Abstract
Background
Tumor enlargement after chemotherapy is considered one of the high-risk factors for local recurrence and survival in osteosarcoma. We hypothesized patients with this risk factor will have similar survival regardless of the development of local recurrence.
Questions/purposes
We asked (1) the prognostic factors for survival in our cohort, (2) how much effect local recurrence has on survival among patients with similar preoperative risk factors, and (3) what prognostic factors are important for survival in these selected patients.
Methods
We analyzed the prognostic factors for survival in 449 patients with extremity osteosarcoma without metastatic disease at initial diagnosis and treatment (38 with local recurrence, 411 without local recurrence). We compared the survival difference between patients with local recurrence (n = 38) and without local recurrence (control, n = 76) matched for age, location, initial tumor volume, and tumor volume change after chemotherapy, and assessed prognostic factors in this subgroup.
Results
In a cohort study, multivariate analysis revealed initial tumor volume, tumor enlargement, inadequate margin, and local recurrence predicted poor survival. In the case-control study, the 10-year metastasis-free survival rates of two groups were 13.1 ± 10.7% and 19.3 ± 9%, respectively. In the case-controlled groups, tumor enlargement and initial tumor volume showed multivariate significance.
Conclusions
Local recurrence has a small impact on survival in patients with high-risk osteosarcoma.
Level of Evidence
Level III, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Keywords: Medicine & Public Health; Conservative Orthopedics; Orthopedics; Sports Medicine; Surgery; Surgical Orthopedics; Medicine/Public Health, general
Introduction
A paradigm in the treatment of osteosarcoma accepts a negative relation between local recurrence (LR) and survival for patients with a primary extremity osteosarcoma. That is, LR predicts poor survival [2, 6, 9, 19, 25]. However, this paradigm may be biased as it is based on results of treatment during the adjuvant chemotherapy era when more than half the patients underwent amputation as compared with current practice in which limb salvage is the norm. There are reports that challenge this concept. For example, Simon et al. [23] reported equivalent survival between patients with amputation and those with limb salvage despite the higher LR rate for patients who have limb salvage.
Currently, in the neoadjuvant era when most patients are treated with limb salvage, another risk factor for LR other than surgical margin is histologic response to chemotherapy [5, 9]. However, at the time of tumor resection, the only controllable factor for LR is surgical margin. For patients with intralesional margins, regardless of the degree of chemotherapeutic response achieved, a secondary operation to prevent LR would be a reasonable approach. On the contrary, for patients with a poor chemotherapeutic response and a risky margin, it is unclear if additional resection including amputation leads to better survival. Moreover, some believe that LR is an expression rather than a factor causing a poor prognosis for patients with tumors with an aggressive biologic behavior [5].
Tumor volume change after preoperative chemotherapy has received little attention as a viable prognostic factor because usually there is not a marked volumetric response. Additionally, although increase in tumor volume is well correlated with a poor histologic response, reduced or stable tumor size cannot guarantee a good response [11, 22]. Therefore, this factor is regarded as one of the parameters that may predict histologic response to preoperative chemotherapy. However, in previous studies, tumor volume increase after preoperative chemotherapy predicted LR and it was independently associated with shortened survival for patients with nonmetastatic osteosarcoma [15, 24]. In view of this, we now consider patients with an increase in tumor size after chemotherapy a high-risk group for LR and poor survival. We hope to further clarify the risk in this high-risk group with respect to LR. We suspect that LR is not a true indicator of risk and that patients with LR would have similar survival regardless of the development of LR. In other words, LR is an expression of tumor aggressiveness and not a prognostic factor for survival in this group.
In this study our primary aim was to determine what prognostic factors were most important for survival of patients with localized extremity osteosarcomas. The second aim was to evaluate whether there is any difference in survival between patients with LR (cases) and those without LR (controls) with similar characteristics before surgery (mainly tumor volume change after chemotherapy). The third aim was to determine the prognostic factors for survival in the high-risk groups.
