Abstract
Purpose
High doses of gabapentin were associated with pancreatic acinar cell tumors in male Wistar rats, but there is little published epidemiological data regarding gabapentin and carcinogenicity. We explored the association between gabapentin and cancer in a United States (US) medical care program, and followed up nominally significant associations in a United Kingdom (UK) primary care database.
Methods
In the US Kaiser Permanente Northern California (KPNC) health system, we performed nested case-control analyses of gabapentin and 55 cancer sites and all-cancer combined using conditional logistic regression. Up to ten controls were matched to each case on year of birth, sex, and year of cohort entry. No other covariates were included in models. Only dispensings for gabapentin 2 years or more prior to index date were considered. Nominally significant associations with an odds ratio > 1.00 and p < 0.05 for three or more dispensings versus no dispensings were followed up by similar nested-case control analyses in the UK General Practice Research Database (GPRD), adjusting for potential indications for gabapentin and risk factors for the specific cancers.
Results
The following analyses had OR>1.00 and p<0.05 for three or more dispensings of gabapentin versus no dispensing (2-year lag) in KPNC, and were also examined in the GPRD: all-cancer, breast, lung and bronchus, urinary bladder, kidney/renal pelvis, stomach, anus/anal canal/anorectum, penis, and other nervous system. These cancers were not statistically significantly associated with gabapentin in the GPRD case-control studies (2-year lag). The GPRD and KPNC studies did not identify a statistically significant increased risk of pancreatic cancer with >2 prescriptions of gabapentin in 2-year lagged analyses.
Conclusions
The epidemiological data in a US cohort with up to 12 years of follow-up and a UK cohort with up to 15 years of follow up do not support a carcinogenic effect of gabapentin use. However, the confidence intervals for some analyses were wide, and an important effect cannot be confidently excluded.
Keywords: Gabapentin, Cancer, Protopathic bias
INTRODUCTION
Gabapentin was approved in the United States (US) as adjunctive therapy for partial seizures in December 1993 and for treatment of post-herpetic neuralgia (PHN) in May 2002. In the United Kingdom (UK), gabapentin was approved as adjunctive therapy for partial seizures on May 1993, and is also indicated for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy (PDN) and PHN in adults. Gabapentin has also been prescribed off-label for other pain conditions,1 and is considered an adjunctive therapy for cancer pain according to the World Health Organization (WHO) analgesic ladder.2
High doses of gabapentin were associated with pancreatic acinar cell tumors in male Wistar rats.3 There is one published epidemiological study of gabapentin and cancer. The association of gabapentin with cancer was evaluated in a case-control screening study of the association of 105 drugs with 55 cancers in Kaiser Permanente Northern California (KPNC), 1994–2006.4 The only cancer that met the screening criteria for possibly increased cancer risk with gabapentin exposure was renal (including renal pelvis) cancer. This association was judged to be likely due to or substantially accentuated by confounding by cigarette smoking and hypertension. The current paper reports the full results of the KPNC study for gabapentin and each cancer.
We also conducted a series of epidemiologic studies using the United Kingdom General Practice Research Database (GPRD) to assess the relationship between gabapentin and (1) pancreatic cancer, based on the rat findings; (2) renal cancer, based on the published KPNC findings; (3) and those cancers with OR>1.00 and p<0.05 for three or more dispensings of gabapentin versus no dispensing of gabapentin in the KPNC study.
METHODS
KPNC Study Population and Methods
The details of the case-control screening study of gabapentin with 55 cancers in Kaiser Permanente Northern California (KPNC), 1994–2006 were published.4 The study was approved by the Kaiser Permanente Institutional Review Board. In brief, KPNC is an integrated health care plan (including comprehensive inpatient and outpatient care and pharmacy services), serving over 3 million current members, comprising about 30% of the residents in the San Francisco Bay area and the Central Valley of California. The membership is fairly representative of the local population except for some under-representation of both extremes of the economic spectrum.
The study design was a nested case-control study within a cohort of 6.6 million KPNC subscribers with drug coverage August 1994-December 31, 2006. Cohort entry began August 1994, or at the time of joining the program and having drug coverage if later than August 1994. Follow-up was through the earlier of (1) December 31, 2006, (2) diagnosis of the cancer of interest, or (3) leaving KPNC for any reason, including death.
Cancer categories were based on invasive cancers at each cancer site (plus non-invasive urinary bladder cancers) listed in the International Classification of Diseases for Oncology, Version 3 (ICD-O-3) except for non-melanoma skin cancers.5 Cancer cases were risk set matched with up to 10 controls by year of birth, sex, and year of cohort entry. The index date was the date of incident cancer for cases. The index date for controls was chosen so as to give the controls equal follow-back time to that of the case for ascertainment of prior use of gabapentin. Conditional logistic regression calculated the odds ratio (OR) as the estimate of the relative risk of cancer associated with 1, 2, and ≥3 prescription dispensings versus no use of gabapentin at least 2-years before the index date. The 2-year lag was defined a priori to control for prediagnostic prescribing for symptoms possibly related to cancer.4, 6 These analyses were not adjusted for covariates other than the matching variables.
GPRD Study Population and Methods
Study Design
Nominally significant associations with OR>1.00 and p<0.05 for three or more dispensings versus no dispensings in KPNC were followed up by similar nested-case control analyses in the GPRD, adjusting for potential indications for gabapentin and the main risk factors for the specific cancers. Pancreatic cancer, though not significant in KPNC, was also evaluated in the GPRD.
GPRD study protocols were approved by the Independent Scientific Advisory Committee (ISAC) of the GPRD and posted to ClinicalTrials.gov prior to analysis (ClinicalTrials.gov Identifiers NCT01138124 and NCT01236053). The results reported herein are from the protocol-specified analyses. The pancreatic and renal studies were conducted initially (NCT01138124), and the additional cancers were subsequently assessed with modifications in the analysis (NCT01236053). The main modification was exclusion of cases and controls with a prior history of cancer before the index date in the analyses of the additional cancers, to limit misclassification of primary cancer diagnosis codes that may be recurrent or metastatic. In the analysis of the additional cancers, back pain was added as a covariate to further control for potential confounding by indication. However, supplemental analyses of gabapentin and the risk of pancreatic and renal cancers were also performed under these modifications (i.e. excluding those with prior history of other cancer before index date and further adjusting for back pain) to allow for comparability of study results.
Data Source
The GPRD, managed by the UK Medicines and Healthcare products Regulatory Agency (MHRA), comprises the entire computerized medical records of a sample of general practitioners (GPs) in England and Wales. All members of the population are registered with a single practice, which centralizes the medical information from GPs, specialist referrals, and hospital attendances. This study is based on data from about a 6% representative sample of the UK population. Data recorded in the GPRD includes demographic information, prescription details, clinical events, preventive care provided, specialist referrals, hospital admissions and their major outcomes. Data are retrieved by means of the Oxford Medical Information Systems (OXMIS) and READ codes.7
Study Cohort
Nested case-control analyses were conducted in a cohort of subjects in the GPRD 1993–2008. Entry into the study cohort began Jan 1, 1993 for all those who were registered in GPRD before that time, and at the time of registration if later than Jan 1, 1993. The ascertainment period for gabapentin use was from Jan 1, 1993 through Dec 31, 2008. The ascertainment period for incident cancers was from Jan 1, 1995 through Dec 31, 2008. Follow-up ended earlier if the respective cancer was diagnosed, or if the subject left the GPRD for any reason including death, whichever came first.
