Abstract
Background
The literature suggests survivorship of unicompartmental knee arthroplasties (UKAs) for spontaneous osteonecrosis of the knee ranges from 93% to 97% at 10 to 12 years. However, these data arise from small series (23 to 33 patients), jeopardizing meaningful conclusions.
Questions/purposes
We determined (1) the longer-term survivorship of UKAs in a larger group of patients with spontaneous osteonecrosis of the knee; (2) their subjective, symptomatic, and functional outcomes; and (3) the percentage of failures and reasons for failures to identify relevant indications, contraindications, and technical parameters for treatment with a modern implant design.
Methods
We retrospectively evaluated all 84 patients with late-stage spontaneous osteonecrosis of the knee who had a medial UKA from 1998 to 2005. All patients had preoperative MRI to confirm the diagnosis, exclude metaphyseal involvement, and confirm the absence of major degenerative changes in the lateral and patellofemoral compartments. The mean age of the patients at surgery was 66 years and mean BMI was 28.9. We conducted Kaplan-Meier survival analysis using revision for any reason as the end point. Minimum followup was 63 months (mean, 98 months; range, 63–145 months).
Results
Ten-year survivorship was 89%. Ten revisions were performed; the most common reasons were subsidence of the tibial component (four) and aseptic loosening of the tibial component (three). No patient underwent revision for progression of osteoarthritis in the lateral or patellofemoral compartments.
Conclusions
Our data suggest spontaneous osteonecrosis of the knee may be an indication for UKA, provided secondary osteonecrosis of the knee is ruled out, preoperative MRI documents the absence of disease in other compartments, and there is no overcorrection in any plane.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Keywords: Medicine & Public Health; Conservative Orthopedics; Orthopedics; Sports Medicine; Surgery; Surgical Orthopedics; Medicine/Public Health, general
Introduction
Spontaneous osteonecrosis of the knee was described by Ahlbäck et al. in 1968 [3] as a typically unicompartmental disease. More recently, Mont et al. [25] reported the limited involvement of the periarticular bone in this disease. Some surgeons believe joint arthroplasty is the only reasonable treatment for late-stage spontaneous osteonecrosis of the knee with secondary joint collapse [1, 23, 25]. UKA seems to be an appropriate procedure [19, 20, 33], particularly for patients older than 65 years with unaffected lateral and patellofemoral compartments [19, 21, 24, 35, 36].
Nevertheless, the literature reports limited data on clinical and radiographic outcomes of UKAs performed for spontaneous osteonecrosis of the knee [9, 13, 17, 24, 29, 30], probably reflecting its low incidence in the general population (0.05%–7% in patients with knee arthroplasties) [33, 34]. These studies report from 23 to 33 patients with mean followups ranging from 3.3 to 10.4 years, only the last two of which [33, 34] suggest survivorship of 93% to 96.7%. A recent systematic literature review of UKA for spontaneous osteonecrosis of the knee in 64 patients noted a mean improvement in the global knee score from 46 to 82, with a mean revision rate of 13%, when followed for 2.5 to 5.5 years [29]. “Poor outcome” was suggested in earlier studies [24, 33], in contradiction to “excellent outcome” when established indications were used in a subsequent study [29]. The better survival in some series [33, 34] cited in the review [29] compared with previous literature [24, 33] might be related to improvements in modern prosthetic designs, less invasive surgical techniques, or more appropriate patient selection (eg, reserving the procedure to knees with strictly unicompartmental joint disease). Thus, the role of UKA in spontaneous osteonecrosis of the knee remains unclear, particularly regarding the indications, contraindications, and technical parameters for treatment with a modern implant design.
We therefore determined (1) the long-term survivorship of UKAs in a larger group of patients with spontaneous osteonecrosis of the knee; (2) WOMAC, Knee Society score (KSS), VAS for self-assessment of pain, Lysholm-Tegner score, and (3) the percentage of failures and possible reasons for failures, either in terms of patient selection (age, BMI, Lysholm-Tegner score for activity level) or surgical technique (femorotibial angle, tibial plateau angle, and posterior tibial slope).
