Abstract
Background
Children with cerebral palsy (CP) use their paretic arm less than normal but have a relative overactivity of wrist flexors, causing an impairing flexed position of the wrist. Voluntary use of a muscle downregulates myosin heavy chain (MyHC) IIx, but it is unclear whether the relative overactivity of wrist flexors and extensors in children with CP affects MyHC expression compared to normal subjects.
Questions/purposes
We therefore asked whether MyHC expression composition differs in wrist flexors compared to extensors in children with CP and in controls and whether it is related to clinical findings.
Methods
We took muscle biopsies from wrist flexors and extensors during hand surgery in children with CP (n = 9) and during open reduction of forearm fractures in control children (n = 5). The expression of the MyHC I, IIa, and IIx isoforms were determined on silver-stained 6% SDS-PAGE.
Results
CP flexors showed a higher proportion of MyHC IIx (40%) than control flexors (16%) and CP extensors (20%). MyHC IIa isoform proportion was lower in CP flexors (27%) than in control flexors (46%) and in CP extensors (45%). MyHC I expression was lower in CP (36%) than in controls (46%) for wrist extensors only.
Conclusions
Both the brain injury in CP and the different demands on flexors and extensors affect the expression of MyHCs. The higher amount of MyHC IIx in CP could be caused by a decreased voluntary use of the hemiplegic arm.
Clinical Relevance
More information on the structural difference between flexors and extensors in normal and spastic muscle could improve the understanding of strain of wrist extensors and possibly the development of flexion contractures in CP.
Keywords: Medicine & Public Health; Conservative Orthopedics; Orthopedics; Sports Medicine; Surgery; Surgical Orthopedics; Medicine/Public Health, general
Introduction
Cerebral palsy (CP) is defined as a disorder of movement and posture caused by a nonprogressive brain injury in the developing fetal or infant brain [24]. The brain injury causes a loss of descending inhibitory impulses to the stretch reflex, resulting in spasticity [12] . Spastic muscle differs from normal in having a larger variability of the fiber area [20] and a higher proportion of Type II muscle fibers [23]. In patients with spasticity of the upper extremity, a flexion contracture of the wrist often develops. Several explanations for the contracture development have been offered. First, spasticity has been suggested as the primary cause of muscle shortening in the flexors [6], although the stretch reflex is often increased in both extensors and flexors. Second, a flexed wrist position in itself may result in contractures [3], impairing the grip even more. Normally, wrist flexors and wrist extensors work in concert; they both stabilize the wrist during grip [1], but in CP the control of the muscle is impaired because of brain damage. The inherent larger physiologic cross-sectional area (and thus strength) of the wrist flexors compared to wrist extensors [15] will then drive the wrist into flexion. Third, there is a likely loss of sarcomeres and the muscle tendon unit is stiffer than normal [21].
When a muscle contracts, the power is generated by myosin interdigitating between actin filaments. Myosins belong to a large family of motor proteins and are composed of two heavy chains (MyHCs) and two light chains. MyHC isoforms determine contracting velocity and endurance. Muscle fibers containing MyHC IIx are fast but fatigable, fibers with MyHC IIa are fast with intermediate endurance, while fibers with MyHC I are slow with a high endurance [19]. Pontén et al. [20] showed children with severe CP have a higher expression of MyHC IIx at the expense of MyHC I in wrist flexors compared to extensors and compared to less severe CP but could not conclude whether this pattern is specific for CP, where the flexors are relatively overactive, or the pattern is typical also for healthy subjects.
A variety of treatments can be offered to the child, such as tendon lengthening, tenotomies, recession of the aponeurosis, transfers of overactive flexors to weaker extensors of the wrist, botulinum toxin injections, stretching, and serial casting [10], all either with a risk of permanent loss of force [14] or with only transient effect [4]. These treatments all have an effect on the passive tension of the muscle and thus affect the signaling from, eg, the cytoskeletal giant spring protein titin to the muscle nuclei. The signaling changes gene and protein expression of, eg, MyHCs in the muscle [26]. As the different MyHC isoforms differ in regard to contractility and endurance and thus have an impact on function, it is important to know the specific MyHC pattern in different muscles in children with and without CP.