Case Definition
The cancer outcomes were defined by READ/OXMIS codes (Supplemental Table 1). The code list for all-cancer was derived from clinical review of all cancer, neoplasm, and oncology related READ/OXMIS codes by William T. Hamilton, MD, FRCP, FRCGP, chair of the early diagnosis subgroup within the UK National Cancer Research Institute, Primary Care Section. All-cancer included metastatic disease, carcinoma in situ, and non-melanotic skin cancers. The codes for specific cancers were reviewed by a physician (MCI). The specific cancers included carcinoma in situ, and excluded metastatic disease to those sites. The index date for the case was the earliest date of diagnosis of the respective cancer in the GPRD.
Control Selection
Patients with a first diagnosis of the respective cancer 1995–2008 were risk set matched with up to 10 controls within the same General Practice for age at cohort entry (within two years), sex, and year of entry into the study cohort (within one year). The index date for controls was set as the date at which the follow-up time from cohort entry was the same as the case. The index date was chosen so as to give the control equal follow-up time to that of the case for ascertainment of use of gabapentin. Cases and controls were required to have at least 2 years of follow-up in the study cohort before their index date. For pancreatic and renal cancer, cases and controls could have a prior diagnosis of another cancer. For the analyses of the other cancers (and supplemental analyses of pancreatic and renal cancer), cases were required to be free of other cancers prior to the index date for the specific cancer, and controls were required to be free of any cancer prior to the index date.
Gabapentin exposure
The exposure of interest was gabapentin use as defined by prescriptions recorded by the general practitioner (British National Formulary Codes). Data on gabapentin prescriptions were obtained for cases and controls from cohort entry to the index date. Exposure to gabapentin was assessed as ever/never, number of prescriptions, cumulative duration, and cumulative dose, from cohort entry to 2-years prior to the index date (2-year lagged exposure). The 2-year lagged exposure attempted to account for protopathic bias (gabapentin prescribing for cancer-related pain or worsening of neuropathic or non-neuropathic pain prior to clinical cancer diagnosis) and latency in carcinogenesis (non-etiological exposure to gabapentin in the period between cancer onset and cancer diagnosis). Tertiles of each exposure measure (prescription, cumulative duration, cumulative dose) for the pancreatic and renal analyses were based on the pooled pancreatic and renal controls; tertiles for the additional cancers were based on the all-cancer controls. Explicit indications for each gabapentin prescription were not recorded in the GPRD.
Covariates
The relevant potential confounders were the consistent risk factors for each cancer (defined a priori) as well as the major indications for gabapentin use in the UK (epilepsy, diabetes, neuropathic pain, back pain). Covariates were assessed up to the index date, unless otherwise specified. The following were controlled by either matching or covariate adjustment in all analyses: Age at cohort entry; sex; calendar year of cohort entry; body mass index (BMI); smoking, diabetes, epilepsy, and neuropathic pain. Covariates for pancreatic cancer included diabetes ≥2 years prior to index date and chronic pancreatitis ≥2 years prior to index date (the lag was incorporated since these can be also be early symptoms of neoplastic destruction of pancreatic tissue).8–10 Covariates for renal cancer included history of hypertension and diuretic use.11 Additional covariates for the other cancers were history of back pain and (1) all-cancer: alcohol consumption; (2) breast: previous hysterectomy, current (within 6 months of index date) or previous hormone replacement therapy; benign breast disease; alcohol consumption;12 (2) lung and bronchus: chronic obstructive pulmonary disease (COPD); alcohol consumption; (3) urinary and bladder – no additional covariates;13 (4) stomach: upper gastrointestinal disorders, acid-suppressing drugs up to 6 months before index date, alcohol consumption;14 (5) anus, anal canal, and anorectum: human immunodeficiency virus (HIV) and human papilloma virus (HPV, anogenital warts, condylomas); (6) penis: HIV; HPV (anogenital warts, condylomas); phimosis/balanitis; (7) other nervous system (defined as malignant meningioma, spinal glioblastoma, and malignant tumors of cranial and peripheral nerves) – no additional covariates.
Analytic methods
Conditional logistic regression models for matched case and control data estimated the crude and multivariable adjusted relative risk (estimated by the odds ratio [OR]) for the respective cancer in relation to gabapentin exposure, conditional on the matching factors. Separate analyses were performed for all-cancer and each individual cancer. Four exposure comparisons (ever versus never, number of prescriptions, duration of exposure, and cumulative exposure) were evaluated, with a 2 year lag from the index date. For the more common cancers (all-cancer, lung, and breast), longer exposures were also assessed (>1 y, >2 y, >3 y cumulative duration versus no exposure). The primary analyses were the multivariable adjusted 2-year lagged models. Adjustments were not made for multiple comparisons. Exploratory models with unlagged exposure (gabapentin usage up to the index date) were performed to gain insight into the extent of protopathic bias (reduction in effect estimates with 2-year lagged exposure compared to unlagged exposure) or sample size effects (widening of confidence intervals with lagged exposure compared to unlagged exposure).
For the comparison of highest tertile of exposure to no exposure in the 2-year lagged analysis, the studies were estimated to have an 80% power (2-sided α=0.05) to detect ORs of 1.20 or greater for all-cancer; 1.62 for lung; 1.78 for breast; 2.34 for bladder; 2.60 for kidney, pancreas and stomach; 7.45 for anus; 10.49 for penis; and 23.45 for other CNS. This assumed a 0.6% overall gabepentin exposure rate and UK Office of National Statistics cancer incidence rates.
RESULTS
Kaiser Permanente Northern California
There were 109,879 exposures to gabapentin in the cohort of 6,608,681 subscribers to KPNC.4 Table 1 reports the ORs of all-cancer and the 55 individual cancers for 1-;, 2-, and ≥3 dispensings of gabapentin versus no dispensing in the 2-year lagged analysis. The following analyses, with adjustment for matching variables only, had OR>1.00 and p<0.05 for three or more dispensings of gabapentin versus no dispensing, and were followed up in the GPRD: 1) all-cancer, OR 1.10 (95% CI 1.02–1.18); 2) breast, OR 1.19 (1.01–1.39); 3) lung and bronchus, OR 1.34 (1.13–1.60); 4) urinary bladder, OR1.41 (1.07–1.87); 5) kidney/renal pelvis, OR 1.71 (1.18–2.47); 6) stomach, OR 1.64 (1.01–2.64); 7) anus, anal canal, and anorectum, OR 2.70 (1.17–6.25); 8) penis, OR 6.68 (1.47–30.28); 9) and other nervous system, OR 6.67 (1.11–39.90).
Table 1.
