Abstract
Background
The controversy surrounding diagnosis of an epithelioid hemangioma (EH), particularly when arising in skeletal locations, stems not only from its overlapping features with other malignant vascular neoplasms, but also from its somewhat aggressive clinical characteristics, including multifocal presentation and occasional lymph node involvement. Specifically, the distinction from epithelioid hemangioendothelioma (EHE) has been controversial. The recurrent t(1;3)(p36;q25) chromosomal translocation, resulting in WWTR1-CAMTA1 fusion, recently identified in EHE of various anatomic sites, but not in EH or other epithelioid vascular neoplasms, suggests distinct pathogeneses.
Question/purposes
We investigated the clinicopathologic and radiologic characteristics of bone and soft tissue EHs in patients treated at our institution with available tissue for molecular testing.
Patients and Methods
Seventeen patients were selected after confirming the pathologic diagnosis and fluorescence in situ hybridization analysis for the WWTR1 and/or CAMTA1 rearrangements. Four patients had multifocal presentation. Most patients with EH of bone were treated by intralesional curettage. None of the patients died of disease and only four patients had a local recurrence.
Results
Our results, using molecular testing to support the pathologic diagnosis of EH, reinforce prior data that EH is a benign lesion characterized by an indolent clinical course with an occasional multifocal presentation and rare metastatic potential to locoregional lymph nodes.
Conclusion
These findings highlight the importance of distinguishing EH from other malignant epithelioid vascular tumors as a result of differences in their management and clinical outcome.
Level of Evidence
Level IV, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Keywords: Medicine & Public Health; Conservative Orthopedics; Orthopedics; Sports Medicine; Surgery; Surgical Orthopedics; Medicine/Public Health, general
Introduction
Vascular tumors encompass a wide histologic spectrum, which includes hemangioma, hemangioendothelioma, angiosarcoma, and their epithelioid variants [16, 22]. Because of major differences in their clinical behavior and consequently in treatment and prognosis, it is paramount to distinguish them effectively and accurately. Epithelioid vascular tumors remain controversial because of their rarity, unusual morphologic features, and unpredictable biologic behavior [14, 16]. Moreover, their classification differs depending on whether they originate in the soft tissue versus bone. The World Health Organization (WHO) classification of soft tissue tumors recognizes three different entities based on their distinct level of malignancy [8] such as EH, benign; EHE, intermediate grade of malignancy, with a metastatic rate of 31% [21]; and angiosarcoma, high grade, with greater than 50% metastatic rates [13]. In contrast, the same edition of WHO classifies bone epithelioid vascular tumors into only two tiers: benign and malignant, EHE and angiosarcoma being included in the same malignant category. This difference in classification stems from the dogmatic belief that malignant skeletal vascular lesions represent a histologic spectrum rather than sharply defined pathologic entities. The terminology and classification applied for the intraosseous epithelioid vascular tumors at the low end of the spectrum have proven particularly controversial [6, 16]. For example, Evans et al. argued that EH is not a distinct clinicopathologic entity but rather a misdiagnosed hemangioendothelioma, a tumor that, unlike hemangioma, has malignant potential [6]. This illustrates the lack of objective diagnostic criteria and the confusion surrounding the classification of this rare subset of vascular tumors. Our opinion and that of others is that the classification of vascular tumors should be uniform regardless of their site of origin [16].
The recent identification of WWTR1-CAMTA1 fusion, as the genetic hallmark of EHE regardless of anatomic location, provides an objective and powerful diagnostic tool that can be used to distinguish EH from EHE in limited biopsy material. [5] The purpose of this study is to evaluate the pathologic, clinical, and radiologic characteristics of EH arising in bone and soft tissue in the first cohort of patients in whom the definitive diagnosis was supplemented by molecular techniques. Our hypothesis is the improved classification can more definitively elucidate the benign biology of EH.