Patients and Methods
To accomplish the aims of this study we performed two analyses. In the first analysis (cohort study), we analyzed prognostic factors for survival. The cohort study included 449 patients (38 patients with LR and 411 patients without LR). The following exclusion criteria were used to extract patients for cohort study. From March 1990 to March 2009, the computerized archives at our institute showed that we treated 779 patients with 779 localized extremity osteosarcomas without LR which were classified as Stage IIB. Of these, 368 patients were excluded because (1) the histologic response to preoperative chemotherapy was not available (84 patients); (2) our treatment protocol was not completed (82 patients); (3) an intralesional procedure was performed (54 patients); (4) followup was less than 36 months (52 patients); (5) the histologic evaluation suggested a low-grade or atypical osteosarcoma (50 patients); (6) an axial location (33 patients); (7) data for preoperative volume change were unavailable (10 patients); and (8) death was not related to the disease (three patients). This left a cohort of 411 patients for retrospective review. During the same period, 98 patients with osteosarcomas with LR were registered. Sixty patients were excluded because: (1) they had Stage III disease at presentation (19 patients); (2) they had a history of an inappropriate procedure, including open reduction and internal fixation at a referral hospital (16 patients); (3) the tumor was in an axial location (13 patients); (4) they underwent primary tumor excision at other institute (six patients); (5) they received no neoadjuvant chemotherapy owing to initial misdiagnosis (three patients); (6) the tumors were surface osteosarcomas (two patients); and (7) they presented with a delayed diagnosis and a pathologic fracture (one patient). This left 38 patients with localized extremity osteosarcomas and LR.
In the second analysis (the case-control study), we selected 76 of these 411 patients as the control group. We compared survival between case (38 patients with LR) and control (76 patients without LR) groups and determined the prognostic factors for survival in the case-controlled groups. Because LR develops with time and is a combined result of histologic response and surgical margin, we presumed the time before definitive resection would be the optimal point when both groups could be assessed in a similar condition. Control group patients were matched with patients in the case group in terms of age, tumor size at presentation, tumor location, and tumor volume change after chemotherapy. All patients in the control group received the same chemotherapeutic protocol initially as the patients in the case group.
All patients underwent staging studies, preoperative chemotherapy, surgery, and postoperative chemotherapy, and were followed as described previously [13]. The independent factors assessed were age, sex, location of primary tumor, plain radiographic pattern, pathologic subtype, pathologic fracture, tumor volume at presentation, tumor volume change before and after chemotherapy, type of operation, surgical margin, and histologic response to preoperative chemotherapy. Additionally, we noted dependent factors such as local and distant disease-free interval and time to death. Surgical margins were assessed using the method of Enneking et al. [7]. Bone and soft tissue margins were determined using pathologic specimens, whereas neurovascular margins were determined by preoperative MRI. An adequate margin was defined as a tumor-free margin greater than 2 cm for bone, the existence of a normal soft tissue cuff enveloping the tumor for soft tissue, and an intact fat plane between the tumor and neurovascular bundle observed on MRI for the neurovascular margin. Any margin status other than adequate was considered an inadequate margin. The final surgical margin was defined as adequate or inadequate based on the weakest margin achieved. The histologic responses of tumors to neoadjuvant chemotherapy were graded using tumor necrosis percentages as Grades III and IV (≥ 90% necrosis), indicating a good response, and Grades I and II (< 90% necrosis), indicating a poor response [7, 20]. Tumor volumes were calculated using MR images, as described by Gobel et al. [8], using the formula: tumor volume = 0.53 × tumor length × tumor width × tumor depth. Tumor volume before and after chemotherapy were calculated from MR images rather than using the special built-in software package installed in the MRI scanner (MathWorks Inc, Natick, MA, USA). We used enhanced T1-weighted and T2-weighted sequences to define the tumor boundary [14]. Tumor volume changes attributable to chemotherapy are expressed as tumor volume ratios, defined as tumor volume after chemotherapy divided by tumor volume before chemotherapy. Patients were assigned to three groups based on tumor volume ratios. Specifically, tumor volume ratios less than 0.95 were classified as decreased, between 0.95 and 1.05 were stable, and greater than 1.05 were increased [11]. After completion of chemotherapy, patients were followed for 2 years with monthly radiographs of the chest and the surgically treated limb. CT of the chest and a whole-body bone scan were performed every 3 months for 2 years. Thereafter, the same protocol was repeated twice yearly up to the fifth year and annually up to the tenth year after surgery.
