Abstract
Background
Reduction of apparent diffusion coefficient (ADC) values in white matter is not always ischaemic in nature.
Methods
We retrospectively analysed our MRI records featuring reduced ADC values in the centrum semiovale without grey matter involvement or significant vasogenic oedema.
Results
Several conditions showed the aforementioned MR findings: moose-horn lesions on coronal images in X-linked Charcot-Marie-Tooth disease; small fronto-parietal lesions in Menkes disease; marked signal abnormalities in the myelinised regions in the acute neonatal form of maple syrup urine disease; strip-like involvement of the corpus callosum in glutaric aciduria type 1; persistent periventricular parieto-occipital abnormalities in phenylketonuria; diffuse signal abnormalities with necrotic evolution in global cerebral anoxia or after heroin vapour inhalation; almost completely reversible symmetric fronto-parietal lesions in methotrexate neurotoxicity; chain-like lesions in watershed ischaemia; splenium involvement that normalises in reversible splenial lesions or leads to gliosis in diffuse axonal injury.
Conclusion
Neuroradiologists must be familiar with these features, thereby preventing misdiagnosis and inappropriate management.
Keywords: Apparent diffusion coefficient (ADC) values, MRI, DWI
Introduction
Diffusion-weighted imaging (DWI) is a relatively new technique in which the image contrast mostly depends on the random microscopic motion of water molecules in tissues. DWI has been primarily applied in acute brain ischaemia, which is characterised in its early phase by cytotoxic oedema. Thanks to its sensitivity in detecting hyperacute ischaemic strokes, DWI has gained a primary role in routine brain MR protocols when dealing with acute neurological syndromes. The frequent utilisation of DWI has unexpectedly revealed peculiar signal abnormalities in several other cerebral diseases, such as abscesses, tumour and head trauma, widening even further the spectrum of brain diseases where this sequence helps to address the diagnosis. The reduction of the apparent diffusion coefficient (ADC) values depends on the disease studied, and it might involve an increase in cell debris, cellularity, intramyelin and axonal oedema.
The neuroradiologist is often challenged by neurologists and paediatricians with neuroimaging studies that document white matter lesions characterised by ADC restriction.
In this pictorial we focused on different conditions (pertaining to adult or child neurology) other than territorial ischaemic stroke where DWI signal abnormalities and ADC value reduction are confined within the white matter.
The DW images throughout the study were performed with a b value of 800 or 1,000 s/mm2.
Genetic and metabolic disorders
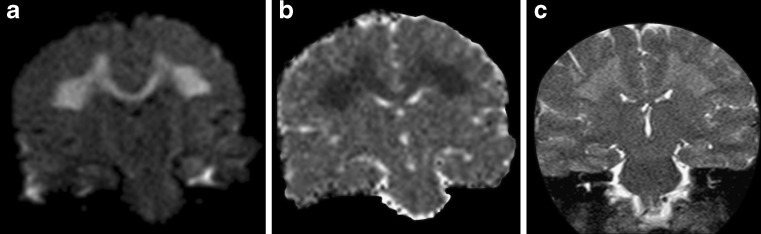
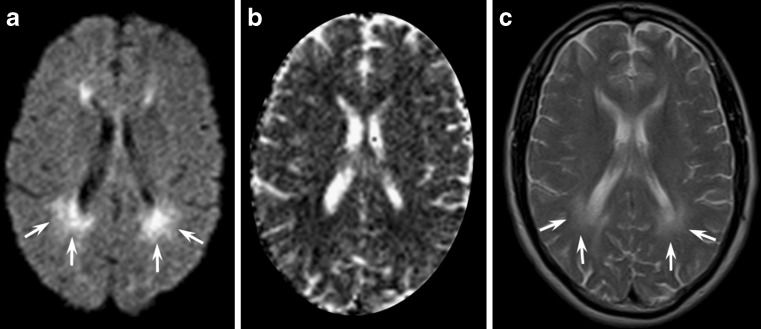
X-linked Charcot-Marie-Tooth disease (CMTX) is the second most common form of inherited motor and sensory demyelinating neuropathy next to the CMT Type 1A; it accounts for approximately 10–20% of all hereditary demyelinating neuropathies. It is associated with different mutations in the protein connexin 32 (CX32), a gap junction protein widely expressed in Schwann cells, oligodendrocytes and neurons [1, 2]. Even if this disease is considered a disorder of the peripheral nervous system, some CX32-mutated subjects might present with acute onset of central nervous system dysfunctions such as hemiparesis, ataxia or aphasia associated with transient white matter changes in the brain. In particular, neuroradiological MR findings in the acute phase show symmetrical, largely confluent cerebral deep white matter signal abnormalities, predominantly in the posterior centrum semiovale, the posterior limb of the internal capsule, the splenium of the corpus callosum (Fig. 1) and sometimes in the middle cerebellar peduncles [1–3]. Characteristically, U fibres are spared and the lesions do not demonstrate contrast enhancement [3]. Bilateral T2 and DWI signal abnormalities might disappear within 1 week or might persist for several months, unrelated to the clinical picture that often appears clearly asymmetric and usually improves rapidly.
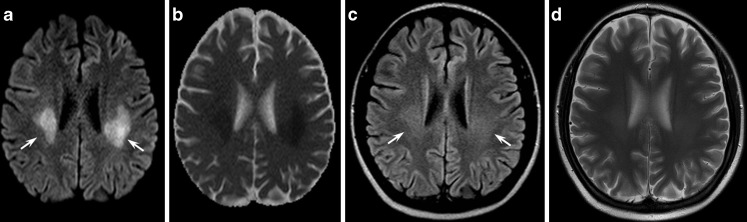
Fig. 1.