Patients and Methods
The population of patients for this retrospective therapeutic case series was derived from 273 patients with medial UKAs, treated between 1998 to 2005, of whom 185 (68%) received a UKA for medial compartment osteoarthritis. The indications for UKA were unicompartmental osteoarthritis or osteonecrosis; age older than 50 years with low-demand activity; BMI less than 35 kg/m2; minimal ROM arc greater than 90°; flexion contracture less than 10°; passively correctable angular deformity less than 15° under spinal anesthesia, and intact ACL and PCL at clinical and MRI evaluations [7, 16, 32]. UKA was considered contraindicated in patients with inflammatory arthritis [28], age younger than 50 years, high activity level, patellofemoral pain, exposed bone in the patellofemoral joint or opposite compartment, and secondary osteonecrosis [7, 16, 25, 29, 31, 32]. From our population, we selected patients with a diagnosis of primary spontaneous osteonecrosis of the knee in the medial compartment on preoperative MRI [11, 22, 25, 35] and intraoperative examination; radiographic diagnosis of Stage 4 osteonecrosis [25–27, 35, 36] with collapse of the medial compartment (Ahlbäck Grades III-IV) [2], and minimum clinical followup of 5 years. We excluded 97 patients with other causes of isolated medial compartment arthritis and those with (1) patellofemoral joint symptoms, (2) lateral joint line pain, (3) previous high tibial osteotomy, (4) morbid obesity (BMI > 40), (5) diagnosis of inflammatory degenerative joint diseases [28], and (6) previous open or arthroscopic medial meniscectomy [31]. The exclusions left 88 patients (32%) for study. The mean age of the patients at surgery was 66 years and mean BMI was 29 (Table 1). At last followup, four patients (one man, three women) had died for reasons unrelated to surgery, leaving 84 patients (30 men, 54 women) available for evaluation, with a minimum followup of 63 months (mean, 98 months; range, 63–145 months) (Table 1). Sixty of the 88 patients (70%) had a minimum followup of at least 10 years. Except for the patients who died, none was lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. The study was approved by our institutional review board. All patients gave informed consent to participate in the study.
Table 1.
Demographics for patients with medial compartment joint space narrowing
| Variable | Value |
|---|---|
| Number of patients | 84 |
| Male/female | 30/54 |
| Age at surgery (years)* | 66 ± 9 (range, 43–84) |
| BMI* | 29 ± 4 (range, 23–40) |
| Followup (months)* | 98 ± 33 (range, 63–145) |
| Pathology site | |
| MFC | 77 |
| MTP | 7 |
| Right/left | 36/48 |
| Ahlbäck grade | |
| IV | 47 |
| III | 37 |
| II | 0 |
| I | 0 |
* Values are expressed as mean ± SD, with range in parentheses; MFC = medial femoral condyle; MTP = medial tibial plateau.
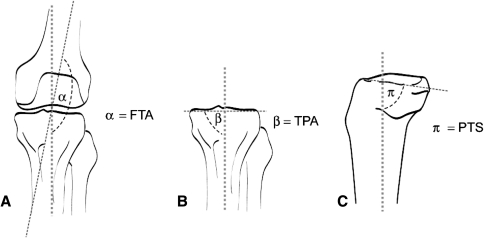
Preoperatively, all patients had weightbearing AP and laterolateral (LL) [18] radiographs. In all knees, spontaneous osteonecrosis involved the medial compartment; in 77 knees, the medial femoral condyle was involved, whereas in seven knees, the medial tibial plateau was involved (Table 1). According to the radiographic classification of knee osteonecrosis as described by Ficat [12] and Mont et al. [25, 26], all patients were diagnosed with Stage 4 spontaneous osteonecrosis of the knee. Using the Ahlbäck classification [2], all patients had Grade III or IV medial joint space narrowing (Table 1). Preoperatively, three observers (SB, NL, TB) with no clinical contact with the patients determined the femorotibial angle (FTA) (Fig. 1A) [14]. The FTA was determined by locating a point 10 cm above and below the joint line, and taking the midpoint of the femur and tibia at these levels. The midpoints of the femur and tibia again were located at the superior edges of the radiograph. The respective two points on the femur and tibia were connected. The angle subtended between these two lines was taken as the FTA. The preoperative tibial plateau angle (TPA) was determined as the angle subtended by the medial tibia plateau on the anatomic axis of the proximal tibia (Fig. 1B) [10]. On the LL radiographs, the posterior tibial slope (PTS) (Fig. 1C) [10] was determined as the angle included between the tangent to medial tibial plateau and anatomic axis of the tibia (interobserver variability, FTA = 0.87, TPA = 0.83, PTS = 0.80), using recognized techniques. All patients also had MRI to document the location and extent of their spontaneous osteonecrosis, exclude any metaphyseal involvement, and assess the absence of degenerative changes in the lateral and patellofemoral compartments [25].
Fig. 1A–C.
Preoperative radiographic measurements of (A) femorotibial angle (FTA), (B) tibial plateau angle (TPA), and (C) posterior tibial slope (PTS) are shown.
All surgeries were performed by one of the three senior authors (MM, SZ, FI) using a minimally invasive quadriceps-sparing technique [4, 5, 8] and the same cemented implant with an all-polyethylene tibial component (Preservation® Uni-Compartmental Knee; DePuy Orthopaedics Inc, Warsaw, IN, USA). We believe a key technical feature is to restore the presumed prepathologic varus alignment (compared with the opposite side), avoiding overcorrection of the varus deformity [38]. In all knees, surgery was performed according to the original surgical technique as suggested by the manufacturer. The minimum thickness for the tibial component (7 mm) was used in 78 knees (93%), whereas the intermediate thickness (9.5 mm) was used in six knees (7%). The maximum polyethylene thickness (11.5 mm) was not used in any knees.