The initial clinicopathologic characteristics of the 38 patients in the case group and 76 patients in the control group were recorded (Table 1), and, as expected, they were well matched for age, initial tumor volume, tumor volume change ratio, and location. The mean tumor volume change ratio for patients in the case and control groups were 1.69 (range, 0.68–4.12) and 1.62 (range, 0.66–4.33), respectively. Although factors such as a plain radiographic pattern, pathologic subtype, and presence of a pathologic fracture, were not used for matching, no differences were observed between the two groups.
Table 1.
Characteristics of 38 patients with local recurrence and 76 patients without recurrence
| Variables | Recurrence (%) | No recurrence (%) | p value |
|---|---|---|---|
| Age (years) | |||
| < 12 | 7 (18.4) | 13 (17.1) | 0.805 |
| ≥ 12 and ≤ 15 | 14 (36.8) | 35 (46.1) | |
| > 15 and ≤ 40 | 15 (39.5) | 24 (31.6) | |
| > 40 | 2 (5.3) | 4 (5.2) | |
| Gender | 56 (73.7) | 0.766 | |
| Male | 27 (71.1) | ||
| Female | 11 (28.9) | 20 (26.3) | |
| Initial tumor volume | |||
| ≤ 150 | 24 (63.2) | 48 (63.2) | 1.000 |
| > 150 | 14 (36.8) | 28 (36.8) | |
| Tumor volume change ratio (mean/range) | 1.69 (0.68–4.12) | 1.62 (0.66–4.33) | 0.533 |
| Decreased | 3 (7.9) | 6 (7.9) | 1.000 |
| Stable | 3 (7.9) | 6 (7.9) | |
| Increased | 32 (84.2) | 64 (84.2) | |
| Location | |||
| Femur | 18 (47.4) | 30 (39.5) | 0.806 |
| Tibia | 12 (31.6) | 24 (31.6) | |
| Humerus | 6 (15.8) | 14 (18.4) | |
| Fibula | 1 (2.6) | 6 (7.9) | |
| Radius | 1 (2.6) | 2 (2.6) | |
| Pattern on plain radiographs | |||
| Mixed | 7 (18.4) | 12 (15.8) | |
| Blastic | 18 (47.4) | 44 (57.9) | |
| Pathologic fracture | |||
| Occurred | 8 (21.1) | 9 (11.8) | 0.193 |
| Did not occur | 30 (78.9) | 67 (88.2) | |
| Pathologic subtype | 27 (71.1) | 61 (80.3) | 0.328 |
| Osteoblastic | |||
| Chondroblastic | 5 (13.2) | 10 (13.2) | |
| Fibroblastic | 3 (7.9) | 3 (3.9) | |
| Telangiectatic | 1 (2.6) | 1 (1.3) | |
| Giant cell rich | 2 (5.2) | 0 (0) | |
| Small cell | 0 (0) | 1 (1.3) | |
| Operation type | |||
| Amputation | 0 (0) | 5 (6.6) | 0.167 |
| Limb salvage | 38 (100) | 71 (93.4) | |
| Surgical margin | 22 (57.9) | 50 (65.8) | 0.116 |
| Wide | |||
| Marginal | 14 (36.8) | 26 (34.2) | |
| Intralesional | 2 (5.3) | 0 (0) | |
| Histologic response | |||
| Good | 6 (15.8) | 19 (25.0) | 0.263 |
| Poor | 32 (84.2) | 57 (75.0) | |
| Final Outcome | |||
| Alive | 13 (34.2) | 35 (46.1) | 0.227 |
| Dead | 41 (53.9) | ||
| Total | 38 (100%) | 76 (100%) | |
For survival analysis, the primary end points used were time to metastasis and time to death; metastasis-free survival was measured from the date of diagnosis to the time of a metastatic event. Overall survival was measured from the date of diagnosis to death. Patients who did not experience a metastatic event (for metastasis-free survival) or who remained alive (for overall survival) were assessed at final followups. The minimum followup was 9 months for the patients in the case (mean, 49 months; range, 9–191 months) and control (mean, 65 months; range, 9–212 months) groups. This study was approved by our Institutional Review Board.
