Abstract
Background
Sacrectomy may offer curative potential for primary sarcomas of the sacrum. However, it is unclear whether and to what extent sacrectomies achieve local control.
Questions/Purpose
We therefore determined the curative potential, walking ability, continence control, and quality of life for children after sacrectomy for primary bone sarcoma.
Methods
We retrospectively reviewed eight children with high-grade primary bone sarcomas treated with sacrectomy. The average age of the patients was 14 years (range, 4–18 years). There were six Ewing sarcomas and two osteosarcomas. Five patients underwent partial and three underwent total sacrectomies with all resection margins free of tumor. We recorded local and distant recurrences, functional (Musculoskeletal Tumor Society [MSTS] score) and oncologic outcome (survival), walking ability, bladder and bowel control, independence, and reintegration in society. The average followup was 80 months (range, 54–204 months).
Results
Six patients were alive without evidence of disease at last followup. Three patients had metastases develop and two died of disease 3 and 25 months postoperative. MSTS scores among survivors averaged 17.5 points (range, 5–25 points). Seven of the eight patients were able to ambulate, two independently. All initially were incontinent, but at last followup, two were continent and two were incontinent only under stress. Complications occurred in seven of eight, five of whom required additional surgery.
Conclusion
Despite the high incidence of neurologic deficits and complications, sacrectomy achieved local control in patients with high-grade bone sarcomas. Our observations suggest long-term survival and adequate quality of life are possible in the pediatric population.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Keywords: Medicine & Public Health; Conservative Orthopedics; Orthopedics; Sports Medicine; Surgery; Surgical Orthopedics; Medicine/Public Health, general
Introduction
Ewing sarcomas and osteosarcomas are the most common primary malignant bone tumors in children, most occurring during the first two decades, with a slight male predilection [15, 18]. Sacral tumors are rare, and account for approximately 6% of all spinal tumors in the pediatric population [19, 24]. Although Ewing sarcomas and osteosarcomas are the most common primary malignancies of the sacrum in children, the lumbosacral region is the most commonly involved [9, 10, 13, 17]. Other malignant tumors of the sacrum include chordoma, Paget’s sarcoma, and multiple myeloma, all rarely seen in children [20].
Current treatment protocols include preoperative and postoperative chemotherapy and local surgical control [4]. Owing to the nonspecific nature of the symptoms (eg, night or rest pain, neurologic symptoms, and/or the presence of a mass), and lack of anatomic barriers or tumor growth into the pelvic region, delayed diagnosis occurs often and the tumor may reach a large size before diagnosis [21, 31]. When feasible, radical or partial sacrectomy may be curative; however, this may result in nerve root sacrifice, potentially causing major neurologic deficits and functional compromise, and creating a large dead space susceptible to infection and difficult to reconstruct.
Owing to the rarity of sacral tumors, the observations on long-term function and survival after sacrectomy for high-grade sarcomas are limited to case reports [22, 28], surgical techniques [8, 22, 31], or studies reporting cohorts that include but are not limited to children or high-grade sarcomas [2, 8, 22, 24–26, 32]. The overall survival after sacrectomy depends on the tumor type, presence of metastasis, and quality of resection (margins). It varies from approximately 20% to 80% at an average of 5 years [8, 24–26, 31, 32]. Among the reported cases of sacrectomy performed in children for primary high-grade bone sarcomas, the surgery has the potential to be curative and has a high complication rate, with overall survival of approximately 80% for patients with localized disease [5, 13, 16, 17, 20].
Based on an exclusively pediatric cohort of patients who underwent sacrectomies for primary high-grade sarcomas of the sacrum, we sought to answer the following questions: (1) Can sacrectomy be curative for primary bone sarcomas of the sacrum in children? (2) Is walking ability preserved in children who have had a sacrectomy? (3) Are bowel and bladder function preserved in children who have had a sacrectomy? (4) Do children who have had a sacrectomy maintain an adequate quality of life based on integration in society and independence (work and studies)?