We therefore asked whether MyHC isoform composition differs in wrist flexors compared to extensors in children with CP and in controls, indicating differences in fatigue and contractile speed. In CP, we also wanted to relate the MyHC composition to functional classifications and clinical findings.
Patients and Methods
We recruited nine children with CP who were planned for tendon transfer surgery (Table 1). Their mean age was 14 years (range, 8–17 years). As controls, we recruited five healthy children who were having an open reduction of a forearm fracture. Power analyses based on our previous study on MyHC IIx suggested this number of participants would be enough for sufficient power [22]. Fracture surgery was performed within 24 hours of the trauma. These patients had their arm immobilized at the emergency room. The mean age was 10 (range, 7–13 years). Biopsy samples were taken from the middle 1/3 of the lower arm, in full vision from muscles in reach from the skin incision needed for the surgeries; the flexor carpi ulnaris (FCU), or in one case the flexor carpi radialis (FCR); the extensor carpi radialis brevis (ECRB), or in two cases, the extensor carpi ulnaris (ECU). In all patients, one flexor and one extensor were included. In control patients, there was no visible injury of the muscles at the biopsy site. The ethical committee of Karolinska Institutet, Stockholm, Sweden, approved the study. Parents and, when possible due to age and mental capacity, children signed an informed consent before surgery.
Table 1.
Patient characteristics.
| Patient | CP/ Control | Sex | Age (years) | Bilateral/ Unilateral | GMFCS | MACS | House | Passive wrist extension (°) | Muscle | MyHC I (%) | MyHC IIa (%) | MyHC IIx (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CP | Male | 16 | Bilateral | 4 | 3 | 5 | 70 | ECRB | 35.6 | 64.4 | 0 |
| FCU | 55.8 | 21.4 | 22.8 | |||||||||
| 2 | CP | Male | 17 | Unilateral | 1 | 1 | 7 | 65 | ECRB | 43.9 | 56.1 | 0 |
| FCU | 19.6 | 27.8 | 52.6 | |||||||||
| 3 | CP | Male | 8 | Unilateral | 1 | 2 | 2 | 40 | ECRB | 47.0 | 36.2 | 16.8 |
| FCU | 33.4 | 37.6 | 29.0 | |||||||||
| 4 | CP | Male | 17 | Bilateral | 5 | 5 | 0 | 0 | ECU | 34.2 | 30.1 | 35.7 |
| FCU | 36.1 | 28.0 | 35.9 | |||||||||
| 5 | CP | Male | 16 | Unilateral | 1 | 2 | 5 | 35 | ECRB | 44.1 | 55.9 | 0 |
| FCU | 36.5 | 31.4 | 32.1 | |||||||||
| 6 | CP | Male | 16 | Bilateral | 5 | 5 | 4 | −50 | ECRB | 34.9 | 32.0 | 33.1 |
| FCU | 24.7 | 25.6 | 49.7 | |||||||||
| 7 | CP | Male | 14 | Unilateral | 1 | 2 | 6 | 85 | ECRB | 24.7 | 38.2 | 37.1 |
| FCU | 39.1 | 25.2 | 35.7 | |||||||||
| 8 | CP | Female | 13 | Bilateral | 3 | 5 | 1 | −5 | ECRB | 29.9 | 44.6 | 25.5 |
| FCU | 29.8 | 24.1 | 46.1 | |||||||||
| 9 | CP | Female | 11 | Unilateral | 1 | 1 | 5 | 65 | ECRB | 25.3 | 43.2 | 31.5 |
| FCU | 23.2 | 21.2 | 55.6 | |||||||||
| 10 | Control | Male | 13 | ECRB | 48.8 | 40.4 | 10.7 | |||||
| ECU | 50.2 | 39.0 | 10.8 | |||||||||
| FCR | 35.4 | 43.4 | 21.2 | |||||||||
| FCU | 30.6 | 50.8 | 18.6 | |||||||||
| 11 | Control | Male | 7 | ECRB | 55.1 | 44.9 | 0 | |||||
| ECU | 59.1 | 40.9 | 0 | |||||||||
| FCR | 53.4 | 39.3 | 7.3 | |||||||||
| FCU | 34.4 | 39.4 | 26.2 | |||||||||
| 12 | Control | Female | 10 | ECU | 48.4 | 51.6 | 0 | |||||
| FCU | 49.1 | 34.4 | 16.5 | |||||||||
| 13 | Control | Female | 10 | ECRB | 45.0 | 55.0 | 0 | |||||
| FCR | 50.1 | 49.9 | 0 | |||||||||
| 14 | Control | Male | 8 | ECRB | 49.3 | 50.7 | 0 | |||||
| FCU | 24.4 | 55.6 | 20.0 |
CP = cerebral palsy; GMFCS = Gross Motor Function Classification System; MACS = Manual Ability Classification System; MyHC = myosin heavy chain; ECRB = extensor carpi radialis brevis; FCU = flexor carpi ulnaris; ECU = extensor carpi ulnaris; FCR = flexor carpi radialis.