Fisher’s exact chi square test and the Student’s t-test were used to identify differences between patients in the case and control groups in terms of clinical characteristics. The Kaplan-Meier method and the log-rank test were used to identify survival differences. Multivariate analysis by Cox proportional hazard model was performed with all factors that showed statistical significance in univariate analysis. Analyses were performed using SPSS® Version 13.0 (SPSS Inc, Chicago, IL, USA), and p values less than 0.05 were considered significant. We hypothesized that 60% or more of the patients in the control group would survive, as compared with 30% or fewer of the patients in the case group, which yielded an odds ratio of 2.0 for the patients in the case group. Based on these assumptions, the cohort study had a statistical power of 93.3% to detect a significant effect among 38 patients in the case group and 411 patients in the control group. The calculated statistical power for the case-control study was 83.8% to detect a significant effect among 38 patients in the case group and 76 in the control group.
Results
In the cohort study, univariate survival analysis on the 449 study patients revealed that initial tumor volume (p = 0.003), tumor volume ratio (p < 0.001), humeral location (p = 0.004), inadequate surgical margin (p < 0.001), poor histologic response (p < 0.001), and local recurrence (p < 0.001) were negatively related to overall survival. Multivariate analysis revealed that initial tumor volume (p = 0.002), tumor volume ratio (p < 0.001), inadequate surgical margin (p = 0.001), and local recurrence (p = 0.001) independently predicted reduced overall survival (Table 2).
Table 2.
Univariate and multivariate analyses of 449 patients
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| 5-year overall survival rate | p value | RR | 95% CI | p value | |
| Age (years) | |||||
| ≤ 15 (n = 225) | 76.2 ± 2.9 | 0.903 | ND | ND | ND |
| > 15 (n = 224) | 76.0 ± 2.9 | ||||
| Sex | |||||
| Male (n = 293) | 74.7 ± 2.6 | 0.219 | ND | ND | ND |
| Female (n = 156) | 78.6 ± 3.4 | ||||
| Pathologic fracture | |||||
| No (n = 406) | 76.9 ± 2.2 | 0.134 | ND | ND | ND |
| Yes (n = 43) | 68.4 ± 7.3 | ||||
| Tumor volume | |||||
| ≤ 150 mL (n = 253) | 81.6 ± 2.5 | 0.003 | 1 | ||
| > 150 mL (n = 196) | 69.0 ± 3.4 | 1.78 | 1.24–2.58 | 0.002 | |
| Tumor volume ratio | |||||
| Decreased or stable (n = 274) | 89.1 ± 1.9 | < 0.001 | 1 | ||
| Increased (n = 175) | 54.2 ± 4.0 | 3.84 | 2.51–5.86 | < 0.001 | |
| Location | |||||
| Other (n = 407) | 77.6 ± 2.1 | 0.004 | 1 | ||
| Humerus (n = 42) | 60.9 ± 7.7 | 1.28 | 0.75–2.17 | 0.371 | |
| Pattern on plain radiograph | |||||
| Lytic (n = 130) | 75.7 ± 3.8 | 0.913 | ND | ND | ND |
| Blastic or mixed (n = 319) | 76.2 ± 2.5 | ||||
| Pathologic subtype | |||||
| Osteoblastic (n = 365) | 75.0 ± 2.3 | 0.656 | ND | ND | ND |
| Other (n = 84) | 80.2 ± 4.7 | ||||
| Surgical margin | |||||
| Wide (n = 394) | 80.7 ± 2.0 | < 0.001 | 1 | ||
| Marginal or intralesional (n = 55) | 41.9 ± 7.1 | 2.13 | 1.38–3.29 | 0.001 | |
| Histologic response | |||||
| Good (n = 196) | 86.3 ± 2.5 | < 0.001 | 1 | ||
| Poor (n = 253) | 67.8 ± 3.0 | 1.39 | 0.87–2.22 | 0.164 | |
| Local recurrence | |||||
| No (n = 411) | 79.7 ± 2.0 | < 0.001 | 1 | ||
| Yes (n = 38) | 35.6 ± 8.2 | 2.32 | 1.44–3.73 | 0.001 | |
| Total | 76.1 ± 2.1 | ||||
CI = confidence interval; ND = not done.