A 23-year-old male patient affected by genetically proven X-linked Charcot-Marie-Tooth disease presenting with acute onset of aphasia and right hemiparesis. Diffusion-weighted (a) and T2-weighted images (c) at admission showed symmetrical confluent hyperintensities in the posterior centrum semiovale and in the splenium of the corpus callosum, with a coronal image resembling “moose horns”. Apparent diffusion coefficient (ADC) maps (b) disclosed a restricted diffusion of water molecules in the corresponding areas
As ADC reduction might reverse after a few months, signal abnormalities cannot be interpreted as simple cytotoxic oedema, which usually lasts less than 2 weeks, while the increase in the magnetisation transfer ratios is not consistent with demyelination or oedema. As gap junction dysfunctions may disrupt the diffusion of small molecules and ions across myelin sheaths, the more likely explanation of the reversible white matter lesions remains myelin splitting and intra-myelin oedema with compression of the extracellular spaces [4].
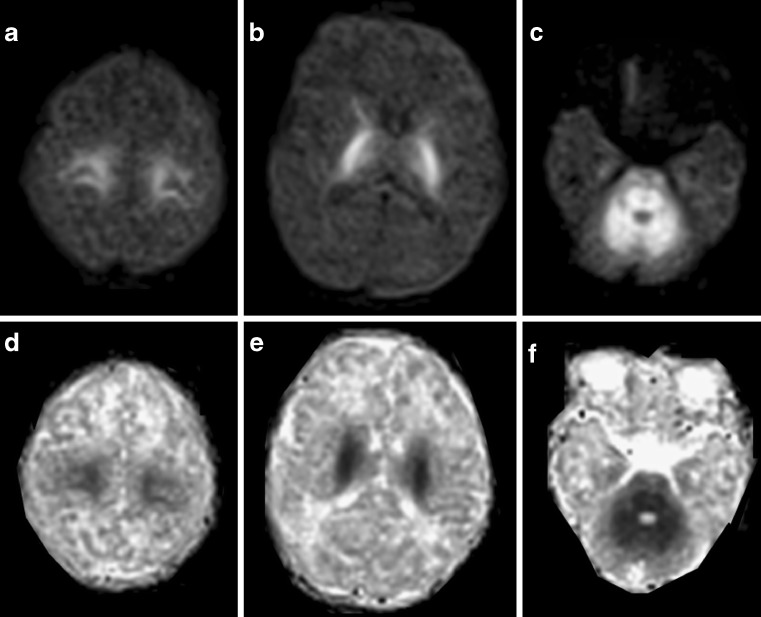
Maple syrup urine disease (MSUD) is a rare autosomic recessive disorder caused by a deficiency of the branched-chain α-keto acid dehydrogenase mitochondrial multi-enzyme complex. A sudden metabolic decompensation with encephalopathy occurs spontaneously or in concomitance with minor illnesses; high concentrations of upstream metabolites are thought to exert the neurotoxic effect. Clinical presentation and outcome are heterogeneous; in the classical phenotype the prognosis is very poor, and untreated surviving children are left with psychomotor retardation, spasticity, generalised dystonia and cerebral blindness. In the classical neonatal form, which accounts for about 80% of cases [4], brain MRI may provide a highly suggestive pattern of lesions with a generalised cerebral oedema and a striking increase in DWI and T2 signal intensity in all areas that are already myelinated at birth (Fig. 2). At microscopic examination the same areas represent myelin splitting and intra-myelin vacuoles. These peculiar histological changes are thought to explain the observed reduction in ADC values and its reversibility. Early diagnosis and treatment may normalise clinical and neuroradiological abnormalities, although inter-current illnesses throughout life may lead to further metabolic derangements [4].
Fig. 2.
Diffusion-weighted axial images (superior row) and ADC axial images (inferior row) obtained in a newborn with a biochemically confirmed classical form of maple syrup urine disease. He was admitted with severe encephalopathy and coma. The myelinated areas shown as symmetrical, markedly high intensity signal on a rather homogeneous brain parenchyma depict the cortico-spinal tract (a, b) and the cerebello-pontine white matter (c). Restricted diffusion of water molecules is well demonstrated by ADC images (d, e, f)
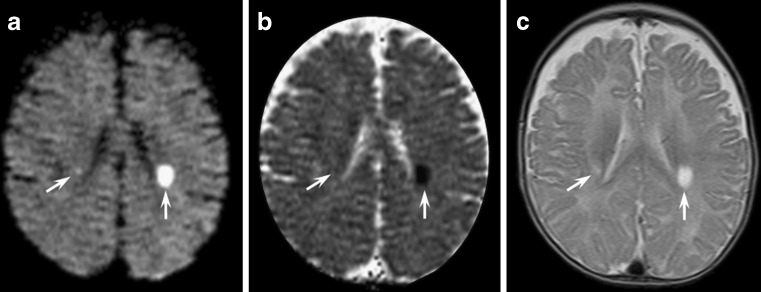
Menkes disease is an X-linked disorder caused by defective copper-transporting adenosine triphosphatase, which results in low levels of intracellular copper. Male children are normal in the first 2–4 months of life, and then present with loss of developmental milestones, hypotonia, seizures and failure to thrive; these patients usually have short, hypopigmented, kinked hair; death often occurs early in childhood. Brain MRI might disclose diffuse brain atrophy, subdural effusion, or haematoma and vascular tortuosity. Asymmetrical localised white matter lesions involving lobar white matter have been described [5, 6]. Recently, Lee et al. [7] reported that white matter lesions are bright on DWI and present decreased ADC values consistent with cytotoxic oedema (Fig. 3).
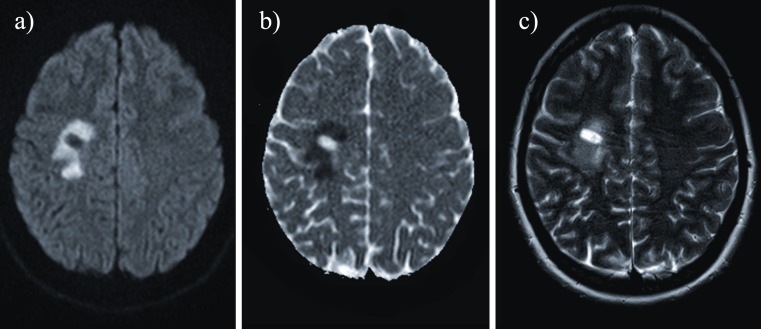
Fig. 3.