Risk of cancer with 1-, 2-, and ≥3 dispensings of gabapentin versus no dispensing in KPNC
| Cancer Site (alphabetical after “Any Cancer”) | GP exposed cases (N) | OR (95%CI) and p-value for 1-, 2-, and ≥3 dispensings of gabapentin versus no dispensings (2-year lag) | ||
|---|---|---|---|---|
| 1 dispensing vs none | 2 dispensings vs none | ≥3 dispensings vs none | ||
| Any Cancer | 1678 | 1.05 (0.96–1.14) p=0.276 | 1.20 (1.06–1.36) p=0.005 | 1.10 (1.02–1.18) p=0.009** |
| Anus/anal canal/Anorectum | 9 | 0.41 (0.06–3.03) p=0.382 | 1.42 (0.17–11.52) p=0.744 | 2.70 (1.17–6.25) p=0.021* |
| Biliary | 7 | Inestimable | 3.35 (1.06–10.57) p=0.040 | 0.81 (0.25–2.63) p=0.726 |
| Bones/joints | 4 | 4.30 (1.11–16.62) p=0.035 | Inestimable | 1.29 (0.16–10.30) p=0.812 |
| Brain | 21 | 1.84 (0.93–3.65) p=0.079 | 0.87 (0.20–3.73) p=0.851 | 1.16 (0.58–2.32) p=0.673 |
| Breast | 352 | 1.09 (0.90–1.31) p=0.375 | 1.35 (1.03–1.78) p=0.032 | 1.19 (1.01–1.39) p=0.038* |
| Cervix uteri | 6 | 0.60 (0.14–2.50) p=0.481 | 1.41 (0.32–6.22) p=0.647 | 0.49 (0.12–2.01) p=0.320 |
| Colon excl. Rectum | 145 | 1.13 (0.85–1.50) p=0.391 | 1.13 (0.73–1.74) p=0.586 | 0.96 (0.75–1.24) p=0.768 |
| Comb mouth-pharynx1 | 9 | 0.91 (0.28–2.96) p=0.874 | Inestimable | 1.14 (0.49–2.68) p=0.760 |
| Corpus uteri | 36 | 0.39 (0.20–0.76) p=0.006 | 0.60 (0.28–1.29) p=0.189 | 0.64 (0.40–1.00) p=0.052 |
| Esophagus | 17 | 0.82 (0.30–2.28) p=0.707 | 2.67 (0.89–8.05) p=0.081 | 1.24 (0.62–2.48) p=0.550 |
| Eye and orbit | 0 | Inestimable | Inestimable | Inestimable |
| Gallbladder | 3 | 1.84 (0.40–8.46) p=0.433 | Inestimable | 0.55 (0.07–4.17) p=0.563 |
| Heart/soft tissue | 12 | 1.19 (0.42–3.38) p=0.748 | 2.31 (0.66–8.12) p=0.190 | 0.99 (0.39–2.48) p=0.978 |
| Hodgkin lymphoma | 10 | 1.93 (0.66–5.63) p=0.228 | 2.95 (0.61–14.19) p=0.178 | 1.96 (0.67–5.75) p=0.222 |
| Kidney/renal pelvis | 70 | 1.20 (0.75–1.94) p=0.450 | 2.48 (1.46–4.24) p=0.001 | 1.71 (1.18–2.47) p=0.004* |
| Larynx | 10 | 1.35 (0.53–3.46) p=0.531 | 2.71 (0.76–9.72) p=0.126 | 0.49 (0.12–2.05) p=0.331 |
| Lip | 8 | 1.11 (0.39–3.15) p=0.841 | 0.67 (0.09–5.08) p=0.695 | 1.11 (0.34–3.69) p=0.861 |
| Liver/intrahep.bile duct | 36 | 2.19 (1.29–3.72) p=0.004 | 0.69 (0.17–2.90) p=0.614 | 1.65 (0.98–2.77) p=0.058 |
| Lung and bronchus | 269 | 0.87 (0.68–1.11) p=0.250 | 1.50 (1.11–2.02) p=0.008 | 1.34 (1.13–1.60) p=0.001** |
| Lymphocytic leukemia | 19 | 1.48 (0.67–3.29) p=0.337 | 1.68 (0.58–4.85) p=0.335 | 1.14 (0.55–2.39) p=0.727 |
| Melanoma | 73 | 0.97 (0.63–1.51) p=0.896 | 1.15 (0.62–2.14) p=0.665 | 1.11 (0.80–1.54) p=0.534 |
| Monocytic leukemia | 0 | Inestimable | Inestimable | Inestimable |
| Multiple myeloma | 27 | 1.46 (0.81–2.63) p=0.211 | 0.32 (0.04–2.30) p=0.255 | 1.11 (0.62–1.97) p=0.730 |
| Myeloid leukemia | 16 | 0.48 (0.15–1.53) p=0.216 | 0.54 (0.13–2.26) p=0.399 | 1.64 (0.86–3.12) p=0.134 |
| Nasopharynx | 0 | Inestimable | Inestimable | Inestimable |
| Non-hodgkin lymphoma | 87 | 1.17 (0.82–1.69) p=0.391 | 1.06 (0.57–1.98) p=0.848 | 1.16 (0.85–1.60) p=0.356 |
| Nose/nasal cavity/middle ear | 2 | 1.01 (0.13–7.88) p=0.994 | Inestimable | 1.43 (0.18–11.62) p=0.739 |
| Other digestive organs | 6 | 2.86 (0.59–13.75) p=0.190 | 6.67 (1.11–39.90) p=0.038 | 4.33 (0.78–24.07) p=0.094 |
| Other endocrine | 1 | Inestimable | Inestimable | 2.50 (0.28–22.37) p=0.413 |
| Other female genital | 1 | Inestimable | Inestimable | 1.24 (0.15–9.88) p=0.842 |
| Other leukemia | 3 | Inestimable | Inestimable | 1.86 (0.54–6.44) p=0.33 |
| Other male genital | 1 | 3.33 (0.35–32.05) p=0.297 | Inestimable | Inestimable |
| Other nervous system | 2 | Inestimable | Inestimable | 6.67 (1.11–39.90) p=0.038* |
| Other skin | 11 | 2.29 (0.92–5.66) p=0.074 | Inestimable | 1.49 (0.57–3.85) p=0.416 |
| Other urinary | 1 | Inestimable | Inestimable | Inestimable |
| Ovary | 23 | 0.94 (0.49–1.80) p=0.849 | 0.62 (0.19–2.00) p=0.426 | 0.77 (0.40–1.46) p=0.417 |
| Pancreas | 46 | 0.74 (0.41–1.34) p=0.318 | 0.24 (0.06–0.96) p=0.043 | 1.23 (0.85–1.78) p=0.282 |
| Penis | 3 | Inestimable | Inestimable | 6.68 (1.47–30.28) p=0.014* |
| Peritoneum/Omentum/Mesentery | 7 | 2.86 (0.76–10.7) p=0.119 | Inestimable | 1.68 (0.58–4.91) p=0.341 |
| Pleura | 1 | Inestimable | Inestimable | Inestimable |
| Prostate | 246 | 1.10 (0.89–1.36) p=0.379 | 1.03 (0.74–1.43) p=0.855 | 0.78 (0.64–0.94) p=0.011† |
| Rectum/Rectosigmoid | 30 | 0.70 (0.39–1.26) p=0.236 | 1.03 (0.52–2.06) p=0.925 | 0.41 (0.21–0.80) p=0.009†† |
| Retroperito-neum | 1 | 1.64 (0.19–14.30) p=0.652 | Inestimable | Inestimable |
| Salivary glands | 8 | 2.55 (0.72–9.06) p=0.147 | Inestimable | 1.67 (0.64–4.36) p=0.298 |
| Small intestine | 8 | 0.82 (0.19–3.48) p=0.789 | 3.00 (0.82–10.88) p=0.096 | 2.05 (0.58–7.33) p=0.268 |
| Stomach | 27 | 0.65 (0.30–1.41) p=0.276 | Inestimable | 1.64 (1.01–2.64) p=0.045* |
| Testis | 3 | Inestimable | Inestimable | 1.82 (0.40–8.20) p=0.437 |
| Thyroid | 26 | 1.06 (0.46–2.48) p=0.886 | 2.16 (0.90–5.22) p=0.087 | 1.51 (0.86–2.66) p=0.155 |
| Trachea | 0 | Inestimable | Inestimable | Inestimable |
| Unspecified | 75 | 1.65 (1.12–2.42) p=0.011 | 1.55 (0.86–2.78) p=0.143 | 1.20 (0.82–1.74) p=0.349 |
| Ureter | 4 | 2.02 (0.44–9.22) p=0.366 | 3.40 (0.35–32.69) p=0.290 | 0.74 (0.10–5.68) p=0.771 |
| Urinary bladder | 100 | 1.20 (0.84–1.71) p=0.309 | 0.70 (0.34–1.43) p=0.325 | 1.41 (1.07–1.87) p=0.015* |
| Uterus | 2 | 4.83 (0.44–53.25) p=0.199 | 5.00 (0.45–55.14) p=0.189 | Inestimable |
| Vagina | 3 | 1.70 (0.20–14.12) p=0.624 | Inestimable | 2.49 (0.53–11.73) p=0.249 |
| Vulva | 3 | 0.54 (0.07–4.14) p=0.554 | Inestimable | 1.11 (0.25–4.84) p=0.895 |
KPNC, Kaiser Permanente Northern California; GP, gabapentin; OR, odds ratio; CI, confidence interval; vs, versus; excl, excluding; intrahep, intrahepatic; comb, combined.