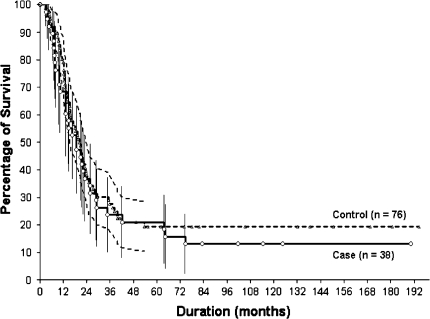
In the case-control study, there was no difference in overall (p = 0.131) or metastasis-free survival (p = 0.511) between the two groups. Ten-year overall survival rates in the case and control groups were 26.3% ± 16.3% and 39.8% ± 12.6% respectively, and metastasis-free survival rates were 13.1% ± 10.7% and 19.3% ± 9% respectively (Fig. 1). Regarding treatment-related factors, no patient in the case group underwent amputation, whereas five (6.6%) in the control group with neurovascular invasion observed on MR images after chemotherapy underwent amputation (p = 0.167). No differences were observed between the two groups in terms of surgical margin (p = 0.116) and the proportion of good responders to preoperative chemotherapy (p = 0.263). At final followup, 13 (34.2%) of the 38 patients in the case group were alive with disease (five patients) or without evidence of disease (eight patients); the remaining 25 died of disease. Thirty-five (46%) of the 76 patients in the control group were alive with disease (five patients) or without evidence of disease (15 patients) or were continuously disease-free (15 patients); the remaining 41 died of disease.
Fig. 1.
Kaplan-Meier estimated survivorship curves show no differences (p = 0.511) between patients in the case (n = 38) and control (n = 76) groups for 10-year metastasis-free survival rates.
For the 114 patients in the case-control study, a large initial tumor volume (p = 0.010), increased tumor volume ratio (p < 0.001), marginal or intralesional margin (p = 0.033), and poor histologic response (p = 0.002) were negatively related to metastasis-free survival on univariate analysis. However, multivariate analysis showed an increased tumor volume ratio (p < 0.001) and large initial tumor volume (p = 0.005) were independently related to metastasis-free survival (Table 3).
Table 3.
Univariate and multivariate analyses of time to metastasis
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| 5-year metastasis-free survival rate | p value | RR | 95% CI | p value | |
| Age (years) | 21.3 ± 5.0 | 0.180 | ND | ND | ND |
| ≤ 15 (n = 69) | |||||
| > 15 (n = 45) | 17.8 ± 5.7 | ||||
| Sex | |||||
| Male (n = 83) | 21.5 ± 4.5 | 0.745 | ND | ND | ND |
| Female (n = 31) | 16.1 ± 6.6 | ||||
| Pathologic fracture | |||||
| No (n = 97) | 21.4 ± 4.2 | 0.567 | ND | ND | ND |
| Yes (n = 17) | 11.8 ± 7.8 | ||||
| Tumor volume | |||||
| ≤ 150 mL (n = 72) | 26.2 ± 5.2 | 0.010 | 1 | ||
| > 150 mL (n = 42) | 9.5 ± 4.5 | 1.81 | 1.19–2.75 | 0.005 | |
| Tumor volume ratio | |||||
| Decreased or stable (n = 18) | 72.2 ± 10.6 | < 0.001 | 1 | ||
| Increased (n = 162) | 10.0 ± 3.1 | 6.23 | 2.67–14.53 | < 0.001 | |
| Location | |||||
| Other (n = 94) | 22.2 ± 4.3 | 0.143 | ND | ND | ND |
| Humerus (n = 20) | 10.0 ± 6.7 | ||||
| Pattern on plain radiograph | |||||
| Lytic (n = 33) | 18.2 ± 6.7 | 0.882 | ND | ND | ND |
| Blastic or mixed (n = 81) | 20.7 ± 4.5 | ||||
| Pathologic subtype | |||||
| Osteoblastic (n = 88) | 21.4 ± 4.4 | 0.755 | ND | ND | ND |
| Other (n = 26) | 15.4 ± 7.1 | ||||
| Surgical margin | |||||
| Wide (n = 72) | 24.9 ± 5.1 | 0.033 | 1 | ||
| Marginal or intralesional (n = 42) | |||||
| Histologic response | 11.4 ± 5.0 | 1.36 | 0.89–2.07 | 0.155 | |
| Good (n = 25) | 39.6 ± 9.9 | 0.002 | 1 | ||
| Poor (n = 89) | 14.5 ± 3.8 | 1.26 | 0.69–2.31 | 0.459 | |
| Local recurrence | |||||
| No (n = 76) | 19.4 ± 4.6 | 0.517 | ND | ND | ND |
| Yes (n = 38) | 21.1 ± 6.6 | ||||
| Total | 20.0 ± 3.8 | ||||
CI = confidence interval; ND = not done.
