Abstract
Metastatic lesions of the pancreas are uncommon, accounting for approximately 2% of pancreatic malignancies. Many tumours involve the pancreas secondarily and may manifest with different clinical and imaging characteristics. Although many patients have widespread disease, isolated metastases can be found. Surgical management is associated with improved survival in these cases. The experience of the pancreatic surgery unit and imaging department of our hospital in many patients presenting with pancreatic metastases is presented, and a review of the recent literature is undertaken.
Main Messages
• The early recognition of secondary pancreatic tumours on US, CT and MRI is extremely important.
• Pancreatic metastases may mimic primary pancreatic adenocarcinoma or induce acute pancreatitis.
• Most pancreatic metastases are discovered on a CT examination performed for follow-up.
Keywords: Pancreas, Metastasis, US, CT, MRI
Introduction
Unlike primary pancreatic carcinoma, metastatic lesions of the pancreas are uncommon and account for approximately 2% of pancreatic malignancies. At autopsy the pancreas has been found to be a site of metastases in a wide range (3–12%) of patients with malignant tumours [1].
A variety of extrapancreatic tumours can involve the pancreas secondarily and may manifest with different clinical and imaging characteristics. The most common primary tumours to give rise to pancreatic metastases are lung cancer, breast cancer, renal cell carcinoma, malignant melanoma, carcinoma of gastrointestinal origin and prostate cancer. Less commonly metastases from osteosarcoma, leiomyosarcoma, chondrosarcoma, and Merkel cell carcinoma, have been reported [2].
Although many patients have widespread disease, isolated metastases can be found. Surgical management seems to be associated with improved survival in these cases [3–5]. Moreover resection is most likely to be warranted in patients presenting after a long disease-free interval following primary cancer treatment, suggesting a biological pattern of slow growth.
The early recognition of secondary pancreatic tumours on ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) is extremely important, so that the patient will benefit from the appropriate treatment according to disease prognosis.
Primary tumours and frequency of pancreatic metastases
Almost any tumour type may metastasize to the pancreas, but in an analysis of a surgical and autopsy database, the majority of tumours found in the surgical database were lymphomas and carcinomas of the stomach, kidney, and lung, whereas in the autopsy database, metastasis was most commonly from the lung, followed by the GI tract and kidney [6].
