Abstract
The prevalence of allergic diseases in children has increased for several decades. We evaluated the correlation between pollen count of weeds and their sensitization rate in Seoul, 1997-2009. Airborne particles carrying allergens were collected daily from 3 stations around Seoul. Skin prick tests to pollen were performed on children with allergic diseases. Ragweed pollen gradually increased between 1999 and 2005, decreased after 2005 and plateaued until 2009 (peak counts, 67 in 2003, 145 in 2005 and 83 grains/m3/day in 2007). Japanese hop pollen increased between 2002 and 2009 (peak counts, 212 in 2006 and 492 grains/m3/day in 2009). Sensitization rates to weed pollen, especially ragweed and Japanese hop in children with allergic diseases, increased annually (ragweed, 2.2% in 2000 and 2.8% in 2002; Japanese hop, 1.4% in 2000 and 1.9% in 2002). The age for sensitization to pollen gradually became younger since 2000 (4 to 6 yr of age, 3.5% in 1997 and 6.2% in 2009; 7 to 9 yr of age, 4.2% in 1997 and 6.4% in 2009). In conclusion, sensitization rates for weed pollens increase in Korean children given increasing pollen counts of ragweed and Japanese hop.
Keywords: Plant Weeds, Pollen, Immunologic Sensitization
INTRODUCTION
For several decades, the prevalence of allergic diseases in children and adolescents has generally increased. Many studies have illustrated this worldwide trend, although there was substantial variability across studies (1-4). There are a number of reports suggesting that the increase of allergic diseases results from environmental change-related risks such as air pollution and climate change. Among allergic diseases, seasonal allergic diseases are expected to increase due to changes in the distribution, quantity and quality of pollen allergens and the duration of pollen season (5). In particular, seasonal allergic rhinitis and other seasonal allergic diseases are also likely to worsen because of the interactions between heavier pollen load and increased air pollution (6, 7).
Some studies have found that recent increases in airborne pollen counts may be due to environmental and climate changes. Specifically, climate change is suggested to impact the amount and season of pollen, as well as the distribution of plant and pollen and other plant attributes (8-10). However, several questions remain regarding the direct relationship between climate change and increased pollen count in each country.
The impact of rising CO2 on ragweed biomass and reproduction has been demonstrated. As such, an increase in pollen exposure can be expected given changes in CO2 and temperature associated with global climate change (11, 12).
Ragweed plants grow along the road and cultivated fields, fallow lands, meadows and building areas. Ragweed growth is carbon dependent, and recent increases in atmospheric CO2 concentration should result in potential increases in pollen production (13). Ragweed is not a native Korean plant, but came from western countries following the growth of economic and commercial trade since the 1950s.
Japanese hop pollen has been reported as one of the major pollen types in East Asian countries. Japanese hop, a weed plant belonging to the Cannabinaceae family, is widespread in both rural and urban areas (14). Mugwort spreads in the temperate and humid zones of the northern hemisphere, European countries as well as parts of Asia. Studies have identified the potential cross-reactivity between ragweed and mugwort pollen allergens (15). In addition, the expansion of ragweed to formerly mugwort-dominant areas is consistent with increases in weed pollen allergies (16). The three aforementioned allergens have recently been considered as major causative allergens in autumn pollinosis in Korea.
Seoul, the capital of Korea, is located in the northern region of the country. It is the country's most important economic, cultural, and political hub and houses a population of 13,000,000, or about one-fifth of the Korean population. Surrounding the Seoul metropolitan area are many satellite cities.
The present study was conducted to determine which allergic diseases have increased due to the aforementioned pollen allergens by analyzing changes in sensitization rates to weed allergens in Korean children and changes in weed pollen counts in Seoul over the last 13 yr.