Hand function in the patients with CP was classified according to House, where 0 is no use of the hand, 1 to 3 different levels of passive helper hand, 4 to 6 different levels of active helper hand, and 7 to 8 a hand that can manipulate [9, 11, 28]. Gross motor function (ambulation ability) was assessed according to the Gross Motor Function Classification System (GMFCS), where GMFCS I refers to independent ambulation without limitations and GMFCS V refers to when a child cannot drive an electric wheelchair by him/herself but has to be driven by someone else [17]. The Manual Ability Classification System (MACS) classifies the child’s ability to handle objects in daily life, where a child in MACS I handles objects easily and successfully, while a child in MACS V does not handle objects and has severely limited ability to perform even limited actions [2]. The maximal passive extension of the wrist was measured with flexed fingers.
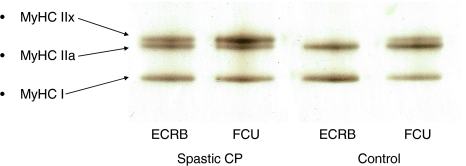
Muscle biopsies were immediately frozen in isopropane cooled with liquid nitrogen. The biopsies were stored at −80°C until analyzed. Depending on the diameter of the biopsy, one to three 10-μm-thick cryosections were used for the analyses. The expression of the MyHC I, IIa, and IIx isoforms was determined on silver-stained 6% SDS-PAGE [13]. The cryosections were placed in a sample buffer (50 μL buffer/section) consisting of 7.43 mL distilled water, 2.1 mL glycerol, 1.4 mL 10% (w/v) SDS, 1.75 mL Tris buffer (0.5 mol/L pH 6.8), 320 μL bromophenol blue solution, 0.0324 g dithiothreitol, and 1 mL leupeptin (5 mg/mL distilled water). The samples were diluted 1:10 and then loaded into each lane of the SDS-PAGE gel system consisting of stacking gel with 4% acryl amide and separating gel with 6% acryl amide and 30% glycerol. The gels were run on a constant voltage of 120 V for a total time of 22 hours and the temperature was kept at 10°C. After the run, the gel was immersed in a fixative consisting of 165 mL ethanol, 285 mL distilled water, and 50 mL glacial acetic acid. After 30 minutes, the gel was transferred to a 10% (v/v) glutaraldehyde solution for 1 hour minimum. After the gel had been washed in distilled water, it was put in a silver stain solution of 31.5 mL 90 mmol/L NaOH, 2.1 mL 14.8 mol/L NH4OH, 6 mL 1.14 mol/L AgNO3, and 110 mL distilled water for about 6 minutes depending on the protein load. After another wash, the gel was then placed in a developer of 500 mL distilled water, 2.5 mL 47.6 mmol/L citric acid, and 250 μL 37% (w/v) formaldehyde in 10% (v/v) methanol. The developing was continued until the protein bands became visible, dark and crisp. In the stained gel, three distinct bands could be separated and identified as MyHC I, IIa, and IIx isoforms, where the MyHC I isoform is the fastest and the MyHC IIx isoform is the slowest migrating protein band (for one example, see Fig. 1). The gels were scanned in a soft laser densitometer (Molecular Dynamics, Sunnyvale, CA, USA). The relative proportion of each MyHC isoform was determined using a densitometry system (ImageQuant™ TL Software v 2003.01; Amersham Biosciences, Uppsala, Sweden). MyHC content measured with immunohistochemistry and by SDS-PAGE (quantified by densitometry) correlates well [16, 22].