Inestimable – no gabapentin-exposed cancer cases or no gabapentin-exposed non-cases at the exposure level, p > 0.97.
Excludes lip, salivary glands and nasopharynx
p<.05, OR>1 for ≥3 dispensings vs no dispensings
p<.01, OR>1 for ≥3 dispensings vs no dispensings
p<.05, OR<1 for ≥3 dispensings vs no dispensings
p<.01, OR<1 for ≥3 dispensings vs no dispensings
General Practice Research Database
Pancreatic Cancer
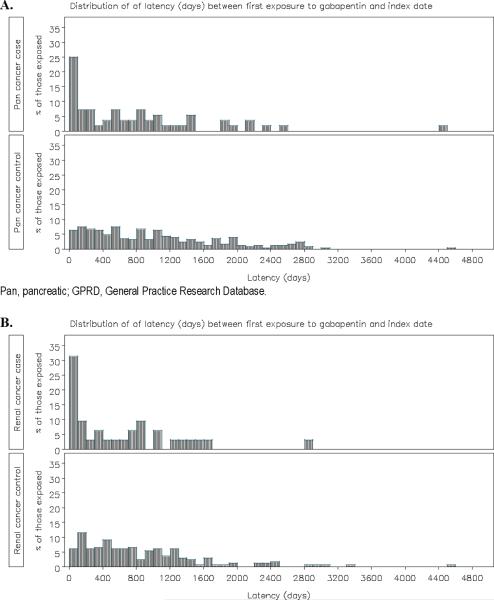
3,149 pancreatic cancer cases were risk set matched to 30,026 controls, with mean duration of follow up from cohort entry of 9.0–9.1 years (Table 2). 1.8% (n=56) of pancreatic cancer cases were exposed to gabapentin, relative to 0.8% (n=253) of the controls. The distribution of time from first gabapentin prescription to index date for the gabapentin-exposed pancreatic cancer cases and controls demonstrates that the first gabapentin prescription in the cancer cases was skewed toward exposure within the 100 days prior to the specific diagnosis code for cancer (Figure 1). In the 2-year lagged exposure window, 0.8% (n=24) of pancreatic cancer cases were exposed to gabapentin, relative to 0.5% (n=143) of the controls (Supplemental Table 2). The OR of pancreatic cancer with gabapentin exposure was sensitive to modeling assumptions (Table 3). Although ever exposure was associated with a significantly increased risk of pancreatic cancer in the unlagged analysis (multivariable adjusted OR 1.82 [95% CI 1.34, 2.46]), the OR was attenuated and not significant in the 2-year lagged exposure analysis (adjusted OR 1.33 [95% CI 0.85, 2.08])(Supplemental Table 3). There was no evidence of a positive dose-response relationship with increasing number of prescriptions, duration of exposure, or cumulative dose; point estimates were consistently lower for the highest exposure tertiles (adjusted ORs 0.87–1.17 relative to no exposure) than the lowest exposure tertiles (adjusted ORs 1.95–2.88), even in the 2-year lagged analysis.
Table 2.
Characteristics of pancreatic cancer cases and controls in the GPRD
| Characteristic | Level | Cases (N) N=3,149 | Cases (%) | Controls (N)4 N=30,026 | Controls (%) |
|---|---|---|---|---|---|
| Age at Index Date (y) | Mean (SD) | 71.9 (11.51) | 71.5 (11.47) | ||
| Gender | Female | 1,603 | 50.9 | 15,316 | 51.0 |
| Male | 1,546 | 49.1 | 14,710 | 49.0 | |
| Duration of follow-up from registration to index date (y) | Mean (SD) | 21.2 (15.38) | 21.9 (15.33) | ||
| Duration of follow-up from cohort entry to index date (y)3 | Mean (SD) | 9.0 (3.90) | 9.1 (3.90) | ||
| Body mass index (kg/m2)1 | Mean (SD) | 26.8 (4.97) | 27.0 (4.94) | ||
| BMI: Missing | 1,917 | 60.9 | 19,419 | 64.7 | |
| Smoking Status | Current smoker | 731 | 23.2 | 4,857 | 16.2 |
| Ex-smoker | 768 | 24.4 | 6,626 | 22.1 | |
| Never smoked | 1,385 | 44.0 | 15,249 | 50.8 | |
| Unknown | 265 | 8.4 | 3,294 | 11.0 | |
| Comorbidities | Diabetes2 | 363 | 11.5 | 2,137 | 7.1 |
| Epilepsy | 53 | 1.7 | 458 | 1.5 | |
| Neuropathic Pain | 737 | 23.4 | 6,616 | 22.0 | |
| Chronic Pancreatitis2 | 13 | 0.4 | 17 | 0.1 |
y, year; SD, standard deviation; BMI, body mass index; GPRD, General Practice Research Database.
1–3 years prior to index date.
2 years prior to index date.
Entry into the study cohort began Jan 1,1993 for all those who were registered in GPRD before that time, and at the time of registration if later than Jan 1,1993.
NOTE: 2,868 (91.1%) of the 3,149 matched cases were 1:10 matched; 181 (5.7%) were 1:4 to 1:9 matched; 100 (3.2%) were 1:1 to 1:3 matched. N= 12 of the GPRD pancreatic cancer cases were unable to be matched.
Figure 1.
Distribution of time from first gabapentin prescription (in days) to index date in gabapentin-exposed pancreatic cancer (A) and renal cancer (B) cases and controls in the GPRD
Table 3.