Discussion
Concerns that the increased use of limb salvage might adversely affect survival have been assuaged [21, 23], but given the poor outcome of patients with LR, surgical oncologists treating patients with tumor enlargement observed on MR images after chemotherapy may have two questions: (1) Can attempts at limb salvage with an unlikely clear surgical margin be justified; and (2) for patients in whom local recurrence actually develops after limb salvage, could the survival rate be improved if the patients had an amputation, and if so, by how much. Although the tumor necrosis rate can be determined only in resected specimens, tumor enlargement after chemotherapy is well correlated with a poor histologic response. Moreover, it predicts LR and less likely survival. Therefore, we questioned the assumption that extending the surgical margin, especially in patients with tumor enlargement after chemotherapy, will minimize LR and thus, will improve survival. In a cohort study, as expected, we reconfirmed the importance of LR on survival. However, in our case-control study no survival difference regardless of the presence or absence of LR was found and LR was not a prognostic factor for survival in this high-risk group.
Ideally, case-control studies should be prospective, but the current topic does not lend itself to prospective study. Furthermore, although we extracted the control group from a relatively large cohort, there is a possibility of selection bias. However, we managed to select twofold matching control cases that meet all four variables simultaneously. Additionally, tumor volumes were calculated from the dimensions measured on the MR images by simplified mathematical formulas rather than by using the built-in software package installed in the MRI scanner. Therefore there is a possibility of underestimation or overestimation. However, Shin et al. [22] reported that tumor volume measured by MRI using the ellipsoid mass formula closely correlated with the volume calculated by the built-in software package in the MRI scanner. Finally, although our pathologist examined the surgical specimens, the adequacy of neurovascular surgical margin was determined by MRI which constitutes a study limitation. LR in osteosarcoma, despite a low reported rate (5%–10%), is a dreadful situation for the patient and the physician [5, 12, 16]. The unique feature of LR is that, theoretically, it is a controllable factor; that is, it can be prevented by extending the surgical margin, and thus, LR generally is regarded as a surgical margin failure. However, although the rate is much lower, LR also occurs in patients with adequate surgical margins, including patients who have had amputations [1, 4, 19]. This phenomenon generally is regarded as a result of poor histologic response to chemotherapy (another factor related to LR) or an error in preoperative radiographic evaluation to decide the surgical margin [17, 18]. The patients in our case-control study had similar high rates of LR between those with adequate (30.5%; 22 of 72) and those with inadequate (38%; 16 of 42) surgical margins. Among the 72 patients with wide margins, 30.5% (22/72) had a good histologic response, whereas 7.1% (3/42) with inadequate margins had a good response.
Regarding the prognostic impact of LR on survival, as in previous studies [4, 5], our cohort study clearly shows LR is a factor independently correlating with reduced overall survival. However, in the case-control study, that powerful prognostic factor lost significance even in univariate analysis. Adverse prognostic factors in a case-control group of 114 patients were large initial tumor volume and tumor volume increase during chemotherapy. Grimer et al. [10], in their subgroup analysis of patients who responded poorly to chemotherapy, compared 24 patients with limb salvage with marginal margins with 40 patients who had undergone amputations. Despite LR developing in nine of the 24 patients with limb salvage compared with none of the 48 who had undergone amputation, neither group of patients did well and there was no difference in survival. These findings parallel our results; that is, poor responders to chemotherapy do poorly regardless of the development of LR. Nevertheless, in our study, patients in the case group showed a tendency toward worse overall survival than patients in the control group, which suggests patients in the case group might belong in a group with the worst prognosis, even among high-risk patients.