Patients and Methods
We retrospectively reviewed the charts of all eight patients who underwent a sacrectomy for a high-grade primary sacral bone sarcoma between 1987 and 2000. We excluded patients (number unknown) who underwent sacrectomy for pelvic tumors extending into the sacrum and patients with soft tissue tumors, metastatic tumors to the sacrum, and patients who did not initially undergo treatment at our institution. The average age of the patients at presentation was 14 years (range, 4–18 years); six patients were male and two were female. All patients presented with pain, usually in the lower back and leg, of an average of 4.1 months duration (range, 1–12 months). Histologically, there were six Ewing sarcomas and two osteosarcomas (Table 1). The average followup was 80 months (range, 3–204 months). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from records and imaging.
Table 1.
Patient characteristics, staging, and treatment history
| Patient number | Age at surgery (years) | Gender | Diagnosis | Metastases at initial diagnosis | MSTS tumor stage | Roots spared (most caudal) | Reconstruction | Metastases after sacrectomy |
|---|---|---|---|---|---|---|---|---|
| 1 | 18 | Male | Ewing sarcoma | None | IIB | R = S1; L = S2 | None | None |
| 2 | 16 | Male | Osteosarcoma | Lung | III | R = L3, S3; L = all | Posterior spinal instrumentation and fusion; bone graft | None |
| 3 | 13.5 | Female | Osteosarcoma | None | IIB | R = S2; L = none | Sacral/iliac wing allograft | None |
| 4 | 13.5 | Male | Ewing sarcoma | Lung | III | R = L4; L = S3 | Cotrel-Dubousset spinal instrumentation and fusion; arthrodesis | None |
| 5 | 13 | Male | Ewing sarcoma | None | IIB | R = S3; L = L4 | Cotrel-Dubousset spinal instrumentation and fusion; bone graft | None |
| 6 | 4 | Female | Ewing sarcoma | Lungs | III | R = S2; L = S1 | None | Pelvis |
| 7 | 16.5 | Male | Ewing sarcoma | Lungs | III | None | Sacral/pelvic allograft | Lungs |
| 8 | 16 | Male | Ewing sarcoma | None | IIB | R = L5; L = L5 | Sacral allograft | Skull |
From a prospectively collected electronic musculoskeletal tumor database we collected the following information: demographics, tumor type, and location. From the medical records we extracted specific information such as tumor staging, resection level, margins, reconstruction procedures, functional outcome, neurologic function, (including motor and sensory levels, ability to walk, and bladder and bowel continence), disease-free survival, event-free survival, and overall survival.
Before surgery, all children underwent staging with MR imaging, CT scan of the chest, and Tc-99 m ethylene diphosphonate bone scan. According to the staging system of Enneking et al. [7], four patients had Stage IIB disease and four had Stage III disease. The four patients with Stage III disease presented with pulmonary metastases, all of which responded to inductive chemotherapy. All patients received neoadjuvant chemotherapy using a standard protocol. Two patients, both with Ewing sarcoma, received radiation therapy. One patient (Patient 6) underwent radiation therapy for pelvic metastases that occurred after sacrectomy, and the other (Patient 7) underwent preoperative radiation as surgery initially was refused.
Five patients underwent partial sacrectomies and three had total sacrectomies; patients with tumors involving S1 or S2 underwent reconstruction surgery after resection. The surgical resection was performed by the senior author (VTT) with assistance by a pediatric general surgeon. Briefly, the technique included an initial anterior approach for exposure of the ventral surface of the sacrum by soft tissue mobilization, which included ligation of the anterior sacral artery and internal iliac vessels when necessary. At that time, all anterior retroperitoneal structures were assessed for tumor involvement. If nerve root sacrifice was anticipated based on preoperative imaging and intraoperative findings, it was performed accordingly at the level of the foramen or just caudal to the tumor mass. The anterior sacral osteotomy then was performed with osteotomes and the space between the sacrum and the vessels and abdominal structures was packed with laparotomy pads. After anterior wound closure, the patient was positioned prone on trunk rolls. The osteotomies of the posterior sacrum, vertebrae, and iliac wing were completed through a posterior approach, with margins based on preoperative imaging studies. Once the tumor was removed and wide margins achieved, reconstruction was performed when needed (Table 1). The average time for surgery was 19 hours (range, 11–32 hours), and the average blood loss was 7000 cc (range, 3000–14,300 cc). Primary closure was possible in all but one patient (Patient 7) who needed a flap. Wide surgical margins (based on inking of the tumor and examinations of several sections) were obtained in all patients (Fig. 1).