A review of resection for metastases to the pancreas found renal cell carcinoma to be the most frequent primary histopathology (62%), followed by non-small-cell lung cancer, and melanoma [7]. In this series, postoperative morbidity was 25%, mortality was 6%, and the overall actuarial survival rate for 2 and 5 years was 62 and 25%, which indicates that resection of metastatic disease to the pancreas is safe and may offer some survival benefit in selected patients.
Other primary malignancies that have been reported to give rise to pancreatic metastases are thyroid, prostate, ovarian, hepatocellular carcinomas, and various types of sarcomas [6, 8, 9].
The interval from diagnosis of an extrapancreatic primary tumour to subsequent detection of a pancreatic metastasis varies but is usually between 1 and 3 years [9]. Metastases from renal cell carcinoma may present many years after resection of the primary tumour. McNichols et al [10] reported that 11% of patients developed metastases 10 or more years after nephrectomy, even with early stage disease. The longest interval was reported by Muranaka [11], who observed pancreatic metastases 27 years after treatment of the primary renal tumour. Rarely, a pancreatic metastasis may be discovered before the distant primary source is known [9].
Clinical symptoms
Pancreatic metastases may directly invade the pancreatic ductal epithelium and thus clinically mimic primary pancreatic adenocarcinoma, or less commonly, induce acute pancreatitis. As a result, clinical symptoms produced by secondary pancreatic tumours are variable and nonspecific, including abdominal pain, back pain, weight loss, nausea, melaena, jaundice, gastrointestinal obstruction, upper gastrointestinal bleeding, and diabetic ketoacidosis [8, 9].
Pancreatic metastatic tumour may also be completely asymptomatic. In a series of 18 patients with metastases to the pancreas, 15 patients were asymptomatic and the pancreatic mass was detected on routine follow-up examination [8]. Clinical signs of acute pancreatitis are rare, although elevated serum amylase levels are often found [11].
Lesion distribution and morphology
Three patterns of metastatic involvement of the pancreas have been described thus far. The first one, reported in 5–10% of patients, is represented by many small nodules, which can coalesce occasionally into larger masses [11, 12]. The attenuation of the neoplastic nodules may be variable and generally depends on the histological type of the causative primary tumour [12].
The second pattern, reported in 15–44% of patients, is given by a diffuse infiltration of the pancreas, which leads to a diffusely enlarged organ, with homogeneous density [11, 12]. However, the diffuse multiple form of metastatic pancreatic involvement may also show central areas of low attenuation [9].