According to the timing of metastasis, patients with LR were categorized into three groups; (1) LR alone, (2) LR followed by metastasis, and (3) metastasis before or synchronous with LR. In two previous studies [5, 9] a majority (79.1%–94.2%) of patients fell into the latter two categories, and those in Category 1 constituted only 5.8% to 20.9% and had the best survival (Table 4). This high survival for patients with LR alone may have been attributable to the prevention of systemic relapse by LR excision. In our series, five patients had LR only (Category 1). Three of them underwent joint-sparing resections; in retrospect, the leading cause of LR in these patients could be surgical margin failure. LR in Category 3 (metastasis before or synchronous with LR) would be a consequence of systemic relapse, and thus, LR would have a limited or negligible role in prognosis. However, the role of LR in Category 2 (LR followed by metastasis) is unclear, as it could play a role in triggering metastasis or simply be a marker of imminent systemic relapse. In our study, five of 11 patients in the case group in Category 2 had inadequate margins and poor histologic response whereas the remaining six patients had wide margins and an equal number of good and poor responses. Owing to the confounding effect of surgical margin and histologic response, we cannot draw any causation between preceding LR and metastasis.
Table 4.
Comparison of studies
| Study | Percent of local recurrence | Initial surgery | Local recurrence according to surgical margin | Local recurrence alone | Local recurrence followed by metastasis | Metastasis before or synchronous to local recurrence | Prognostic factor | ||
|---|---|---|---|---|---|---|---|---|---|
| Amputation | Limb salvage | adequate | inadequate | ||||||
| Current study | 8.4% (38/449) | 0 (0%) | 38 (100%) | 30.5% (22/72) | 38% (16/42) | 5 (13%) | 11 (30%) | 22 (57%) | Tumor volume (> 150 cc); tumor volume ratio increase |
| Weeden et al. [25] | 8.0% (42/559) | 4 (10%) | 38 (90%) | – | – | – | – | – | Local recurrence < 18 months |
| Bacci et al. [3] | 6.0% (34/570) | 4 (11.7%) | 30 (88.3%) | 4.7%† | 21.9%† | 2 (5.8%) | 32 (94.2%) | Surgical margin | |
| Rodriguez-Galindo et al. [19] | 6.5% (26*/397) | 20 (76.9%) | 6 (23.1%) | – | – | – | – | – | Surgical margin; recurrence < 2 years |
| Grimer et al. [9] | 10.8% (96/886) | 6 (6.3%) | 90 (93.7%) | – | – | 20 (20.9%) | 37 (38.5%) | 39 (40.6%) | Metastasis at time of local recurrence |
| Bacci et al. [4] | 5.4% (61/1126) | 10 (16.4%) | 51 (83.6%) | 3.6% (37/1019) | 23.5% (24/102) | – | – | – | Surgical margin; histologic response |
* Seven of 26 presented with metastasis; †5-year local recurrence rate.
Although local recurrence is a powerful negative prognostic factor on survival, in high-risk patients with an increase in tumor volume after chemotherapy, local recurrence has a small impact on survival.
Acknowledgment
We thank to Minkyo Song, MD, at Seoul National University for technical assistance of statistical analysis.
Footnotes
The authors certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might constitute a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
The authors certify that their institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Korea Cancer Center Hospital.
References
- 1.Andreou D, Bielack SS, Carrle D, Kevric M, Kotz R, Winkelmann W, Jundt G, Werner M, Fehlberg S, Kager L, Kuhne T, Lang S, Dominkus M, Exner GU, Hardes J, Hillmann A, Ewerbeck V, Heise U, Reichardt P, Tunn PU. The influence of tumor- and treatment-related factors on the development of local recurrence in osteosarcoma after adequate surgery: an analysis of 1355 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Ann Oncol. 2011;22:1228–1235. doi: 10.1093/annonc/mdq589. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Lari S, Mercuri M, Donati D, Longhi A, Forni C, Bertoni F, Versari M, Pignotti E. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84:88–92. doi: 10.1302/0301-620X.84B1.12211. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, Salduca N, Versari M, Smith KV. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–38. doi: 10.1016/S0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F, Serra M, Briccoli A, Balladelli A, Picci P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96:118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: the experience of a single institution with 44 patients. Cancer. 2006;106:2701–2706. doi: 10.1002/cncr.21937. [DOI] [PubMed] [Google Scholar]