Material and Methods
We searched the surgical pathology files of Memorial Sloan-Kettering Cancer Center for the diagnosis of EH arising from bone and soft tissue from 1991 to 2011. In each case, the diagnosis of EH was confirmed by reviewing available histologic slides and the positivity for CD31 endothelial marker by a dedicated expert musculoskeletal pathologist (CRA). Fluorescence in situ hybridization (FISH) was performed in retrospect in all cases for the presence of WWTR1/CAMTA1 rearrangements to confirm the histologic diagnosis and exclude the possibility of EHE [5]. As reported previously, we obtained BAC clones from the BACPAC Resources Center of the Children’s Hospital of Oakland Research Institute (http://bacpac.chori.org) [5]. Probe preparation and FISH analysis were performed on paraffin-embedded, 4-μm-thick tissue sections, as previously described [1]. However, in five tumors, FISH was deemed unsuccessful as a result of a decalcification procedure. The remaining 12 specimens were negative for signal abnormalities in WWTR1 and CAMTA1genes by FISH (Fig. 1). Thus a total of 17 patients were included in the study after pathologic rereview and confirmation of diagnosis.
Fig. 1.
Three-color fluorescence in situ hybridization (FISH) shows no break-apart (split signal) in the region of 3q25.1-25.3; orange (RP11-690M20, RP11-718M13); green (RP11-286N6, RP11-157D5, RP11-64O23); or red (RP11-615G7, RP11-152K5, RP11-880C10).
We obtained demographic, clinical treatment, and followup information from the patients’ medical records (Institutional Review Board WA0697-10) (Table 1). The patients had distinctive demographics and tumor distribution. There was a strong predilection for men (13:4), and their age ranged from 18 to 81 years (mean, 43 years). The anatomic distribution of EH was as follows: 10 tumors in bone, five in soft tissue, and two involved bone and soft tissue. Most tumors occurred in the extremities (arm, two; hand, three; tibia, one; foot, five) followed by the trunk (rib, two; vertebra, one; axilla, one; neck, one; clavicle, one). Four patients presented with multiple sites of involvement, two in the hand and one each in the foot and in the head and neck. Multifocality was restricted to a regional location and typically involved bone and soft tissue in almost all cases. Followup information was available for all 17 patients with a minimum of 6 months (mean, 62 months; range, 6–162 months).
Table 1.
Clinicopathologic data for patients with epithelioid hemangiomas
| Patient number | Age (years) | Sex | Location | Multifocal versus solitary | Treatment | Outcome (months) |
|---|---|---|---|---|---|---|
| 1* | 31 | M | Bone (foot, metatarsal) | Solitary | Surgery (intralesional margin) | NED (51) |
| 2* | 20 | M | Bone (foot, metatarsal) | Solitary | Surgery (intralesional margin) | NED (31) |
| 3 | 40 | M | Bone (foot, metatarsal) | Solitary | Surgery (intralesional margin) | NED1 (22) |
| 4* | 59 | M | Bone (foot, cuneiform) | Solitary | Surgery (intralesional margin) | NED (9) |
| 5* | 23 | M | Bone (rib) | Solitary | Surgery (wide margin) | NED (68) |
| 6* | 41 | M | Bone (rib) | Solitary | Surgery (wide margin) | NED1 (67) |
| 7* | 81 | M | Bone (clavicle) | Solitary | Surgery (intralesional margin) | NED (6) |
| 8 | 50 | M | Bone (vertebra) | Solitary | Surgery (intralesional margin) and radiation | DOO (16) |
| 9 | 34 | F | Bone (tibia) | Solitary | Surgery (intralesional margin) | NED1 (114) |
| 10 | 18 | M | Bone (hand) | Multifocal (scaphoid and trapezium) | Surgery (wide margin) | NED (156) |
| 11 | 49 | M | Bone and soft tissue (hand) | Multifocal (index and middle fingers) | Surgery (intralesional margin) | NED (48) |
| 12* | 35 | M | Soft tissue (head and neck) | Multifocal (bone and soft tissue) | Surgery (marginal margin), radiation and chemotherapy | NED1 (240) |
| 13* | 56 | F | Bone and soft tissue (foot) | Multifocal (metatarsal) | Surgery (wide margin) | NED (66) |
| 14* | 63 | M | Soft tissue (arm) | Solitary | Biopsy | AWD (64) |
| 15* | 38 | M | Soft tissue (arm) | Solitary | Surgery (marginal margin) | NED (58) |
| 16* | 63 | F | Soft tissue (hand) | Solitary | Surgery (marginal margin) | NED (67) |
| 17* | 38 | F | Soft tissue (axilla) | Solitary | Surgery (wide margin) | NED (162) |
* Negative for WWTR1/CAMTA1 fluorescence in situ hybridization (remaining test failure as a result of decal); M = male; F = female; NED = no evidence of disease; NED1 = no evidence of disease after local or distant recurrence; DOO = dead of other causes; AWD = alive with disease.