The last pattern, reported in 50–75% of cases, is given by a solitary mass with an occasional hypodense focus on contrast-enhanced CT, due to tumour necrosis (Figs. 1, 2, 3) [11, 12]. However, the hypodensity may not be apparent when the metastasis is small, but may only appear as the tumour becomes larger [9].
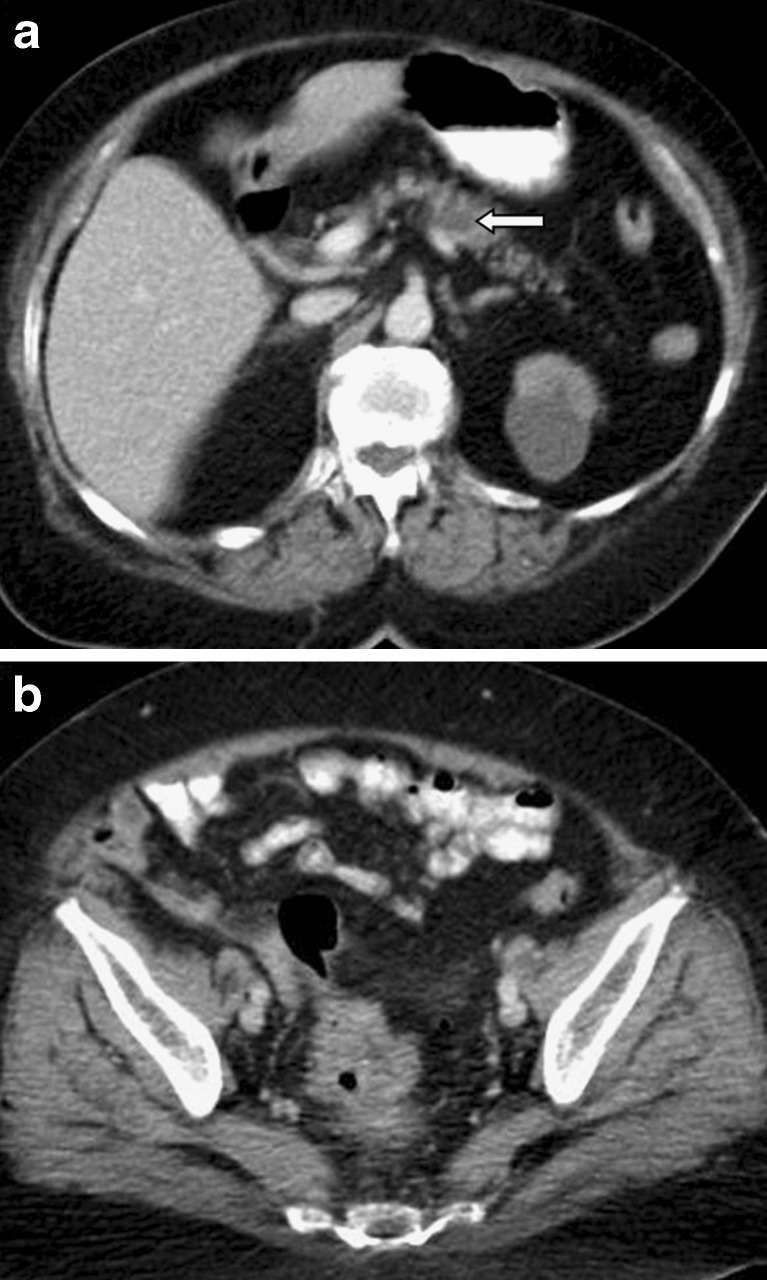
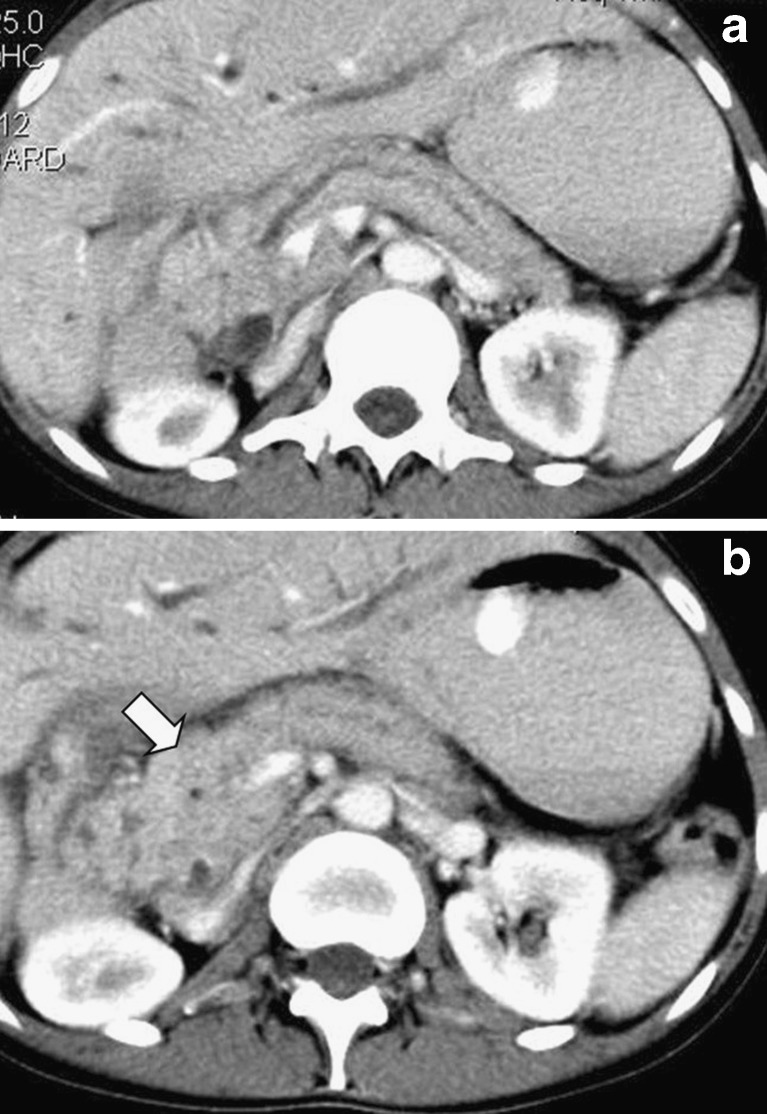
Fig. 1.
A solitary pancreatic metastasis is seen on this contrast-enhanced CT image (a), as a hypodense lesion with peripheral contrast enhancement (arrow) in a 70-year-old man presenting with rectal bleeding. The primary neoplasm in the sigmoid colon is also evident (b)
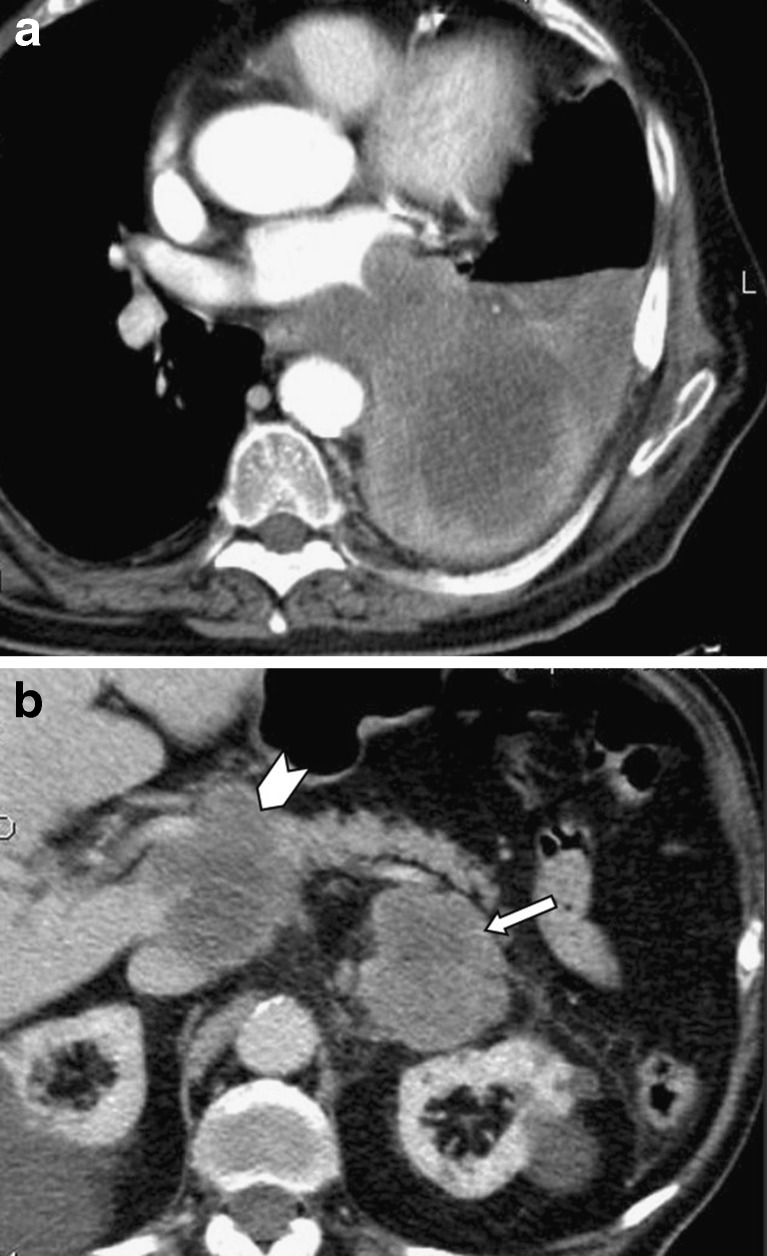
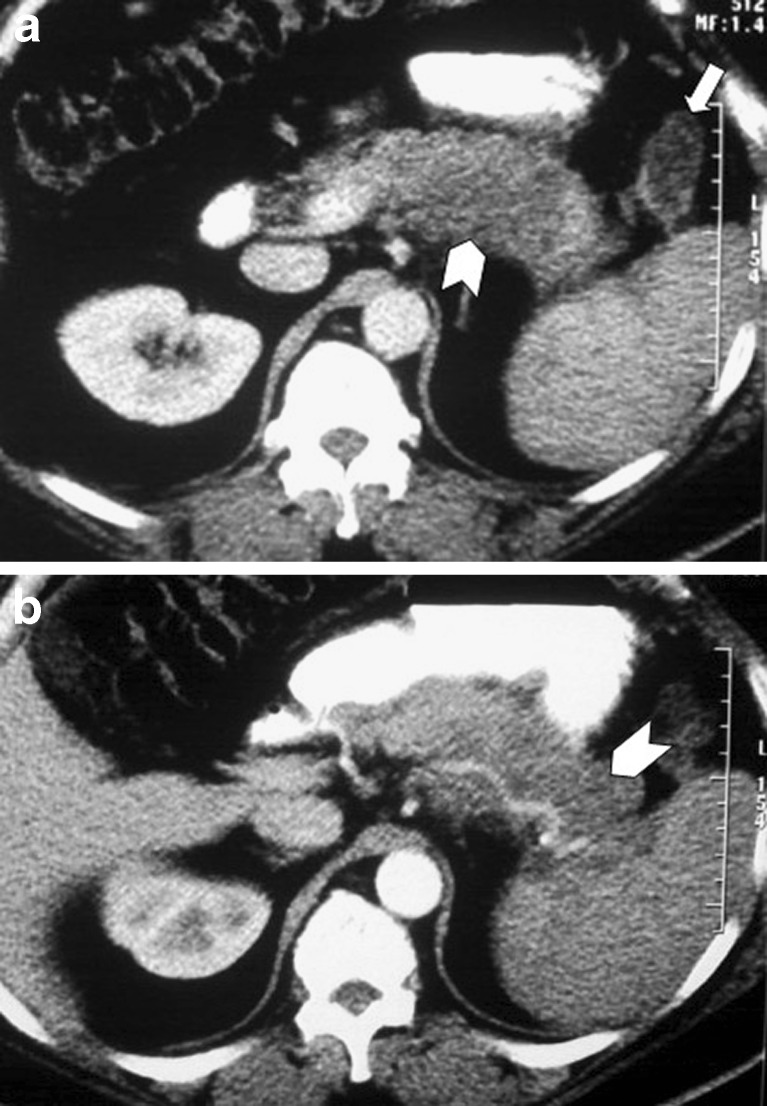
Fig. 2.
A 65-year-old man suffering from lung cancer that caused atelectasis of the left lower lobe (a). Left adrenal metastasis (arrow), as well as hypodense metastastic infiltration of the pancreatic head (arrowhead) are also seen (b). Note that both the vessels and the pancreatic duct appear normal
Fig. 3.
A gastric cancer (arrow) with nodal dissemination (arrowheads) is visible on this contrast-enhanced CT image (a) in a 63-year-old woman presenting with anemia. A liver metastasis (arrow) as well as a small hypodense heterogenous pancreatic head lesion (arrowhead) is also demonstrated (b)
The location within the pancreas may be responsible for some of the physical signs and symptoms. If located in the head, the mass may easily cause obstruction of the bile duct with subsequent jaundice or obstruction of the pancreatic duct, which may in turn cause atrophy of the body and tail and a beaded appearance of the dilated Wirsung’s duct [13]. In this group of patients the highest incidence of elevation of serum amylase is found, probably because of the acinar destruction caused by the obstructed flow in the Wirsung’s duct.
Muranaka et al [11] have suggested an extended classification of localised and multifocal forms (Fig. 4), as well as diffuse enlargement (Figs. 5, 6) without consideration of density characteristics. This classification was adopted by Kelekis et al [14] who first described the MR appearance of pancreatic metastases.
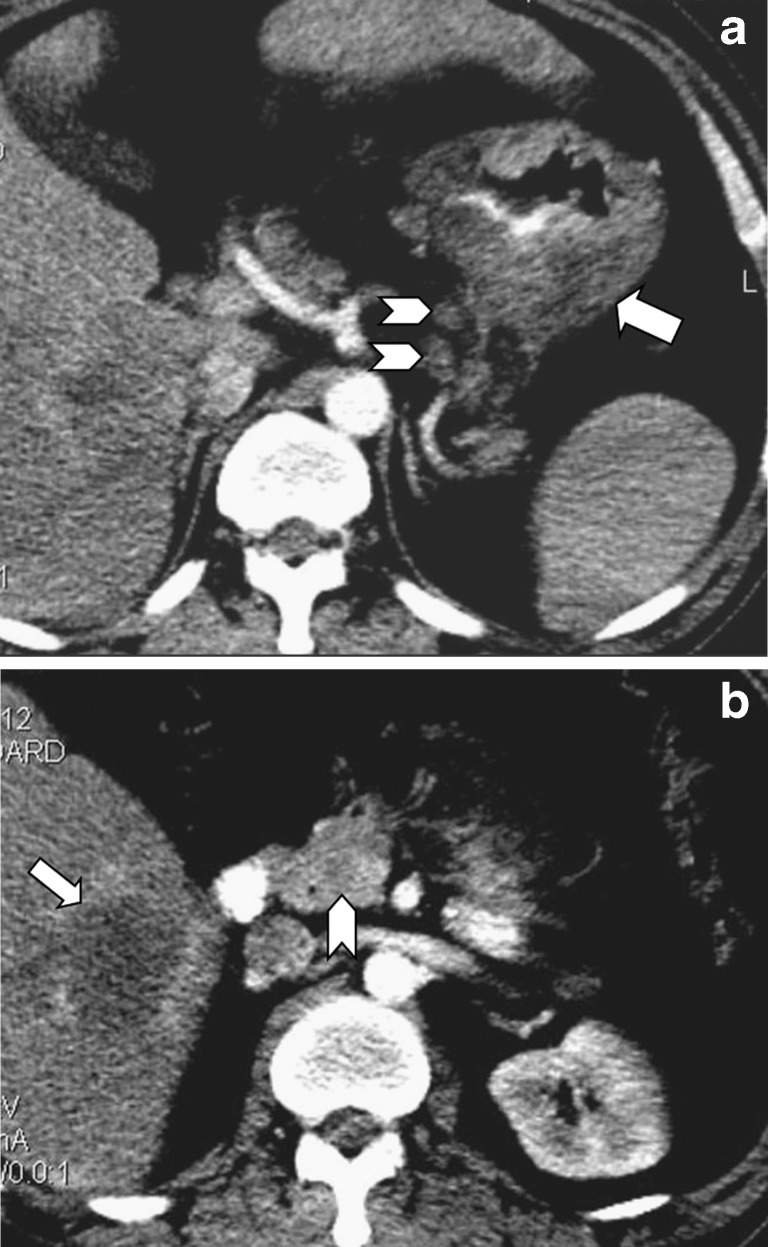
Fig. 4.
Two metastatic lesions are depicted on contrast-enhanced CT in the pancreatic tail (arrow in a) and head (arrow in b), in a 70-year-old male patient suffering from lung cancer, 1 year after chemotherapy. Right adrenal metastasis (arrowhead in a) as well as a peripancreatic lymph node (arrowhead in b) are also demonstrated
Fig. 5.