MATERIALS AND METHODS
Pollen count
Airborne particles containing allergens were collected daily from 3 pollen collecting stations (Hanyang University Hospital, the National Institute of Meteorological Research and Hanyang University Guri Hospital) for 13 yr (between July 1, 1997 and December 31, 2009) using a 7-day-Burkard sampler (Burkard Manufacturing Co. Ltd., Hertfordshire, UK). The collected samples were sent to Hanyang University Guri Hospital, which was charged with identifying and counting the pollen in separate aluminum cases. The samples were stained with Calberla's fuchsin dye (10 mL of glycerin, 20 mL of 95% Alcohol, 30 mL of distilled water and 0.2 mL basic fuchsin) and counted under a light microscope (× 400). The counts of airborne pollen grains were calculated and recorded per m3. The start of main pollen season (MPS) was defined as the day on which 1 grain/m3 was reached when the air contained 3 or more grains/m3/day for 5 consecutive days. The end of main pollen season was determined as the last day on which 3 grains/m3/day was recorded when the air presented concentrations below this level for 5 consecutive days (17). Mean pollen counts in Seoul were calculated by averaging the pollen counts of the 3 collecting stations, as the distance between the three stations was less than 20 km.
Sensitization rate to allergenic pollen
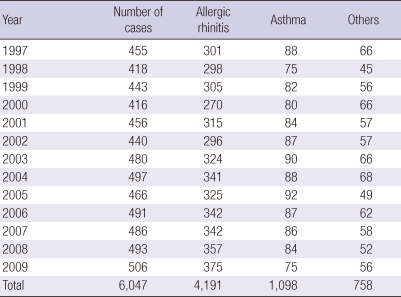
To assess the sensitization rate to allergenic pollen, data was collected from pediatric patients under 15 yr of age who suffered from allergic rhinitis, asthma, and allergic conjunctivitis. Data was collected from 3 hospitals where skin allergy tests were feasible. These patients did not exhibit any positive results in previous allergy tests or laboratory examinations such as skin tests and allergy blood tests. The number of subjects totaled 6,047 and included those with allergic rhinitis (n = 4,191), asthma (n = 1,098), and others (n = 758). Data was analyzed annually for the years between 1997 and 2009 (Table 1).
Table 1.
Number of cases with allergic diseases who were recruited from 3 pollen collecting stations* around Seoul during the period 1997-2009
*Hanyang University Hospital, National Institute of Meteorological Research and Hanyang University Guri Hospital.
Skin prick tests were conducted to evaluate the sensitization rate to various pollen allergens. They were performed with allergen extracts of birch, alder, oak, timothy, rye, orchard grasses, ragweed, mugwort, and Japanese hop (ALK, Copenhagen, Denmark, and Center Lab, Port Washington, NY, USA). Histamine phosphate was used as a positive control (10 mg/mL) and diluents, which was an unbuffered saline containing 0.03% human serum albumin (ALK), was used as a negative control. A positive reaction to skin prick tests was defined when wheals of more than one-half the area of histamine reaction was obtained by using commercial aeroallergen extracts standardized in biological units (BU).
Comparisons of the annual sensitization rates to pollen allergens according to age
To assess the sensitization rates to pollen allergens, the allergens were sorted into 3 representative pollen allergens that were included in the skin test extracts: tree, grass and weed pollen allergens. Birch, alder, and oak pollen represented tree pollen allergens. Timothy, rye and orchard grasses represented grass pollen allergens. Ragweed, mugwort, and Japanese hop represented weed pollen allergens.
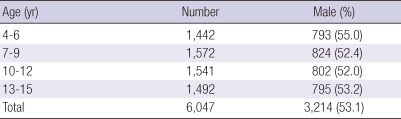
To obtain a more accurate distribution of the sensitization rates to pollen allergens in children, patients were divided into 4 age groups: 1) 4 to 6 yr; 2) 7 to 9 yr; 3) 10 to 12 yr; and 4) 13 to 15-yr-old (Table 2). The 1 to 3-yr-old group was excluded due to a low incidence and prevalence. Annual sensitization rates to pollen allergens were compared between the 4 age groups.
Table 2.