Risk of pancreatic cancer with gabapentin exposure in the GPRD
| Exposure Model | Without 2 year lag | With 2 year lag | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)1 | Unadjusted OR (95% CI) | Adjusted OR (95% CI)1 | |
| Gabapentin (Ever vs Never) | 2.04 (1.51, 2.75) | 1.82 (1.34, 2.46) | 1.52 (0.98, 2.36) | 1.33 (0.85, 2.08) |
| Gabapentin Prescriptions 2,3 | ||||
| Tertile1 vs Never | 2.82 (1.86, 4.28) | 2.53 (1.66, 3.85) | 2.75 (1.51, 5.03) | 2.44 (1.32, 4.49) |
| Tertile2 vs Never | 1.80 (0.97, 3.36) | 1.65 (0.87, 3.11) | 0.55 (0.17, 1.78) | 0.48 (0.15, 1.57) |
| Tertile3 vs Never | 1.35 (0.76, 2.42) | 1.17 (0.65, 2.11) | 1.31 (0.58, 2.97) | 1.11 (0.48, 2.57) |
| Duration of exposure (mo) 4,5 | ||||
| Tertile1 vs Never | 3.15 (2.04, 4.86) | 2.88 (1.85, 4.46) | 2.75 (1.47, 5.13) | 2.45 (1.31, 4.62) |
| Tertile2 vs Never | 1.68 (0.94, 2.99) | 1.50 (0.83, 2.69) | 0.82 (0.29, 2.28) | 0.72 (0.26, 2.01) |
| Tertile3 vs Never | 1.34 (0.75, 2.39) | 1.15 (0.64, 2.07) | 1.11 (0.49, 2.51) | 0.94 (0.41, 2.15) |
| Cumulative Exposure (g) 6,77 | ||||
| Tertile1 vs Never | 2.90 (1.88, 4.47) | 2.65 (1.71, 4.11) | 2.19 (1.12, 4.25) | 1.95 (0.99, 3.81) |
| Tertile2 vs Never | 1.87 (1.05, 3.32) | 1.64 (0.92, 2.95) | 1.42 (0.64, 3.16) | 1.21 (0.54, 2.72) |
| Tertile3 vs Never | 1.34 (0.75, 2.39) | 1.15 (0.64, 2.08) | 1.01 (0.42, 2.41) | 0.87 (0.36, 2.12) |
OR, odds ratio; CI, confidence interval; vs, versus; mo, months; g, grams; GPRD, General Practice Research Database.
Adjusted for smoking, body mass index, diabetes, chronic pancreatitis, neuropathic pain, epilepsy
The tertiles for number of prescriptions in the analysis without 2 year lag are: Tertile 1 (1–2). Tertile 2 (3–8). Tertile3 (9–218).
The tertiles for number of prescriptions in the analysis with 2 year lag are: Tertile 1 (1–2). Tertile 2 (3–10). Tertile 3(11–191).
The tertiles for cumulative duration in the analysis without 2 year lag are: Tertile 1 (0.01 – 1.55 mo). Tertile 2 (1.56 – 6.44 mo). Tertile 3 (6.45 – 78.36 mo).
The tertiles for cumulative duration in the analysis with 2 year lag are: Tertile 1 (0.01 – 1.78 mo). Tertile 2 (1.79 – 7.20 mo). Tertile 3 (7.21 – 64.13 mo)
The tertiles for cumulative dose in the analysis without 2 year laq are: Tertile 1 (0.01 – 33.6 g). Tertile 2 (33.7 – 185.0 g). Tertile 3 (185.1 – 7500.2 g).
The tertiles for cumulative dose in the analysis with 2 year lag are: Tertile 1 (0.01 – 39.0 g) Tertile 2 (39.1 – 210.0 g). Tertile 3 (210.1 – 5623.8 g).
Renal Cancer
1,981 renal cancer cases were risk set matched to 19,046 controls, with mean duration of follow up from cohort entry of 9.4–9.5 years (Table 4). 1.6% (n=32) of renal cancer cases were exposed to gabapentin, relative to 0.9% (n=166) of the controls. Similar to the gabapentin-exposed pancreatic cancer cases, the distribution of gabapentin exposure was skewed toward exposure within the 100 days prior to the specific diagnosis code for renal cancer (Figure 1). In the 2-year lagged exposure window, 0.7% (n=13) of renal cancer cases were exposed to gabapentin, relative to 0.4% (n=76) of the controls (Supplemental Table 4). The OR of renal cancer with gabapentin exposure was sensitive to modeling assumptions, particularly given the low numbers of gabapentin exposed cases and controls in the tertile analyses (Table 5). Ever exposure was associated with a significantly increased risk of renal cancer in the unlagged analysis (adjusted OR 1.68 [95% CI 1.13, 2.49]); the point estimate was similar in the 2-year lagged analysis (adjusted OR 1.63 [0.89, 2.97])(Supplemental Table 5). Dose-response estimates were limited by the low numbers of exposed cases, especially in the 2-year lagged exposure analyses.
Table 4.
Descriptive characteristics of renal cancer cases and controls in the GPRD
| Characteristic | Level | Cases (N) N=1,981 | Cases (%) | Controls (N)3 N=19,046 | Controls (%) |
|---|---|---|---|---|---|
| Age at Index Date (y) | Mean (SD) | 67.1 (12.68) | 66.7 (12.67) | ||
| Gender | Female | 759 | 38.3 | 7,273 | 38.2 |
| Male | 1,222 | 61.7 | 11,773 | 61.8 | |
| Duration of follow-up from registration to index date (y) | Mean (SD) | 21.3 (15.44) | 21.3 (14.91) | ||
| Duration of follow-up from cohort entry to i ndex date (y)2 | Mean (SD) | 9.4 (4.10) | 9.5 (4.09) | ||
| Body mass index (kg/m2)1 | Mean (SD) | 27.8 (5.29) | 27.3 (4.92) | ||
| BMI: Missing | 1,150 | 58.1 | 11,989 | 62.9 | |
| Smoking Status | Current smoker | 476 | 24.0 | 3,555 | 18.7 |
| Ex-smoker | 524 | 26.5 | 4,589 | 24.1 | |
| Never smoker | 867 | 43.8 | 9,121 | 47.9 | |
| Unknown | 114 | 5.8 | 1,781 | 9.4 | |
| Comorbidities | Hypertension | 1,159 | 58.5 | 9,113 | 47.8 |
| Diuretic use | 875 | 44.2 | 6,396 | 33.6 | |
| Diabetes | 240 | 12.1 | 1,598 | 8.4 | |
| Epilepsy | 33 | 1.7 | 303 | 1.6 | |
| Neuropathic Pain | 428 | 21.6 | 4,018 | 21.1 |
y, year; SD, standard deviation; BMI, body mass index; GPRD, General Practice Research Database.
1–3 years prior to index date.
Entry into the study cohort began Jan 1, 1993 for all those who were registered in GPRD before that time, and at the time of registration if later than Jan 1, 1993.
NOTE: 1,821 (91.9%) of the 1,981 matched cases were 1:10 matched; 107 (5.4%) were 1:4 to 1:9 matched; 53 (2.7%) were 1:1 to 1:3 matched. N=7 of the overall renal cancer cases were unable to be matched.
Table 5.