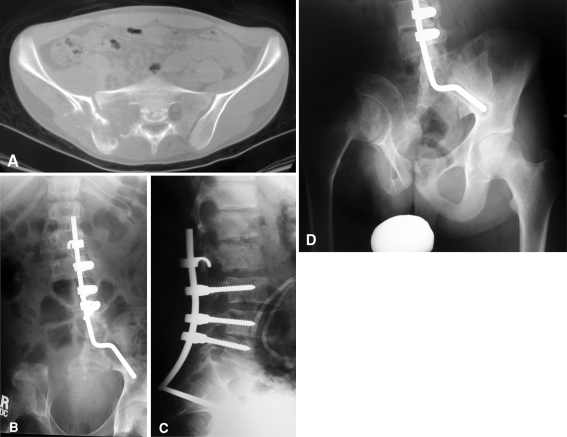
Fig. 1A–D.
A16-year-old male presented with a 3-month history of increasing pain in the lower back and right hip. (A) A CT scan of the lumbosacral spine and pelvis showed an aggressive, ill-defined, lytic lesion of the hemisacrum, sacrum ala, and ilium. After the biopsy, osteogenic sarcoma was confirmed and the patient underwent inductive chemotherapy, followed by surgical resection. (B) AP and (C) lateral radiographs of the lumbosacral spine show the resection, followed by instrumentation. (D) With time, compensatory scoliosis developed owing to pelvic obliquity that did not need formal treatment. (Images reproduced with permission from Children’s Orthopaedic Center, Children’s Hospital Los Angeles, Los Angeles, CA, USA.)
Postoperatively, all patients received appropriate pain control and physical therapy. The physical therapy differed if reconstruction was needed and if there was iliac involvement. In general, mobilization started as soon as the patient was comfortable and if there was no contraindication, the patients received gait training using an assistive device. All patients received postoperative chemotherapy and two (Patients 7 and 8) also received postoperative radiation.
We typically evaluated patients on the following schedule: 2 weeks postoperatively, followed by 6 weeks, 3 months, then every 3 months for 1 year, 4 months another year, and every 6 months subsequently. We evaluated function using to the MSTS criteria described by Enneking et al. [6]: the score varied from 0 to 30 (best result) and incorporated the clinician’s evaluation for pain, function, emotional acceptance, need for support, and walking ability (0 to 5 points in each category). We subjectively documented quality of life based on patients’ psychological and occupational status, integration in society, and independence. Complications were recorded and classified according to Dindo et al. [3]. Minor complications were managed clinically and major complications needed surgical intervention.
Results
At last followup six of the eight patients were alive with no evidence of disease, and two patients had died of distant disease progression. Three of the patients had late metastatic disease; two of these three had lung metastases at presentation that initially responded to chemotherapy, but one (Patient 7) refused postoperative chemotherapy and eventually died of disease 25 months postoperative. The other patient (Patient 6) had pelvic metastases develop 3 months after surgery, which was treated successfully with chemotherapy and radiotherapy. Another patient (Patient 8) had skull metastases develop during the postoperative chemotherapy regimen and died of disease 3 months after surgery.
The average MSTS score was 17.5 (range, 5–25) for the six surviving patients, whereas the two patients who died had scores of 0 and 2 (Table 2). All but two patients experienced some sensory loss postoperatively. Two patients were able to ambulate independently, five required the use of a cane or crutch, and one was wheelchair-bound after attempted reconstruction failed and the patient had spinal-pelvic discontinuity.
Table 2.
