neuronal cells with siRNA against the HIF-1α gene abrogated pyruvate-induced cytoprotection against oxygen-glucose deprivation. Secondly, incubation with soluble EPOR to bind EPO attenuated pyruvate’s cytoprotective effect, although some residual cytoprotection was noted. These results substantiate the hypothesis (Figure 6) that HIF-1α stabilization and, consequently, EPO induction were pivotal to the pyruvate-induced cerebroprotection.
Figure 6. Proposed pyruvate-induced cerebroprotective mechanisms.
See text for details. ROS, reactive oxygen species; PHD, prolyl hydroxylase; α-KG, α-ketoglutarate; VHL, von Hippel-Lindau tumor-suppressor protein; (Ub)n, polyubiquitinylation.
Soluble EPOR did not completely block pyruvate-induced cytoprotection, suggesting the involvement of additional, EPO-independent mechanisms. Indeed, HIF-1 activates expression of a variety of protective factors,25,26 that enable cells to survive hypoxic or ischemic insults. Conceivably, these proteins may confer some cytoprotection even when EPO signaling is disabled. Furthermore, a neuronal EPOR isoform, which forms heterodimers with CD131, has been shown to mediate cytoprotection but not hematopoiesis.27 Because EPO is a ligand of both classical and neuronal EPOR isoforms, the use of sEPOR to bind EPO cannot differentiate the potential contributions of the two EPOR isoforms to pyruvate’s cytoprotection.
Although mounting evidence of EPO’s cerebroprotection has been documented in stroke models, EPO’s limited ability to cross the BBB necessitates high dosages for stroke treatment, which might impose hypercoagulability and increased blood viscosity.3,4 Pyruvate readily traverses the BBB and enters the brain parenchyma via monocarboxylate transporters (MCT) in the BBB12 and plasma membranes.13 In rats, MCA occlusion induced a rapid and substantial increase of MCT in neurons, astrocytes, and vascular endothelium, which could increase the capacity for pyruvate uptake.28 Upon entering the brain parenchyma, pyruvate may stabilize HIF-1α in both neurons and astrocytes, thereby driving endogenous EPO synthesis. The endogenous EPO could then activate EPOR in a paracrine or autocrine fashion,29 avoiding the rheological consequences of high doses of exogenous EPO.
Induction of EPO signaling might not be pyruvate’s sole neuroprotective mechanism. A natural intermediary metabolite, pyruvate could function as an energy-yielding fuel for ATP production, and as an antioxidant that neutralizes reactive oxygen and nitrogen intermediates30 and enhances reducing power of the glutathione antioxidant system.31 Given the tight coupling between cerebral blood flow and cerebral metabolism, it is conceivable that the vascular component of the neurovascular unit could also be involved in the protective mechanisms of pyruvate against ischemic stroke. Other pharmacological antioxidants that traverse the BBB also have been found to decrease lesion volume in rodent focal cerebral ischemia.32,33 Nevertheless, the fact that HIF-1α siRNA and sEPOR abrogated pyruvate cytoprotection implicates EPO as an essential component of the protective mechanism, and suggests that, without EPO, pyruvate’s energy-yielding and antioxidant properties may not be sufficient to defend brain from severe ischemia or hypoxia.
Limitations
In this study, the longer-term effects of pyruvate on neuroprotection were not examined. In this regard, intravenous pyruvate administration during cardiopulmonary resuscitation afforded substantial neuroprotection 3d later34 and protracted pyruvate-induced protection against focal cerebral ischemia was reported.35 Other HIF-1-activated cytoprotective proteins could act independently of or in collaboration with EPO to protect brain from ischemia-reperfusion.