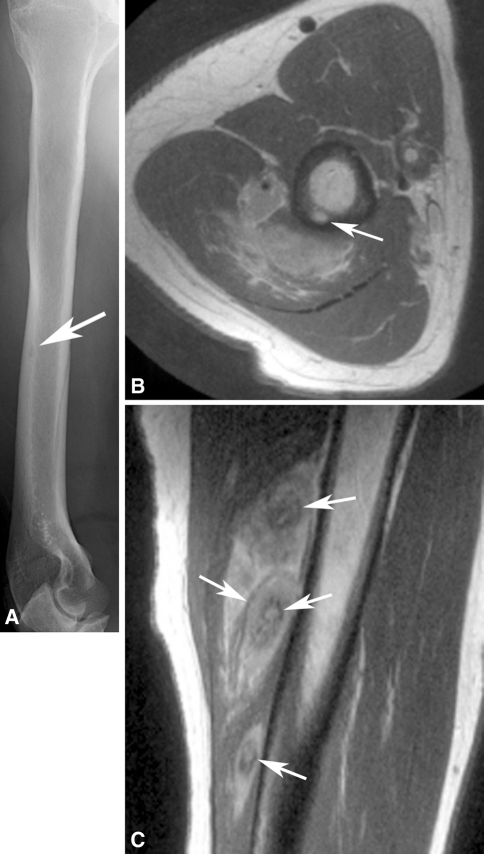
Radiologic images were available for 10 patients and were reviewed by an expert musculoskeletal tumor radiologist (DMP). The first metatarsal bone was involved in two patients (Fig. 2), and one patient each had involvement of the anterior ninth rib (Fig. 3), clavicle, T2 and T3 vertebrae, and multiple bones in a foot (Fig. 4). In the four patients with EH of soft tissue with available radiology, the lesion was located in the triceps muscle in two (Fig. 5) and one each had involvement of the flexor tendons of a hand and an index finger (Table 2).
Fig. 2A–B.
(A) A radiograph shows the first metatarsal has been replaced and expanded by a multiseptated lytic lesion. No gross calcified matrix is evident in the lesion. An interrupted periosteal reaction was present on the radiograph obtained 16 months earlier (not shown) when the lesion was much smaller. (B) A coronal noncontrast CT image through the forefoot of the same patient shows marked expansion of the first metatarsal with extensive cortical destruction and several thin intralesional septa. The attenuation of the tumor is slightly lower than that of muscle.
Fig. 3A–B.
(A) The en bloc resection specimen shows a diffusely hemorrhagic cut surface lesion expanding the rib. (B) A contrast-enhanced CT image of the same patient shows a multiseptated, expansile lytic lesion in the anterior portion of the right ninth rib, indenting and causing low-attenuation presumed to be reactive edema in the subjacent liver.
Fig. 4.
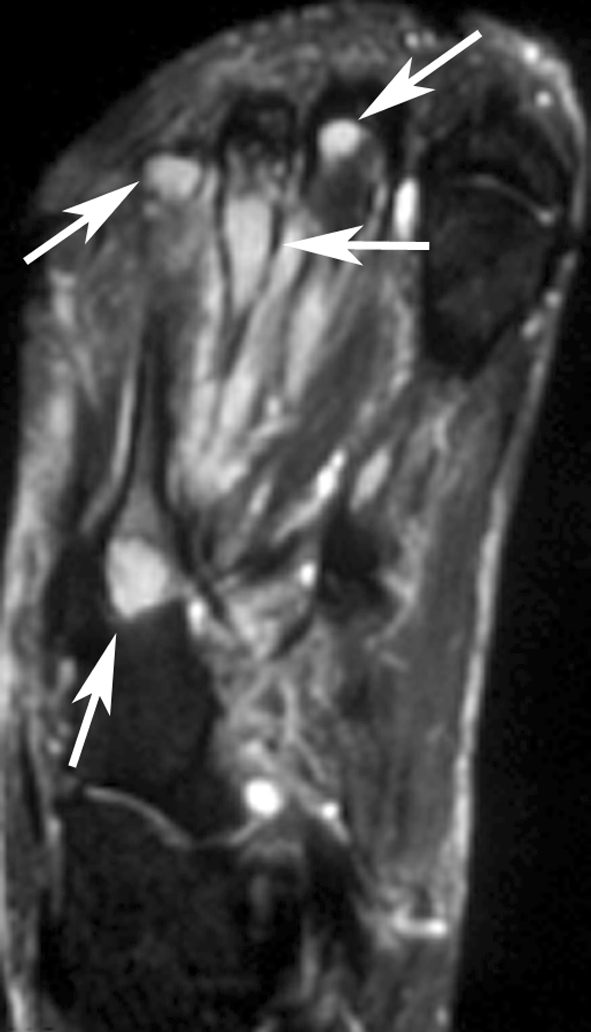
An axial T2-weighted MR image shows tumor deposits as areas of bright signal in several bones of foot (arrows).
