Abstract
Background:
Similarities between the age-specific incidence pattern of testicular germ cell tumours (TGCTs) and the age-specific incidence pattern of cancers of viral origin prompted us to evaluate the relationship between common infections occurring during childhood or young adult life and TGCT using existing data from the US Servicemen's Testicular Tumor Environmental and Endocrine Determinants (STEED) case–control study.
Methods:
TGCT cases diagnosed between 2002 and 2005 (n=767) were matched on age, race and serum draw date to at least one control (n=929).
Results:
None of the infections evaluated were associated with TGCT risk. Further, a meta-analysis of mumps and mumps orchitis or orchitis infection did not support an association with TGCT (mumps pooled odds ratio (OR): 1.03, 95% confidence interval (CI): 0.89–1.20; mumps orchitis or orchitis pooled OR: 1.80, 95% CI: 0.74–4.42).
Conclusion:
Based on our evaluation of childhood and early life infections and meta-analyses of mumps and mumps orchitis and/or orchitis, TGCT does not appear to be associated with common childhood infections.
Keywords: testicular germ cell tumours, childhood infections, mumps, orchitis, case–control
Testicular germ cell tumours (TGCTs) are the most common cancer among young men in many countries (Chia et al, 2010). There are few established risk factors beyond age, race/ethnicity, adult height, history of cryptorchidism and family history of TGCT (McGlynn and Cook, 2009). The great majority of TGCTs arise in young men between the ages of 15 and 40 years. Although there has been much interest in a possible perinatal aetiology of these young adult tumours, non-perinatal factors may also have a role. In addition, the age-specific incidence pattern of TGCT resembles that of some cancers with viral aetiology, such as young-adult non-Hodgkin's lymphoma (NHL) (Newell et al, 1984; Algood et al, 1988).
Mumps, a viral disease, is manifested by inflammation of the salivary glands (Plotkin and Rubin, 2008). In the absence of vaccination, most persons will be infected by this disease in young adulthood (Plotkin and Rubin, 2008) and up to 37% of post-pubertal males will develop orchitis. A number of studies support an association between post-pubertal mumps and/or orchitis and TGCT (Mills et al, 1984; Brown et al, 1987; Swerdlow et al, 1987), whereas evidence linking TGCT and childhood mumps is largely null (Henderson et al, 1979; Loughlin et al, 1980; Coldman et al, 1982; Moss et al, 1986; Brown et al, 1987; Haughey et al, 1989; Stone et al, 1991; UK Testicular Cancer Study Group, 1994; Petridou et al, 1997).
Using existing data from the US Servicemen's Testicular Tumor Environmental and Endocrine Determinants (STEED) study, we evaluated the association between common childhood infections and TGCT. In addition, using published reports in the literature, we conducted a meta-analysis of mumps and/or orchitis and risk of TGCT.
Materials and methods
Study population
Participants in the STEED study were enrolled between 2002 and 2005 (McGlynn et al, 2007). Briefly, men between 18 and 45 years of age who had at least one serum sample stored in the US Department of Defense Serum Repository (DoDSR, Silver Spring, MD, USA) were eligible for the study. Men who developed TGCT while on active duty were eligible to participate as cases, whereas men who did not develop TGCT were eligible to participate as controls. TGCT diagnoses were limited to classic seminoma or nonseminoma (embryonal carcinoma, yolk sac carcinoma, choriocarcinoma, teratoma and mixed germ cell tumour). Eligible controls (n=928) were individually matched to cases (n=767) based on age at diagnosis (within 1 year), ethnicity (white, black and other) and date when serum was donated (within 30 days). Informed consent was obtained from all the participants. The study was approved by the institutional review boards of the National Cancer Institute (Bethesda, MD, USA) and the Walter Reed Army Institute of Research (Silver Spring, MD, USA).
The study questionnaire elicited information on risk factors for TGCT, as well as self-reported history of common childhood infections and age at onset (mumps, chicken pox including varicella or shingle, measles, roseola or Sixth disease and mononucleosis). The questionnaire also ascertained a self-reported history of orchitis, inflammation and/or swelling of the testicles prior to the reference date and the age at onset of orchitis. It also included a self-reported history of urinary tract infections, inflammation in the groyne area and sexually transmitted infections (chlamydia, genital herpes, gonorrhoea and syphilis).