An 8-month-old child affected by Menkes disease. Axial diffusion- (a) and T2-weighted images (c) disclosed bilateral asymmetric oval bright white matter lesions in the centrum semiovalis (arrows). ADC maps (b) of the lesions showed low intensity signal
As in CMT and MSUD disease, the pathogenesis of these findings is uncertain. According to Hsich et al. [8], the tortuosity of the vessels might produce erratic and turbulent blood flow, thus predisposing to deep white matter ischaemia [8]. Alternatively, brain tissue abnormalities may be related to a deficiency in ATP7A mitochondrial copper-containing enzyme [9] with consequent energy failure.
Glutaric aciduria type 1 (GA-1) is a rare autosomal recessive disorder caused by deficiency of the mitochondrial enzyme glutaryl CoA dehydrogenase, necessary for the metabolism of three essential amino acids: lysine, hydroxylysine and tryptophan. The metabolic blocking of these amino acids results in increased levels of glutaric acid and 3-hydroxy glutaric acid. Because excessive glutaric acid is probably toxic to the striatal cells, atrophy in these nuclei occurs with age [10]. Patients may be asymptomatic or present with acute metabolic decompensation and striatal necrosis, which often leads to severe dyskinesia and cognitive impairment.
Magnetic resonance imaging findings in GA-1 include wide cerebrospinal fluid spaces anterior to the temporal lobes and within the sylvian fissure [11], subdural collections and brain atrophy. Oedema and increased T2 signal intensity within both caudate heads and lentiform nuclei are seen during metabolic derangement followed by necrosis and shrinking of these structures [10].
In older children as well as in asymptomatic patients, a peculiar and persistent DWI hyperintensity of white matter with a peculiar strip-like involvement of the corpus callosum has been noted. Within white matter lesions, ADC values are reduced (Fig. 4).
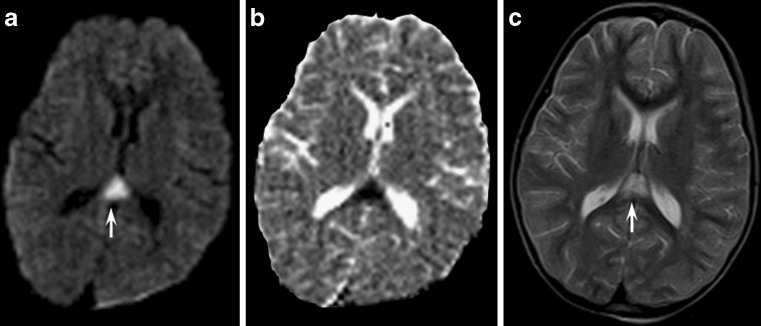
Fig. 4.
Axial diffusion- (a) and T2-weighted images (c) of an asymptomatic 26-year-old patient with type 1 glutaric aciduria demonstrating the peculiar strip-like involvement of the corpus callosum due to signal abnormalities in the median genu of the corpus callosum (arrows) and in periventricular white matter (arrowhead). ADC maps (b) revealed mildly reduced values in the affected areas
No convincing, definitive explanation has yet been found for the long persistence of these signal abnormalities. Spongiform changes in the white matter sparing U fibres have been demonstrated histopathologically [10].
Phenylketonuria (PKU) is a severe autosomal recessive disorder due to phenylalanine hydroxylase deficiency [4, 12, 13] that strongly benefits from early diagnosis and dietary treatment. Brain MR examination of adolescents and adults discloses white matter abnormalities both in untreated PKU patients and those treated early.
Typically, white matter lesions are occipital-parietal periventricular signal abnormalities on T2 sequences and are related to phenylalanine blood concentrations without significant correlation with the neurological impairment.
Recently, restricted diffusion of water molecules has been reported in the areas of white matter abnormalities [4, 12, 14, 15], appearing hyperintense on DWI in an otherwise rather homogeneously grey brain parenchyma (Fig. 5). These findings have been interpreted as cytotoxic oedema because of a reduction in the Na+K+-ATPase activity [16] or alternatively as dysmyelination with intra-myelin oedema [16], but the long persistence of the signal abnormalities does not support these hypotheses [16].
Fig. 5.
Magnetic resonance images obtained in an asymptomatic, phenylketonuric adult who was treated early (plasmatic phenylalanine levels of 729 μmol/l n.v. 44–66 μmol/l). Axial diffusion-weighted (a) ADC maps (b) and T2-weighted images (c) showed periventricular white matter signal abnormalities predominantly in the parieto-occipital regions (arrows)
Vascular conditions
Watershed infarctions account for 0.7–3.2% of all strokes [17] and may be confined to the deep white matter. In the acute phase, MR examination may reveal peri-ventricular round foci of restricted diffusion, with a “string of pearls appearance” within the deep white matter (Fig. 6) [17]. Despite a multi-focal bilateral appearance, all these lesions functionally represent a single vascular territory between superficial branches and deep perforators of cerebral arteries. This territory is particularly vulnerable in conditions of severely impaired cerebral blood flow such as prolonged hypotensive events, cardiac arrest with resuscitation and Moyamoya disease.
Fig. 6.
A 53-year-old male patient affected by dissection of the left internal carotid artery. Axial diffusion (a) and T2-weighted images (c) revealed hyperintense foci in the subcortical watershed territories in a characteristic parasagittal chain-like distribution (arrows). ADC maps disclosed the cytotoxic nature of the lesions (b)
On MRI lesions might also be unilateral in the case of an ipsilateral internal carotid artery occlusion or tight stenosis or main intracranial artery stenosis, especially when intracranial collateral flow is not compensatory (e.g. because of the incompleteness of the circle of Willis).
Contrary to patients with territorial infarction, patients with WI often do have a history of transient ischaemic attacks, generally have better outcomes, and usually do not benefit from thrombolysis.