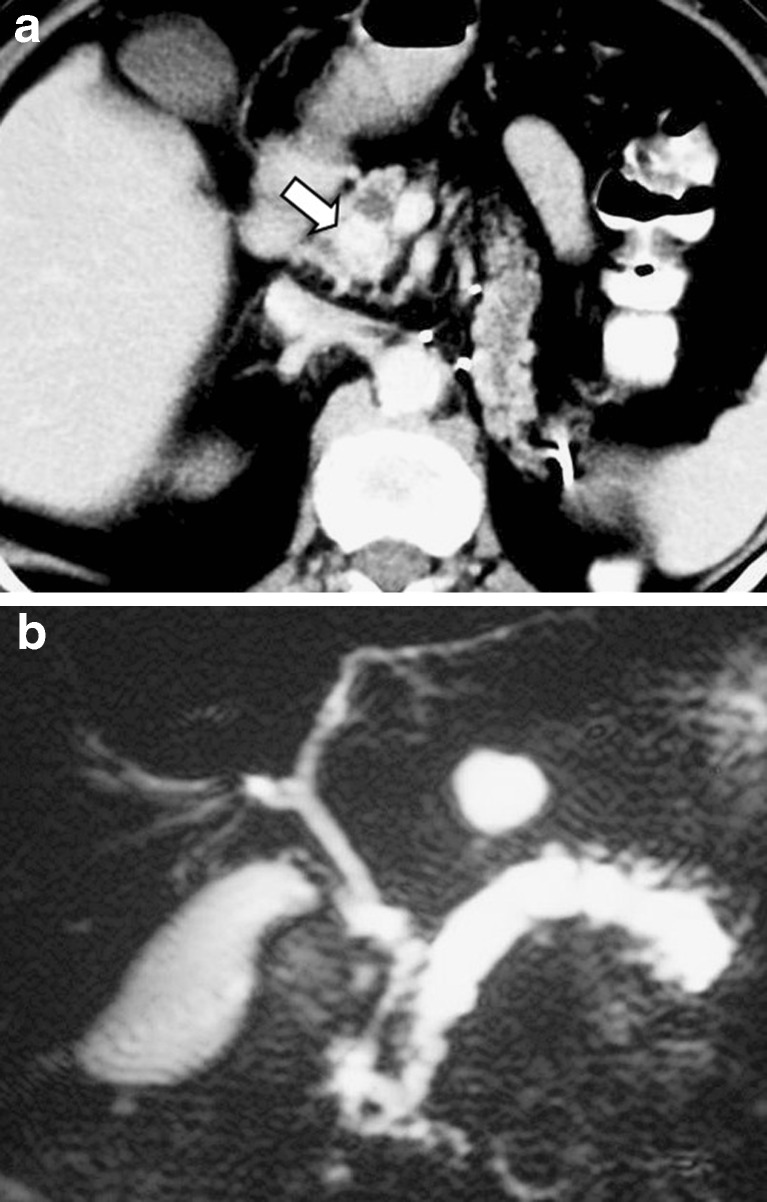
A 35-year-old woman who underwent liver surgery due to hepatocellular carcinoma. Two years later, the follow-up CT scan revealed intrahepatic biliary dilatation and slight pancreatic duct dilatation (a) due to metastatic infiltration of the pancreatic head (arrow in b) proven by EUS-FNA
Fig. 6.
A 55-year-old man, 8 months after left hemicolectomy due to a low-differentiated cancer. Tumour recurrence is evident (arrow in a), as well as an extensive pancreatic body and tail diffuse infiltration (arrowheads in a and b)
Imaging methods and findings
The diagnosis of a metastasis to the pancreas begins with suspicion based on the history of a relevant cancer. Most pancreatic metastases are firstly discovered on a CT examination performed for follow-up of patients with history of primary malignancy. However, all imaging studies such as dynamic contrast-enhanced CT scans, magnetic resonance imaging (MRI), endoscopic ultrasound (EUS) and contrast-enhanced US may support that suspicion, especially if multiple tumours are noted [15].
On US examination, metastases appear as solid hypoechogenic masses located within the pancreatic parenchyma [16]. Cysts are generally not a feature. EUS may discover small pancreatic metastases after negative CT scans [17]. Pancreatic metastases are more likely to have well-defined margins compared to primary ductal adenocarcinoma that usually presents with irregular margins, while there is no statistically significant difference concerning tumour size, echogenicity, and location [17].
EUS-guided FNA is safe and accurate for the diagnosis of pancreatic metastases from different primary carcinomas. However, effective sampling requires techniques that differ from those used for other solid pancreatic masses. Use of immunocytochemistry, when available, may help to confirm a suspected diagnosis (Figs. 7, 8) [18].
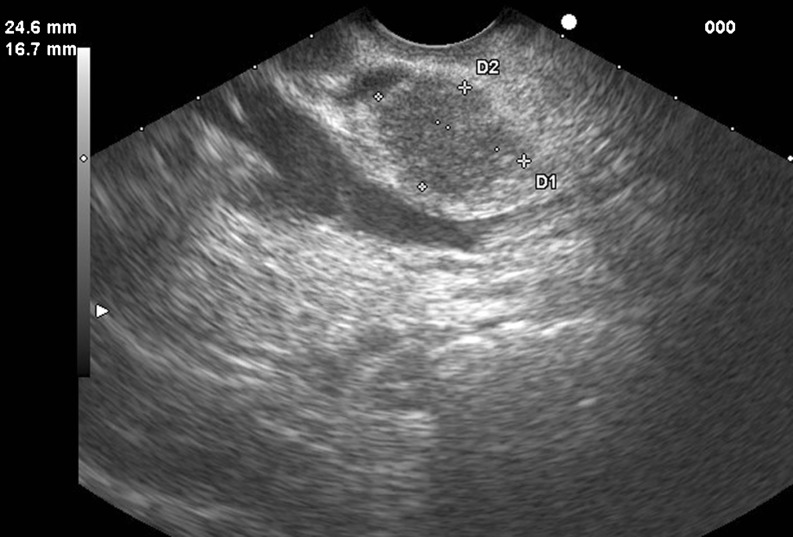
Fig. 7.
EUS image showing a large metastatic lesion from renal cell cancer confirmed by FNA, cytology, and immunohistochemical analysis, in a 56-year-old male presenting with jaundice
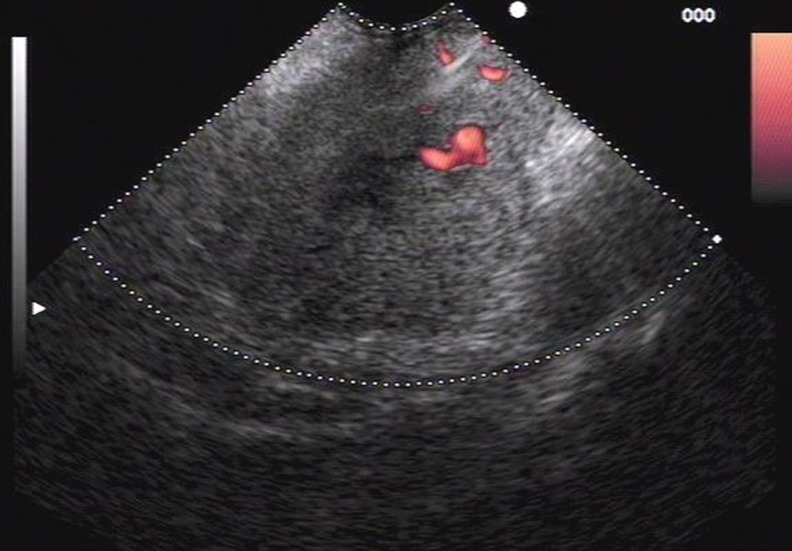
Fig. 8.