Fig. 5A–C.
(A) The radiograph shows a tiny permeative, lytic focus in the posterior cortex of the midhumeral shaft (arrow). No calcifications are evident in nearby soft tissues. (B) An axial proton density MR image shows heterogeneous tumor deposits in the triceps muscle and an intracortical tumor deposit (arrow). (C) A sagittal proton density MR image shows multinodular tumor deposits with low-signal intensity inner rings (arrows), possibly related to hemosiderin deposition.
Table 2.
Summary of radiologic findings in 10 patients with available imaging
| Patient number | Location/size (cm) | Radiographs | CT* | MRI |
|---|---|---|---|---|
| Bone | ||||
| 1 | First metatarsal/6.0 cm | Lytic, expansile, multiseptated, fills entire bone | Lytic, well-defined, expansile, septated, with cortical destruction; | Well-defined; small nodule extends through cortex; |
| < muscle (without contrast) | > muscle on T2, STIR | |||
| 2 | First metatarsal/3.8 cm | Lytic, expansile, geographic, septated; thinned cortex | ||
| 5 | Anterior ninth rib/4.3 cm | Lytic, expansile, septated | Lytic, well-defined, expansile, septated, with cortical destruction; | |
| enhances > muscle | ||||
| 7 | Clavicle, midshaft/2.0 cm | Lytic, geographic, septated, cortical destruction; | ||
| mild thick, smooth periosteal reaction | ||||
| 8 | T2 and T3 vertebra/4.1 cm | Lytic, geographic, collapse | Lytic, well-defined, septated, with cortical destruction; | Well-defined, in body and posterior elements, with cord compromise; |
| < muscle (without contrast) | ≤ muscle on T1; ≤ muscle on T2; < , = , and > muscle on STIR | |||
| Enhances = muscle | ||||
| 13 | Multiple phalanges, metatarsals, tarsals/1.7 cm | Well-defined, with marrow edema around some lesions; | ||
| soft tissue edema; | ||||
| = muscle on T1; > muscle on T2 | ||||
| Soft tissue | ||||
| 14 | Triceps muscle/12 cm | Lytic, permeative focus | Well-defined | Well-defined, with one satellite lesion; |
| 8-mm intracortical lytic deposit; | 8-mm intracortical lytic deposit; | |||
| < mMuscle precontrast; = muscle postcontrast; > muscle on delayed images; | mixed signal with low-signal inner rings on proton density; | |||
| ipsilateral axillary adenopathy, up to 2.0 × 1.6 cm | > muscle on gradient echo; low-signal centers | |||
| 15 | Triceps muscle/5.3 cm | Well-defined, with mild soft tissue edema; | ||
| slightly > muscle on T1; > muscle on T2, with dark rings; | ||||
| enhances = vessels | ||||
| 16 | Hand flexor tendons/3.5 cm | Unremarkable | Well-defined; | |
| = muscle on T1; = and > muscle on STIR, with dark rings; | ||||
| moderate enhancement | ||||
| 11 | Index finger/2 cm | Erosion of middle phalanx | ||
* For CT, comparisons to muscle refer to attenuation values; for MRI, comparisons refer to signal intensities; STIR = short tau inversion recovery.