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression adjusting for matching factors and TGCT risk factors (cryptorchidism, family history of testicular cancer and adult height). Sensitivity analyses were conducted, whereby study participants with self-reported age at infection within 1 year of the reference age were excluded. Analyses were conducted of all TGCT together and for seminoma and nonseminoma using SAS 9.1 software (SAS Institute Inc., Cary, NC, USA). All tests were two-sided, with P-value <0.05 considered statistically significant.
Meta-analysis
PubMed (http://www.ncbi.nlm.nih.gov/) was searched in a systematic manner for all observational studies of mumps, orchitis or mumps/orchitis and TGCT through 1 August 2011, using the following keywords: TGCT, testicular cancer, mumps, mumps orchitis AND orchitis, or their combinations. The references of all publications were searched for additional studies, and the PubMed option ‘related articles’ was used to search for potentially relevant articles.
Meta-analyses using fixed-effect methods and DerSimonian and Laird random-effects methods (DerSimonian and Laird, 1986) were performed in STATA v.11.2 (StataCorp., College Station, TX, USA). Summary ORs were compared and the I2 value and its 95% uncertainty intervals were used to assess the consistency of the study-specific estimates (Higgins et al, 2003). Publication bias was evaluated using a funnel plot of the log OR against the s.e. of the log OR. Small-study effects were tested using the Egger and Begg's tests and sensitivity analyses were conducted, whereby each study was omitted in turn and the summary estimate recalculated.
Results
The distributions of selected characteristics of the study population are provided in Table 1. Cases were more likely than controls to report a history of cryptorchidism (adjusted OR: 3.31; 95% CI: 1.84–5.97).
Table 1. Characteristics of study participants stratified by case-control status and TGCT histology, STEED Study, 2002–2005.
|
Controls (n=928)
|
All TGCT (n=767)
|
Seminoma (n=324)
|
Nonseminoma (n=442)
|
|||||
|---|---|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | n | %a | |
| Age (years) | ||||||||
| <25 | 318 | 34.3 | 278 | 36.2 | 61 | 18.8 | 216 | 48.9 |
| 25–29 | 277 | 29.8 | 217 | 28.3 | 97 | 29.9 | 120 | 27.1 |
| 30–34 | 174 | 18.8 | 137 | 17.9 | 78 | 24.1 | 59 | 13.3 |
| 35–39 | 120 | 12.9 | 101 | 13.2 | 64 | 19.8 | 37 | 8.4 |
| 40+ | 39 | 4.2 | 34 | 4.4 | 24 | 7.4 | 10 | 2.3 |
| Race | ||||||||
| White | 788 | 84.9 | 647 | 84.4 | 260 | 80.2 | 387 | 87.6 |
| Black | 35 | 3.8 | 22 | 2.9 | 12 | 3.7 | 10 | 2.3 |
| Other | 105 | 11.3 | 98 | 12.8 | 52 | 16.0 | 45 | 10.2 |
| Reference height (cm) | ||||||||
| <172.73 | 260 | 15.3 | 157 | 9.3 | 62 | 3.7 | 95 | 5.6 |
| 172.73–177.80 | 257 | 15.2 | 223 | 13.2 | 98 | 5.8 | 125 | 7.4 |
| 177.81–182.88 | 240 | 14.2 | 197 | 11.6 | 87 | 5.1 | 110 | 6.5 |
| >182.88 | 171 | 10.1 | 188 | 11.1 | 77 | 4.5 | 110 | 6.5 |
| Cryptorchidism | ||||||||
| Yes | 16 | 1.7 | 41 | 5.3 | 11 | 3.4 | 30 | 6.8 |
| No | 912 | 98.3 | 726 | 94.7 | 313 | 96.6 | 412 | 93.2 |
| First- or second-degree family history of testicular cancer | ||||||||
| Yes | 14 | 1.5 | 33 | 4.3 | 17 | 5.2 | 16 | 3.6 |
| No | 915 | 98.5 | 734 | 95.7 | 307 | 94.8 | 426 | 96.4 |
Abbreviations: STEED=Servicemen's Testicular Tumor Environmental and Endocrine Determinants; TGCT=testicular germ cell tumour.
Percents may not sum to 100 because of missing values.