Functional and oncologic outcomes
| Patient number | Functional result (maximum 30 points) | Patient status | Followup (months) | Minor complications | Major complications |
|---|---|---|---|---|---|
| 1 | 23/30 | NED | 54 | Gluteal muscle tear | None |
| 2 | 18/30 | NED | 100 | None | Hardware failure and deep wound infections leading to sepsis; needed partial hardware removal |
| 3 | 5/30 | NED | 96 | None | Chronic sacral decubitus, hardware infection |
| 4 | 17/30 | NED | 204 | Superficial wound infection | Sacral decubitus |
| 5 | 17/30 | NED | 55 | Perianal skin breakdown, superficial wound infections | None |
| 6 | 25/30 | NED | 116 | None | None |
| 7 | 0/30 | DOD | 25 | None | Acute arterial bleeding from hypogastric remnant, deep wound infection leading to sepsis |
| 8 | 2/30 | DOD | 3 | Superficial wound infection | Infected allograft, spinal shock syndrome |
NED = no evidence disease; DOD = dead of disease
Postoperative, all patients initially were incontinent, resulting in urinary tract infections secondary to neurogenic bladder in five patients. At last followup, two patients were completely continent, two experienced some leaking or incontinence only under stress, and four remained completely incontinent, managed with self-catheterization.
According to their self-assessment at the long-term clinic assessment, all patients finished school and performed at a nearly normal level, including working full time.
Complications occurred in seven of the patients (Table 2). There were four minor complications (Grades I and II) that resolved with observation and conservative treatment, and five major complications (Grades III, IV, and V) [3].
Discussion
Primary high-grade bone sarcomas of the sacrum are rare, particularly in children. Although osteosarcoma and Ewing sarcoma are the most common primary bone malignancies in children, less than 10% of the cases involve the spine and approximately half of those are in the sacrum [13, 14, 23]. The current treatment for osteosarcoma and Ewing sarcoma include neoadjuvant and adjuvant chemotherapy, with local control performed by surgical resection [4]. However, it is unclear whether and to what extent sacrectomy with wide excision provides local disease control. In this study we sought to answer the following questions regarding the treatment of primary bone sarcoma of the sacrum in children: (1) Can sacrectomy be curative? (2) Can children retain their walking ability after sacrectomy?; (3) Can they retain bowel and bladder function? (4) Can they be an active part of the community and be able to finish their studies or work?
The limitations of this study relate mostly to the rare nature of the problem. First, we lacked a control group with patients who did not undergo surgery (specifically Ewing sarcoma is radiosensitive and therefore some centers may recommend radiation as local control). Second, this study was retrospective and therefore we had no preestablished protocol for management of these patients; rather, this treatment was on a case-to-case basis. Third, the cohort was small; however, that reflects the rare nature of this condition.
The disease-free survival rate after sacrectomy for high-grade sarcomas is unclear. A sampling of studies in the literature for disease-free survival gives an average of 51% (range, 25%–67%) at an average of 58.9 months followup (range, 1–174 months) after sacrectomy for various tumors, mainly chordomas [4, 6, 16]. Our patients’ disease-free survival rate of 75% suggests that, specifically for high-grade sarcomas in children, sacrectomy has the potential to be curative (Table 3). However, chemotherapy is critical in the treatment of Ewing sarcoma and osteosarcoma [4, 23, 30], therefore sacrectomy without effective chemotherapy most likely would be insufficient to achieve disease-free status. The poorer oncologic outcome of the two patients who died does not reflect a lack of efficacy of sacrectomy, but rather tumor biology and external factors. One patient waited more than a year after initial onset of symptoms before seeking treatment at our institution compared with an average duration of 3 months for the other patients. By presentation, the patient already was paraplegic and incontinent and elected to undergo surgery as a last resort after chemotherapy and radiation therapy failed. The second patient did not respond to neoadjuvant chemotherapy as evidenced by the near zero-percent tumor necrosis after resection with free margins.
Table 3.