Fig. 1.
A polyacrylamide gel with bands containing MyHC I, IIa, and IIx from a wrist extensor and a wrist flexor from one child with CP and one control is shown. The MyHC I isoform is the fastest and the MyHC IIx isoform is the slowest migrating protein band.
The expression of MyHC I, IIa, and IIx was expressed as a percentage of total MyHCs in each muscle. Differences between wrist flexors and wrist extensors were tested as related samples with Wilcoxon signed-ranks test. Differences between CP muscles and control muscles were tested with the Mann-Whitney test. Spearman’s rank correlation coefficient was used to test for correlation between MyHC isoform percentage and passive wrist extension or the GMFCS, MACS, and House classifications. We used PASW® Statistics 18.0 (IBM Corp, Armonk, NY) for all analyses.
Results
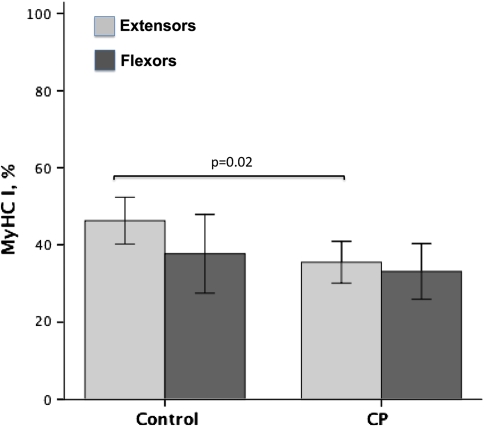
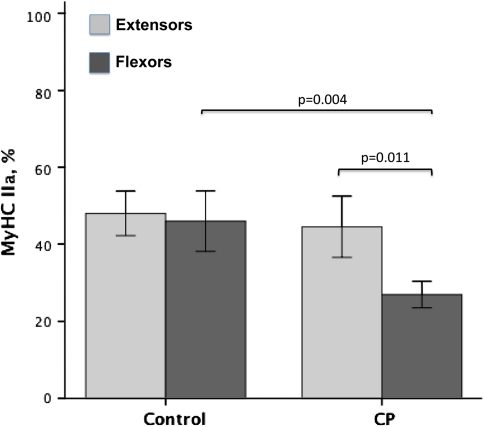
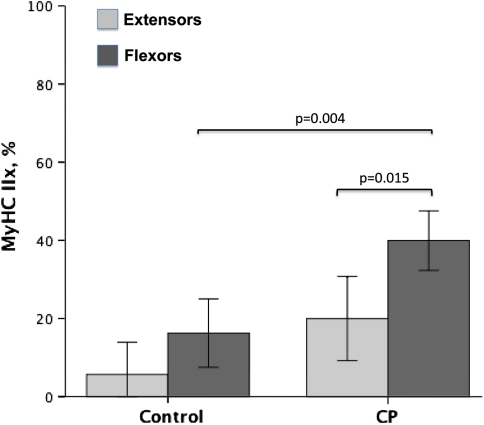
The expression of the MyHC IIx isoform was higher in CP flexors (40%) compared to controls (16%) (p = 0.004) and compared to CP extensors (20%) (p = 0.015) (Fig. 2). In some cases, more than 50% of the myosins were of the MyHC IIx type in wrist flexors in patients with CP. Three patients with CP and three of the controls had no MyHC IIx in the extensors, and one of the controls had no MyHC IIx in the flexor either (Table 1). The expression of the MyHC IIa isoform in wrist flexors was lower (p = 0.004) in CP muscles (27%) than in control muscles (46%). There was also lower (p = 0.001) expression of MyHC IIa in flexors (27%) than in extensors (45%) in CP (Fig. 3). MyHC I isoform expression was lower (p = 0.02) in CP (36%) than in controls (46%) for wrist extensors only (Fig. 4).