- 6.Chi SN, Conklin LS, Qin J, Meyers PA, Huvos AG, Healey JH, Gorlick R. The patterns of relapse in osteosarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2004;42:46–51. doi: 10.1002/pbc.10420. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 8.Gobel V, Jurgens H, Etspuler G, Kemperdick H, Jungblut RM, Stienen U, Gobel U. Prognostic significance of tumor volume in localized Ewing’s sarcoma of bone in children and adolescents. J Cancer Res Clin Oncol. 1987;113:187–191. doi: 10.1007/BF00391442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimer RJ, Sommerville S, Warnock D, Carter S, Tillman R, Abudu A, Spooner D. Management and outcome after local recurrence of osteosarcoma. Eur J Cancer. 2005;41:578–583. doi: 10.1016/j.ejca.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Grimer RJ, Taminiau AM, Taminiau AM, Cannon SR. Surgical Subcommittee of the European Osteosarcoma Intergroup. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br. 2002;84:395–400. doi: 10.1302/0301-620X.84B3.12019. [DOI] [PubMed] [Google Scholar]
- 11.Holscher HC, Bloem JL, Vanel D, Hermans J, Nooy MA, Taminiau AH, Henry-Amar M. Osteosarcoma: chemotherapy-induced changes at MR imaging. Radiology. 1992;182:839–844. doi: 10.1148/radiology.182.3.1535905. [DOI] [PubMed] [Google Scholar]
- 12.Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, Gobel U, Helmke K, Jundt G, Kabisch H, Kevric M, Klingebiel T, Kotz R, Maas R, Schwarz R, Semik M, Treuner J, Zoubek A, Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 13.Kim MS, Cho WH, Song WS, Lee SY, Jeon DG. Time dependency of prognostic factors in patients with stage II osteosarcomas. Clin Orthop Relat Res. 2007;463:157–165. doi: 10.1097/BLO.0b013e318142b27d. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Jeon DG. Tumor necrosis rate adjusted by tumor volume change is a better predictor of survival of localized osteosarcoma patients. Ann Surg Oncol. 2008;15:906–914. doi: 10.1245/s10434-007-9779-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Jung ST, Jeon DG. Effect of increases in tumor volume after neoadjuvant chemotherapy on the outcome of stage II osteosarcoma regardless of histological response. J Orthop Sci. 2009;14:292–297. doi: 10.1007/s00776-009-1334-y. [DOI] [PubMed] [Google Scholar]
- 16.Nathan SS, Gorlick R, Bukata S, Chou A, Morris CD, Boland PJ, Huvos AG, Meyers PA, Healey JH. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107:1607–1616. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- 17.Picci P, Sangiorgi L, Bahamonde L, Aluigi P, Bibiloni J, Zavatta M, Mercuri M, Briccoli A, Campanacci M. Risk factors for local recurrences after limb-salvage surgery for high-grade osteosarcoma of the extremities. Ann Oncol. 1997;8:899–903. doi: 10.1023/A:1008230801849. [DOI] [PubMed] [Google Scholar]
- 18.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Galindo C, Shah N, McCarville MB, Billups CA, Neel MN, Rao BN, Daw NC. Outcome after local recurrence of osteosarcoma: the St. Jude Children’s Research Hospital experience (1970–2000) Cancer. 2004;100:1928–1935. doi: 10.1002/cncr.20214. [DOI] [PubMed] [Google Scholar]
- 20.Rosen G, Marcove RC, Huvos AG, Caparros BI, Lane JM, Nirenberg A, Cacavio A, Groshen S. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983;106(suppl):55–67. doi: 10.1007/BF00625054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur: a long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Shin KH, Moon SH, Suh JS, Yang WI. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res. 2000;376:200–208. doi: 10.1097/00003086-200007000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 24.Song WS, Jeon DG, Kong CB, Cho WH, Koh JS, Lee JA, Yoo JY, Jung ST, Shin DS, Lee SY. Tumor volume increase during preoperative chemotherapy as a novel predictor of local recurrence in extremity osteosarcoma. Ann Surg Oncol. 2011;18:1710–1716. doi: 10.1245/s10434-010-1536-8. [DOI] [PubMed] [Google Scholar]
- 25.Weeden S, Grimer RJ, Cannon SR, Taminiau AH, Uscinska BM. European Osteosarcoma Intergroup. The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer. 2001;37:39–46. doi: 10.1016/S0959-8049(00)00362-2. [DOI] [PubMed] [Google Scholar]