Abstract
Patients with type 2 diabetes, particularly women, are at risk for heart failure. Myocardial substrate metabolism derangements contribute to cardiac dysfunction in diabetic animal models. The purpose of this study was to determine the effects of diabetes and sex on myocardial metabolism and diastolic function in humans, separate from those of obesity.
Thirty-six diabetic subjects (22 women) and 36 non-diabetic, body mass index (BMI)-matched subjects (21 women) underwent positron emission tomography (myocardial metabolism) and echocardiography (structure, function). Myocardial blood flow and oxygen consumption (MVO2) were higher in women than men (P=0.003 and <0.0001, respectively). Plasma fatty acid (FA) levels were higher in diabetics (vs. obese, P<0.003) and sex and diabetes status interacted in its prediction (P=0.03). Myocardial FA utilization, oxidation, and esterification were higher and percent FA oxidation lower in diabetics (vs. obese, P=0.0004, P=0.007, P=0.002, P=0.02). FA utilization and esterification were higher and percent FA oxidation lower in women (vs. men, P=0.03, P=0.01, P=0.03). Diabetes and sex did not affect myocardial glucose utilization, but myocardial glucose uptake/plasma insulin was lower in the diabetics (P=0.04). Left ventricular relaxation was lower in diabetics (P<0.0001) and in men (P=0.001), and diabetes and sex interacted in its prediction (P=0.03).
Sex, type 2 diabetes, or their interaction affect myocardial blood flow, MVO2, FA metabolism, and relaxation separate from obesity’s effects. Sexually dimorphic myocardial metabolic and relaxation responses to diabetes may play a role in the known cardiovascular differences between men and women with diabetes.
INTRODUCTION
The prevalence of type 2 diabetes mellitus in the United States has increased dramatically over the past 30 years, paralleled by increases in the prevalence of obesity (1–3). Both entities are associated with significant cardiovascular morbidity and mortality, with cardiovascular disease as the leading cause of death in diabetics (4, 5). Both obesity and type 2 diabetes are predictors of left ventricular (LV) dysfunction, independent of associated co-morbidities such as coronary artery disease and hypertension (3). Diabetes also increases the mortality risk in patients with LV dysfunction. However, diabetes does not affect men and women in exactly the same way. Women with diabetes have both a higher risk of developing heart failure (4) and a higher risk of dying from heart failure than men with diabetes (6). However, the reasons for sex differences in diabetic cardiovascular disease remain poorly understood.
Results of studies in animal models of diabetes suggest that derangements in myocardial substrate metabolism contribute to the development of cardiac dysfunction and heart failure in diabetes. Specifically, excessive fatty acid (FA) uptake, oxidation and/or storage contribute directly to cardiac dysfunction in these models (7–9). In addition, excessive dependence on FA metabolism in the diabetic heart may impair the heart’s ability to cope with superimposed conditions that require a switch towards glucose metabolism (such as ischemia). Few previous studies evaluated myocardial substrate metabolism rates in patients with type 2 diabetes but without significant coronary artery disease (10, 11). Even fewer measured myocardial fatty acid metabolism (12), and none have evaluated the combined effect of sex and diabetes on myocardial metabolism and function. The results of previous studies of diabetes’ effects on myocardial substrate metabolism in men may not automatically be extrapolated to women with diabetes, given the major effect of sex on myocardial metabolism and blood flow (13,14). It is also not completely clear to what extent obesity, which frequently accompanies type 2 diabetes, may be affecting the differences in myocardial FA metabolism noted between diabetics and nondiabetic controls. The purpose of the present study was to test the hypothesis that both type 2 diabetes and female sex increase the heart’s FA metabolism at the expense of glucose metabolism and that diabetes and sex interact in the determination of cardiac relaxation. To this end, we quantified myocardial metabolism using positron emission tomography (PET), as well as cardiac structure and function using echocardiography in well-phenotyped obese men and women with type 2 diabetes and in body mass index-matched, non-diabetic controls.
METHODS AND PROCEDURES
Study population
Thirty-nine obese, non-diabetic, and 33 diabetic subjects were studied. All subjects completed a comprehensive evaluation that included a history, physical examination, routine blood tests, and a fasting lipid profile. Non-diabetic subjects underwent a 2-hr glucose tolerance test to exclude diabetes mellitus. Subjects with diabetes were treated with metformin with or without a sulfonylurea, were required to be naïve to exogenous insulin, have well-controlled indices of glycemia (glycosylated hemoglobin, HbA1c <7%), be non-smokers, normotensive with or without therapy (defined as a systolic blood pressure <140 mm Hg and a diastolic pressure <90 mm Hg), and have normal total cholesterol (<220 mg/dL) and low-density lipoprotein cholesterol (<160 mg/dL) levels. Subclinical coronary artery disease and other forms of cardiac disease were excluded with a normal electrocardiogram, echocardiogram, and in the diabetics a normal rest/stress echocardiogram. Subjects were excluded who had persistent hypertension despite therapy, a history of coronary artery or other cardiac disease, a history of smoking cigarettes within the last 12 months, or who were pregnant or lactating. Subjects underwent dual-energy x-ray absorptiometry (DXA, Hologic, WDR-1000/w) for determination of body composition. The Human Research Protection Office (Institutional Review Board), the General Clinical Research Center, and the Radioactive Drug Research Committee at the Washington University School of Medicine all approved this study. All subjects signed informed consent before enrollment.