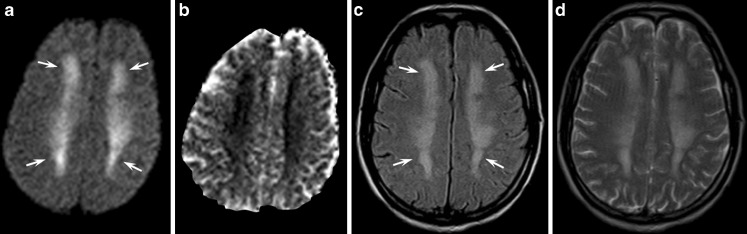
Grey matter is known to be more vulnerable to diffuse hypoxic-ischaemic injury than neighbouring white matter [18], but recent reports have highlighted that white matter is more sensitive to ischaemia than previously thought and may be primarily involved, sometimes in an isolated fashion [19, 20]. Global hypoxic-ischaemic encephalopathy of the adult is generally secondary to prolonged cardiac arrest, cardiac arrhythmias, drug overdose, respiratory failure or carbon monoxide poisoning [21].
Even though prominent symmetrical, restricted diffusion abnormalities (Fig. 7) have been described in the early stages of diffuse hypoxic-ischaemic injury [21], white matter abnormalities typically appear in the late-subacute period (14–20 days) and may normalise thereafter.
Fig. 7.
Magnetic resonance images obtained during the subacute stage of CO intoxication in a 47-year-old man with a history of heroin abuse found unconscious at home. Diffusion-weighted (a), FLAIR (c) and T2-weighted images (d) disclosed prominent, bilateral and symmetrical confluent hyperintensities in the centrum semiovalis that were more evident in the fronto-parietal lobes (arrows). On ADC maps (b), white matter lesions appeared moderately hypointense due to reduced water diffusion. As CO intoxication and heroin-associated spongiform leukoencephalopathy may produce similar MRI findings, both events could have resulted in this peculiar MR picture in our patient
Histologically, diffuse white matter abnormalities on MR imaging have correlated with deep white matter vacuolar degeneration and with adjacent axonal injury in normal-appearing white matter [22].
The outcome is generally poor with only a minority of patients regaining independent function [23].
Iatrogenic conditions
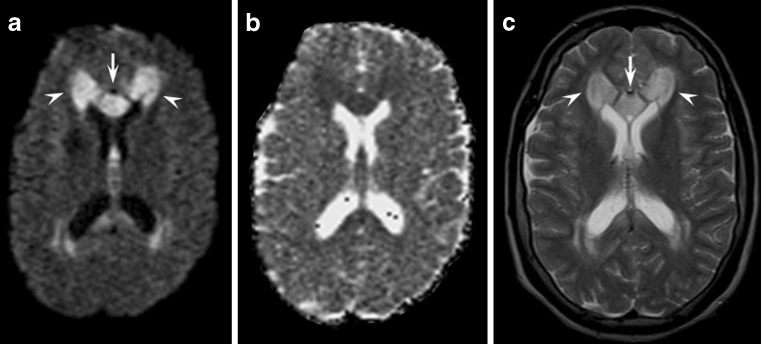
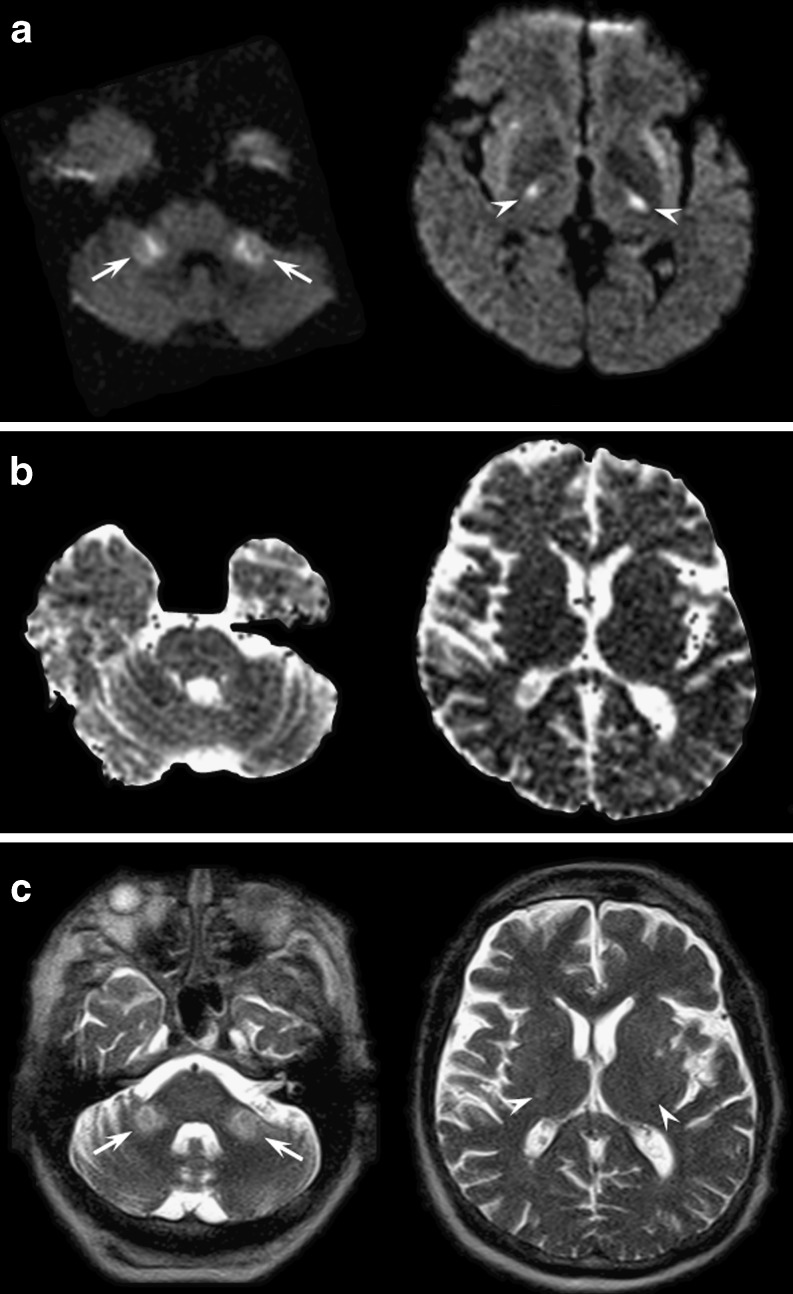
Pontine and extrapontine myelinolysis is a rare condition elicited by hypo- or hyper-natraemia, especially when an inappropriate (i.e. too fast) correction occurs.