Comparison of demographics and outcome*
| Study | Number of patients younger than 18 years | Average age – years (range) | Number of primary sarcomas (types) | Overall survival (at 5 years) |
|---|---|---|---|---|
| Ilaslan et al.# [13] | 67 (N/A) | 19 (4–54) | 67 (EWS) | 60% |
| Ilaslan et al.# [14] | 41 (N/A) | 27 (8–80) | 41 (OGS) | N/A |
| Kelley et al. [16] | 32 (20) | 42 (7–76) | Eight (OGS and EWS) | N/A |
| Sar & Eralp [26] | 22 (5) | 26 (17–63) | Two (EWS and chondrosarcoma) | One of two sarcomas |
| Ozaki et al. [23] | 15 (N/A) | 17 (5–55) | 15 (OGS) | 30% |
| Zileli et al. [32] | 11 (N/A) | 42 (14–71) | Five (OGS and chondrosarcoma) | Two of five sarcomas |
| Wuisman et al. [31] | Nine (N/A) | 44 (17–70) | Three (OGS and chondrosarcoma) | Six of nine including nonsarcomas |
| Current study | Eight (8) | 14 (4–18) | Eight (OGS and EWS) | 75% |
* After sacrectomy for primary sarcomas of the sacrum in patients younger than 18 years at time of disease onset; studies included have at least one or more pediatric patients with a primary sacral sarcoma; OGS = osteosarcoma; EWS = Ewing sarcoma; # = mostly descriptive/epidemiologic study; N/A = not available.
Sacrectomy is a demanding and risky procedure. There is a high incidence of postoperative complications, including potentially permanent neurologic deficits in sensation, motor capabilities, and bladder and bowel continence [1, 22, 25, 26, 29]. Neurologic compromise or deficit is a well-known result after sacrectomy. In this series, no patients experienced the saddle-like anesthesia described in other series after complete sacral nerve root sacrifice [5, 18]. Others have reported that approximately 90% of patients retain the ability to ambulate, with or without external support, after sacrectomy [8, 12]. Our results are similar to those findings.
Bladder and bowel incontinence often occur after sacrectomy [1, 5, 16, 18]. Most patients with unilateral preservation of the S2 nerve root are able to retain continence [12], but these cut-off points are not absolute and there are exceptions [8]. Accordingly, although all of our patients had at least transitory bladder incontinency, at followup, two patients who had retained the S2 nerve root unilaterally had continence and one patient who retained the nerve roots from S3 down unilaterally had bladder incontinence but was able to retain bowel continence with diet control.
Despite the high risk of complications and enduring neurologic deficits, sacrectomy is often the best definitive treatment (eg, local disease control) for high-grade sacral sarcomas [23, 31]. We achieved local control in all of our patients. Furthermore, although all patients had some degree of neurologic deficit, all six long-term survivors continued their education and worked full time. Our observations show that despite their neurologic deficits and the associated psychological impact, these patients are not only free of disease, but also active participants in society.
The rate of postoperative complications after sacrectomy is high, ranging from 32% to 67%. Of these, the most common is wound infection [8, 22, 24, 25, 27]. Our rate of postoperative complications (88%) is higher than those reported in some other studies [2, 8, 22, 25, 26, 31, 32], although this may be attributable to our relatively small sample size. Infection also was the most common complication seen in this cohort. Of the three patients with no or only minor complications, two of the three (67%) had not undergone any reconstruction surgery; the other five patients with major complications underwent complex reconstruction surgery. Tumors located below the S2 nerve root were resected without reconstruction [11]. Only patients with a resection level above the S2 nerve root, either bilaterally or unilaterally, needed reconstruction surgery [8, 25, 32]. Therefore, the need for reconstruction, based on the exact level of the tumor, may be considered a risk factor for development of complications.
Our study includes one of the largest, exclusively pediatric, series of sacrectomies for primary sarcomas of the sacrum. Based on our observations, we believe that sacrectomy along with systemic treatment has the ability of being curative. Children who undergo this procedure have a high chance of retaining walking ability, bowel and bladder control, and likely will reintegrate in society.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
The authors certify that their institution has approved the publication of this study that all the investigations were conducted in conformity with ethical principles of research, and informed consent was obtained.