Experimental procedure
Subjects were admitted to the Inpatient Research Unit of the Clinical Research Unit the evening before the positron emission tomography (PET) study. At 6 PM the night before, all subjects ingested a standard meal containing 12 kcal/kg body weight for non-obese subjects and 12 kcal/kg adjusted body weight for obese subjects (adjusted body weight = ideal body weight + [(actual body weight – ideal body weight) × (0.25)]. The following morning, after subjects fasted overnight (12 hours), two 18- or 20- gauge catheters were inserted into both antecubital veins for radiopharmaceutical injections and blood sampling.
PET Imaging Protocol and Analysis. All studies were performed on a commercially available tomograph (Siemens ECAT 961 HR and ECAT 962 HR+, Siemens Medical Systems, Knoxville, Tennessee). Studies were performed under resting conditions starting at 8 AM and ending at approximately 1:30 PM to avoid circadian variations in myocardial metabolism. Measurements of myocardial blood flow, myocardial oxygen consumption (MVO2), and glucose and fatty acid metabolism were obtained with PET tracers 15O-water, 1-11C-acetate, 1-11C-glucose, and 1-11C-palmitate, respectively. In conjunction with well-validated compartmental modeling schemes, measurements of myocardial blood flow (mL/g/min), MVO2, (μmol/g/min) fractional glucose uptake (mL/g/min) and utilization (nmol/g/min), and fractional FA uptake (mL/g/min), utilization and oxidation (nmol/g/min) were performed as previously reported.(15–18) Fractional glucose and FA uptake represents the myocardial extraction fraction of glucose or FA, corrected for blood flow. Glucose and FA utilization reflect the total uptake of substrate by multiplying the fractional uptake by the substrate concentration in the blood. In order to measure intramyocardial FA metabolism, we used compartmental modeling to subdivide FA utilization into myocardial FA oxidation and FA “esterification.” The latter represents all FA utilization, which does not undergo immediate oxidation.
Measurements of plasma insulin, substrates, and insulin resistance
Blood samples were obtained at predetermined intervals during the PET study to measure plasma substrates (glucose, fatty acids, and lactate), and insulin levels. Glucose and lactate levels were measured using a commercially available glucose-lactate analyzer (YSI, Yellow Springs, Ohio). For plasma free fatty acid level determination, blood was collected in tubes containing EDTA. Samples were immediately centrifuged to obtain plasma, which was stored at −80°C until batch analysis. Plasma fatty acid levels were determined using an enzymatic colorimetric method (NEFA-C, Wako Chemicals USA, Richmond, VA). Plasma insulin levels were measured by radioimmunoassay (Linco Research Inc., St Charles, Missouri). Homeostasis model assessment (HOMA) of insulin resistance was calculated as described by Matthews et al. (19). Plasma levels of 11CO2 values and 11C-lactate were measured to correct for the arterial input function during compartmental modeling of the myocardial kinetics of the various metabolic tracers.
Echocardiography
Subjects underwent echocardiographic evaluation immediately following 1-11C-acetate injection and subsequent PET imaging (for measurement of MVO2). Cardiac chamber size and function was measured as previously described (20). Tissue Doppler was used to measure systolic function (Sm) and diastolic relaxation (Em), also as previously described. Cardiac output was calculated = time-velocity integral x LV outflow tract area. Cardiac minute work per gram = (mean arterial pressure x cardiac output)/LV mass. LV efficiency = workg/MVO2 as described in detail in our previous publication (13).
Statistical analysis
T-tests were used to compare outcome measures across sex while one-way analysis of variance provided an assessment of group differences. Subgroup analyses (e.g., between obese men and women) were performed using unpaired t-tests or Wilcoxon tests, as was appropriate. Variables with skewed data were log-transformed as appropriate. Two-factor analysis of variance was used to evaluate the adjusted effect of group and sex on the outcome measures. These were performed initially with the interaction term present. If the interaction was not significant, the significance of group and sex was evaluated after removing the interaction term from the model. The association between a predefined set of potential covariates and the outcome measures was evaluated using Pearson correlation coefficients. Stepwise regression analyses, which required that group and sex remain in the model, then provided a determination of a best set of predictors of selected outcomes. Variables included as potential predictors in these models were those that had a significant univariate correlation with the outcome. In addition to group and sex, variables retained by the stepwise procedure included those that had a P value less than 0.1. All analyses were performed using version 9 of SAS®. A P value of <0.05 was considered to be significant.
RESULTS
Baseline characteristics. Table 1
Table 1.