After surgery, static quadriceps exercises and continuous passive motion were started on the first postoperative day, after drain removal and dressing, to achieve 90° knee flexion over 2 to 3 days. Walking with two crutches with weightbearing as tolerated was started on the same day and continued for 30 days, after which one crutch was used for 15 to 20 days until passive extension was possible. Electrostimulation for quadriceps strengthening was performed for 2 weeks after suture removal. The patients performed unsupervised physiotherapy at home and generally started full weightbearing at approximately 45 days. Patients received complete clinical evaluations at 30, 60, and 90 days and then yearly after surgery at which time they were evaluated for pain, swelling, ROM, and ability to rise from a chair and to climb stairs. At the last followup patients evaluated their pain using a 0 to 10 VAS. Three different fellows (BS, ACP, MN) with no previous contact with the patients obtained KSS [15] and WOMAC scores [6], and a Lysholm-Tegner score [37]. The interobserver variability of the KSS is reportedly 0.87 and that of the WOMAC 0.86. We obtained radiographs at 30 days and then yearly after surgery.
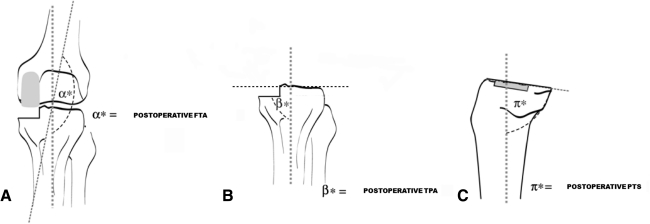
Three observers (SB, NL, TB) independently measured FTA (Fig. 2A), TPA (Fig. 2B), and PTS (Fig. 2C) on new AP and LL short-film weightbearing radiographs at the postoperative observation point. The postoperative FTA was determined as described previously [14], whereas the postoperative TPA was measured as the angle subtended by the tangent to the plane of medial tibial plateau resection and the proximal anatomic axis of the tibia [10]. The postoperative PTS was ascertained as the angle included by the tangent to the medial tibial plateau resection plane and the anatomic axis of the tibia in the sagittal plane [10]. We assumed an FTA greater than 175° to be a varus knee, an FTA between 170° and 175° a normal knee, and an FTA less than 170° a valgus knee. Moreover, we assumed a TPA greater than 90° to be a valgus knee and a TPA less than 90° a varus knee.
Fig. 2A–C.
Postoperative radiographic measurements of (A) femorotibial angle (FTA), (B) tibial plateau angle (TPA), and (C) posterior tibial slope (PTS) are shown.
All patients with persistent pain on the medial joint line underwent revision surgery after greater than 12 months after UKA. Revision was performed with a primary implant (PFC® Sigma® RP; DePuy Orthopaedics Inc) in seven knees and with a constrained modular design (PFC® Sigma® TC3; DePuy Orthopaedics Inc) in three knees (Table 2). The constrained modular design was used for the patient with the medial tibial fracture, for the patient with prosthetic infection, and for one of the patients with aseptic loosening of the tibial component. In all these knees, it was necessary to use stems to achieve optimal distal and proximal fixation. Revision was performed as a single-stage procedure in all patients, except for the patient with a prosthetic infection, where a temporary spacer was used and revision was performed in a second stage.
Table 2.
Data for patients with revision for any reason
| Variable | Value |
|---|---|
| Number of patients | 10 |
| Male/female | 4/6 |
| Age at surgery (years)* | 65 ± 7 (range, 56–71) |
| BMI* | 29 ± 3 (range, 25–35) |
| Time to revision (months)* | 26 ± 23 (range, 12–60) |
| Pathology site | |
| MFC | 10 |
| MTP | 0 |
| Right/left | 5/5 |
| Reason for revision | |
| Tibial subsidence | 4 |
| Tibial loosening | 3 |
| Femoral loosening | 1 |
| MTF | 1 |
| Infection | 1 |
| Implant used for revision | |
| Primary | 7 |
| Constrained | 3 |
* Values are expressed as mean ± SD, with range in parentheses; MFC = medial femoral condyle; MTP = medial tibial plateau; MTF = medial tibial fracture.