Supplementary Material
Acknowledgments
Research support: This work was supported by National Institutes of Health grants R01NS054687 (SY), R01NS054651 (SY), R01NS076975 (RM) and T32 AG020494 (MR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure / Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dame C, Juul SE, Christensen RD. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate. 2001;79:228–235. doi: 10.1159/000047097. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Pierce LJ, Chen GL, Chang KT, Spangrude GJ, Prchal JT. Erythropoietin receptor signaling regulates both erythropoiesis and megakaryopoiesis in vivo. Blood Cells Mol Dis. 2010;44:1–6. doi: 10.1016/j.bcmd.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortega LM, Contreras G. The clinical impact of the physiological effects of erythropoietin and erythropoietin-stimulating agents on the incidence of malignancy, and hypertension: beyond anaemia. Nefrologia. 2009;29:288–294. doi: 10.3265/Nefrologia.2009.29.4.5282.en.full. [DOI] [PubMed] [Google Scholar]
- 5.Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 7.Siren AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101:271–276. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 8.Juul SE, Yachnis AT, Rojiani AM, Christensen RD. Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Pediatr Dev Pathol. 1999;2:148–158. doi: 10.1007/s100249900103. [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Kim YH, Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukushima M, Lee SM, Moro N, Hovda DA, Sutton RL. Metabolic and histologic effects of sodium pyruvate treatment in the rat after cortical contusion injury. J Neurotrauma. 2009;26:1095–1110. doi: 10.1089/neu.2008.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryou MG, Flaherty DC, Hoxha B, Sun J, Gurji H, Rodriguez S, et al. Pyruvate-fortified cardioplegia evokes myocardial erythropoietin signaling in swine undergoing cardiopulmonary bypass. Am J Physiol Heart Circ Physiol. 2009;297:H1914–H1922. doi: 10.1152/ajpheart.01213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller LP, Oldendorf WH. Regional kinetic constants for blood-brain barrier pyruvic acid transport in conscious rats by the monocarboxylic acid carrier. J Neurochem. 1986;46:1412–1416. doi: 10.1111/j.1471-4159.1986.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 13.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993;264:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 14.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Wu CL, Chen SD, Yin JH, Hwang CS, Yang DI. Erythropoietin and sonic hedgehog mediate the neuroprotective effects of brain-derived neurotrophic factor against mitochondrial inhibition. Neurobiol Dis. 2010;40:146–154. doi: 10.1016/j.nbd.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Lamprecht W HF. Pyruvate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1983. pp. 570–578. [Google Scholar]
- 19.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 20.Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott S, Busse L, McCaffery I, Rossi J, Sinclair A, Spahr C, et al. Identification of a sensitive anti-erythropoietin receptor monoclonal antibody allows detection of low levels of EpoR in cells. J Immunol Methods. 2010;352:126–139. doi: 10.1016/j.jim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 23.Sutter CH, Laughner E, Semenza GL. Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci U S A. 2000;97:4748–4753. doi: 10.1073/pnas.080072497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasselblatt M, Ehrenreich H, Siren AL. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol. 2006;18:132–138. doi: 10.1097/00008506-200604000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem. 2002;277:39728–39738. doi: 10.1074/jbc.M204619200. [DOI] [PubMed] [Google Scholar]
- 26.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 27.Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci U S A. 2008;105:10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Vannucci SJ, Philp NJ, Simpson IA. Monocarboxylate transporter expression in the spontaneous hypertensive rat: effect of stroke. J Neurosci Res. 2005;79:139–145. doi: 10.1002/jnr.20312. [DOI] [PubMed] [Google Scholar]
- 29.Lappin TR, Maxwell AP, Johnston PG. EPO's alter ego: erythropoietin has multiple actions. Stem Cells. 2002;20:485–492. doi: 10.1634/stemcells.20-6-485. [DOI] [PubMed] [Google Scholar]
- 30.Mallet RT, Sun J. Antioxidant properties of myocardial fuels. Mol Cell Biochem. 2003;253:103–111. doi: 10.1023/a:1026009519783. [DOI] [PubMed] [Google Scholar]
- 31.Tejero-Taldo MI, Caffrey JL, Sun J, Mallet RT. Antioxidant properties of pyruvate mediate its potentiation of beta-adrenergic inotropism in stunned myocardium. J Mol Cell Cardiol. 1999;31:1863–1872. doi: 10.1006/jmcc.1999.1020. [DOI] [PubMed] [Google Scholar]
- 32.Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK. N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res. 2003;971:1–8. doi: 10.1016/s0006-8993(03)02244-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Tanaka J, Yang L, Yang L, Sakanaka M, Hata R, et al. Protective effect of vitamin E against focal brain ischemia and neuronal death through induction of target genes of hypoxia-inducible factor-1. Neuroscience. 2004;126:433–440. doi: 10.1016/j.neuroscience.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 34.Sharma AB, Barlow MA, Yang SH, Simpkins JW, Mallet RT. Pyruvate enhances neurological recovery following cardiopulmonary arrest and resuscitation. Resuscitation. 2008;76:108–119. doi: 10.1016/j.resuscitation.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi JS, Kim TY, Kyu Kim D, Koh JY. Systemic pyruvate administration markedly reduces infarcts and motor deficits in rat models of transient and permanent focal cerebral ischemia. Neurobiol Dis. 2007;26:94–104. doi: 10.1016/j.nbd.2006.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.