Relative risk of renal cancer with gabapentin exposure in the GPRD
| Exposure Model | Without 2 year lag | With 2 year lag | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)1 | Unadjusted OR (95% CI) p-value | Adjusted OR (95% CI)1 | |
| Gabapentin (Evervs Never) | 1.79 (1.22, 2.63) | 1.68 (1.13, 2.49) | 1.65 (0.91, 2.99) | 1.63 (0.89, 2.97) |
| Gabapentin Prescriptions2,3 | ||||
| Tertile1 vs Never | 2.53 (1.44, 4.44) | 2.34 (1.32, 4.14) | 2.18 (0.90, 5.32) | 2.14 (0.87, 5.28) |
| Tertile2 vs Never | 1.89 (0.98, 3.63) | 1.77 (0.92, 3.43) | 1.40 (0.49, 4.01) | 1.39 (0.48, 4.02) |
| Tertile3 vs Never | 0.87 (0.35, 2.19) | 0.83 (0.33, 2.11) | 1.32 (0.39, 4.47) | 1.30 (0.38, 4.47) |
| Duration of exposure (mo)4,5 | ||||
| Tertile1 vs Never | 3.24 (1.79, 5.85) | 2.90 (1.59, 5.28) | 2.54 (1.03, 6.25) | 2.40 (0.96, 5.98) |
| Tertile2 vs Never | 1.35 (0.69, 2.65) | 1.33 (0.68, 2.63) | 0.95 (0.29, 3.12) | 0.99 (0.30, 3.29) |
| Tertile3 vs Never | 1.19 (0.54, 2.63) | 1.10 (0.50, 2.45) | 1.70 (0.58, 4.96) | 1.63 (0.55, 4.84) |
| Cumulative Exposure (g) | ||||
| Tertile1 vs Never | 1.89 (0.92, 3.88) | 1.75 (0.85, 3.63) | 2.03 (0.78, 5.34) | 2.05 (0.77, 5.46) |
| Tertile2 vs Never | 2.44 (1.42, 4.22) | 2.34 (1.35, 4.07) | 1.78 (0.68, 4.69) | 1.70 (0.64, 4.53) |
| Tertile3 vs Never | 0.99 (0.42, 2.29) | 0.91 (0.39, 2.13) | 1.15 (0.35, 3.82) | 1.15 (0.34, 3.86) |
OR, odds ratio; CI, confidence interval; vs, versus; mo, months; g, grams; GPRD, General Practice Research Database.
Adjusted for smoking, body mass index, hypertension, diuretic use, diabetes, neuropathic pain, and epilepsy.
The tertiles for number of prescriptions in the analysis without 2 vearlaq are: Tertile 1 (1–2). Tertile 2 (3–8). Tertile 3. (9–218).
The tertiles for number of prescriptions in the analysis with 2 yearlag are: Tertile 1 (1–2). Tertile 2 (3–10). Tertile 3 (11–191).
The tertiles for cumulative duration in the analysis without 2 yearlag are: Tertile 1 (0.01 – 1.55 mo). Tertile 2 (1.56 – 6.44 mo). Tertile 3 (6.45 – 78.36 mo).
The tertiles for cumulative duration in the analysis with 2 year lag are: Tertile 1 (0.01 – 1.78 mo). Tertile 2 (1.79 – 7.20 mo). Tertile 3 (7.21 – 64.13 mo).
The tertiles for cumulative dose in the analysis without 2 yearlag are: Tertile 1 (0.01 – 33.6g). Tertile 2 (33.7 – 185.0 g). Tertile 3 (185.1 – 7500.2 g).
The tertiles for cumulative dose in the analysis with 2 year lag are: Tertile 1 (0.01 – 39.0g) Tertile 2 (39.1 – 210.0 g). Tertile 3 (210.1 – 5623.8 g).
Other Cancers
The additional cancers in the KPNC study nominally (p<0.05) associated with gabapentin were not statistically significantly associated with gabapentin in the GPRD case-control studies. The results based on ever/never exposure and tertiles of duration of exposure (2-year lag) are shown in Table 6; the results were similar for tertiles of number of prescriptions and of cumulative dose (supplementary results available at GSK Clinical Study Register15 and Supplemental Tables 6–15). There was a trend (p=0.054) for association of the highest tertile of gabapentin cumulative duration (>5.56 months 2-year lagged exposure) with breast cancer (OR 1.49, 95% CI 0.99–2.04). Longer cumulative durations of gabapentin exposure were assessed (>1 y, >2 y, or >3 y) for the more common cancers—all cancer, lung, and breast; long durations of gabapentin exposure were not significantly associated with increased risk of these cancers. The results for breast cancer were OR 1.48 (95% CI 0.92, 2.39) for >1y duration, OR 1.62 (95% CI 0.79, 3.29) for >2y duration, and OR 0.76 (95% CI 0.18, 3.21) for >3y duration.
Table 6.
OR of cancer with gabapentin exposure versus no exposure in the GPRD
| Cancer | Cases, N Age, y (SD) GP exposed, N (%) | Controls, N Age, y (SD) GP exposed, N (%) | Adjusted OR (95% CI), for cancer based on cumulative duration of gabapentin exposure relative to never exposure to gabapentin (2-year lagged analysis) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ever vs Never | Tertile 1 (0.01–1.38 mo) vs Never | Tertile 2 (1.39–5.56 mo) vs Never | Tertile 3 (>5.56 mo) vs Never | >1 y vs Never | >2 y vs Never | >3 y vs Never | |||
| All cancers1,2 | 179,138 66.3 (15.12) 804 (0.4%) |
1,710,950 65.8 (15.07) 6,507 (0.4%) |
1.07 (0.99,1.15) | 1.03 (0.90,1.17) | 1.10 (0.96,1.25) | 1.07 (0.95,1.22) | 1.09 (0.92,1.29) | 1.16 (0.89,1.52) | 1.05 (0.69,1.60) |
| Breast1,2,3 | 19,564 61.9 (14.04) 96 (0.5%) |
188,924 61.4 (13.87) 725 (0.4%) |
1.22 (0.99, 1.52) | 1.08 (0.73, 1.59) | 1.19 (0.82, 1.72) | 1.42 (0.99, 2.04) | 1.48 (0.92, 2.39) | 1.62 (0.79, 3.29) | 0.76 (0.18, 3.21) |
| Lung and bronchus1,2,4 | 10,855 70.8 (10.43) 55 (0.5%) |
102,836 70.4 (10.38) 402 (0.4%) |
0.94 (0.69, 1.27) | 1.05 (0.64, 1.73) | 0.93 (0.52, 1.66) | 0.84 (0.50, 1.39) | 0.79 (0.39, 1.58) | 1.13 (0.37, 3.43) | 1.87 (0.21, 16.86) |
| Urinary Bladder1 | 4,600 71.0 (11.59) 15 (0.3%) |
43,559 70.6 (11.52) 144 (0.3%) |
0.84 (0.49, 1.44) | 0.80 (0.32, 2.05) | 0.67 (0.24, 1.85) | 1.06 (0.44, 2.52) | |||
| Stomach1,2,5 | 1,877 73.5 (11.19) 5 (0.3%) |
17,853 73.0 (11.06) 70 (0.4%) |
0.54 (0.21, 1.34) | 0.00 (no exposed cases) | 1.07 (0.24, 4.84) | 0.70 (0.21, 2.32) | |||
| Anus, anal canal, anorectum1,6 | 212 65.1 (15.20) 4 (1.9%) |
2,067 64.5 (14.86) 6 (0.3%) |
3.39 (0.71, 16.17) | 21.47 (1.78, 258.8) | 1.00 (0.03, 32.21) | 0.93 (0.06, 13.87) | |||
| Penis1,6,7 | 148 63.6 (14.26) 1 (0.7%) |
1,396 62.9 (14.04) 2 (0.1%) |
2.90 (0.24, 34.69) | 4.63 (0.28, 77.51) | Inestimable (no exposed cases or controls) | 0.00 (no exposed cases) | |||
| Other Nervous System Cancers1 | 38 58.5 (19.22) 0 (0%) |
380 58.5 (19.08) 0 (0%) |
Inestimable (no exposed cases or controls) | Inestimable (no exposed cases or controls) | Inestimable (no exposed cases or controls) | Inestimable (no exposed cases or controls) | |||
| SUPPLEMENTARY ANALYSES | |||||||||
| Pancreas1,8 | 2,155 71.6 (11.52) 20 (0.9%) |
20,382 71.1 (11.47) 86 (0.4%) |
1.68 (1.00, 2.82) | 3.25 (1.55, 6.83) | 0.61 (0.14, 2.62) | 1.32 (0.54, 3.21) | |||
| Renal and renal pelvis1,9 | 1,272 65.8 (12.60) 9 (0.7%) |
12,167 65.4 (12.59) 53 (0.4%) |
1.33 (0.64, 2.75) | 1.73 (0.49, 6.15) | 1.10 (0.32, 3.75) | 1.29 (0.37, 4.58) | |||
GP, gabapentin; mo, month; y, year; vs, versus; SD, standard deviation; OR, odds ratio; CI, confidence interval; GPRD, General Practice Research Database.