Results
Characteristic histologic features and the absence of gene rearrangements by FISH distinguished EH from other vascular tumor types. Morphologically, the tumors showed a lobulated or multinodular growth (Fig. 6), with a pushing border in the medullary cavity or soft tissues. Although present in variable degrees, all lesions showed vasoformative properties, forming ‘mature’ vessels with open lumens containing erythrocytes (Fig. 6A–B). This was particularly evident at the periphery of the lesions, where well-defined small vessels were lined by epithelioid endothelial cells (Fig. 6B–C). In the center of the lesion or of the tumor lobules, the plump, polygonal endothelial cells were rather packed and arranged in solid sheets, thus simulating more aggressive vascular tumors (Fig. 6E). Also quite distinct was the hobnailed appearance of the endothelial cells lining larger caliber vessels (Fig. 6D) bulging in a characteristic ‘tombstone’ pattern. The tumor cells often contained abundant, densely eosinophilic cytoplasm (Fig. 6E). In some tumors, however, the cytoplasm had a more fine, feathery, or foamy appearance, resembling that of histiocytic cells (Fig. 6F). Scattered multinucleated giant cells, reminiscent of osteoclast-type giant cells, also were seen intermixed. Intracytoplasmic vacuoles were seen but typically were not a predominant feature (Fig. 6G). The nuclei frequently were enlarged with irregular nuclear contours and occasional grooves. The cytologic atypia was typically mild, but sometimes focal moderate nuclear pleomorphism, including multilobated nuclei or pseudonuclear inclusions, were observed (Fig. 6F). A high degree of cytologic atypia was consistently absent as was the presence of increased mitotic activity or necrosis. Mitotic figures were consistently less than one per 10 high-power fields. In only two patients (one in the foot and the other in the axilla) was the tumor associated with a brisk eosinophilic infiltrate. Although most tumors were purely epithelioid, tumors in two patients also had large areas of spindling composed of short fusiform or oval, bland endothelial cells lining (Fig. 6H). One tumor showed abundant extravasated erythrocytes microscopically (Fig. 6E), correlating with the dark, hemorrhagic appearance noted grossly (Fig. 3A). Vascular invasion was identified in only one patient (Fig. 6I).
Fig. 6A–I.
Histologic analysis showed (A) a lobular growth pattern of the lesion with extension outside the cortex in adjacent soft tissue (Stain, hematoxylin & eosin; original magnification, ×40); (B) mature vascular lumen formation with eosinophils infiltrating the adjacent stroma (Stain, hematoxylin & eosin; original magnification, ×100); (C) vasoformative properties at the periphery of the lesion, with larger caliber vessels lined by epithelioid cells (Stain, hematoxylin & eosin; original magnification, ×100); (D) hobnailed endothelial cells protruding in the lumen in a characteristic ‘tombstone’ appearance (Stain, hematoxylin & eosin; original magnification, ×100); (E) the central portion of the lesion typically had more solid growth with sheets of epithelioid cells with densely eosinophilic cytoplasm and lacked obvious vessel formation. Occasionally abundant erythrocyte extravasation was seen (Stain, hematoxylin & eosin; original magnification, ×200); (F) epithelioid cells with a more foamy, vacuolated cytoplasm and focal, moderate pleomorphism and pseudonuclear inclusions (Stain, hematoxylin & eosin; original magnification, ×400); (G) intracytoplasmic vacuoles but typically these were not a predominant feature (Stain, hematoxylin & eosin; original magnification, ×200); (H) occasional areas of a bland spindle cell component (Stain, hematoxylin & eosin; original magnification, ×200); and (I) vascular invasion in one patient who had lymph node spread (Stain, hematoxylin & eosin; original magnification, ×100).
On radiographs the bone lesions often appeared lytic, septated, and expansile. (Fig. 2A; Table 2) The mean size of the bone lesions was 3.7 cm (range, 1.7-6 cm). One lesion showed a smooth, thick periosteal reaction. CT showed well-defined, septated, lytic lesions with cortical destruction and bony expansion (Figs. 2B, 3B). The lesions were well defined by MRI, being hypointense or isointense to muscle on T1-weighted images, and hyperintense on T2-weighted images (Fig. 4). In one patient, a small nodule of tumor extended through a region of destroyed cortex into adjacent soft tissues. Preoperative embolization images available for one patient showed an intense blush in the tumor. The mean size of the soft tissue lesions was 5.7 cm (range, 2–12 cm). Radiographs showed a small, lytic intracortical focus in the humerus of one patient (Fig. 5A) and erosion of a phalanx in another; no lesion was evident in the third. MRI showed the mass to be well defined with an unusual pattern of low-signal inner rings on proton density, T2-weighted, and gradient echo images in three of three patients (Fig. 5C). A satellite lesion was observed in one patient and a small intracortical deposit in another (Fig. 5B).