Fig. 2.
A graph shows the expression of MyHC I as a percentage of total MyHCs in wrist extensors and wrist flexors from children with CP and controls. Error bars represent ± 2 standard errors. Differences are noted with exact p values.
Fig. 3.
A graph shows the expression of MyHC IIa as a percentage of total MyHCs in wrist extensors and wrist flexors from children with CP and controls. Error bars represent ± 2 standard errors. Differences are noted with exact p values.
Fig. 4.
A graph shows the expression of MyHC IIx as a percentage of total MyHCs in wrist extensors and wrist flexors from children with CP and controls. Error bars represent ± 2 standard errors. Differences are noted with exact p values.
MyHC isoform expression showed no correlation with the passive extension of the wrist or the GMFCS, MACS, and House classifications.
Discussion
When treating patients with CP, one realizes flexors and extensors react differently to the altered signaling from the lesioned brain, resulting in a flexed position and eventually flexion contractures. Clinically, we have the impression that the contractility of the wrist extensors differs from that of the flexors. We therefore investigated MyHC composition in wrist flexors and extensors, compared it between children with and without CP, and attempted to relate the results to clinical findings.
There are some major limitations of the study. First, the controls were somewhat younger (mean age, 10 years; range, 7–13 years) than the children with CP (mean age, 14 years; range, 8–17 years). The reason is, at our unit, we treat trauma patients up to the age of 15 years, while children with CP are treated up to the age of 18 years. Myosin expression is different during early infanthood and childhood [27], but although we are unaware of any data to support the contention, we suspect there would be no age-related differences between these slightly different age groups in this study. Second, as we were allowed to take biopsies only from muscles that were directly accessible during surgery, different muscles were taken in different children. All muscles in the human body have their specific phenotype, but the FCU and FCR and the ECRB and ECU, respectively, have similar function and architecture and could therefore be grouped. Third, using this SDS-PAGE method, small amounts of developmental myosins are difficult to differentiate from the other MyHCs. Our previous studies using immunohistochemical techniques have shown occurrence of few very small fibers expressing developmental myosins in wrist flexors compared to extensors in CP and in spastic biceps compared to control [20, 23]. These small cells were interpreted as signs of ongoing regeneration, not of immaturity, as all larger cells expressed only mature MyHCs. The net amount of developmental myosin in spastic flexors was thus very small and would, if present, be hidden in this type of SDS-PAGE.
Our data confirm the results of our earlier immunohistochemical study [23], finding more MyHC IIx in flexors than in extensors in children with CP, but now we have also compared these results with typically developing children. The upregulation of MyHC IIx we found in patients with CP could be caused by the relative inactivity of the whole paretic arm during, eg, walking and manual activities [18]. This explanation is contradicted by the observation that spastic muscles often are involuntarily activated not only as a part of the stretch reflex but also when the child is trying to move another part of the body or as a part of a startle reaction. The disuse factor could thus be related to only voluntary muscle action. We have previously shown the percentage of MyHC IIx is higher when the functional impairment and spasticity are more severe [22], suggesting voluntary activation downregulates MyHC IIx more than involuntary spastic contractions. In an attempt to understand the actual activation pattern of the upper limb in children with CP during reaching and grasping, EMG studies have been performed in other studies [25]. Different techniques have been used, varying from intradermal to surface or fine-wire electrodes recording muscle activity during voluntary, eg, dorsal extension and flexion of the wrist [25]. The results have differed greatly among studies and subjects, but a general finding has been involuntary and, for the task, nonfunctional cocontractions of different muscles. In one study, compared to typically developed control children, children with CP had a higher mean power frequency of the EMG signal in wrist flexors [5]. This was interpreted as a relatively higher contribution of Type II muscle fibers in children with CP than in control children, which correlates well with our results. It is thus plausible that an altered general activation pattern in CP is the main influence for the atypical MyHC expression. Greig and Wells [7] have investigated the muscle activity in the forearm when applying external forces to the hand during a pinch grip in healthy subjects. Both the perceived exertion and the muscle activity, measured as a percentage of maximum voluntary activation, were higher in the wrist extensors compared to the wrist FCR, which could be explained by the fact that the flexors of the lower arm have a larger physiologic cross-sectional area and are thus inherently stronger than the extensors [1, 15]. EMG recordings during backhand strokes in tennis have shown similar results [8].