Mumps and orchitis
There was no association between history of mumps without orchitis and TGCT (OR: 0.98; 95% CI: 0.76–1.27) (Table 2). Overall history of orchitis was associated with an increased risk of TGCT (OR: 2.38; 95% CI: 1.56–3.63), however, this association was limited to orchitis diagnosed within one calendar year of the reference date (OR: 23.16; 95% CI: 5.53–96.99). Orchitis diagnosed more than 1 year prior to the reference age was not associated with risk (OR: 1.17; 95% CI: 0.71–1.94).
Table 2. Association of mumps, orchitis and testicular germ cell tumours according to histology: STEED Study 2002–2005.
| Controls |
All Cases
|
Seminoma
|
Nonseminoma
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | ORa | (95% CI) | n (%) | ORa | (95% CI) | n (%) | ORa | (95% CI) | |
| Mumps | ||||||||||
| Yes | 256 (27.6) | 194 (25.3) | 0.98 | (0.76, 1.27) | 103 (31.8) | 0.89 | (0.63, 1.25) | 91 (20.6) | 1.04 | (0.75, 1.44) |
| No | 610 (65.7) | 486 (63.4) | 1.00 | Reference | 184 (56.8) | 1.00 | Reference | 302 (68.3) | 1.00 | Reference |
| Orchitis | ||||||||||
| Yes | 36 (3.9) | 68 (8.9) | 2.38 | (1.56, 3.63) | 22 (6.8) | 1.52 | (0.86, 2.71) | 46 (10.4) | 3.09 | (1.93, 4.93) |
| No | 889 (95.7) | 692 (90.2) | 1.00 | Reference | 300 (92.6) | 1.00 | Reference | 391 (88.5) | 1.00 | Reference |
| Orchitis stratified by age at infection with respect to reference age | ||||||||||
| Within 1 year | 2 (< 0.1) | 36 (4.7) | 23.16 | (5.53, 96.99) | 13 (4.0) | 19.99 | (4.3, 92.73) | 23 (5.2) | 23.58 | (5.45, 101.99) |
| >1 year | 34 (3.7) | 32 (4.4) | 1.17 | (0.71, 1.94) | 9 (2.8) | 0.60 | (0.28, 1.32) | 23 (5.2) | 1.72 | (0.98, 3.03) |
| No | 889 (95.7) | 692 (90.2) | 1.00 | Reference | 300 (92.6) | 1.00 | Reference | 391 (88.5) | 1.00 | Reference |
Abbreviations: CI=confidence interval; OR=odds ratio; STEED=Servicemen's Testicular Tumor Environmental and Endocrine Determinants.
Adjusted for matching factors, cryptorchidism, adult height and family history of testicular cancer.
We further evaluated mumps and orchitis occurring around puberty (⩾10 years of age) as the testes undergo maturation and peripubertal infection accompanied with inflammation may be an aetiologically relevant event. Orchitis at ⩾10 years of age (OR: 1.12; 95% CI: 0.67–1.90) and mumps infection at ⩾10 years of age were not associated with TGCT (OR: 1.34; 95% CI: 0.71–2.51) (Supplementary Table 1).
Common childhood infections, genital conditions and genital tract infections
History of measles, chicken pox, roseola/sixth disease and mononucleosis were not associated with TGCT overall or by histological type (Supplementary Table 2). Similarly, self-reported inflammation in the groyne area was not associated (OR: 1.20; 95% CI: 0.83–1.74), nor were there patterns suggesting that urinary tract infection or sexually transmitted infections were associated.
With the exception of results presented above for orchitis infection where the age at diagnosis of orchitis was within 1 year of reference age, the ORs for association between infection and TGCT remained unchanged in sensitivity analyses excluding participants with self-reported age at infection within 1 year of the reference age.
Meta-analysis of the association between mumps, orchitis and TGCT
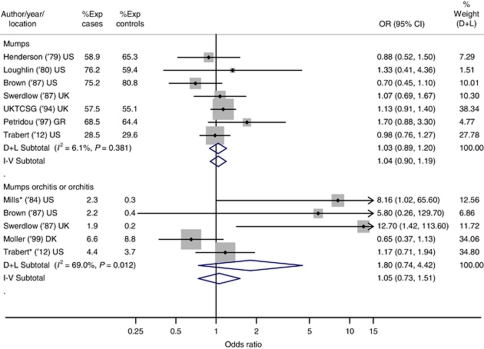
The pooled summary OR based on the random-effects model for mumps was 1.03 (95% CI: 0.89–1.20); suggesting no association with TGCT (Figure 1). The consistent fixed- and random-effects pooled ORs and I2 of 6.1% indicate very little heterogeneity among the six studies (P-value: 0.38).