Adjusted for body mass index, smoking, diabetes, epilepsy, neuropathic pain, back pain
Additionally adjusted for alcohol consumption
Additionally adjusted for previous hysterectomy, current or previous hormone replacement therapy, and benign breast disease
Additionally adjusted for chronic obstructive pulmonary disease
Additionally adjusted for upper gastrointestinal disorders and acid-suppressing drugs up to 6 months before index date
Additionally adjusted for human immunodeficiency virus and human papilloma virus (anogenital warts, condylomas)
Additionally adjusted for phimosis/balanitis
Additionally adjusted for chronic pancreatitis > 2 years prior to index date
Additionally adjusted for hypertension and diuretic use
Supplemental analyses of pancreatic and renal cancer were reported for comparability (Table 6). The numbers of incident pancreatic and renal cancer cases were reduced by approximately 1/3 after excluding those with prior history of other cancers or non-specific cancer codes before their index date. Ever exposure to gabapentin (2-year lag) was marginally associated with an increased risk of pancreatic cancer (adjusted OR 1.68 [95% CI 1.00, 2.82] relative to no exposure). The highest tertiles of gabapentin exposure were not significantly associated with risk of pancreatic cancer (point estimates of OR 1.00–1.36 relative to no exposure). Ever exposure to gabapentin and the highest tertiles of exposure were not significantly associated with renal cancer.
DISCUSSION
Gabapentin was approved in the US and UK in 1993. Despite being prescribed for epilepsy and pain, the exposure rate in the general population is low (<1%).4 Therefore, large population-based studies are required to accrue sufficient exposure and outcomes for safety studies, especially for rare outcomes such as pancreatic cancer. The GPRD and KPNC cohorts are well suited to study gabapentin and carcinogenicity. These data sources include large numbers of drug recipients with complete cancer follow-up, often long-term, in a comprehensive health care system. They contain objective data on the prescribing or dispensing of prescriptions, without recall bias.
Pancreatic and Renal Cancer
The relationship between gabapentin and pancreatic and renal cancer was sensitive to modeling assumptions. Increased risks of cancer were observed with lower exposures, or shorter duration of exposures, and closer to the index diagnosis of cancer. Relative risks were attenuated toward unity with the lag analysis, and there was no evidence of a dose response relationship with increasing numbers of prescriptions, cumulative dose, or duration of use. The pattern of increasing OR for cancer with very short gabapentin exposures (<2 months), lack of a positive dose response, and decreasing OR with longer exposures is not a pattern consistent with a mutagenic or non-mutagenic carcinogen.16 The decreasing ORs with lagged analysis and with increasing exposure suggests that protopathic bias is inflating the OR.17, 18 Protopathic bias (reverse causation) occurs when a pharmaceutical agent is prescribed for an early manifestation of a disease that has not yet been diagnostically detected or formally recorded in the medical record.19, 20 Elements of protopathic bias were observed in studies of proton pump inhibitors and H2-antagonists with risk of gastric cancer, and of acetaminophen with cancer mortality.17, 18, 21 In these examples, elevations in cancer risk diminished with duration of follow-up, returning to baseline with long-term use of the drug.
A plausible explanation is that individuals are prescribed gabapentin for cancer-related pain or worsening of other non-neuropathic or neuropathic pain in the context of pancreatic cancer, prior to the specific cancer diagnosis being recorded in the GPRD. Gabapentin is considered an adjunctive treatment for cancer pain in the World Health Organization (WHO) analgesic ladder.2 Indeed, the first gabapentin prescription in the cancer cases was skewed toward exposure within the 100 days prior to the specific diagnosis code for cancer (Figure 1). Excluding exposure in the time period before the index date (lagged exposure) can control for protopathic bias, but misspecification of the lag period may result in spurious association from residual protopathic bias, or reduce power for detecting associations. A 2-year lagged exposure was incorporated in all analyses a priori based on recommendations for addressing protopathic bias for drugs that may be used to treat early symptoms of cancer, and for comparability of the results across cancers in GPRD and KPNC;6 however, the optimal specification of the lag period is likely to vary across cancer types based on different symptoms, pain characteristics, time courses, and treatment patterns.18 For instance, retroperitoneal cancers such as pancreatic or renal may be more likely to present as back pain that would serve as the protopathic stimulus for gabapentin treatment.22
Taken together, the GPRD and KPNC studies did not identify a statistically significant increased risk of pancreatic cancer with >2 prescriptions of gabapentin in 2-year lagged analyses.
Other cancers
The additional cancers in the KPNC study nominally associated with gabapentin (p<0.05) were not statistically significantly associated with gabapentin in the 2-year lag analysis of the GPRD case-control studies. There was a suggestion of association between gabapentin and breast cancer based on the OR point estimates. In the KPNC, the OR of breast cancer for ≥3 dispensings versus no dispensing of gabapentin was 1.19 (1.01–1.39), p=0.03. In the GPRD, the adjusted OR of breast cancer for 3–10 prescriptions versus no prescriptions was OR 1.17 (0.76, 1.81), p = 0.47; for >10 prescriptions the OR was 1.36 (0.95, 1.96) p = 0.09.
Given the low overall gabapentin exposure frequency, statistical power was limited for the evaluation of the effect of long durations or very high cumulative exposures, especially for the less common cancers such as anal cancer, penile cancer, and other nervous system cancers. Nonetheless, ever exposure to gabapentin was not statistically significantly associated with these less common cancers.