Magnetic resonance imaging might show a characteristic lesion, namely a trident-shaped signal change within the pons with sparing of the tegmentum (pontine myelinolysis) or symmetrical bilateral signal changes of the middle cerebellar peduncles, of the cortico-spinal tract and of the splenium of the corpus callosum (extrapontine myelinolysis) (Fig. 8) [17]. It might be difficult to differentiate extrapontine myelinolysis from Wallerian degeneration [24]. In fact, several case reports of pontine stroke present with signal abnormalities in the middle cerebellar peduncles [25]. However, in Wallerian degeneration signal abnormalities are usually fainter compared with those of extrapontine myelinolysis. Furthermore, an associated pontine lesion is the key to a definitive diagnosis of Wallerian degeneration of the ponto-cerebellar tracts [26].
Fig. 8.
Axial diffusion-weighted (a), ADC maps (b) and T2-weighted (c) images at the level of the pons (left images) and basal ganglia (right images) obtained in a 54-year-old man who developed impaired consciousness after rapid correction of severe hyponatraemia. Diffusion-weighted and T2-weighted images revealed white matter hyperintensities that were more evident in the middle cerebellar peduncles (arrows) and in the cortico-spinal tract (arrowheads) consistent with osmotic extrapontine myelinolysis. ADC values were mildly decreased
Despite the term “myelinolysis”, several cases of bilateral basal ganglia involvement have been reported. DWI may show a variable decrease in ADC values, which probably depends on the timing of the MR examination and on the severity of white matter involvement.
Following an initial stage of diffuse encephalopathy, the neurological sequelae are usually characterised by diffuse cognitive deficits and extrapyramidal or cortico-bulbo-spinal signs; however, a complete remission of neurological symptoms can also be achieved. Variable T2 hyperintense lesions with increased ADC values are usually found on follow-up examinations, although complete regression of the MR lesions is possible.
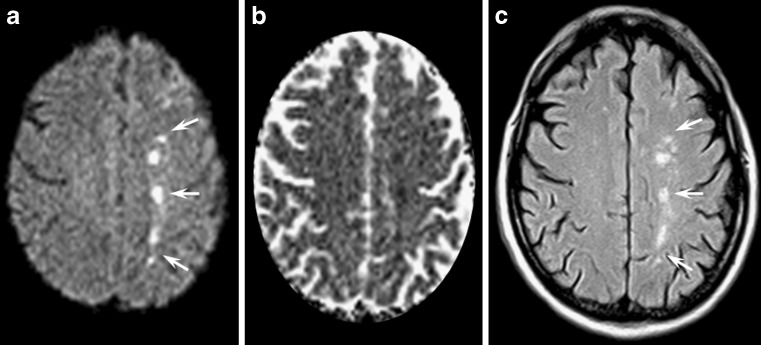
Methotrexate (MTX) is a cycle-specific antimetabolite that inhibits the enzyme dihydrofolate reductase and is used for the treatment of oncological disorders. MTX crosses the blood-brain barrier and can be administered intravenously as well as intrathecally to eradicate leukaemic cells from the CNS and to prevent CNS recurrence in acute lymphocytic leukaemia. Acute MTX neurotoxicity has been described in 3–10% of cases and varies with dose, frequency and route of administration [27–29]. The temporal relationship between acute MTX neurotoxicity and induction ranges from 2 to 127 weeks [28], but it is most often seen 10–11 days after intrathecal administrations of MTX. From a clinical point of view acute MTX neurotoxicity may mimic a stroke and present with aphasia or hemiparesis. Conventional MR imaging shows bilateral T2 hyperintense lesions within the deep fronto-parietal white matter [28]. DWI identifies the white matter involvement earlier than other sequences (Fig. 9) and reveals a variable decrease of ADC values probably due to the derangement of folate and homocysteine metabolism. DWI abnormalities and clinical symptoms usually resolve within a few days even when T2 abnormalities persist.
Fig. 9.
This 14-year-old girl affected by acute lymphocytic leukaemia and treated with intrathecal MTX acutely developed hemiparesis and aphasia. Axial diffusion-weighted (a), FLAIR (c) and T2-weighted images (d) showed bilateral T2 hyperintense lesions within the deep fronto-parietal white matter (arrows). ADC maps (b) presented reduced diffusivity. The neurological deficits and the DWI/ADC abnormalities resolved completely within 1 week, while slight T2 hyperintensities persisted on follow-up examinations
Non-iatrogenic toxicity
Inhalation of heroin vapour, a practice known as “chasing the dragon”, can cause a specific leukoencephalopathy. The pathophysiological mechanism seems to be selectively activated by substances originating during the heating of heroin. Symptoms usually occur within some days after the last heroin consumption and are first dominated by cerebellar deficits.
Characteristically, heroin-induced leukoencephalopathy appears as symmetrical T2 and DWI hyperintense lesions involving the cerebellar white matter, the posterior limbs of the internal capsule, the splenium of the corpus callosum and the posterior cerebral white matter [30]. Sub-cortical U fibres are usually spared (Fig. 7) [30]. A decrease in the ADC values and the absence of contrast enhancement further characterise the affected areas, whereas a spongiform demyelination is considered the histological substrate. Clinical and neuroradiological pictures may slowly improve if the patients are treated promptly; otherwise manifest severe neurological impairment and death occur.
Traumatic condition
Diffuse axonal injury (DAI) accounts for 50% of all primary intra-axial traumatic brain injuries. These lesions can be both haemorrhagic and non-haemorrhagic, and are caused by differential linear and rotational accelerations of the cortex in relation to the underlying deep brain structures.