We did not find any correlation between MyHC isoform composition and the functional classifications (GMFCS, MACS, or House) or to the severity of contracture. Either the study group was too small to show any correlation or other factors could have a strong influence on the MyHC expression, explaining the large variation among subjects. These factors could be different frequency and intensity of the use of the arm in daily life, altered signaling from the brain resulting in different kinds of spasticity and stiffness, or perhaps yet other trophic factors released from the motor nerve that have an effect on the gene expression and, in turn, protein expression in the muscle. The former two could be tested with accelerometers and standardized spasticity/stiffness tests using calibrated apparatus, respectively, and the latter could be investigated by taking muscle biopsies and correlating with clinical findings. How all these factors interact is truly complex and a wide array of aspects of the muscle should be investigated. This study presents one aspect of muscle alterations in CP and gives reason to proceed with more hypotheses on three important problems: motor control and weakness and contracture formation.
Acknowledgment
The authors thank Professor Lars Larsson for providing laboratory facilities and for valuable advice.
Footnotes
One or more of the authors have received funding from Stiftelsen Promobilia (EP, SG), Riksförbundet Rörelsehindrade Barn och Ungdomars forskningsstiftelse (EP), Stiftelsen Sunnerdahls Handikappfond (EP), Norrbacka-Eugeniastiftelsen (EP, SG), Jerringfonden (EP, SG), Sällskapet Barnavård (EP, SG), Stiftelsen Samariten (EP), and through the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet (EP, SG).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
The surgery was performed at the Department of Pediatric Orthopedic Surgery at Astrid Lindgren Children’s Hospital and the laboratory work was performed at the Department of Clinical Neurophysiology, Uppsala University.
References
- 1.Chow JW, Carlton LG, Lim YT, Shim JH, Chae WS, Kuenster AF. Muscle activation during the tennis volley. Med Sci Sports Exerc. 1999;31:846–854. doi: 10.1097/00005768-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–554. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 3.Farmer SE, James M. Contractures in orthopaedic and neurological conditions: a review of causes and treatment. Disabil Rehabil. 2001;23:549–558. doi: 10.1080/09638280010029930. [DOI] [PubMed] [Google Scholar]
- 4.Fattal-Valevski A, Sagi L, Domenievitz D. Botulinum Toxin A injections to the upper limbs in children with cerebral palsy: duration of effect. J Child Neurol. 2011;26:166–170. doi: 10.1177/0883073810376446. [DOI] [PubMed] [Google Scholar]
- 5.Feltham MG, Ledebt A, Deconinck FJ, Savelsbergh GJ. Assessment of neuromuscular activation of the upper limbs in children with spastic hemiparetic cerebral palsy during a dynamical task. J Electromyogr Kinesiol. 2010;20:448–456. doi: 10.1016/j.jelekin.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Fergusson D, Hutton B, Drodge A. The epidemiology of major joint contractures: a systematic review of the literature. Clin Orthop Relat Res. 2007;456:22–29. doi: 10.1097/BLO.0b013e3180308456. [DOI] [PubMed] [Google Scholar]
- 7.Greig M, Wells R. A systematic exploration of distal arm muscle activity and perceived exertion while applying external forces and moments. Ergonomics. 2008;51:1238–1257. doi: 10.1080/00140130802037289. [DOI] [PubMed] [Google Scholar]
- 8.Hatch GF, 3rd, Pink MM, Mohr KJ, Sethi PM, Jobe FW. The effect of tennis racket grip size on forearm muscle firing patterns. Am J Sports Med. 2006;34:1977–1983. doi: 10.1177/0363546506290185. [DOI] [PubMed] [Google Scholar]