Continuous variables (ie, age, BMI, range of flexion, FTA, TPA, PTS, VAS pain score, KSS, WOMAC, Lysholm-Tegner) were expressed as arithmetic mean ± SD and minimum to maximum ranges. We performed survival analysis using the Kaplan-Meier method, with a 95% CI, using revision for any reason as the end point. The 10 patients whose UKAs were revised were compared with the patients with no implant revision to determine statistical differences between selected variables (FTA, TPA, VAS, ROM, Lysholm-Tegner score, BMI, and age). To evaluate differences between failure and success cohorts, Fisher’s exact test was used to compare nominal variables (gender) and the Mann-Whitney U test was used to compare continuous variables (FTA, PTA, PTS, BMI, KSS, WOMAC, VAS, ROM, Lysholm-Tegner). Statistical analyses were performed using SPSS® Version 16 (SPSS Inc, Chicago, IL, USA).
Results
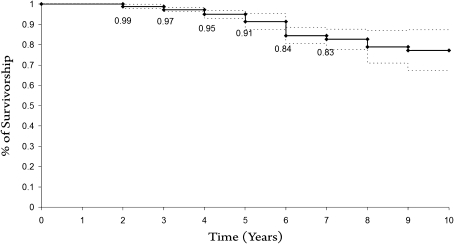
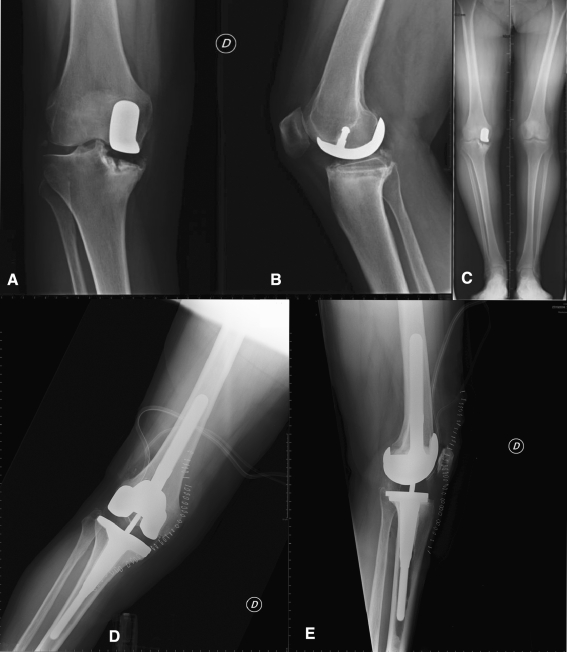
The 10-year Kaplan-Meier survivorship with revision for any reason as the end point was 89% ± 2% (Fig. 3). The four patients who died before final evaluation had no clinical symptoms of implant failure or radiographic signs of loosening at last followup. Ten of the 84 patients (12%) underwent revision. Subsidence of the tibial component in four knees (Fig. 4A–C), aseptic loosening of the tibial component in three, aseptic loosening of the femoral component in one, medial tibial fracture in one, and prosthetic infection in one were the reasons for revision (Fig. 4D–E). Mean time from UKA to revision was 26.4 months and no patient experienced failure of their knee greater than 5 years after surgery (Table 2).
Fig. 3.
The 10-year Kaplan-Meyer survival analysis with revision for any reason as the end point showed a survival rate of 89%. The 95% confidence interval was 89.6 to 103.5 months.
Fig. 4A–E.
(A) AP, (B) lateral, and (C) long-leg radiographs of a patient with subsidence of the medial tibial plateau and increased postoperative posterior tibial slope are shown. The patient underwent revision surgery after 18 months from the index surgery owing to persistent pain on the medial side. (D) AP and (E) lateral radiographs show the knee after the revision procedure. The reimplantation was performed with a one-stage procedure using a constrained implant with uncemented stems for the femoral and tibial components. Owing to bone loss on the medial tibial side, a metal augment was used.
Postoperatively, the average KSS was 87.1 ± 13.8, WOMAC score was 12 ± 10.3 (Table 3), and VAS was 1.7 ± 2.5 (0–3) (Table 4), whereas the average Lysholm-Tegner score for postoperative activity was 50.8 ± 6.25(45–63). The postoperative VAS pain score and ROM were lower in the revision group than in the survivors, with similar changes observed in KSS and WOMAC (Table 5).
Table 3.
Clinical scores for KSS and WOMAC
| Variable | Postoperative* | Interobserver correlation coefficient between classes | Intraobserver correlation coefficient between classes |
|---|---|---|---|
| WOMAC | 12 ± 10.3 (0–44) | 0.86 | 0.93 |
| KSS | 87.1 ± 13.8 (45–100) | 0.87 | 0.91 |
* Values are expressed as mean ± SD, with range in parentheses; KSS = Knee Society score; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Table 4.