Besides multi-focal punctuate haemorrhages at the fronto-temporal cortico-medullary junction, deep grey matter and upper brainstem, isolated DWI signal abnormalities of the corpus callosum may also be found. The splenium and the undersurface of the posterior body are mainly involved (Fig. 10) [31] due to the presence of the falx cerebri.
Fig. 10.
Axial MR images obtained in a child with a head injury at the level of the lateral ventricles. The midline of the splenium of the corpus callosum presented diffusion-weighted (a) and T2-weighted (c) hyperintensity (arrows) with reduced ADC values on ADC maps (b) consistent with diffuse axonal injury
Signal abnormalities are thought to be due to traumatic axonal stretching with consequent depolarisation and cellular swelling [31]. ADC abnormalities may also represent Wallerian degeneration secondary to proximal neuronal death or axonal disconnection [29] and may therefore appear in the subacute phase.
Most DAI lesions (80%) are microscopic [31] so that those visible on CT or MRI are just the tip of the iceberg: this fact might explain the poor correlation between neuroimaging and the clinical picture after a head trauma [32].
Demyelinating disorders
Multiple sclerosis (MS) is a chronic, multifocal, immunomediated inflammatory demyelinating and degenerative disorder of the central nervous system of unknown aetiology. MS lesions, known as plaques, typically appear as periventricular, ovoid white matter abnormalities perpendicular to the ventricles, along the path of the deep medullary veins.
On MRI the plaques are T2 hyperintense and show variable contrast enhancement. DWI usually discloses high ADC values in the plaque core due to prevailing inflammatory changes with vasogenic oedema or gliosis with myelin and axonal loss. Reduced ADC values may be found peripherally in acute MS lesions (Fig. 11) [33]. Electron microscopy studies have shown the presence of inflammatory infiltrates of lymphocytes and lipid-engulfing macrophages, swollen oligodendroglia, which could account for the ADC decrease [33]. Very rarely, acute plaques might present a homogeneous reduction in ADC values, which could suggest a diagnosis of stroke rather than an acute episode of MS [34]. However, according to our experience, concomitant lesions with increased ADC values are typically detected in these cases [34] and the cytotoxic-like lesions usually present components of increased ADC values as well [33, 34].
Fig. 11.
A 30-year-old female patient affected by relapsing-remitting multiple sclerosis (MS) presenting with a large demyelinating plaque in the right centrum semiovale. This atypical MS lesion appears hyperintense on diffusion-weighted (a) and T2-weighted (c) axial images with predominant ADC restriction (b)
Other conditions
Focal reversible lesions of the splenium (RSL) of the corpus callosum (Fig. 12) have been recently reported in several miscellaneous conditions such as viral encephalitis, encephalopathies, seizures, antiepileptic and anticancer drug administration [35, 36]. A few DWI studies [36, 37] have shown transitory restricted diffusion, but its aetiology and pathophysiology remain enigmatic. As a matter of fact, the proposed excitotoxic mechanism due to increased extracellular glutamate with consequent intramyelinic oedema [36] does not fully explain its detection in poorly myelinated brain such as in newborns with mitochondrial genetic disorders [38].
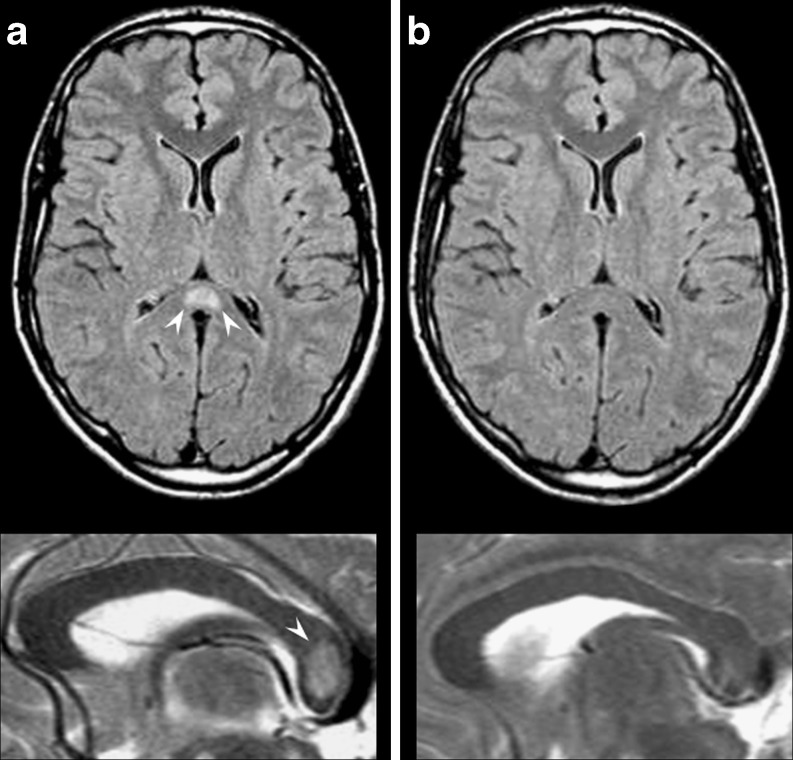
Fig. 12.
A 32-year-old male patient admitted with partial seizures secondary to antiepileptic drug withdrawal. Axial FLAIR (superior row) and sagittal T2-weighted images (inferior row). At onset MRI examination (a) revealed increased signal intensity in the central area of the splenium of the corpus callosum (white arrowheads); 1 month later (b) the lesion had disappeared
Clinically, RSL can present with seizures, confusion, ataxia, drowsiness, coma, headache and delirium, while the expected, specifically splenium-related deficits (visual simultanagnosia, hemialexia, unilateral agraphia and unilateral apraxia) are often difficult to evaluate.
Isolated corpus callosum lesions without concomitant parenchymal lesions usually regress rapidly, leading to complete clinical and neuroradiological recovery regardless of their cause [35, 36].