- 9.House JH, Gwathmey FW, Fidler MO. A dynamic approach to the thumb-in palm deformity in cerebral palsy. J Bone Joint Surg Am. 1981;63:216–225. [PubMed] [Google Scholar]
- 10.Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619–1631. doi: 10.1016/S0140-6736(04)16207-7. [DOI] [PubMed] [Google Scholar]
- 11.Koman LA, Williams RM, Evans PJ, Richardson R, Naughton MJ, Passmore L, Smith BP. Quantification of upper extremity function and range of motion in children with cerebral palsy. Dev Med Child Neurol. 2008;50:910–917. doi: 10.1111/j.1469-8749.2008.03098.x. [DOI] [PubMed] [Google Scholar]
- 12.Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30:1303–1313. doi: 10.1212/wnl.30.12.1303. [DOI] [PubMed] [Google Scholar]
- 13.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieber RL, Bodine-Fowler SC. Skeletal muscle mechanics: implications for rehabilitation. Phys Ther. 1993;73:844–856. doi: 10.1093/ptj/73.12.844. [DOI] [PubMed] [Google Scholar]
- 15.Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. J Hand Surg Am. 1990;15:244–250. doi: 10.1016/0363-5023(90)90103-X. [DOI] [PubMed] [Google Scholar]
- 16.Norman H, Kandala K, Kolluri R, Zackrisson H, Nordquist J, Walther S, Eriksson LI, Larsson L. A porcine model of acute quadriplegic myopathy: a feasibility study. Acta Anaesthesiol Scand. 2006;50:1058–1067. doi: 10.1111/j.1399-6576.2006.01105.x. [DOI] [PubMed] [Google Scholar]
- 17.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 18.Pette D. The adaptive potential of skeletal muscle fibers. Can J Appl Physiol. 2002;27:423–448. doi: 10.1139/h02-023. [DOI] [PubMed] [Google Scholar]
- 19.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Ponten E, Friden J, Thornell LE, Lieber RL. Spastic wrist flexors are more severely affected than wrist extensors in children with cerebral palsy. Dev Med Child Neurol. 2005;47:384–389. doi: 10.1017/S0012162205000757. [DOI] [PubMed] [Google Scholar]
- 21.Ponten E, Gantelius S, Lieber RL. Intraoperative muscle measurements reveal a relationship between contracture formation and muscle remodeling. Muscle Nerve. 2007;36:47–54. doi: 10.1002/mus.20780. [DOI] [PubMed] [Google Scholar]
- 22.Ponten E, Lindstrom M, Kadi F. Higher amount of MyHC IIX in a wrist flexor in tetraplegic compared to hemiplegic cerebral palsy. J Neurol Sci. 2008;266:51–56. doi: 10.1016/j.jns.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 23.Ponten EM, Stal PS. Decreased capillarization and a shift to fast myosin heavy chain IIx in the biceps brachii muscle from young adults with spastic paresis. J Neurol Sci. 2007;253:25–33. doi: 10.1016/j.jns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 25.Samilson RL, Morris JM. Surgical improvement of the cerebral-palsied upper limb: electromyographic studies and results of 128 operations. J Bone Joint Surg Am. 1964;46:1203–1216. [PubMed] [Google Scholar]
- 26.Smith LR, Ponten E, Hedstrom Y, Ward SR, Chambers HG, Subramaniam S, Lieber RL. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med Genomics. 2009;2:44. doi: 10.1186/1755-8794-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens L, Bastide B, Maurage CA, Dupont E, Montel V, Cieniewski-Bernard C, Cuisset JM, Vallee L, Mounier Y. Childhood spinal muscular atrophy induces alterations in contractile and regulatory protein isoform expressions. Neuropathol Appl Neurobiol. 2008;34:659–670. doi: 10.1111/j.1365-2990.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 28.Waters PM, Zurakowski D, Patterson P, Bae DS, Nimec D. Interobserver and intraobserver reliability of therapist-assisted videotaped evaluations of upper-limb hemiplegia. J Hand Surg Am. 2004;29:328–334. doi: 10.1016/j.jhsa.2003.10.014. [DOI] [PubMed] [Google Scholar]