Subject Characteristics
| Obese | T2DM | P values | |||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Obese vs. T2DM | Men vs. Women | Interaction sex X group | |
| N | 10 | 29 | 12 | 21 | |||
| Age (yrs) | 35±6 | 31±6 | 58±9 | 53±11 | <0.0001 | 0.04 | 0.89 |
| Weight (kg) | 171±11 | 104±20 | 115±21 | 102±15a | 0.81 | 0.61 | 0.98 |
| BMI (kg/m2) | 37±2 | 39±7 | 37±5 | 38±5 | 0.63 | 0.28 | 0.75 |
| Fat Mass (kg) | 37.4±6.5 | 43.9±14.3a | 36.7±10.5 | 40.8±13.1 | 0.57 | 0.12 | 0.72 |
| Percent Fat (%) | 33±4 | 49±14b | 34±6 | 45±3c | 0.42 | <0.0001 | 0.32 |
| Total Cholesterol (mg/dL) | 195±38 | 175±39 | 144±21 | 166±30a | 0.008 | 0.86 | 0.02 |
| Triglycerides (mg/dL) | 240±115 | 96±53b | 151±50 | 135±46 | 0.99 | <0.0001 | 0.0002 |
| LDL (mg/dL) | 115±34 | 106±31 | 70±20 | 95±36a | 0.006 | 0.35 | 0.04 |
| HDL (mg/dL) | 37±7 | 49±13d | 44±11 | 49±7 | 0.47 | 0.003 | 0.16 |
| Hemoglobin A1c (%) | 6.6±0.6 | 6.4±0.6 | 0.65 | NA | |||
| Insulin (μU/mL) | 14.9±4.8 | 9.2±4.8e | 20.4±11.1 | 15.7±10.5 | 0.003 | 0.02 | 0.81 |
| Glucose (μmol/mL) | 5.2±0.4 | 4.7±0.4e | 6.3±1.6 | 6.9±1.9 | <0.0001 | 0.99 | 0.12 |
P<0.05,
P<0.0001,
P<0.0005,
P<0.01,
P<0.005 for differences between men and women in subgroup analysis (e.g., obese men versus obese women)
BMI = body mass index, LDL = low-density lipoprotein, HDL = high-density lipoprotein, NA = not applicable; insulin and glucose measurements are from the time of the PET study.
The diabetic subjects were slightly older than the obese subjects. Importantly, weight and body mass index, fat%, fat mass, plasma triglycerides and high-density lipoprotein (HDL), and sex of the subjects were not different between the groups. Total cholesterol and low-density lipoprotein (LDL) values were slightly lower in the diabetic subjects than the obese. Of note, 69% of the diabetics were taking HMG-CoA reductase inhibitor therapy in order to have their cholesterol levels near that of the obese controls. As expected, the diabetic subjects had higher glucose, and insulin than the obese. Table 2. Lists all statin, metabolic, and vasoactive medication use among the diabetics divided by sex. (The obese were not taking any of these medications). There were no significant differences between the diabetic men and women’s use of hypoglycemic or antihypertensive agents. More diabetic women were taking statins or any lipid-lowering agent compared with the men.
Table 2.
Medications of the Type 2 Diabetic Patients
| Women (total N=33) | Men (total N=16) | P value | |
|---|---|---|---|
| Metformin N (%) | 31 (94%) | 15 (94%) | NS |
| Any sulfonylurea | 7 (21%) | 7 (44%) | NS |
| Acarbose | 1 (3%) | 1 (6%) | NS |
| ACE-inhibitor or ARB | 19 (58%) | 8 (50%) | NS |
| Antihypertensive Rx (other than ACE-inhibitor or ARB) | 13 (39%) | 3 (19%) | NS |
| Any antihypertensive Rx | 24 (73%) | 8 (50%) | NS |
| Statin | 27 (82%) | 7 (44%) | 0.007 |
| Any lipid lowering Rx | 27 (82%) | 8(50%) | 0.02 |
NS = Not significant; ACE = angiotensin converting enzyme; ARB = Angiotensin receptor blocker; Rx = drug
Regarding sex effects, the women in both groups had higher HDL and lower triglycerides than the men. The women also had lower insulin than the men, despite having a higher fat mass and percent fat. Diabetes status and sex interacted in the prediction of triglycerides, total cholesterol, and LDL. In a subgroup analysis of the obese, the women had lower insulin resistance than the men (HOMA 1.88±2.33 vs. 3.25±4.41, respectively P<0.01).
Hemodynamics, cardiac structure, function, and efficiency. Table 3
Table 3.