Strengths and limitations
The study should be interpreted in the context of its strengths and limitations. Many strengths of the study are methodological, including the nesting of the case-control study within a population-based cohort of patients in KPNC and the GPRD. In KPNC, occurrence of cancer was ascertained through KPNC's Cancer Registry, which covers all subscribers and contributes to the local Surveillance, Epidemiology, and End Results (SEER) program. The accuracy of clinical data and cancer diagnoses was good in previous validation studies in the GPRD.23–25 However, the index date of cancer based on the record of a specific diagnostic code by the general practitioner generally occurs after cancer symptoms, and may also be recorded after diagnosis by a specialist. This raises the possibility of protopathic bias—gabapentin prescription for cancer-related pain prior to the formal diagnosis in the GPRD.2
Imprecision in the measurement of potential confounders and unmeasured confounders (e.g. alcohol use) may result in residual confounding; this includes missing data in BMI and smoking, and the parameterization of smoking status, hypertension, and diuretic usage. Given the low overall gabapentin exposure frequency, statistical power was limited for the evaluation of the effect of long durations or very high cumulative exposures. Follow-up may not be long enough to detect a carcinogenic effect with a latency of more than a few years. Misclassification of exposure was possible for analyses of cumulative duration or cumulative dosage given missing data on daily dose or quantity dispensed; misclassification was likely to be non-differential with respect to cases and controls, and could weaken our ability to detect associations.
Conclusion
Studies in two large healthcare systems—KPNC in the US with up to 12 years of follow-up and GPRD in the UK with up to 15 years of follow-up—do not support a statistically significant association of >2 prescriptions of gabapentin and pancreatic cancer in the 2-year lag analyses. Furthermore, the nominal gabapentin associations with other cancers in the KPNC study were not statistically significant in the GPRD study in the 2-year lag analyses. However, the confidence intervals for some analyses were wide, and an important effect cannot be confidently excluded.
Supplementary Material
Key Points
Studies in two large healthcare systems—Kaiser Permanente Northern California (KPNC) in the United States and the General Practice Research Database (GPRD) in the United Kingdom—do not support a statistically significant association between gabapentin exposure (>2 prescriptions with a 2-year lag) and pancreatic cancer.
Nominal gabapentin associations with other cancers in KPNC were not statistically significant in the GPRD.
Evidence of protopathic bias (gabapentin prescribing for cancer-related pain prior to the formal diagnostic record of cancer) was observed for pancreatic and renal cancer in the GPRD.
Acknowledgments
Sponsors/Acknowledgments The KPNC study was supported by Grant R01 098838 from the National Cancer Institute to Dr. Habel. The GPRD study was performed by GlaxoSmithKline. The code list for all-cancer was derived from clinical review of all cancer, neoplasm, and oncology related READ/OXMIS codes by William T. Hamilton, MD, FRCP, FRCGP, chair of the early diagnosis subgroup within the UK National Cancer Research Institute, Primary Care Section. The clinical review was supported by GlaxoSmithKline.
Footnotes
Conflicts of Interest M Irizarry, D Webb, N Boudiaf, and J Logie are stock-holding employees of GlaxoSmithKline. GSK is developing gabapentin enacarbil, a transported prodrug of gabapentin, for treatment of restless legs syndrome.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm. 2003;9(6):559–568. doi: 10.18553/jmcp.2003.9.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raphael J, Ahmedzai S, Hester J, et al. Cancer pain: part 1: Pathophysiology; oncological, pharmacological, and psychological treatments: a perspective from the British Pain Society endorsed by the UK Association of Palliative Medicine and the Royal College of General Practitioners. Pain Med. 2010;11(5):742–764. doi: 10.1111/j.1526-4637.2010.00840.x. [DOI] [PubMed] [Google Scholar]
- 3.Sigler RE, Gough AW, de la Iglesia FA. Pancreatic acinar cell neoplasia in male Wistar rats following 2 years of gabapentin exposure. Toxicology. 1995;98(1–3):73–82. doi: 10.1016/0300-483x(94)02966-x. [DOI] [PubMed] [Google Scholar]
- 4.Friedman GD, Udaltsova N, Chan J, Quesenberry CP, Jr., Habel LA. Screening pharmaceuticals for possible carcinogenic effects: initial positive results for drugs not previously screened. Cancer Causes Control. 2009;20:1821–1835. doi: 10.1007/s10552-009-9375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute . SEER Program Coding and Staging Manual. National Cancer Institute; Bethesda, MD: 2007. [Google Scholar]
- 6.Habel LA, Friedman GD. Pharmaceuticals Other than Hormones. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. 2nd ed Oxford Univ. Press; Oxford: 1996. pp. 489–506. [Google Scholar]
- 7.Lawson DH, Sherman V, Hollowell J. The General Practice Research Database. Scientific and Ethical Advisory Group. QJM. 1998;91(6):445–452. doi: 10.1093/qjmed/91.6.445. [DOI] [PubMed] [Google Scholar]
- 8.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6(12):699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 9.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134(1):95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipworth L, Tarone RE, Lund L, McLaughlin JK. Epidemiologic characteristics and risk factors for renal cell cancer. Clin Epidemiol. 2009;1:33–43. doi: 10.2147/clep.s4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaye JA, Meier CR, Walker AM, Jick H. Statin use, hyperlipidaemia, and the risk of breast cancer. Br J Cancer. 2002;86(9):1436–1439. doi: 10.1038/sj.bjc.6600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye JA, Myers MW, Jick H. Acetaminophen and the risk of renal and bladder cancer in the general practice research database. Epidemiology. 2001;12(6):690–694. doi: 10.1097/00001648-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Lindblad M, Lagergren J, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):444–450. doi: 10.1158/1055-9965.EPI-04-0467. [DOI] [PubMed] [Google Scholar]
- 15.Risk of cancer in patients exposed to gabapentin in the GPRD. GSK Clinical Trial Register. 2011 http://www.gsk-clinicalstudyregister.com/
- 16.Breslow NE, Day NE. Modelling the relationship between risk, dose and time. In: Breslow NE, Day NE, editors. Statistical Methods in Cancer Research Volume II: The Design and Analysis of Cohort Studies. International Agency for Research on Cancer; Lyon, France: 1987. pp. 232–271. [Google Scholar]
- 17.Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sorensen HT, Blot WJ. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther. 2002;9(3):199–205. doi: 10.1097/00045391-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Tamim H, Monfared AA, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf. 2007;16(3):250–258. doi: 10.1002/pds.1360. [DOI] [PubMed] [Google Scholar]
- 19.Feinstein AR, Horwitz RI. An algebraic analysis of biases due to exclusion, susceptibility, and protopathic prescription in case-control research. J Chronic Dis. 1981;34(8):393–403. doi: 10.1016/0021-9681(81)90038-2. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case-control studies. Am J Med. 1980;68(2):255–258. doi: 10.1016/0002-9343(80)90363-0. [DOI] [PubMed] [Google Scholar]
- 21.Johnson AG, Jick SS, Perera DR, Jick H. Histamine-2 receptor antagonists and gastric cancer. Epidemiology. 1996;7(4):434–436. doi: 10.1097/00001648-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 23.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jick H, Jick S, Derby LE, Vasilakis C, Myers MW, Meier CR. Calcium-channel blockers and risk of cancer. Lancet. 1997;349(9051):525–528. doi: 10.1016/S0140-6736(97)80084-0. [DOI] [PubMed] [Google Scholar]
- 25.Meier CR, Derby LE, Jick SS, Jick H. Angiotensin-converting enzyme inhibitors, calcium channel blockers, and breast cancer. Arch Intern Med. 2000;160(3):349–353. doi: 10.1001/archinte.160.3.349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.