Clinical and radiographic results for all patients
| Variable | Preoperative* | Postoperative* | Difference* | p value | Interobserver correlation coefficient between classes | Intraobserver correlation coefficient between classes |
|---|---|---|---|---|---|---|
| Maximum active knee flexion | 102° ± 5° (90°–130°) | 130° ± 10° (100°–139°) | 28° ± 5° | < 0.001 | 0.81 | 0.90 |
| VAS pain score | 8.6 ± 1.6 (7–10) | 1.7 ± 2.5 (0–3) | 5.7 ± 0.9 | < 0.001 | 0.82 | 0.91 |
| FTA | 179.5° ± 3° (171°–185°) | 177° ± 2.9° (169°–185°) | 2.5° ± 0.1° | < 0.001 | 0.87 | 0.95 |
| TPA | 87.3° ± 1.9° (82°–90°) | 85.2° ± 3.6° (74°–91°) | 2.1° ± 1.7° | < 0.001 | 0.83 | 0.90 |
| PTS | 83° ± 3.7° (range, 74°–90°) | 82.1° ± 3.8° (range, 72°–89°) | 0.9° ± 0.1° | 0.06 | 0.80 | 0.91 |
* Values are expressed as mean ± SD, ranges in parentheses; FTA = femorotibial angle; TPA = tibial plateau angle; PTS = posterior tibial slope.
Table 5.
Comparison of survivors and patients who had revision surgery
| Parameter | Survivor group | Revision group | p value |
|---|---|---|---|
| Number of patients | 10 | 74 | |
| Male/female | 4/6 | 26/48 | 1.0000 |
| Age at time of surgery (years) | 65 ± 6 (range, 56–71) | 65 ± 9 (range, 43–84) | 0.9000 |
| BMI | 28 ± 2 | 29 ± 3 | 0.9022 |
| MFC/MTP | 10/0 | 68/7 | 0.9998 |
| Variable | Postoperative | Postoperative | |
| WOMAC | 89.4 ± 10.1 | 69.2 ± 6.9 | < 0.001 |
| KSS | 90.6 ± 10.0 | 54.3 ± 5.4 | < 0.001 |
| VAS pain score | 2.3 ± 1.9 | 7.7 ± 1.5 | < 0.001 |
| Lysholm-Tegner | 50.2 ± 6.1 | 55.7 ± 7.5 | 0.0886 |
| Maximum active knee flexion | 120° ± 9° | 106o ± 5° | < 0.001 |
* Values are expressed as mean ± SD, with range in parentheses; BMI = body mass index; MFC = medial femoral condyle; MTP = medial tibial plateau; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; KSS = Knee Society score; VAS = visual analog scale.
The differences in postoperative PTS in the survivors and the revision group were 0.5° ± − 2.7° and 4.2° ± − 2.5°, respectively, suggesting an overcorrection of 3.7° in the revision group (p = 0.002). There was no difference in the two groups with relation to age, BMI (Table 5), preoperative activity as measured by Lysholm-Tegner score, FTA, and TPA (Table 6). VAS pain score after revision of UKA, compared with after UKA, was improved (p = 0.001) from 8.4 ± 1.4 to 4.4 ± 3.7.
Table 6.
Comparison of survivor and revision groups
| Survivor group | Revision group | p value | |||
|---|---|---|---|---|---|
| Variable | Preoperative | Postoperative | Preoperative | Postoperative | |
| FTA | 178° ± 3° | 178° ± 5° | 180° ± 3° | 177° ± 3° | 0.8904 |
| TPA | 87° ± 2° | 84° ± 6° | 87° ± 2° | 85° ± 3° | 0.1961 |
| PTS | 3° ± 2° | 7° ± 5° | 7° ± 4° | 8° ± 4° | 0.2646 |
* Values are expressed as mean ± SD, with range in parentheses; FTA = femorotibial angle; TPA = tibial plateau angle; PTS = posterior tibial slope.
Discussion
The literature suggests survivorship of UKA for spontaneous osteonecrosis of the knee ranges from 93% to 96.7% at 10 to 12 years [29]. However, these data arise from series reporting 23 to 33 patients, jeopardizing meaningful conclusions. Thus, the indications, contraindications, and technical parameters in treating spontaneous osteonecrosis of the knee with a modern implant design are unclear. We determined (1) the long-term survivorship of UKAs in a larger group of patients with spontaneous osteonecrosis of the knee; (2) WOMAC, KSS, VAS for self-assessment of pain, Lysholm-Tegner score, and (3) the percentage of failures and reasons for failures.
Our study had several limitations. First, the size and depth of the lesions were not documented in our study. However, it seems less likely they would impact the results as the patients selected all had Ahlbäck Grades III or IV osteonecrosis, with bone to bone contact, and we used a cemented onlay implant positioned on the tibial cortical rim, with a nonresurfacing technique, where the implant did not rest on subchondral bone. Second, we were unable to conduct a wear analysis to determine whether and possibly how polyethylene wear was related to altered PTS. Third, we had no control group undergoing TKA for this group of patients with spontaneous osteonecrosis of the knee. However, the survivorship obtained with UKA in our series is comparable to the survivorship reported for TKA [33].