Hemodynamics, LV Structure, Efficiency, & Systolic Function
| Obese | T2DM | P values | |||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Obese vs. Diabetics | Men vs. Women | Interaction sex X group | |
| Hemodynamics | |||||||
| Systolic blood pressure (mmHg) | 138±9 | 119±13a | 141±17 | 130±17 | 0.0004 | 0.002 | 0.36 |
| Heart rate (bpm) | 70±11 | 70±9 | 63±9 | 67±9 | 0.96 | 0.83 | 0.41 |
| Rate-pressure product (mmHg*bpm) | 9623±2009 | 8327±1377b | 8913±1884 | 8751±1792 | 0.03 | 0.09 | 0.22 |
| LV structure | |||||||
| LV mass (g) | 223±42 | 171±40c | 204±43 | 160±38d | 0.24 | <0.0001 | 0.71 |
| LV mass index (g/m2) | 90±17 | 80±11b | 97±29 | 80±13b | 0.59 | 0.002 | 0.41 |
| LV efficiency and systolic function | |||||||
| LV efficiency (%) | 16.3±4.4 | 12.4±5.0 | 17.7±9.6 | 13.8±5.4 | 0.35 | 0.02 | 0.98 |
| LV ejection fraction (%) | 58±6 | 64±6d | 61±4 | 63±6 | 0.93 | 0.02 | 0.10 |
| Cardiac output (L/min) | 4.5±0.6 | 5.0±1.0 | 5.4±1.3 | 4.7±1.3 | 0.77 | 0.61 | 0.06 |
| Cardiac index (L/min/m2) | 1.9±0.4 | 2.4±0.4 | 2.6±0.7 | 2.5±0.6 | 0.08 | 0.30 | 0.06 |
| Cardiac work (mmHg*[L/min]) | 0.28±0.04 | 0.35±0.10 | 0.35±0.15 | 0.37±0.13 | 0.38 | 0.15 | 0.42 |
| Sm (cm/s) | 8.1±1.2 | 8.9±1.2 | 8.2±0.7 | 8.3±1.2 | 0.14 | 0.14 | 0.30 |
P<0.0005,
P<0.05,
P<0.005,
P<0.01 for differences between men and women in subgroup analysis (e.g., obese men versus obese women)
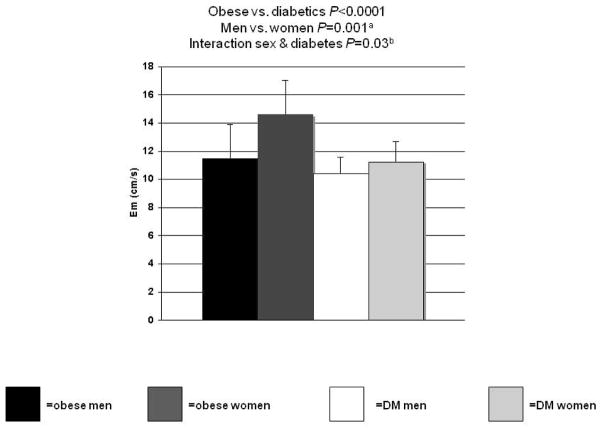
The diabetic subjects and their obese controls were similar in resting heart rate, LV mass, LV mass index, efficiency, and all measures of systolic function. The diabetics also had higher systolic blood pressure, and consequently, a higher rate-pressure product, despite 53% of them taking ACE inhibitor therapy. In contrast with the lack of an effect of diabetes on systolic function, LV relaxation (as measured by Em) was lower in the diabetics (Figure 1). This lower relaxation rate in the T2DM subjects was despite the fact that several of the diabetic subjects were taking angiotensin converting enzyme inhibitors or other antihypertensive therapy (while none of the obese were), and antihypertensive drugs improve LV relaxation (21). This suggests that the negative effect of T2DM on LV relaxation may be even greater.
Figure 1.
Effects of sex and diabetes (DM) on left ventricular relaxation. aP<0.005 for the subgroup comparison between obese men and women. bP=0.03 for the interaction between sex and diabetes status even after adding left ventricular mass index, mean arterial pressure, and rate-pressure product to a multivariate model.
Consistent with well-known sex effects, the women had lower LV mass and mass index than the men (22). The women also had lower LV efficiency yet slightly higher ejection fractions than the men (even though all subjects had an ejection fraction in the normal range) (Table 3). LV relaxation was higher in the women compared with the men (Figure 1). In subgroup analysis, the obese women had better LV relaxation than the men; there was no difference between the T2DM men and T2DM women. In addition, diabetes status and sex interacted in the prediction of Em since diabetic status lowered the Em much more in the women than in the men (shown graphically in Figure 1). This interaction between diabetic status and sex in the prediction of Em was still significant in a multivariate analysis (P=0.03) that included the other parameters, which significantly correlated with Em (i.e., age, LV mass index, systolic blood pressure, rate-pressure product, and mean arterial pressure). It is unlikely that the sex effect on LV relaxation is due to differences in antihypertensive therapy because the obese men had a much lower LV relaxation than the obese women (and neither was taking antihypertensive therapy). In addition, there were no differences in antihypertensive therapy between the men and women with T2DM. Moreover, the interaction between sex and T2DM is at least as robust as we found because some of the women with T2DM were taking antihypertensive treatment (which should improve their diastolic function), thus suggesting that the magnitude of the difference between obese and T2DM women’s LV relaxation rates are, if anything, even greater.