This work was performed at Children’s Hospital Los Angeles, Los Angeles, CA, USA.
References
- 1.Davidge KM, Eskicioglu C, Lipa J, Ferguson P, Swallow CJ, Wright FC. Qualitative assessment of patient experiences following sacrectomy. J Surg Oncol. 2010;101:447–450. doi: 10.1002/jso.21517. [DOI] [PubMed] [Google Scholar]
- 2.Dickey ID, Hugate RR, Jr, Fuchs B, Yaszemski MJ, Sim FH. Reconstruction after total sacrectomy: early experience with a new surgical technique. Clin Orthop Relat Res. 2005;438:42–50. doi: 10.1097/01.blo.0000180054.76969.41. [DOI] [PubMed] [Google Scholar]
- 3.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominkus M, Darwish E, Funovics P. Reconstruction of the pelvis after resection of malignant bone tumours in children and adolescents. Recent Results Cancer Res. 2009;179:85–111. doi: 10.1007/978-3-540-77960-5_8. [DOI] [PubMed] [Google Scholar]
- 5.Dormans JP, Pill SG. Benign and malignant tumors of the spine in children. Spine: State of Arts Review. 2000;14:263–279. [Google Scholar]
- 6.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 7.Enneking WF, Spanier S, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. 1980. Clinical Orthop Relat Res. 2003;415:4–18. doi: 10.1097/01.blo.0000093891.12372.0f. [DOI] [PubMed] [Google Scholar]
- 8.Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, Suki D, Gallia GL, Garonzik I, Gokaslan ZL. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–122. doi: 10.3171/spi.2005.3.2.0111. [DOI] [PubMed] [Google Scholar]
- 9.Grubb MR, Currier BL, Pritchard DJ, Ebersold MJ. Primary Ewing’s sarcoma of the spine. Spine (Phila Pa 1976) 1994;19:309–313. doi: 10.1097/00007632-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto M, Akabane Y, Tate E. Ewing’s sarcoma of the sacrum. Radiat Med. 1999;17:451–453. [PubMed] [Google Scholar]
- 11.Hugate RR, Jr, Dickey I, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop Relat Res. 2006;450:82–88. doi: 10.1097/01.blo.0000229331.14029.44. [DOI] [PubMed] [Google Scholar]
- 12.Hulen CA, Temple HT, Fox WP, Sama AA, Green BA, Eismont FJ. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am. 2006;88:1532–1539. doi: 10.2106/JBJS.D.02533. [DOI] [PubMed] [Google Scholar]
- 13.Ilaslan H, Sundaram M, Unni KK, Dekutoski MB. Primary Ewing’s sarcoma of the vertebral column. Skeletal Radiol. 2004;33:506–513. doi: 10.1007/s00256-004-0810-x. [DOI] [PubMed] [Google Scholar]
- 14.Ilaslan H, Sundaram M, Unni KK, Shives TC. Primary vertebral osteosarcoma: imaging findings. Radiology. 2004;230:697–702. doi: 10.1148/radiol.2303030226. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto Y. Diagnosis and treatment of Ewing’s sarcoma. Jpn J Clin Oncol. 2007;37:79–89. doi: 10.1093/jjco/hyl142. [DOI] [PubMed] [Google Scholar]
- 16.Kelley SP, Ashford RU, Rao AS, Dickson RA. Primary bone tumours of the spine: a 42-year survey from the Leeds Regional Bone Tumour Registry. Eur Spine J. 2007;16:405–409. doi: 10.1007/s00586-006-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozlowski K, Barylak A, Campbell J, Hoeffel JD, Beluffi G, Masel J, Panuel M, Pelizza A, Taccone A, Arico M. Primary sacral bone tumours in children (report of 16 cases with a short literature review) Australas Radiol. 1990;34:142–149. doi: 10.1111/j.1440-1673.1990.tb02830.x. [DOI] [PubMed] [Google Scholar]