The management used for EH varied considerably, but the clinical course was consistently benign. None of the patients died of disease, including the four patients with multifocal presentation of EH and four patients with local recurrence (Tables 1, 3). Treatment ranged from biopsy alone to amputation. Eight patients with bone lesions were treated with intralesional curettage. Three patients, two with rib involvement and one with a carpal bone lesion, underwent segmental resection of their tumors. In contrast, all patients with EH of soft tissue underwent excision with narrow or wide margins, except for one patient in whom only biopsy of the tumor was done. This latter patient was a 63-year-old man diagnosed with an arm EH who did not show disease progression after 64 months of followup. Two patients, including one with T3 vertebral involvement and the other with multifocal head and neck lesions, received radiation therapy. The first patient died of radiotherapy complications, with severe radiation-induced dysphagia. The second patient had locoregional lymph node metastases and received brain radiation and systemic therapy without clinical improvement. The four patients who presented with multiple sites of disease required sequential treatment but all have survived (Table 3).
Table 3.
Clinical information and followup for four patients with multifocal EH
| Patient number | Age/ gender | Anatomic site of multifocal disease | Surgical Resection | Other therapy | Last followup (months) |
|---|---|---|---|---|---|
| 1 | 18/M | Right hand (scaphoid & trapezium bones) | Segmental resection (wide margins) | None | NED (156) |
| 2 | 49/M | Index and middle finger (bone & soft tissue) | Several lesions were excised | Remainder of lesions treated with laser therapy | NED (48) |
| 3 | 35/M | Mandible, skull bones, facial soft tissue, and locoregional lymph node involvement | Marginal excision only for recurrent disease | Chemotherapy (doxorubicin & Edatrexate); whole brain 3000 cGy (good response); 4500 cGy for local recurrence | NED (240) |
| 4 | 56/F | Midfoot and the forefoot (bone & soft tissue) | Transtarsal amputation | None | NED (66) |
NED = no evidence of disease.
Discussion
This study’s major limitation is its descriptive nature. The rarity of musculoskeletal vascular tumors mandates that such a study be retrospective to accrue sufficient cases for meaningful analysis. There is no comparison group owing to the small denominator of vascular tumors and elimination of nonEH diagnoses by the newly available FISH markers for other vascular tumors. Some of the histopathologic and radiologic features found in EH might also be seen in the other tumors, so they should not be considered specific for EH. Despite the fact that our series is relatively small, it reinforces a similar outcome as reported by Nielsen et al. [14], using molecular techniques to enhance the degree of confidence in pathologic subclassification, particularly to exclude an EHE diagnosis, in tumors lacking obvious mature vessel formation, or when confronted with limited material. The clinical information, with multifocal presentation, aggressive radiologic appearance, and/or lymph node metastasis, can be seen in both tumor types and should not be used as the gold standard to favor one diagnosis over the other. In fact, although most patients received conservative treatment, their long-term prognosis was excellent, and none of them died of disease. By contrast, 23% of patients with WWTR1-CAMTA1-positive EHE died of disease [5].
Our findings suggest EH is a benign tumor with a good prognosis. Based on our experience, EH of bone can be treated effectively with intralesional curettage, whereas EH of soft tissue can be treated with marginal/wide excision. There was no conclusive benefit of chemotherapy or radiation in any of our patients. Although imaging helps in the diagnosis of intraosseous hemangioma and usually excludes the need for biopsy, it is ineffective in the diagnosis of EH and its closest mimics because these entities lack characteristic radiologic features [10, 18]. The presence of regional multifocal involvement may be the only clue indicating a diagnosis of a vascular tumor [20, 22]. Morphologic and immunohistochemical features remain the cornerstone of diagnosis of vascular tumors and their epithelioid variants. The differential diagnosis of EH includes EHE and epithelioid angiosarcoma. Because of their epithelioid appearance, epithelioid vascular neoplasms also might be misdiagnosed as metastatic carcinoma. Antibodies against certain endothelial markers may differentiate vascular tumors from metastatic carcinomas [3, 12]. Furthermore, features that distinguish EH from epithelioid angiosarcoma include the absence of anaplasia or considerable cytologic atypia, brisk mitotic activity, and necrosis [3]. The more difficult distinction is encountered between EH and EHE as a result of considerable overlap at the cytologic level with epithelioid cells showing well-defined cell borders and abundant, densely eosinophilic cytoplasm. A mild degree of cytologic atypia can be seen in both entities. The presence of a peculiar myxochondroid or densely sclerotic stroma in EHE and the presence, at least focally, of mature vessels with open lumen formation in EH are the only consistent distinctive histologic features between these two entities. However, recent identification of consistent WWTR1-CAMTA1 fusion in EHE, but not in other epithelioid vascular tumors, promises a more objective subclassification [5]. As such, none of the EH tumors with adequate material included in this study was positive for this abnormality. Distinguishing these two entities is critical, because EHE exhibits a more aggressive clinical course than EH with higher rates of multifocality and distant spread [4, 15].