Myocardial blood flow, MVO2, glucose metabolism
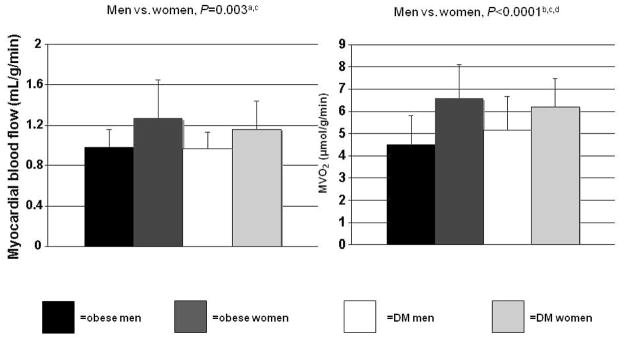
Myocardial blood flow and MVO2 were not different between the diabetics and the obese (Figure 2). However, the women had significantly higher myocardial blood flow and MVO2 than the men. Moreover, in multivariate models that included age, diabetes status, and the sex-diabetes interaction, female sex still predicted higher myocardial blood flow (P<0.005) and MVO2 (P<0.0001). Subgroup analysis showed differences between the T2DM men and women’s blood flow and MVO2, and between the obese men ad women’s MVO2 (Figure 2). Average values for fractional uptake of myocardial glucose and the overall utilization were not different between groups or between the women and the men (Table 4). There was also no significant group-sex interaction in the prediction of myocardial glucose metabolism. The ratio of myocardial glucose uptake to plasma insulin level was lower in the diabetics (than the obese), mostly due to their higher insulin levels since myocardial glucose uptake was not different.
Figure 2.
Effects of sex on myocardial blood flow and oxygen consumption (MVO2). No effect of diabetes (DM). After adjustment for age, aP=0.005; bP<0.0001. Subgroup analysis: cP<0.05 for diabetic men vs. women, dP<0.001 for obese men vs. women.
Table 4.
Myocardial Glucose Metabolism
| Obese | T2DM | P values | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Obese vs. T2DM | Men vs. Women | |
| Glucose uptake (mL/g/min) | 0.034±0.025 | 0.031±0.024 | 0.028±0.017 | 0.022±0.012 | 0.10 | 0.43 |
| Glucose utilization (nmol/g/min) | 179±138 | 146±115 | 175 ±111 | 141 ± 82 | 0.83 | 0.25 |
| Glucose uptake/plasma insulin*1000 ([mL/g/min]/[μU/mL]) | 2.2±2.0 | 5.5±7.2 | 1.7±2.1 | 1.5±1.6 | 0.04 | 0.21 |
No significant differences in subgroup analyses.
Myocardial FA metabolism
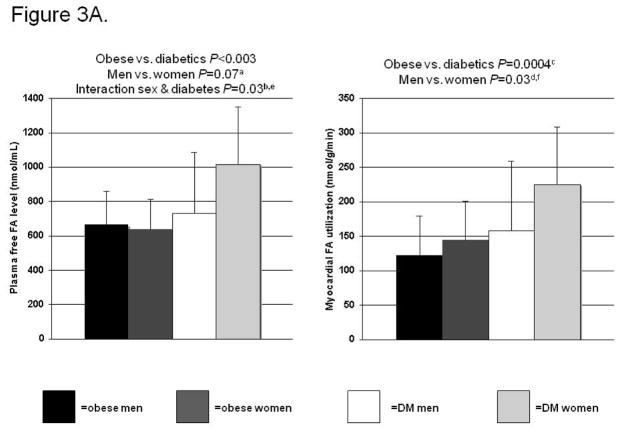
Plasma free FA levels were higher in the diabetics than the obese (Figure 3A, left panel), and FA levels were higher in the women than the men. Plasma free FA levels were higher in the T2DM women than the men. In addition, sex and diabetes status interacted in their correlation with plasma FAs, such that diabetes more markedly increased FA levels in the women than in the men. This interaction was still significant in multivariate analysis in which age, fat-free mass, systolic blood pressure were added to the model (P=0.04). Myocardial FA utilization was also significantly higher in the diabetics than the obese controls and higher in the women than the men (Figure 3A, right panel). In the multivariate model, that included age, diabetes status, and diabetes status-sex interaction, only sex was an independent predictor of FA utilization (P=0.007).
Figure 3.
Figure 3A. Effects of sex and diabetes on plasma fatty acid (FA) levels and myocardial FA utilization. No interaction sex & diabetes (DM) on FA utilization. After adjustment for age, aP=0.02 and bP=0.03, cP=0.33, dP=0.008. Subgroup analyses: eP=0.03 and fP<0.06 for diabetic men vs. women, P not significant for obese men vs. women.