Age and sex of cases in the present study
Statistical analysis
We examined and compared the annual variation in weed pollen counts. Statistical analysis for annual variation was performed by using basic statistics of anomaly data. The sensitization rates to pollen allergens released by tree, grass and weed plants were evaluated and compared between age groups by using time series analysis. The correlation between the sensitization rate to pollen allergen and annual pollen count of each plant were analyzed by using multiple regression models. Analyses were conducted using the Statistical Package for Social Sciences, SPSS 11.5 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The protocol and consent forms of this study were reviewed and approved by the institutional review board of Hanyang University Guri Hospital (2011-029). Informed oral consent was obtained from all parents and/or participants because additional invasive procedures, with the exception of skin prick tests, were not required.
RESULTS
The distribution of allergenic pollen in Seoul
Results show that there are two peak pollen-scattering seasons in Korea. On average, the first peak is from the beginning of April to the mid-June, and the second one is from the mid-August to the end of September. On October, the pollen collection markedly decreased. In spring, pollination of tree pollen was high in Seoul. Alder pollen started to pollinate in the mid-February. It was followed by birch, juniper and poplar in March; and oak, elm, and willow in April and May. In autumn, pollination of weeds was high. Mugwort began to pollinate in the beginning of August, followed by ragweed, Japanese hops, English plantain, dock and lambsquarters in August and September, and continued to pollinate up to the mid-October.
Increases in annual weed pollen counts
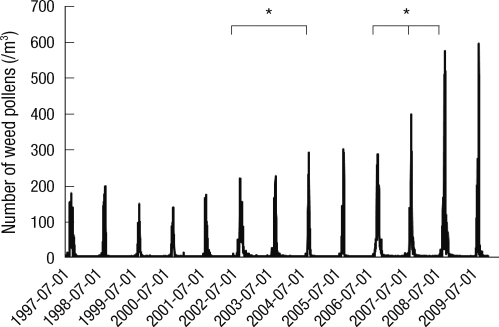
Weed pollen grains were mostly collected from the mid-August to the end of September. Among these pollen grains, ragweed, mugwort and Japanese hop were most commonly collected in Seoul. The peak count of weed pollen in autumn was 174 grains/m3 in 1997, but this abruptly increased to 218 grains/m3 in 2002, to 277 grains/m3 in 2004 (P < 0.05), and 286 grains/m3 in 2006 to 576 grains/m3 in 2008 (P < 0.05) (Fig. 1).
Fig. 1.
Annual weed pollen counts from 1997 to 2009 in Seoul. Weed pollen included ragweed, Japanese hop and mugwort which are common weed plants in Korea. *P < 0.05.
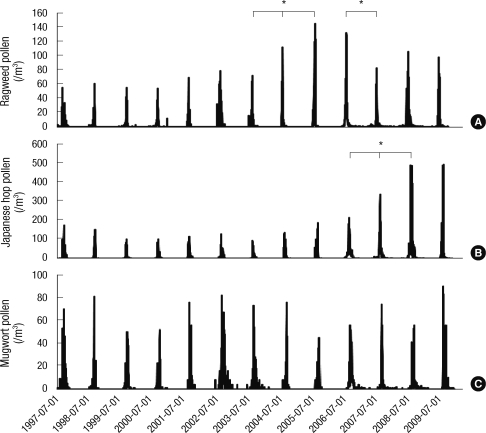
Interestingly, among weed pollen, ragweed pollen counts increased each year, especially since 2000 (peak pollen counts: 67 grains/m3/day in 2003, 112 grains/m3/day in 2004 and 145 grains/m3/day in 2005) (P < 0.05). Pollen counts then decreased and plateaued until 2009 (peak pollen counts: 132 grains/m3/day in 2006 and 83 grains/m3/day in 2007) (P < 0.05). Counts of Japanese hop pollen also gradually increased each year since 2002 and peak pollen counts significantly increased from 212 grains/m3/day in 2006 to 492 grains/m3/day in 2009 (P < 0.05). On the other hand, counts of mugwort pollen did not change significantly until 2009 (Fig. 2).
Fig. 2.
Changes in annual weed pollen counts from 1997 to 2009 in Seoul. (A) Ragweed. (B) Japanese hops. (C) Mugwort. *P < 0.05.