EUS image revealing a hypoechoic metastatic lesion from non-small-cell lung cancer confirmed by FNA, cytology, and immunohistochemical analysis, in a 66-year-old male presenting with abdominal pain
The introduction of contrast-enhanced ultrasonography (CEUS) has lead to great developments in the diagnostic capabilities of ultrasound. The study of the pancreas is a new and promising application of CEUS. CEUS offers advantages over conventional US for identifying pancreatic lesions or for characterising pancreatic lesions already visible at US [19]. The results of this technique are better in metastases from hypervascular tumours such as renal cell cancer, neuroendocrine tumours, or hepatocellular carcinoma.
Circumscribed lesions (single or multifocal) appear isodense or, more often, slightly hypodense on unenhanced CT [1, 20]. Bulging of the gland contour is occasionally seen. After i.v. contrast medium administration, a peripheral rim of enhancement is usually demonstrated and a central area of low attenuation may be clearly visible (Fig. 1). Rim enhancement is especially common in lesions larger than 1.5 cm in size, whereas smaller lesions sometimes demonstrate homogeneous enhancement. In these cases, splenic vein involvement may be observed. In portal and delayed phase images, the lesions demonstrate a rapid wash-out between 60 and 120 s [8, 9, 15].
It should be emphasised that metastases from many primary hypovascular tumours (lung, colonic, gastric cancers) often present as hypoattenuated lesions compared to the normal enhanced pancreas after i.v. injection, while metastases from primary hypervascular tumours (HCC, thyroid, renal cell cancers) also display increased vascularity on contrast-enhanced CT.
On MR imaging, pancreatic lesions typically appear hypointense compared with normal gland tissue on unenhanced T1-weighted images, both with and without fat saturation. On T2-weighted images, the lesions have heterogeneous or moderately hyperintense signal [14]. Hypointense nodules are sometimes visible on T2-weighted images, especially in the diffusely enlarged type. After i.v. paramagnetic contrast administration, a rim of enhancement is usually visible in larger lesions and homogeneous enhancement is typically demonstrated in smaller tumours, as seen on CT scans.
The transition from uniform enhancement in small metastases to ring enhancement in larger metastases relates to the blood supply to these tumours. The metastases parasitize blood supply from the surrounding organ, which will result in a uniform tumour blush in small lesions [21, 22]. In larger lesions, the peripheral aspect of the tumour receives a better blood supply than the more hypovascular central portion, which results in a predominant ring enhancement pattern on early postcontrast images.
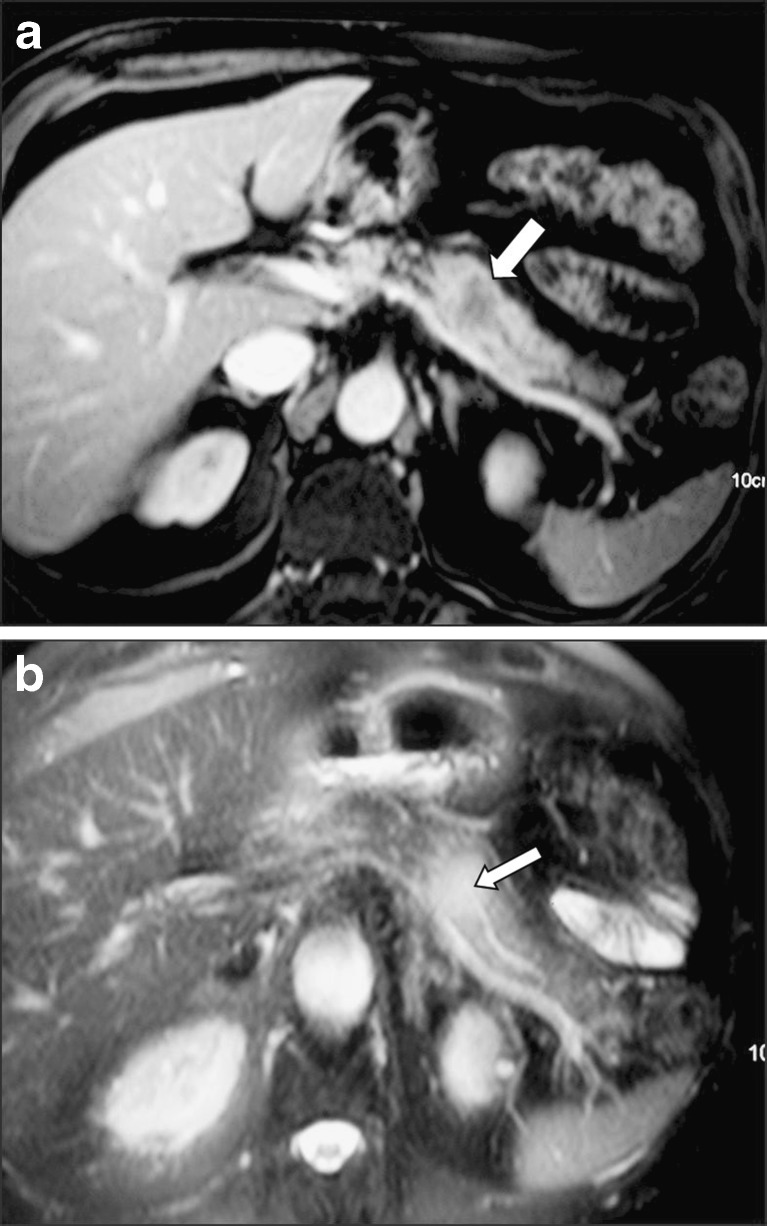
Dilatation of the main pancreatic duct is not a common finding, but when present, differential diagnosis from primary pancreatic cancer is extremely difficult (Fig. 9). An observation we have made, not previously reported, is the presence of the “duct penetrating sign,” a feature that has been so far related only to focal pancreatitis (Fig. 10). The explanation could be that primary pancreatic cancer in fact represents an adenocarcinoma of the duct itself, while metastatic lesions arise outside the ducts and may only affect them secondarily by compression or rarely infiltration.