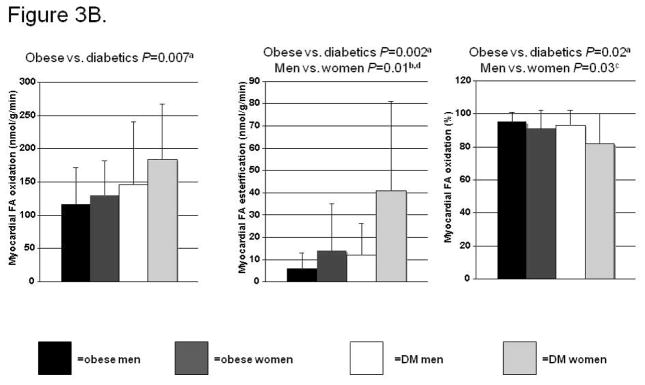
Figure 3B. Effects of sex and diabetes (DM) on myocardial fatty acid (FA) oxidation, esterification, and %oxidation. After adjustment for age, aP=NS, bP=0.0060, cP=0.03. Subgroup analyses not significantly different for FA oxidation; for FA esterification, dP<0.06 for diabetic men vs. women, not significant for obese men vs. women; for %FA oxidation, no significant differences in subgroup analyses.
When overall FA utilization was broken down into its components, FA oxidation, and FA “esterification,” we found that they were both higher in the diabetics compared with the obese (Figure 3B, left and middle panels). However, the increase in esterification in the diabetics was proportionately higher than the increase in oxidation, resulting in a lower percentage FA oxidation in the diabetics (Figure 3B right panel). Sex also affected myocardial FA esterification and percent oxidation, with the women having higher esterification, and consequently, lower percent oxidation. In multivariate analyses, sex trended towards being an independent predictor of higher myocardial FA oxidation (P=0.08); female sex independently predicted higher FA esterification (P=0.005), and lower percent FA oxidation (P=0.03). This higher level of FA esterification, oxidation, and overall utilization in the women, which is especially notable in those with T2DM, is despite the fact that more of the women (than men) with T2DM were taking lipid-lowering therapy, which should if anything decrease lipid delivery to the heart.
DISCUSSION
Our study’s results demonstrate that diabetes and sex have major, independent, and intertwined effects on myocardial metabolism and LV relaxation in humans. Relatively few previous studies evaluated myocardial metabolism rates in patients with type 2 diabetes but without significant coronary artery disease (10,11); even fewer measured myocardial fatty acid metabolism (12); and none have evaluated the combined effect of sex and diabetes on myocardial metabolism and function. We found that type 2 diabetes affects the heart’s myocardial metabolic phenotype even after accounting for the obesity that frequently accompanies diabetes. Diabetes resulted in the heart utilizing, oxidizing, and esterifying more FAs than in obese controls. However, despite the increase in oxidation rates, the diabetic heart oxidized proportionately less extracted FA than the obese heart. Diabetes also resulted in the heart relaxing less well than in obesity alone. Our study’s results also show that men’s and women’s hearts do not appear to respond to diabetes in exactly the same way. Sex relates to myocardial blood flow, MVO2, FA utilization, FA esterification, percent FA oxidation, and relaxation. Furthermore, female sex independently predicts higher myocardial blood flow, MVO2, and FA utilization. Diabetes and sex interact in the prediction of plasma free FA levels and LV relaxation. This interaction also independently predicted LV relaxation in multivariate analysis, such that women with diabetes had much worse relaxation than their nondiabetic counterparts than the men with diabetes had compared with their counterparts.
Even though traditionally abnormalities in glucose metabolism were thought to be the primary metabolic derangement in diabetes, abnormalities in myocardial FA metabolism contribute to the development of cardiac dysfunction and heart failure in animal models of type 2 diabetes (7–9). The results from the current study builds upon the findings of a previous study, which showed that men with type 2 diabetes have increased FA uptake and oxidation (12) by demonstrating that FA metabolism is increased to even a greater extent in diabetic women. Our finding that diabetes, independent of obesity, increases fatty acid esterification rate and decreases the percent fatty acid oxidation is consistent with preliminary findings of a study with a less well-defined cohort of diabetic patients (23). This suggests that the excessive amount of lipid deposits found in the myocardium of diabetic humans (24, 25) is due in part to excessively high esterification rates. Thus, it appears that although the myocardium’s capacity to oxidize fatty acids is up-regulated, it cannot ‘keep up’ with the excessive rate of FA utilization, which is similar to results from studies in animal models of diabetes mellitus that showed accumulation of FAs in the heart and their detrimental consequences (8,9).
In addition, our study is the first to demonstrate that female sex independently predicts higher myocardial FA utilization, esterification, and lower percent FA oxidation in humans, even after including diabetes in the multivariate modeling. These findings add to those of myocardial metabolism studies in non-diabetic humans that showed women have higher FA metabolism rates (13,14). The interaction between sex and diabetes in predicting plasma FA level highlights the complicated nature of their combined effects and influences myocardial FA metabolism because substrate availability is a primary driver of substrate metabolism. The higher plasma free FA levels in the women compared with men is likely partly attributable to a higher %fat mass as opposed to intrinsically higher lipolytic rates per the same amount of fat mass in women (26, 27). However, even after accounting for differences in %fat mass, female sex was still an independent predictor of the level of FA metabolism. Consequently, other mechanisms must also be contributing to the observed sex-reated differences in myocardial FA metabolism. There are several lines of evidence to support the role of estrogen in modulating myocardial FA metabolism and function. In both liver and skeletal muscle, estrogen decreases glucose oxidation and increases FA oxidation (28, 29). In healthy postmenopausal women, chronic estrogen replacement therapy increases myocardial FA uptake and oxidation, (and progesterone attenuates this effect) thus providing evidence for a similar estrogen effect in the heart (30). Whether these sex- and diabetes-related alterations in myocardial FA metabolism contribute to increased heart failure and worse outcome in humans requires a future longitudinal study.