As much as 25% of EH might be multifocal [2, 16, 19]. Among 50 reported patients with EHs in bone [14], most presented with a solitary lesion, but nine (18%) had lesions involving more than one bone, similar to the 25% multifocal presentation in the current series. Even more controversial is the rare propensity of EH to metastasize to regional lymph nodes [9]. Evans et al. [6] responded that EH is not a distinct clinicopathologic entity, but rather a misdiagnosed hemangioendothelioma, a tumor with malignant potential. Nielsen et al. [14] countered that EH is a benign, pathologically distinct entity, citing the illustrations from the 2003 series by Evans et al. of 13 cases of so-called “hemangioendotheliomas” show in fact characteristics of EH. In the series by Evans et al., three patients received aggressive chemotherapy and three underwent forequarter amputation, hip disarticulation, and internal hemipelvectomy, respectively [6].
In the current study, two tumors initially were diagnosed as either hemangioendothelioma, not otherwise specified, or EHE. The two tumors designated as hemangioendothelioma occurred in the foot and were composed of a mixture of epithelioid and spindle cell components lacking a considerable degree of cytologic atypia. Because this heterogeneous morphology was not initially recognized as a diagnostic pattern of EH, it can cause confusion with more aggressive malignant neoplasms. However, a similar histologic appearance was described by Keel and Rosenberg under the term “hemorrhagic epithelioid and spindle cell hemangioma” [11]. This benign tumor, with propensity for multifocal presentation and acral bone involvement, showed some distinctive features from the typical EH, including abundant extravasated erythrocytes and an admixture of epithelioid cells with bland spindle cells arranged in short fascicles. Nielsen et al. later acknowledged that this lesion is in fact a histologic variant of EH [14]. Our findings support the hypothesis that occasional examples of EH might contain areas of spindling or abundant hemorrhage and should not be used against the diagnosis of EH. Thus, the term ‘hemangioendothelioma, not otherwise specified’ of bone remains a nebulous, most likely ‘wastebasket’ diagnosis and should be avoided as much as possible.
All but two tumors in the current series lacked any stromal eosinophils, suggesting that bone and soft tissue EH might have distinct pathogenesis compared with the skin counterparts. Initially described as “angiolymphoid hyperplasia with eosinophilia” [17] as a result of its heavy eosinophilic infiltrate, the neoplastic etiology of EH occurring in the skin has been questioned by some authors, who speculate that it might instead be a reactive process owing to its prominent inflammatory infiltrate and angiocentric distribution around a larger vessel with evidence of mural damage and often associated trauma [7].
It is important to distinguish EH from other epithelioid vascular tumors because of the major differences in their management and clinical outcome. Testing for the presence of WWTR1/CAMTA1 rearrangements by FISH can be used in difficult diagnoses or for tumors with a multifocal/aggressive clinical presentation. It remains controversial if the presence of multiple organ involvement (bone, soft tissue, lymph node) is merely a manifestation of multicentricity rather than locoregional spread in an otherwise benign lesion.
Acknowledgments
We thank Jesse Galle for assistance with clinical followup and Milagros Soto and Lionel Santibañez for editorial assistance.