Figure 1.
Summary odds ratios (ORs) and 95% confidence intervals (CIs) for the association between mumps (top), mumps orchitis or orchitis (bottom) and TGCT. Summary ORs and 95% CIs are reported based on fixed-effect (I–V) and random-effects (D+L) meta-analytic models. All statistical tests were two-sided. ‘% Weight’ describes the weighting each study contributes to the random-effects summary OR. The dot on each square represents the study-specific OR (also listed in the ‘OR (95% CI)’ column), and the size of the shaded square is illustrative of the random-effects study weighting. The horizontal lines represent the 95% CIs; the lines ending in an arrow indicate that this interval transcends the region plotted. The diamonds represent the summary ORs and 95% CIs for the mumps group (first subtotal), the mumps orchitis or orchitis group (second subtotal) and overall (last). For the ‘mumps orchitis or orchitis’ plot, studies denoted with an ‘*’ included orchitis cases only. Abbreviations: DK=Denmark; GR=Greece; UK=United Kingdom; UKTCSG=United Kingdom Testicular Cancer Study Group; US=United States; Yr=year study published.
For mumps orchitis or orchitis, the random-effects model yielded a pooled OR of 1.80 (95% CI: 0.74–4.42). The disparate fixed- and random-effects pooled ORs and I2 of 69.0% (P-value=0.01) suggested considerable heterogeneity across study-specific ORs for the five studies. Baujat's plot indicated that the two recent studies contributed most to the heterogeneity (Moller and Skakkebaek, 1999 and the current study). When these studies were removed, the I2 value dropped to 0.0% (P-value: 0.91) and the pooled ORs for fixed- and random-effects models became identical (OR (95% CI): 9.06 (2.3–35.2)) and indicative of an increased risk of TGCT.
Discussion
History of orchitis, mumps and other childhood infections were not risk factors for TGCT in this study.
As summarised in the meta-analysis of mumps orchitis or orchitis, increased risk of TGCT was observed in three prior studies (Mills et al, 1984; Brown et al, 1987; Swerdlow et al, 1987) and a null association was observed in the current study and another recent study conducted by Moller and Skakkebaek (1999). These earlier studies, however, are based on small sample sizes (n=8, 6 and 5 exposed cases, respectively), whereas the latter studies included 32 and 34 exposed cases, respectively. Not included in the meta-analysis were three studies that did not provide case/control counts or risk estimates, two of which reported an increased risk with orchitis (Beard et al, 1977) and one reported a null association (Stone et al, 1991).
Common childhood infections, inflammation in the groyne area, history of urinary tract infection and sexually transmitted infections were not associated with TGCT. The lack of association with childhood infectious diseases, including mumps, was consistent with most published reports (Henderson et al, 1979; Loughlin et al, 1980; Coldman et al, 1982; Moss et al, 1986; Brown et al, 1987; Haughey et al, 1989; Stone et al, 1991; UK Testicular Cancer Study Group, 1994; Petridou et al, 1997). Our meta-analysis of mumps included six studies in addition to the current study (Henderson et al, 1979; Loughlin et al, 1980; Brown et al, 1987; Swerdlow et al, 1987; UK Testicular Cancer Study Group, 1994; Petridou et al, 1997); all of these studies reported a null association. Four additional studies also reported a null association, however, these studies were not included in the meta-analysis because of the lack of case/control counts or risk estimates (Coldman et al, 1982; Moss et al, 1986; Haughey et al, 1989; Stone et al, 1991). Most authors reported, as we do, a lack of association between sexually transmitted infections and TGCT (Coldman et al, 1982; Moss et al, 1986; Brown et al, 1987; Swerdlow et al, 1987).
Strengths of the current study include the large sample size, high response proportion (91% of cases and 81% of controls), and that cases and controls were drawn from the same well-defined population (McGlynn et al, 2007). Further, the study included only pathologically confirmed TGCT. As with all case–control studies, however, limitations include the reliance on participant recall, which may be more selective among cases. We attempted to address this issue with sensitivity analyses that excluded self-reported infection that occurred in the calendar year prior to the reference date.