Results from animal studies demonstrate diabetes increased MVO2, and this study’s finding that FA oxidation was increased suggest that MVO2 should be increased (7, 31, 32). However, diabetes mellitus did not have an independent effect on overall MVO2, which was somewhat surprising. Part of the explanation for a lack of a significant effect of diabetes on MVO2 may be that the effect of diabetes is drowned out by the major effect of sex on MVO2 (13, 33). In addition, obesity has a significant effect on MVO2 (especially in women) (14), which may make any additional signal from diabetes difficult to detect. Lastly, the oxidation of other possible substrates (e.g., lactate) may contribute to the lack of effect on overall MVO2.
Our finding that fasting myocardial glucose utilization was no different between subjects with and those without type 2 diabetes may seem unexpected since diabetes makes the body insulin resistant. However, whether type 2 diabetes makes the human heart insulin resistant is controversial with some studies demonstrating insulin resistance (34) and others not (35). There is agreement though, that myocardial glucose utilization is very low in the fasting state in general. This makes it technically more difficult to determine a clinically significant difference in myocardial glucose utilization between type 2 diabetic patients and controls. Thus, many studies have employed hyperinsulinemic-euglycemic clamp to evaluate myocardium’s glucose utilization (10, 34). However, it is also important to demonstrate that type 2 diabetes does not appear to have a major effect on resting myocardial glucose metabolism in a normal, everyday, physiologic condition, such as after an overnight fast. Our study’s finding that type 2 diabetes had a lower myocardial glucose utilization per plasma insulin does suggest that there is some degree of myocardial insulin resistance in type 2 diabetes, which is compatible with the findings of Cook et al., and others (10, 34).
While it was not surprising that diabetes had a detrimental effect on relaxation (36, 37), our data showing that sex and diabetes interact on the prediction of cardiac relaxation is a novel finding. Although we did not study the change from obesity to diabetes, it is interesting to speculate that since the obese men have worse insulin resistance and higher insulin levels (than the obese women) that this may negatively impact their LV relaxation more (than the women), so that the decrease in relaxation from obesity to diabetes is not as dramatic in men as it is in women. This builds upon the findings from an epidemiologic study that showed that sex modulates the effects of insulin resistance on cardiac structure (38). There are also studies showing that diastolic function changes with many other conditions (e.g., aging) are modulated by sex (39, 40). The possible mechanisms for the effects of sex (and diabetes) on diastolic function have recently been reviewed (40). It is tempting to speculate that some of the known sex-related differences in diabetic cardiovascular disease may perhaps be related to sex-related metabolic and lusitropic changes rather than simply due to other sex-related differences in co-morbidity prevalence and diagnostic approaches.
Study limitations
This was a cross-sectional cohort study. A longitudinal study of the transition between obesity to type 2 diabetes was not done, so conclusions regarding the effects of a transition between the two cannot be made. In addition, although the diabetic and obese groups were similar, there were some differences, e.g., the former were older. Thus, we adjusted for age in all multivariate analyses. Most diabetic subjects also received antihypertensive and statin therapy per clinically applicable guidelines, to make their blood pressures and lipid levels more comparable to those of the controls. However, since all samples were processed identically then at a minimum the relative differences among the groups we studied should be correct. The PET approach for quantifying myocardial FA metabolism does not account for the contribution of FA metabolism from exogenous or endogenous triglycerides. This study did not designed to address the effects of all possible hormones, adipokines, and substrates on myocardial metabolism although it would be interesting to build upon the current research’s findings by investigating these molecules’ effects in future studies. In addition, although plasma was rapidly separated by centrifugation and stored at −80 deg C until analysis for free FA levels, no additional measures (e.g., use of the neurotoxin paraoxon) were taken to prevent in vitro lipolysis. Thus, the fact that free FA levels were relatively high in the present study, especially in the diabetic women, may be partially artifactual. However, since all samples were processed identically then at a minimum the relative differences among the groups studied should be correct. Lastly, this study was not designed to address (and so can not make conclusions on) the effects of obesity, T2DM, and sex on myocardial metabolism and function during multiple feeding states (e.g., immediate postprandial) - in addition to 12 hours postprandial - since patients with T2DM may have differences in gastric emptying and absorption.
Clinical Implications
Our study demonstrates that diabetes, sex, and their interaction affect myocardial metabolism and diastolic function, are such that women with diabetes often manifest the worst cardiometabolic profile and that diabetes has a disproportionately greater detrimental effect on diastolic function in women. The greater impact of diabetes on LV metabolism and diastolic function in women may help to explain the increased relative risk of heart failure in diabetic women compared with men (4). Our results also suggest that sex may modulate the response to any therapeutic strategies aimed at metabolic modulation as a means of improving function. The relevance of our study’s findings assumes an increased significance in the face of the alarming growth of diabetes and heart failure in the US and worldwide.