Conclusions
The literature ripens with diversified causes of areas of ADC restriction in the brain parenchyma: a few genetic, metabolic, iatrogenic, traumatic, infectious and vascular conditions can present with selective involvement of white matter with DWI hyperintense lesions and decreased ADC values. The neuroradiologist is often unnerved by the diagnostic challenges suggested by the clinician, as clinical history and course are not always disease-specific. In this setting, some suggestive or specific DWI MR patterns may provide powerful clues that can help in addressing the diagnosis and in tailoring treatment (Table 1).
Table 1.
DWI MR patterns that can help with the diagnosis. CCi: Corpus callosum involvement, CSTi: cortico-spinal tract involvement, CSi: involvement of the centra semiovalia
| Disease | Age | CCI | CSTi | CSi | IWMi | Neuroradiologic features | Other features | Evolution |
|---|---|---|---|---|---|---|---|---|
| CMT X-linked | Young adult | ++ | - | ++ | + | Moose-horn appearance | Stroke-like onset | Reversible |
| MSUD | Newborn | - | ++ | ++ | ++ | Already myelinated areas | Severe metabolic decompensation | Variable recovery |
| MD | Infant | - | - | ++ | - | Oval/drop-shaped lesions | Severe metabolic decompensation | Persistent T2 lesions |
| GA-1 | Child/adult | ++ | - | ++ | -+ | Strip-like appearance of corpus callosum | Also in asymptomatic patients | Persistent on T2 and DWI |
| PKU | Child/adult | + | - | ++ | -+ | Prominent parieto-occipital involvement | Also in asymptomatic patients | Persistent on T2 and DWI |
| WI | Elderly | - | - | ++ | - | Chain-like appearance | Ipsilateral severe carotid stenosis | Persistent T2 lesions |
| GCA | Adult/elderly | -+ | -+ | ++ | - | Extensive bilateral involvement of centrum semiovale | Evocative clinical history | Late necrotic evolution |
| Extrapontine myelinolysis | Adult/elderly | + | -+ | + | ++ | Natrium serum level abnormalities | Variable recovery | |
| MTX | Child | - | - | ++ | - | Posterior part of centrum semiovale | Clinical history of MTX administration | Reversible |
| Inhalation of heroin vapour | Young adult | -+ | + | ++ | + | Extensive bilateral involvement of centrum semiovale | Clinical history of drug abuse | Persistent T2 lesions |
| Diffuse axonal injury | Any | ++ | -+ | -+ | -+ | Splenium and undersurface of corpus callosum | Clinical history of head trauma | Persistent T2 lesions |
| Reversible lesions of the splenium | Any | ++ | - | + | - | Splenium | Miscellaneous conditions | Reversible |
References
- 1.Schelhaas HJ, Engelen BG, Gabreëls-Festen AA, Hageman G, Vliegen JH, Knaap MS, Zwarts MJ. Transient cerebral white matter lesions in a patient with connexin 32 missense mutation. Neurology. 2002;59:2007–2008. doi: 10.1212/01.wnl.0000038390.29853.46. [DOI] [PubMed] [Google Scholar]
- 2.Hanemann CO, Bergmann C, Senderek J, Zerres K, Sperfeld AD. Transient, recurrent, white matter lesions in X-linked Charcot-Marie-Tooth disease with novel connexin 32 mutation. Arch Neurol. 2003;60:605–609. doi: 10.1001/archneur.60.4.605. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RA, Simon EM, Marks HG, Scherer SS. The CNS phenotype of X-linked Charcot-Marie-Tooth disease: more than a peripheral problem. Neurology. 2003;61:1475–1478. doi: 10.1212/01.wnl.0000095960.48964.25. [DOI] [PubMed] [Google Scholar]
- 4.Knaap MS, Valk J. Maple Syrup Urine Disease. Magnetic Resonance of Myelination and Myelin Disorders. Berlin New York: Springer, Heidelberg; 2005. pp. 311–319. [Google Scholar]
- 5.Santos LM, Teixeira CD, Vilanova LC, et al. Menkes disease: case report of an uncommon presentation with white matter lesions. Arq Neuropsiquiatr. 2011;59:125–127. doi: 10.1590/S0004-282X2001000100027. [DOI] [PubMed] [Google Scholar]
- 6.Blaser SI, Berns DH, Ross JS, Lanska MJ, Weissman BM. Serial MR studies in Menkes disease. J Comput Assist Tomogr. 1989;13:113–115. doi: 10.1097/00004728-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Lee ES, Ryoo JW, Choi DS, Cho JM, Kwon SH, Shin HS. Diffusion-weighted MR imaging of unusual white matter lesion in a patient with Menkes disease. Korean J Radiol. 2007;8:82–85. doi: 10.3348/kjr.2007.8.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsich GE, Robertson RL, Irons M, Soul JS, Plessis AJ. Cerebral infarction in Menkes' disease. Pediatr Neurol. 2000;23:425–428. doi: 10.1016/S0887-8994(00)00207-1. [DOI] [PubMed] [Google Scholar]
- 9.Kaler SG. Menkes disease. Adv Pediatr. 1994;41:263–304. [PubMed] [Google Scholar]
- 10.Harting I, Neumaier-Probst E, Seitz A, et al. Dynamic changes of striatal and extrastriatal abnormalities in glutaric aciduria type I. Brain. 2009;132:1764–1782. doi: 10.1093/brain/awp112. [DOI] [PubMed] [Google Scholar]
- 11.Brismar J, Ozand PT. CT and MR of the brain in the diagnosis of organic acidemias. Experiences from 107 patients. Brain Dev. 1994;16:104–124. doi: 10.1016/0387-7604(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 12.Dezortova M, Hajek M, Tintera J, Hejcmanova L, Sykova E. MR in phenylketonuria-related brain lesions. Acta Radiol. 2001;42:459–466. doi: 10.1080/028418501127347179. [DOI] [PubMed] [Google Scholar]
- 13.Koch R, Moats R, Guttler F, Guldberg P, Nelson MJ. Blood-brain phenylalanine relationships in persons with phenylketonuria. Pediatrics. 2000;106:1093–1096. doi: 10.1542/peds.106.5.1093. [DOI] [PubMed] [Google Scholar]