The potential of UKAs to treat spontaneous osteonecrosis of the knee has generated considerable controversy (Table 7). A literature review of UKA in patients with spontaneous osteonecrosis of the knee showed a cumulative revision rate of 13% and an improved KSS from a mean preoperative value of 46 to a mean postoperative value of 82 [29]. However, survivorship seems to have changed in recent studies [29, 30, 34] in comparison to previous literature. An earlier study noted a failure rate of 12.5% at 5.5 years [24], whereas a recent study had a 96.7% 12-year survival rate [30]. The improvement in survivorship in subsequent literature could reflect better patient selection on the basis of low BMI or exclusion of secondary osteonecrosis and documentation of size of lesion by preoperative MRI rather than intraoperative inspection. A previous report [33] of superior KSS with TKAs compared with UKAs performed for spontaneous osteonecrosis of the knee included secondary osteonecrosis and did not compare size of the lesion among patients with failed results and survivors. Two recent studies suggest comparable results for UKAs in patients with spontaneous osteonecrosis of the knee versus those for patients with osteoarthritis [17, 34]. The overall 10-year survivorship in our study was 89% for medial UKAs performed for late-stage spontaneous osteonecrosis of the knee. However, excluding the failed results attributable to postoperative infection and posttraumatic fracture, probably only eight (8.91%) were related to errors in patient selection or surgical technique, leading to progression of the lesion, loosening, or polyethylene wear.
Table 7.
Literature comparison of UKAs performed in patients with spontaneous and secondary osteonecrosis of the knee
| Study | Number of patients | Male/female | Age (years)* | BMI* | Followup (years)* | Secondary ON | Site of disease | Sizing of lesion | Preoperative MRI | Survival analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Marmor [24] | 32 (2 bilateral) | 10/22 | 68 (range, 34–84) | NA | 5.5 (range, 2–16) | 2 steroid-induced | 31 MFC; | NA | NA | NA |
| 1 MTP; | ||||||||||
| 2 LFC; | ||||||||||
| Langdown et al. [17] | 27 UKA ON (2 bilateral) versus 26 UKA OA (2 bilateral) | 5/22 | 73 (range, 43–88) | NA | 5.2 (range, 1–13) | NA | 26 MFC; | NA | NA | NA |
| 3 MTP | ||||||||||
| Radke et al. [33] | 23 UKA ON versus 16 TKA ON | 3/20 | 70 (range, 54–80) | Weight, 71 kg (range, 56–104) | 3.3 (6 patients); | 1 postradiation therapy; | All medial compartment | Radiographic | NA | NA |
| 4.7 (10 patients); | 3 corticosteroid therapy | |||||||||
| 10.4 (10 patients) | ||||||||||
| Parratte et al. [30] | 30 patients (1 bilateral) | 10/20 | 71 (range, 51–88) | 27 (range, 18–35) | 7 (range, 3–16) | 10 (33%) (7 steroid-induced; 3 barotraumatic) | 29 medial compartment; | NA | 26 patients (86%) | 96.7% at 12 years with revision for any reason or radiographic loosening as end point |
| 2 lateral compartment | ||||||||||
| Servien et al. [34] | 33 UKA ON versus 35 UKA OA | 10/23 | 74 (range, 42–89) | 25 ± 4 | 5.1 (range, 2–11.5) | NA | 33 MFC | NA | Not systematically provided | 93% at 10 years with removal and/or conversion to TKA as end point |
* Values are expressed as mean or mean ± SD, ranges in parentheses; †patients were divided into three groups for univariate ANOVA; UKA = unicompartmental knee arthroplasty; ON = osteonecrosis; UKA ON = UKA performed for osteonecrosis; UKA OA = UKA performed for osteoarthritis; TKA ON = TKA performed for osteonecrosis; MFC = medial femoral condyle; MTP = medial tibial plateau; LFC = lateral femoral condyle; NA = not available.
To explore these reasons for failure, the patients with no implant revision and those with revision for any reason were compared for patient selection criteria (age, BMI, activity level documented by Lysholm-Tegner score) and surgical technique (FTA, TPA, and PTS). We found an increase in PTS in our patients with revisions. There were no differences detected in the current study with respect to the other parameters. Although a PTS of 7° is reportedly beneficial when a UKA is performed in knees with an ACL deficiency [13], we found no comparable data reported in the previous literature for PTS. Along with patient selection, it is vital not to overcorrect the PTS, a factor that also probably applies to FTP and TPA.