Acknowledgments
The authors gratefully acknowledge Kristin O'Callaghan’s editorial assistance and Ava Ysaguirre’s assistance with manuscript preparation. This work was funded by grants PO1-HL13851, RO1-HL073120, UL1 RR024992 (CTSA), 5 P60 DK020579 (Diabetes Research and Training Center), HD145902 (Building Interdisciplinary Research in Women’s Health), and DK 56341 (Clinical Nutrition Research Unit) from the National Institutes of Health (Bethesda, MD).
Footnotes
DISCLOSURES
The authors have no conflicts to disclose.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–327. [PubMed] [Google Scholar]
- 2.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Aban IB, Vaccarino V, et al. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–1590. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 8.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 10.Ohtake T, Yokoyama I, Watanabe T, et al. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–463. [PubMed] [Google Scholar]
- 11.van der Meer RW, Rijzewijk LJ, de Jong HW, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 12.Rijzewijk LJ, van der Meer RW, Lamb HJ, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 13.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. J Nucl Cardiol. 2007;14:573–581. doi: 10.1016/j.nuclcard.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson LR, Soto PF, Herrero P, et al. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging. 2008;1:424–433. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann SR, Herrero P, Markham J, Weinheimer CJ, Walsh MN. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol. 1989;14:639–652. doi: 10.1016/0735-1097(89)90105-8. [DOI] [PubMed] [Google Scholar]
- 16.Buck A, Wolpers HG, Hutchins GD, et al. Effect of carbon-11-acetate recirculation on estimates of myocardial oxygen consumption by PET. J Nucl Med. 1991;32:1950–1957. [PubMed] [Google Scholar]
- 17.Herrero P, Kisrieva-Ware Z, Dence CS, et al. PET measurements of myocardial glucose metabolism with 1–11C-glucose and kinetic modeling. J Nucl Med. 2007;48:955–964. doi: 10.2967/jnumed.106.037598. [DOI] [PubMed] [Google Scholar]
- 18.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. 1996;37:1723–1730. [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Peterson LR, Waggoner AD, Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SD, Janardhanan R, Verma A, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 22.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 23.Ungar I, Gilbert M, Siegel A, Blain JM, Bing RJ. Studies on myocardial metabolism. IV. Myocardial metabolism in diabetes. Am J Med. 1955;18:385–396. doi: 10.1016/0002-9343(55)90218-7. [DOI] [PubMed] [Google Scholar]
- 24.Alavaikko M, Elfving R, Hirvonen J, Jarvi J. Triglycerides, cholesterol, and phospholipids in normal heart papillary muscle and in patients suffering from diabetes, cholelithiasis, hypertension, and coronary atheroma. J Clin Pathol. 1973;26:285–293. doi: 10.1136/jcp.26.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 26.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009;17:1872–1877. doi: 10.1038/oby.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab. 2001;281:E1333–9. doi: 10.1152/ajpendo.2001.281.6.E1333. [DOI] [PubMed] [Google Scholar]
- 28.Matute ML, Kalkhoff RK. Sex steroid influence on hepatic gluconeogenesis and glucogen formation. Endocrinology. 1973;92:762–768. doi: 10.1210/endo-92-3-762. [DOI] [PubMed] [Google Scholar]
- 29.Kendrick ZV, Ellis GS. Effect of estradiol on tissue glycogen metabolism and lipid availability in exercised male rats. J Appl Physiol. 1991;71:1694–1699. doi: 10.1152/jappl.1991.71.5.1694. [DOI] [PubMed] [Google Scholar]
- 30.Herrero P, Soto PF, Dence CS, et al. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J Nucl Cardiol. 2005;12:574–581. doi: 10.1016/j.nuclcard.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 32.Vik-Mo H, Mjos OD. Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am J Cardiol. 1981;48:361–365. doi: 10.1016/0002-9149(81)90621-4. [DOI] [PubMed] [Google Scholar]
- 33.Peterson LR, Herrero P, McGill J, et al. Fatty acids and insulin modulate myocardial substrate metabolism in humans with type 1 diabetes. Diabetes. 2008;57:32–40. doi: 10.2337/db07-1199. [DOI] [PubMed] [Google Scholar]
- 34.Cook SA, Varela-Carver A, Mongillo M, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utriainen T, Takala T, Luotolahti M, et al. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia. 1998;41:555–559. doi: 10.1007/s001250050946. [DOI] [PubMed] [Google Scholar]
- 36.Scheuermann-Freestone M, Madsen PL, Manners D, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 37.Boyer JK, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–875. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 39.Okura H, Takada Y, Yamabe A, et al. Age- and gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging. 2009;2:41–46. doi: 10.1161/CIRCIMAGING.108.809087. [DOI] [PubMed] [Google Scholar]
- 40.Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis. 2007;49:241–251. doi: 10.1016/j.pcad.2006.08.011. [DOI] [PubMed] [Google Scholar]