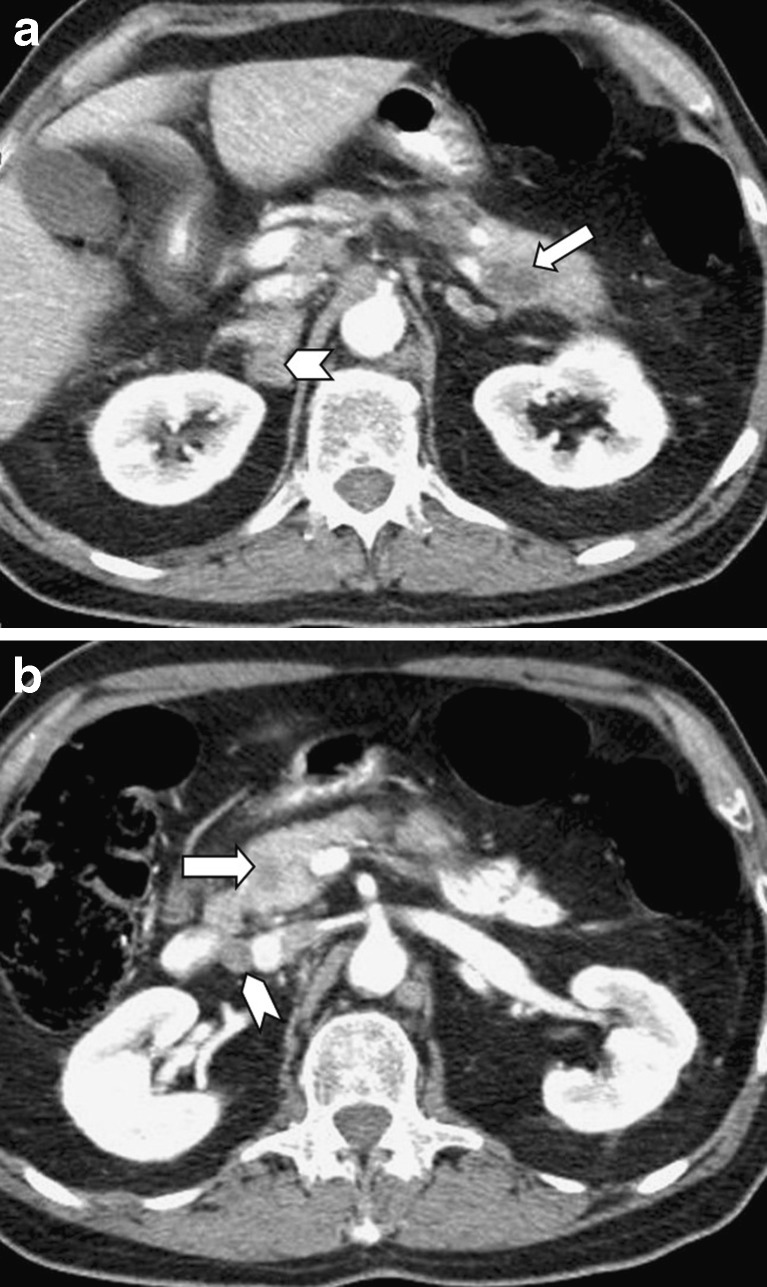
Fig. 9.
A 63-year-old woman who underwent left nephrectomy due to renal cell carcinoma. Three years later, the follow-up CT scan revealed a metastatic lesion with intense contrast enhancement in the uncinate process of the pancreas (arrow in a), causing dilatation of the main pancreatic duct in the body and tail that was evaluated by MRCP (b)
Fig. 10.
A 70-year-old man who underwent surgery for lung cancer. Six months later, he developed a solitary metastatic lesion in the pancreatic body evaluated by CT (arrow in a) and MRI (b). Note that the pancreatic duct “penetrates” the metastatic lesion with no evidence of infiltration on the T2-weighted image (arrow in b)
It should be noted that an appropriate imaging protocol is essential for the diagnosis of pancreatic metastases. This is of particular importance for metastases from renal cell carcinomas that demonstrate intense early enhancement both at CT and MRI, a pattern that reflects hypervascularity of viable tumour tissue and is the main differential diagnostic feature from the hypovascular pancreatic adenocarcinoma (Fig. 9) [8, 9, 14]. Such metastases may fail to be appreciated in the delayed phase. Therefore in patients with suspected renal cell carcinoma metastases to the pancreas, early-phase scanning after i.v. contrast administration should be performed [23].
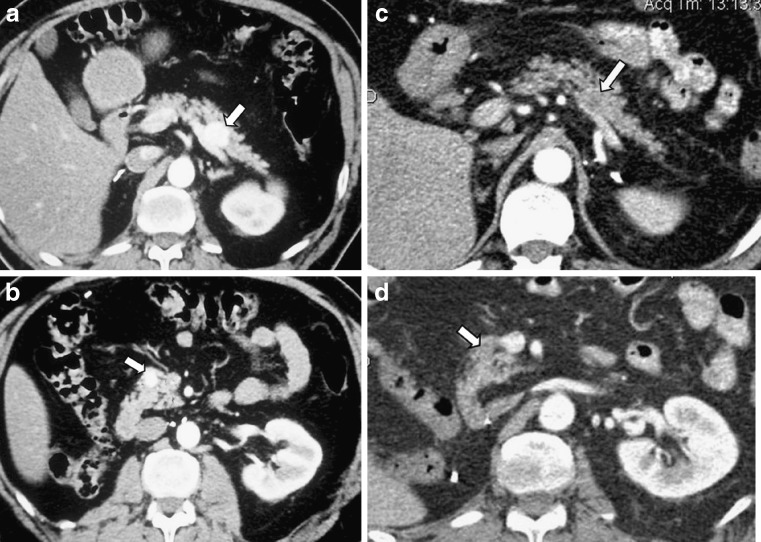
Although the solitary type is the most common in pancreatic metastases from renal cell carcinoma, multifocality of the lesions is not unusual, ranging from 20 to 45% in different reports (Fig. 11) [24–28]. Multifocal lesions may also be found in cases of lung cancer or colonic cancer metastases. Usually all pancreatic lesions present the same imaging features, but in some cases where small and larger metastases co-exist, some differences may be noticed in all imaging modalities and differential diagnostic problems may arise.
Fig. 11.
A 48-year-old man with a history of right nephrectomy due to renal cell cancer. Two years after, he developed two metastatic lesions in the pancreatic body and head that were hyperdense after contrast enhancement (arrows in a and b). He received targeted chemotherapy and the follow-up CT scan revealed a very good response proven by the reduced vascularity (arrows in c and d)
Differential diagnosis and pitfalls
In most cases the diagnosis of secondary pancreatic tumours, along with the differential diagnosis of primary pancreatic adenocarcinomas, can still be difficult, due to a multiplicity of factors. Firstly, CT can occasionally fail to show subtle differences in attenuation between normal pancreatic tissue and nonnecrotic or noncystic neoplastic tissue; this issue becomes especially crucial when confronting small metastatic nodules which do not alter the normal contour of the pancreas, and this deception can only occasionally be prevented by dynamic CT [12].
In addition, one of the patterns of pancreatic involvement is a diffuse homogeneous infiltration of the organ, which then appears simply enlarged, but without any sign of focal disease, a deceiving appearance both at US and CT [11, 12]. This appearance, quite similar to a diffuse lymphomatous infiltration, may present serious difficulties in the differential diagnosis, especially if accompanied by a visible involvement of the locoregional lymph nodes.
Also, nonhyperfunctioning islet cell carcinoma can be indistinguishable from some metastatic cancers to the pancreas that do not closely resemble the common ductal type of pancreatic adenocarcinoma. In particular, metastasis from renal cell carcinoma shares morphologic and imaging features with nonhyperfunctioning islet cell carcinoma. Both types of tumour are typically hypervascular but are subject to central necrosis and cystic degeneration [29–32]. Although the imaging features of the metastatic lesions along with a history of prior nephrectomy for renal cell carcinoma aid in postulating the diagnosis, histological verification is mandatory.