Footnotes
One or more of the authors received funding from PO1 CA047179-15A2 (CRA), P50 CA 140146-01 (CRA), the Maynard Orthopaedic Research Fund, and Associazione per la Ricerca e la Cura dei Tumori dell’Apparato Locomotore (CE).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, Edelman M, Rosenberg AE, Nielsen GP, Dal Cin P, Fletcher CD. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors: a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande V, Rosenberg AE, O’Connell JX, Nielsen GP. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol. 2003;27:709–716. doi: 10.1097/00000478-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Deyrup AT, Montag AG. Epithelioid and epithelial neoplasms of bone. Arch Pathol Lab Med. 2007;131:205–216. doi: 10.5858/2007-131-205-EAENOB. [DOI] [PubMed] [Google Scholar]
- 4.Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32:924–927. doi: 10.1097/PAS.0b013e31815bf8e6. [DOI] [PubMed] [Google Scholar]
- 5.Errani C, Zhang L, Sung SY, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans HL, Raymond AK, Ayala AG. Vascular tumors of bone: astudy of 17 cases other than ordinary hemangioma, with an evaluation of the relationship of hemangioendothelioma of bone to epithelioid hemangioma, epithelioid hemangioendothelioma, and high-grade angiosarcoma. Hum Pathol. 2003;34:680–689. doi: 10.1016/S0046-8177(03)00249-1. [DOI] [PubMed] [Google Scholar]
- 7.Fetsch JF, Weiss SW. Observations concerning the pathogenesis of epithelioid hemangioma (angiolymphoid hyperplasia) Mod Pathol. 1991;4:449–455. [PubMed] [Google Scholar]
- 8.Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classidfication of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. [Google Scholar]
- 9.Floris G, Deraedt K, Samson I, Brys P, Sciot R. Epithelioid hemangioma of bone: a potentially metastasizing tumor? Int J Surg Pathol. 2006;14:9–15. doi: 10.1177/106689690601400102. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Saifuddin A, Briggs TW, Flanagan AM. Subperiosteal hemangioendothelioma of the femur. Skeletal Radiol. 2006;35:793–796. doi: 10.1007/s00256-005-0060-6. [DOI] [PubMed] [Google Scholar]
- 11.Keel SB, Rosenberg AE. Hemorrhagic epithelioid and spindle cell hemangioma: a newly recognized, unique vascular tumor of bone. Cancer. 1999;85:1966–1972. doi: 10.1002/(sici)1097-0142(19990501)85:9<1966::aid-cncr13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Kleer CG, Unni KK, McLeod RA. Epithelioid hemangioendothelioma of bone. Am J Surg Pathol. 1996;20:1301–1311. doi: 10.1097/00000478-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683–697. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen GP, Srivastava A, Kattapuram S, Deshpande V, O’Connell JX, Mangham CD, Rosenberg AE. Epithelioid hemangioma of bone revisited: a study of 50 cases. Am J Surg Pathol. 2009;33:270–277. doi: 10.1097/PAS.0b013e31817f6d51. [DOI] [PubMed] [Google Scholar]
- 15.O’Connell JX, Kattapuram SV, Mankin HJ, Bhan AK, Rosenberg AE. Epithelioid hemangioma of bone: a tumor often mistaken for low-grade angiosarcoma or malignant hemangioendothelioma. Am J Surg Pathol. 1993;17:610–617. doi: 10.1097/00000478-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell JX, Nielsen GP, Rosenberg AE. Epithelioid vascular tumors of bone: a review and proposal of a classification scheme. Adv Anat Pathol. 2001;8:74–82. doi: 10.1097/00125480-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia: a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781–796. doi: 10.1016/S0190-9622(85)70098-9. [DOI] [PubMed] [Google Scholar]
- 18.Shah ZK, Peh WC, Shek TW, Wong JW, Chien EP. Hemangioendothelioma with an epithelioid phenotype arising in hemangioma of the fibula. Skeletal Radiol. 2005;34:750–754. doi: 10.1007/s00256-005-0912-0. [DOI] [PubMed] [Google Scholar]
- 19.Sung MS, Kim YS, Resnick D. Epithelioid hemangioma of bone. Skeletal Radiol. 2000;29:530–534. doi: 10.1007/s002560000254. [DOI] [PubMed] [Google Scholar]
- 20.Vermaat M, Vanel D, Kroon HM, Verbeke SL, Alberghini M, Bovee JV, Bloem JL. Vascular tumors of bone: imaging findings. Eur J Radiol. 2011;77:13–18. doi: 10.1016/j.ejrad.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 21.Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol. 1986;3:259–287. [PubMed] [Google Scholar]
- 22.Wenger DE, Wold LE. Malignant vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol. 2000;29:619–631. doi: 10.1007/s002560000261. [DOI] [PubMed] [Google Scholar]