As hypothesised in the introduction, we evaluated infectious agents and risk of TGCT based on similarities in age-specific incidence patterns for TGCT and cancers with infectious etiologies. Specifically, NHL also occurs in young men, and studies have shown that some cases are the result of an infectious agent following the paralytic polio model. Given the epidemiologic similarities between TGCT and NHL, Newell et al (1984) hypothesised that the paralytic polio model may be relevant to TGCT aetiology (Newell et al, 1984). However, our data suggest that, unlike NHL, TGCT does not appear to be associated with common childhood infections.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
The authors declare no conflict of interest.
Supplementary Material
References
- Algood CB, Newell GR, Johnson DE (1988) Viral etiology of testicular tumors. J Urol 139(2): 308–310 [DOI] [PubMed] [Google Scholar]
- Beard CM, Benson Jr RC, Kelalis PP, Elveback LR (1977) Incidence of malignant testicular tumors in the population of Rochester, Minnesota, 1935 through 1974. Mayo Clin Proc 52(1): 8–10 [PubMed] [Google Scholar]
- Brown LM, Pottern LM, Hoover RN (1987) Testicular cancer in young men: the search for causes of the epidemic increase in the United States. J Epidemiol Community Health 41(4): 349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, McGlynn KA (2010) International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev 19(5): 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldman AJ, Elwood JM, Gallagher RP (1982) Sports activities and risk of testicular cancer. Br J Cancer 46(5): 749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3): 177–188 [DOI] [PubMed] [Google Scholar]
- Haughey BP, Graham S, Brasure J, Zielezny M, Sufrin G, Burnett WS (1989) The epidemiology of testicular cancer in upstate New York. Am J Epidemiol 130(1): 25–36 [DOI] [PubMed] [Google Scholar]
- Henderson BE, Benton B, Jing J, Yu MC, Pike MC (1979) Risk factors for cancer of the testis in young men. Int J Cancer 23(5): 598–602 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414): 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin JE, Robboy SJ, Morrison AS (1980) Risk factors for cancer of the testis. N Engl J Med 303(2): 112–113 [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Cook MB (2009) Etiologic factors in testicular germ-cell tumors. Future Oncol 5(9): 1389–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn KA, Sakoda LC, Rubertone MV, Sesterhenn IA, Lyu C, Graubard BI, Erickson RL (2007) Body size, dairy consumption, puberty, and risk of testicular germ cell tumors. Am J Epidemiol 165(4): 355–363 [DOI] [PubMed] [Google Scholar]
- Mills PK, Newell GR, Johnson DE (1984) Testicular cancer associated with employment in agriculture and oil and natural gas extraction. Lancet 1(8370): 207–210 [DOI] [PubMed] [Google Scholar]
- Moller H, Skakkebaek NE (1999) Risk of testicular cancer in subfertile men: case-control study. BMJ 318(7183): 559–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss AR, Osmond D, Bacchetti P, Torti FM, Gurgin V (1986) Hormonal risk factors in testicular cancer. A case-control study. Am J Epidemiol 124(1): 39–52 [DOI] [PubMed] [Google Scholar]
- Newell GR, Mills PK, Johnson DE (1984) Epidemiologic comparison of cancer of the testis and Hodgkin′s disease among young males. Cancer 54(6): 1117–1123 [DOI] [PubMed] [Google Scholar]
- Petridou E, Roukas KI, Dessypris N, Aravantinos G, Bafaloukos D, Efraimidis A, Papacharalambous A, Pektasidis D, Rigatos G, Trichopoulos D (1997) Baldness and other correlates of sex hormones in relation to testicular cancer. Int J Cancer 71(6): 982–985 [DOI] [PubMed] [Google Scholar]
- Plotkin S, Rubin S (2008) Mumps vaccine. In: Vaccines, Plotkin S, Orenstein W, Offit P (eds) pp 435–465. Elsevier: Philadelphia, PA, USA [Google Scholar]
- Stone JM, Cruickshank DG, Sandeman TF, Matthews JP (1991) Laterality, maldescent, trauma and other clinical factors in the epidemiology of testis cancer in Victoria, Australia. Br J Cancer 64(1): 132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ, Huttly SR, Smith PG (1987) Testicular cancer and antecedent diseases. Br J Cancer 55(1): 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Testicular Cancer Study Group (1994) Social, behavioural and medical factors in the aetiology of testicular cancer: results from the UK study. Br J Cancer 70(3): 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.