Finally, hemorrhagic-necrotising pancreatitis can imitate the appearance of multiple pancreatic metastases, as it presents preserved organ parenchyma and necrotic hypoechoic areas side by side. Because pancreatic metastases can also give rise to severe pancreatitis with development of necrotic tracts [33], differential diagnosis is extremely difficult in these cases.
However, CEUS, CT, and MRI may demonstrate features that aid in distinguishing metastatic from primary cancer of the pancreas. For example, the enhancement of tumour tissue reflects a degree of vascular perfusion that is not typical of primary pancreatic adenocarcinoma. Whereas ductal adenocarcinoma of the pancreas typically appears as a uniformly nonenhancing mass at contrast-enhanced CT [34], a large proportion of pancreatic metastases enhance perceptibly, either heterogeneously or, less commonly, homogeneously. Metastases from renal cell carcinomas are often seen as hyperattenuating masses, often with nonenhancing internal components. This pattern, which reflects hypervascularity of viable tumour tissue and diminished or absent perfusion of necrotic components, is also typical of primary renal cell carcinomas [29].
Metastatic pancreatic tumours may also have certain imaging features that are more characteristic of their extrapancreatic sources than of primary pancreatic cancer. Multiplicity of tumours within the pancreas, although relatively common in metastasis to the pancreas, is not characteristic of primary pancreatic carcinoma. Coexisting metastases at sites that are not typically involved with adenocarcinoma of pancreatic origin, such as the skeleton or adrenal glands, should also suggest the possibility of metastatic disease of the pancreas. Metastasis may also be more likely when there is a large tumour of the pancreatic head without a dilated bile duct and when the retropancreatic fat is not obliterated in a patient with a large pancreatic mass [15].
Intratumoural calcification is not characteristic of primary ductal adenocarcinoma of the pancreas, although it has been reported as a rare occurrence in that relatively common tumour [30]. Calcification occurs more frequently in some of the less common types of primary pancreatic neoplasia such as islet cell carcinoma [31, 32].
Focal infiltration of the pancreas in Hodgkin and non-Hodgkin lymphomas has been described in the US literature thus far exclusively as large, solitary, hypoechoic, space-occupying lesions [35, 36]. The presence of para-aortic lymph nodes and the fact that the underlying disease usually is already known at the time pancreatic lesions are demonstrated facilitate differential diagnosis.
Treatment
Pancreatic metastases are rare and the possible benefit of surgical treatment is not clearly defined. However, surgical resection can be performed safely in patients with isolated pancreatic metastases from colorectal cancer and in selected patients with associated extrapancreatic disease. Although long-term survival is rare, surgery should be included, whenever possible, in the multimodality approach to this disease [37].
In patients with pancreatic metastases from renal cell cancer (RCC) who have only limited disease, complete resection of all lesions can be successfully performed with a low rate of complications [38]. It seems that lesion size and the number of metastases may predict survival after resection, but sufficient data are not available. In a retrospective study involving 220 patients, it was shown that survival after resection of RCC with isolated metastasis to the pancreas is favorable [39]. However, a more detailed analysis considering outcomes without surgery for each primary tumour site is needed before the value of this aggressive surgical approach can be completely assessed in the general occurrence of pancreatic metastasis.
Sunitinib recently showed clinical efficacy in patients with advanced renal cell carcinoma. According to the results of a recent study [40] it seems that sunitinib could be also effective in patients with pancreatic metastasis (Fig. 11). This raises the question of whether patients with metastatic renal cell carcinoma limited to the pancreas may derive greater clinical benefit from anti-angiogenic agents, rather than from aggressive surgical resection. However, surgery remains the only potential cure in patients with isolated pancreatic metastasis.
Conclusion
Pretherapeutic diagnosis of a secondary pancreatic tumour is essential for proper patient management. In certain cases prolonged survival may be achieved with successful surgical resection. Radiologists should be familiar with the different imaging appearances of pancreatic metastases and be aware that they may encounter this diagnosis not only in cases of multiple pancreatic lesions but also when a single atypical lesion is found even incidentally. In patients with a history of a malignant tumour, a newly diagnosed mass in the pancreas should raise the suspicion of metastatic disease. In doubtful cases the absence of vessel infiltration and/or the normal pancreatic duct appearance favors the diagnosis of pancreatic metastasis.
References
- 1.Rumancik WM, Megibow AJ, Bosniak MA, Hilton S. Metastatic disease to the pancreas: evaluation by computed tomography. JCAT. 1984;8:829–834. doi: 10.1097/00004728-198410000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Rubin E, Dunham WK, Stanley RJ. Pancreatic metastases in bone sarcomas: CT demonstration. JCAT. 1985;9:886–888. doi: 10.1097/00004728-198509000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Sperti C, Pasquali C, Liessi G, Pinciroli L, Decet G, Pedrazzoli S. Pancreatic resection for metastatic tumours to the pancreas. J Surg Oncol. 2003;83:161–166. doi: 10.1002/jso.10262. [DOI] [PubMed] [Google Scholar]
- 4.Crippa S, Angelini C, Mussi C, Bonardi C, Romano F, Sartori P, Uggeri F, Bovo G. Surgical treatment of metastatic tumours to the pancreas: a single center experience and review of the literature. World J Surg. 2006;30:1536–1542. doi: 10.1007/s00268-005-0464-4. [DOI] [PubMed] [Google Scholar]
- 5.Hung JH, Wang SE, Shyr YM, Su CH, Chen TH, Wu CW (2011) Resection for secondary malignancy of the pancreas. Pancreas (in press) [DOI] [PubMed]
- 6.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumours of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527–535. doi: 10.1007/s00428-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 7.Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002;9:675–679. doi: 10.1007/BF02574484. [DOI] [PubMed] [Google Scholar]
- 8.Scatarige JC, Horton KM, Sheth S, Fishman EK. Pancreatic parenchymal metastases: observations on helical CT. Am J Roentgenol. 2001;176:695–699. doi: 10.2214/ajr.176.3.1760695. [DOI] [PubMed] [Google Scholar]
- 9.Merkle EM, Boaz T, Kolokythas O, et al. Metastases to the pancreas. Br J Radiol. 1998;71:1208–1214. doi: 10.1259/bjr.71.851.10434919. [DOI] [PubMed] [Google Scholar]