Survivorship of UKAs in patients with spontaneous osteonecrosis of the knee seems variable in different studies [13, 17, 24, 29, 30, 33, 34], yet certain parameters can be identified that can favorably influence it. Secondary osteonecrosis probably should be a contraindication owing to the propensity for subsequent multicompartment involvement. The involvement of other compartments should be checked even in spontaneous osteonecrosis of the knee with preoperative MRI. We believe UKA, with its bone-preserving nature and preservation of natural kinematics, is a better solution than TKA for spontaneous osteonecrosis of the knee, which is a unicompartmental disease, without soft tissue imbalance, ligament involvement, and malalignment.
Acknowledgments
We thank Angelo Canales Passalacqua, MD, Marco Nitri, MD, Nicola Lopomo, Engineer, and Tommaso Bonanzinga, MD for help in clinical followup. We also thank Bharat Sharma, MD and Simone Bignozzi, Engineer for help with revision of this document.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Rizzoli Orthopaedic Institute, Bologna, Italy.
References
- 1.Aglietti P, Insall JN, Buzzi R, Deschamps G. Idiopathic osteonecrosis of the knee: aetiology, prognosis and treatment. J Bone Joint Surg Br. 1983;65:588–597. doi: 10.1302/0301-620X.65B5.6643563. [DOI] [PubMed] [Google Scholar]
- 2.Ahlbäck S. Osteoarthrosis of the knee: a radiographic investigation. Acta Radiol Diagn (Stockh). 1968;277(suppl):7–72. [PubMed] [Google Scholar]
- 3.Ahlbäck S, Bauer GC, Bohne WH. Spontaneous osteonecrosis of the knee. Arthritis Rheum. 1968;11:705–733. doi: 10.1002/art.1780110602. [DOI] [PubMed] [Google Scholar]
- 4.Argenson JN, Chevrol-Benkeddache Y, Aubaniac JM. Modern unicompartmental knee arthroplasty with cement: a three to ten-year follow-up study. J Bone Joint Surg Am. 2002;84:2235–2239. [PubMed] [Google Scholar]
- 5.Argenson JN, Parratte S, Flecher X, Aubaniac JM. Unicompartmental knee arthroplasty: technique through a mini-incision. Clin Orthop Relat Res. 2007;464:32–36. doi: 10.1097/BLO.0b013e3180986da7. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy N, Goldsmith CH, Buchanan WW, Campbell J, Duku E. Prior score availability: observations using the WOMAC osteoarthritis index. Br J Rheumatol. 1991;30:150–151. doi: 10.1093/rheumatology/30.2.150. [DOI] [PubMed] [Google Scholar]
- 7.Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg. 2008;16:9–18. doi: 10.5435/00124635-200801000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bruni D, Iacono F, Russo A, Zaffagnini S. Marcheggiani Muccioli GM, Bignozzi S, Bragonzoni L, Marcacci M. Minimally invasive unicompartmental knee replacement: retrospective clinical and radiographic evaluation of 83 patients. Knee Surg Sports Traumatol Arthrosc. 2010;18:710–717. doi: 10.1007/s00167-009-0895-9. [DOI] [PubMed] [Google Scholar]
- 9.Cartier P, Gaggiotti G. Jully JL [Primary osteonecrosis of the medial femoral condyle: unicompartmental or total replacement?][in French] Int Orthop. 1988;12:229–235. doi: 10.1007/BF00547168. [DOI] [PubMed] [Google Scholar]
- 10.Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):108–115. doi: 10.1016/j.arth.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Ecker ML, Lotke PA. Spontaneous osteonecrosis of the knee. J Am Acad Orthop Surg. 1994;2:173–178. doi: 10.5435/00124635-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Ficat RP. Idiopathic bone necrosis of the femoral head: early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 13.Hernigou P, Bove JC, Goutallier D. Idiopathic osteonecrosis of the medial femoral condyle: treatment with tibial osteotomy or unicompartmental arthroplasty] [in French] Rev Chir Orthop Reparatrice Appar Mot. 1988;74:232–237. [PubMed] [Google Scholar]
- 14.Hopgood P, Martin CP, Rae PJ. The effect of tibial implant size on post-operative alignment following medial unicompartmental knee replacement. Knee. 2004;11:385–388. doi: 10.1016/j.knee.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 16.Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop. 2007;78:128–135. doi: 10.1080/17453670610013538. [DOI] [PubMed] [Google Scholar]