- 18.Lahl M, Fisher VL, Laschinger K. Ewing’s sarcoma family of tumors: an overview from diagnosis to survivorship. Clin J Oncol Nurs. 2008;12:89–97. doi: 10.1188/08.CJON.89-97. [DOI] [PubMed] [Google Scholar]
- 19.Lam CH, Naqib MG. Nonteratomatous tumors in the pediatric sacral region. Spine (Phila Pa 1976) 2002;27:E284–E287. doi: 10.1097/00007632-200206010-00023. [DOI] [PubMed] [Google Scholar]
- 20.Llauger J, Palmer J, Amores S, Bague S, Camins A. Primary tumors of the sacrum: diagnostic imaging. AJR Am J Roentgenol. 2000;174:417–424. doi: 10.2214/ajr.174.2.1740417. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo T, Sugita T, Sato K, Hotta T, Tsuchiya H, Shimose S, Kubo T, Ochi M. Clinical outcomes of 54 osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005;68:375–381. doi: 10.1159/000086978. [DOI] [PubMed] [Google Scholar]
- 22.Ohata N, Ozaki T, Kunisada T, Morimoto Y, Tanaka M, Inoue H. Extended total sacrectomy and reconstruction for sacral tumor. Spine (Phila Pa 1976) 2004;29:E123–E126. doi: 10.1097/01.BRS.0000115140.19829.4B. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki T, Flege S, Liljenqvist U, Hillmann A, Delling G, Salzer-Kuntschik M, Jurgens H, Kotz R, Winkelmann W, Bielack SS. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069–1077. doi: 10.1002/cncr.10258. [DOI] [PubMed] [Google Scholar]
- 24.Randall RL, Bruckner J, Lloyd C, Pohlman TH, Conrad EU., 3rd Sacral resection and reconstruction for tumors and tumor-like conditions. Orthopedics. 2005;28:307–313. doi: 10.3928/0147-7447-20050301-17. [DOI] [PubMed] [Google Scholar]
- 25.Sahakitrungruang C, Chantra K, Dusitanond N, Atittharnsakul P, Rojanasakul A. Sacrectomy for primary sacral tumors. Dis Colon Rectum. 2009;52:913–918. doi: 10.1007/DCR.0b013e3181a0d932. [DOI] [PubMed] [Google Scholar]
- 26.Sar C, Eralp L. Surgical treatment of primary tumors of the sacrum. Arch Orthop Trauma Surg. 2002;122:148–155. doi: 10.1007/s00402-001-0356-5. [DOI] [PubMed] [Google Scholar]
- 27.Sciubba DM, Nelson C, Gok B, McGirt MJ, McLoughlin GS, Noggle JC, Wolinsky JP, Witham TF, Bydon A, Gokaslan ZL. Evaluation of factors associated with postoperative infection following sacral tumor resection. J Neurosurg Spine. 2008;9:593–599. doi: 10.3171/SPI.2008.9.0861. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel DA, Richardson WJ, Scully SP, Harrelson JM. Long-term survival following total sacrectomy with reconstruction for the treatment of primary osteosarcoma of the sacrum: a case report. J Bone Joint Surg Am. 1999;81:848–855. doi: 10.2106/00004623-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Todd LT, Jr, Yaszemski MJ, Currier BL, Fuchs B, Kim CW, Sim FH. Bowel and bladder function after major sacral resection. Clin Orthop Relat Res. 2002;397:36–39. doi: 10.1097/00003086-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Weber K, Damron TA, Frassica FJ, Sim FH. Malignant bone tumors. Instr Course Lect. 2008;57:673–688. [PubMed] [Google Scholar]
- 31.Wuisman P, Lieshout O, Sugihara S, Dijk M. Total sacrectomy and reconstruction: oncologic and functional outcome. Clin Orthop Relat Res. 2000;381:192–203. doi: 10.1097/00003086-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Zileli M, Hoscoskun C, Brastianos P, Sabah D. Surgical treatment of primary sacral tumors: complications associated with sacrectomy. Neurosurg Focus. 2003;15:E9. [PubMed] [Google Scholar]