- 10.McNichols DW, Segura JW, Deweerd JH. Renal cell carcinoma. Long term survival and late recurrence. J Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 11.Muranaka T, Teshima K, Honda H, Nanjo T, et al. Computed tomography and histologic appearance of pancreatic metastases from distant sources. Acta Radiol. 1989;30:615–619. doi: 10.3109/02841858909174725. [DOI] [PubMed] [Google Scholar]
- 12.Ferrozzi F, Bova D, Campodonico F, Chiara FD, Passari A, et al. Pancreatic metastases: CT assessment. Eur Radiol. 1997;7:241–245. doi: 10.1007/s003300050144. [DOI] [PubMed] [Google Scholar]
- 13.Muranaka T. Morphologic changes in the body of the pancreas secondary to a mass on the pancreatic head. Acta Radiol. 1990;31:483–488. [PubMed] [Google Scholar]
- 14.Kelekis NL, Semelka RC, Siegelman ES. MRI of pancreatic metastases from renal cancer. J Comput Assist Tomogr. 1996;20:249–253. doi: 10.1097/00004728-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Boudghene FP, Deslandes PM, LeBlanche AF, et al. US and CT imaging features of intrapancreatic metastases. J Comput Assist Tomogr. 1994;18:905–910. doi: 10.1097/00004728-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Varytimiadis K, Kalaitzakis E, Salla C, Ghika E, Pandazopoulou A, Karoumpalis I. Pancreatic metastasis of thymic neuroendocrine carcinoma: is there a role for endoscopic ultrasound?: report of a case and review of the literature. Pancreas. 2009;38:230–232. doi: 10.1097/MPA.0b013e3181816700. [DOI] [PubMed] [Google Scholar]
- 17.Witt J, Jowell P, Leblanc J, McHenry L, McGreevy K, Cramer H, Volmar K, Sherman S, Gress F. EUS-guided FNA of pancreatic metastases: a multicenter experience. Gastrointest Endosc. 2005;61:689–696. doi: 10.1016/S0016-5107(05)00287-7. [DOI] [PubMed] [Google Scholar]
- 18.McIntire M, Siziopikou K, Patil J, Gattuso P. Synchronous metastases to the liver and pancreas from a primary neuroendocrine carcinoma of the breast diagnosed by fine-needle aspiration. Diagn Cytopathol. 2008;36:54–57. doi: 10.1002/dc.20753. [DOI] [PubMed] [Google Scholar]
- 19.D’Onofrio M, Zamboni G, Faccioli N, Capelli P, Pozzi Mucelli R. Ultrasonography of the pancreas: contrast-enhanced imaging. Abdom Imaging. 2007;32:171–181. doi: 10.1007/s00261-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 20.Charnsangavej C, Whitley NO. Metastases to the pancreas and peripancreatic lymph nodes from carcinoma of the right side of the colon: CT findings in 12 patients. AJR Am J Roentgenol. 1993;160:49–52. doi: 10.2214/ajr.160.1.8416644. [DOI] [PubMed] [Google Scholar]
- 21.Larson RE, Semelka RC, Bagley AS, et al. Hypervascular malignant liver lesions: comparison of various MR imaging pulse sequences and dynamic CT. Radiology. 1994;192:393–399. doi: 10.1148/radiology.192.2.8029403. [DOI] [PubMed] [Google Scholar]
- 22.Lin G, Lunderquist A, Hagerstrand I, et al. Postmortem examination of the blood supply and vascular pattern of small liver metastases in man. Surgery. 1984;96:517–526. [PubMed] [Google Scholar]
- 23.Ng CS, Loyer EM, Iyer RB, David CL, DuBrow RA, Charnsangavej C. Metastases to the pancreas from renal cell carcinoma: findings on three-phase contrast-enhanced helical CT. AJR Am J Roentgenol. 1999;172:1555–1559. doi: 10.2214/ajr.172.6.10350288. [DOI] [PubMed] [Google Scholar]
- 24.Sohn TA, Yeo CJ, Cameron JL, Nakeeb A. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg. 2001;5:346–351. doi: 10.1016/S1091-255X(01)80060-3. [DOI] [PubMed] [Google Scholar]
- 25.Law CH, Wei AC, Hanna SS, et al. Pancreatic resection for metastatic renal cell carcinoma: presentation, treatment, and outcome. Ann Surg Oncol. 2003;10:922–926. doi: 10.1245/ASO.2003.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Mecho S, Quiroga S, Cuellar H, Sebastia C. Pancreatic metastasis of renal cell carcinoma: multidetector CT findings. Abdom Imaging. 2009;34:385–389. doi: 10.1007/s00261-008-9391-9. [DOI] [PubMed] [Google Scholar]
- 27.Bassi C, Butturini G, Falconi M, Sargenti M, et al. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br J Surg. 2003;90:555–559. doi: 10.1002/bjs.4072. [DOI] [PubMed] [Google Scholar]
- 28.Thompson L, Heffess C. Renal cell carcinoma to the pancreas in surgical pathology material. A clinicopathological study with 12 cases with a review of the literature. Cancer. 2000;89:1076–1088. doi: 10.1002/1097-0142(20000901)89:5<1076::AID-CNCR17>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Zagoria RJ, Wolfman NT, Karstaedt N, et al. CT features of renal cell carcinoma with emphasis on relation to tumour size. Invest Radiol. 1990;25:261–266. doi: 10.1097/00004424-199003000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Ward EM, Stephens DH, Sheedy PF., II Computed tomographic characteristics of pancreatic carcinoma: an analysis of 100 cases. RadioGraphics. 1983;3:547–565. [Google Scholar]
- 31.Eelkema EA, Stephens DH, Ward EM, Sheedy PF., II CT features of nonfunctioning islet cell carcinoma. AJR Am J Roentgenol. 1984;143:943–948. doi: 10.2214/ajr.143.5.943. [DOI] [PubMed] [Google Scholar]
- 32.Buetow PC, Parrino TV, Buck JL, et al. Islet cell tumours of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol. 1995;165:1175–1179. doi: 10.2214/ajr.165.5.7572498. [DOI] [PubMed] [Google Scholar]
- 33.Niccolini DG, Graham JH, Banks PA. Tumour-induced acute pancreatitis. Gastroenterology. 1976;71:142–145. [PubMed] [Google Scholar]
- 34.Freeny PC, Marks WM, Ryan JA, Traverso LW. Pancreatic ductal adenocarcinoma: diagnosis and staging with dynamic CT. Radiology. 1988;166:125–133. doi: 10.1148/radiology.166.1.2827228. [DOI] [PubMed] [Google Scholar]
- 35.Carroll BA, Ta HN. The ultrasonic appearance of extranodal abdominal lymphoma. Radiology. 1980;136:419–425. doi: 10.1148/radiology.136.2.7403517. [DOI] [PubMed] [Google Scholar]
- 36.Glazer HS, Lee JKT, Balfe DM, et al. Non-Hodgkin lymphoma: computed tomographic demonstration of unusual extranodal involvement. Radiology. 1983;149:211–217. doi: 10.1148/radiology.149.1.6225145. [DOI] [PubMed] [Google Scholar]
- 37.Sperti C, Pasquali C, Berselli M, Frison L, Vicario G, Pedrazzoli S. Metastasis to the pancreas from colorectal cancer: is there a place for pancreatic resection? Dis Colon Rectum. 2009;52:1154–1159. doi: 10.1007/DCR.0b013e31819f7397. [DOI] [PubMed] [Google Scholar]
- 38.Volk A, Kersting S, Konopke R, Dobrowolski F, Franzen S, Ockert D, Grutzmann R, Saeger HD, Bergert H. Surgical therapy of intrapancreatic metastasis from renal cell carcinoma. Pancreatology. 2009;9:392–397. doi: 10.1159/000181174. [DOI] [PubMed] [Google Scholar]
- 39.Sweeney AD, Wu MF, Hilsenbeck SG, Brunicardi FC, Fisher WE. Value of pancreatic resection for cancer metastatic to the pancreas. J Surg Res. 2009;156:189–198. doi: 10.1016/j.jss.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Medioni J, Choueiri TK, Zinzindohoué F, Cho D, Fournier L, Oudard S. Response of renal cell carcinoma pancreatic metastasis to sunitinib treatment: a retrospective analysis. J Urol. 2009;181:2470–2475. doi: 10.1016/j.juro.2009.02.020. [DOI] [PubMed] [Google Scholar]