- 17.Langdown AJ, Pandit H, Price AJ, Dodd CA, Murray DW, Svärd UC, Gibbons CL. Oxford medial unicompartmental arthroplasty for focal spontaneous osteonecrosis of the knee. Acta Orthop. 2005;76:688–692. doi: 10.1080/17453670510041772. [DOI] [PubMed] [Google Scholar]
- 18.LaValley MP, McLaughlin S, Goggins J, Gale D, Nevitt MC, Felson DT. The lateral view radiograph for assessment of the tibiofemoral joint space in knee osteoarthritis: its reliability, sensitivity to change, and longitudinal validity. Arthritis Rheum. 2005;52:3542–3547. doi: 10.1002/art.21374. [DOI] [PubMed] [Google Scholar]
- 19.Lotke PA, Abend JA, Ecker ML. The treatment of osteonecrosis of the medial femoral condyle. Clin Orthop Relat Res. 1982;171:109–116. [PubMed] [Google Scholar]
- 20.Lotke PA, Battish R, Nelson CL. Treatment of osteonecrosis of the knee. Instr Course Lect. 2001;50:483–488. [PubMed] [Google Scholar]
- 21.Lotke PA, Ecker ML. Osteonecrosis-like syndrome of the medial tibial plateau. Clin Orthop Relat Res. 1983;176:148–153. [PubMed] [Google Scholar]
- 22.Lotke PA, Ecker ML. Osteonecrosis of the knee. J Bone Joint Surg Am. 1988;70:470–473. [PubMed] [Google Scholar]
- 23.Low K, Mont MA, Hungerford DS. Steroid-associated osteonecrosis of the knee: a comprehensive review. Instr Course Lect. 2001;50:489–493. [PubMed] [Google Scholar]
- 24.Marmor L. Unicompartmental arthroplasty for osteonecrosis of the knee joint. Clin Orthop Relat Res. 1993;294:247–253. [PubMed] [Google Scholar]
- 25.Mont MA, Baumgarten KM, Rifai A, Bluemke DA, Jones LC, Hungerford DS. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82:1279–1290. doi: 10.2106/00004623-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Mont MA, Hungerford DS. Osteonecrosis of the shoulder, knee, and ankle. In: Urbaniak JR, Jones JP, editors. Osteonecrosis: Etiology, Diagnosis, and Treatment. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1997. pp. 429–436. [Google Scholar]
- 27.Motohashi M, Morii T, Koshino T. Clinical course and roentgenographic changes of osteonecrosis in the femoral condyle under conservative treatment. Clin Orthop Relat Res. 1991;266:156–161. [PubMed] [Google Scholar]
- 28.Mullaji AB, Sharma A, Marawar S. Unicompartmental knee arthroplasty: functional recovery and radiographic results with a minimally invasive technique. J Arthroplasty. 2007;22(4 suppl 1):7–11. doi: 10.1016/j.arth.2006.12.109. [DOI] [PubMed] [Google Scholar]
- 29.Myers TG, Cui Q, Kuskowski M, Mihalko WM, Saleh KJ. Outcomes of total and unicompartmental knee arthroplasty for secondary and spontaneous osteonecrosis of the knee. J Bone Joint Surg Am. 2006;88(suppl 3):76–82. doi: 10.2106/JBJS.F.00568. [DOI] [PubMed] [Google Scholar]
- 30.Parratte S, Argenson JN, Dumas J, Aubaniac JM. Unicompartmental knee arthroplasty for avascular osteonecrosis. Clin Orthop Relat Res. 2007;464:37–42. doi: 10.1097/BLO.0b013e31812f7821. [DOI] [PubMed] [Google Scholar]
- 31.Patel DV, Breazeale NM, Behr CT, Warren RF, Wickiewicz TL, O’Brien SJ. Osteonecrosis of the knee: current clinical concepts. Knee Surg Sports Traumatol Arthrosc. 1998;6:2–11. doi: 10.1007/s001670050064. [DOI] [PubMed] [Google Scholar]
- 32.Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87:1488–1492. doi: 10.1302/0301-620X.87B11.16324. [DOI] [PubMed] [Google Scholar]
- 33.Radke S, Wollmerstedt N, Bischoff A, Eulert J. Knee arthroplasty for spontaneous osteonecrosis of the knee: unicompartimental vs bicompartimental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2005;13:158–162. doi: 10.1007/s00167-004-0551-3. [DOI] [PubMed] [Google Scholar]
- 34.Servien E, Verdonk PC, Lustig S, Paillot JL, Kara AD, Neyret P. Medial unicompartmental knee arthroplasty for osteonecrosis or osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2008;16:1038–1042. doi: 10.1007/s00167-008-0617-8. [DOI] [PubMed] [Google Scholar]
- 35.Soucacos PN, Johnson EO, Soultanis K, Vekris MD, Theodorou SJ, Beris AE. Diagnosis and management of the osteonecrotic triad of the knee. Orthop Clin North Am. 2004;35:371–381. doi: 10.1016/j.ocl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle: classification and treatment. Clin Orthop Relat Res. 1997;341:82–89. [PubMed] [Google Scholar]
- 37.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 38.Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 suppl 2):2–3. doi: 10.1016/j.arth.2005.03.029. [DOI] [PubMed] [Google Scholar]