- 14.Vermathen P, Robert-Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magn Reson Med. 2007;58:1145–1156. doi: 10.1002/mrm.21422. [DOI] [PubMed] [Google Scholar]
- 15.Phillips MD, McGraw P, Lowe MJ, Mathews VP, Hainline BE. Diffusion-weighted imaging of white matter abnormalities in patients with phenylketonuria. AJNR Am J Neuroradiol. 2001;22:1583–1586. [PMC free article] [PubMed] [Google Scholar]
- 16.Citton V, Manara R, Burlina A, et al. Diffusion weighted imaging (DWI) and white matter abnormalities (WMa) in early-treated patients with phenylketonuria (PK) Neuroradiol. 2007;49:S75–S162. doi: 10.1007/s00234-007-0285-2. [DOI] [Google Scholar]
- 17.Osborn AG. Stroke. In: Osborn AG, editor. Diagnostic Neuroradiology. St. Louis: Mosby; 1994. [Google Scholar]
- 18.Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH., Jr Differential regional vulnerability in transient focal cerebral ischemia. Stroke. 1982;13:339–346. doi: 10.1161/01.STR.13.3.339. [DOI] [PubMed] [Google Scholar]
- 19.Hirano A, Zimmerman HM. Some new pathological findings in the central myelinated axon. J Neuropathol Exp Neurol. 1971;30:325–336. doi: 10.1097/00005072-197107000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Schwedemberg TH. Leukoencephalopathy following carbon monoxide asphyxia. J Neuropathol Exp Neurol. 1959;18:597–608. doi: 10.1097/00005072-195910000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Chalela JA, Wolf RL, Maldjian JA, et al. MRI identification of early white matter injury in anoxic-ischaemic encephalopathy. Neurology. 2001;56:481–485. doi: 10.1212/wnl.56.4.481. [DOI] [PubMed] [Google Scholar]
- 22.Ryan A, Molloy FM, Farrell MA, et al. Fatal toxic leukoencephalopathy: clinical, radiological and necropsy findings in two patients. J Neurol Neurosurg Psychiatry. 2005;76:1014–1016. doi: 10.1136/jnnp.2004.047134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinney AM, Teksam M, Felice R, et al. Diffusion-weighted imaging in the setting of diffuse cortical laminar necrosis and hypoxic-ischemic encephalopathy. AJNR Am J Neuroradiol. 2004;25(10):1659–1665. [PMC free article] [PubMed] [Google Scholar]
- 24.Musson R, Romanowski C. Restricted diffusion in Wallerian degeneration of the middle cerebellar peduncles following pontine infarction. Pol Przegl Radiol. 2010;75(4):38–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Bermejo Garcés R, Cabada Giadas T, Bacaicoa Saralegui MC, et al. Bilateral wallerian degeneration of the pontocerebellar fibers secondary to pontine stroke: a report of 4 cases. Radiologia. 2010;52(1):71–75. doi: 10.1016/j.rx.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Uchino A, Sawada A, Takase Y, et al. Symmetrical lesions of the middle cerebellar peduncle: MR imaging and differential diagnosis. Magn Reson Med Sci. 2004;3:133–140. doi: 10.2463/mrms.3.133. [DOI] [PubMed] [Google Scholar]
- 27.Winick NJ, Bowman WP, Kamen BA, et al. Unexpected acute neurologic toxicity in the treatment of children with acute lymphoblastic leukemia. J Natl Cancer Inst. 1992;19:252–256. doi: 10.1093/jnci/84.4.252. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney DH, Jr, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy—a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 29.Rubnitz JE, Relling MV, Harrison PL, et al. Transient encephalopathy following high-dose methotrexate treatment in childhood acute lymphoblastic leukemia. Leukemia. 1998;12(8):1176–1181. doi: 10.1038/sj.leu.2401098. [DOI] [PubMed] [Google Scholar]
- 30.Keogh CF, Andrews GT, Spacey SD, et al. Neuroimaging features of heroin inhalation toxicity: "chasing the dragon.". AJR Am J Roentgenol. 2003;180:847–850. doi: 10.2214/ajr.180.3.1800847. [DOI] [PubMed] [Google Scholar]
- 31.Osborn AG. Diffuse axonal injury. In: Osborn AG, editor. Diagnostic Neuroradiology. St. Louis: Mosby; 1994. [Google Scholar]
- 32.Scheid R, Walther K, Guthke T, Preul C, Cramon DY. Cognitive sequelae of diffuse axonal injury. Arch Neurol. 2006;63(3):418–424. doi: 10.1001/archneur.63.3.418. [DOI] [PubMed] [Google Scholar]
- 33.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol. 1999;20(8):1491–1499. [PMC free article] [PubMed] [Google Scholar]
- 34.Rosso C, Remy P, Creange A, Brugieres P, Cesaro P, Hosseini H. Diffusion-weighted MR imaging characteristics of an acute strokelike form of multiple sclerosis. AJNR Am J Neuroradiol. 2006;27(5):1006–1008. [PMC free article] [PubMed] [Google Scholar]
- 35.Gallucci M, Limbucci N, Paonessa A, et al. Reversible focal splenial lesions. Neuroradiology. 2007;49:541–544. doi: 10.1007/s00234-007-0235-z. [DOI] [PubMed] [Google Scholar]
- 36.Maeda M, Tsukahara H, Terada H, et al. Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol. 2006;33:229–236. doi: 10.1016/S0150-9861(06)77268-6. [DOI] [PubMed] [Google Scholar]
- 37.Bulakbasi N, Kocaoglu M, Tayfun C, et al. Transient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. AJNR Am J Neuroradiol. 2006;27(9):1983–1986. [PMC free article] [PubMed] [Google Scholar]
- 38.Takanashi J, Meda M, Hayashi M, et al. A neonate showing a reversible splenial lesion. Arch Neurol. 2005;62:1481–1882. doi: 10.1001/archneur.62.9.1481. [DOI] [PubMed] [Google Scholar]