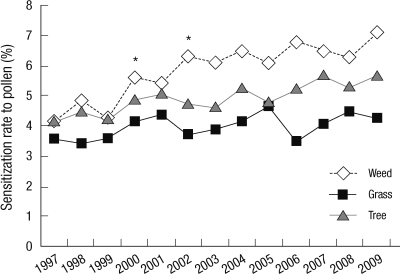
Changes in the annual sensitization rates to pollen in children
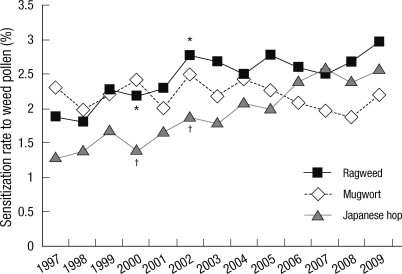
Among the three groups of pollen allergens (weed, tree and grass), sensitization rates to weed and tree gradually increased. Among them, sensitization rates to weed pollen were 4.1% in 1999, 6.3% in 2001 and 7.1% in 2009 (P < 0.05). Sensitization rates to tree pollen were 4.2% in 1997, 5.1% in 2002, and 5.7% in 2007. There was no significant increase in sensitization rates to grass plants (Fig. 3). Among weed pollen, sensitization rates to ragweed and Japanese hop increased significantly between 2000 and 2002. Sensitization rates remained high after 2002. Sensitization rate to ragweed was 2.2% in 2000 and 2.8% in 2002. Sensitization rate to Japanese hop was 1.4% in 2000 and 1.9% in 2002 (Fig. 4).
Fig. 3.
Annual sensitization rates to pollens from 1997 to 2009 in Seoul. *P < 0.05.
Fig. 4.
Annual sensitization rates to ragweed, mugwort and Japanese hop. *P < 0.05 for ragweed pollen between 2000 and 2002; †P < 0.05 for Japanese hop between 2000 and 2002.
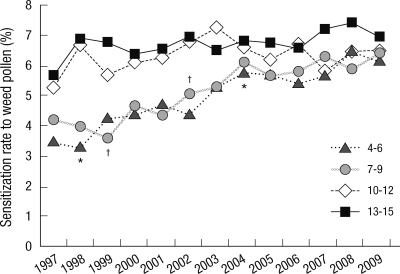
Changes in the annual sensitization rate to allergenic pollen according to age
In the 4 to 6 yr age group, sensitization rates increased from 3.5% in 1997 to 5.3% in 2004, and to 6.2% in 2009. In the 7 to 9 yr age group, sensitization rates increased from 4.2% in 1997 to 6.1% in 2004, and to 6.4% in 2009. Although the 10 to 12 yr age group exhibited a slight increase in sensitization rates from 5.3% in 1997 to 6.5% in 2009, but the rates were lower than those exhibited before 2001. In the 13 to 15 yr age group, sensitization rates increased slightly from 1997 (5.7%) to 2009 (6.9%), but did not change significantly over the 13-yr period (Fig. 5).
Fig. 5.
Annual sensitization rates to weed pollen allergens according to age. Weed pollen included ragweed, Japanese hop and mugwort. *P < 0.05 between 1998 and 1999, and between 2002-2004 in 4-6 yr of age; †P < 0.05 between 1999 and 2000 in 7-9 yr of age.
DISCUSSION
The present study shows that since 2000, counts of ragweed pollen increased rapidly in Seoul through 2005, plateaued since 2006, and then decreased in 2007. Sensitization rates to weed allergens have increased significantly in children between 1999 and 2002. Annual sensitization rates to pollen have increased in children living in Seoul, especially for children less than 9 yr of age.
In line with observations of a significant increase in weed pollen, sensitization rates to weed pollen in children have increased proportionately over the same period. As to allergic rhinitis, the Korean version of the International Study of Asthma and Allergies in Childhood (ISAAC) of Korean children reported the nationwide prevalence of allergic rhinitis in 1995, 2000, and 2006. The prevalence of "diagnosis of allergic rhinitis, ever" in a study of more than 25,000 elementary students was 15.5%, 20.4%, and 28.4% in 1995, 2000, and 2006, respectively. In addition, the prevalence of "treatment of allergic rhinitis, last 12 months" was 11.8%, 15.1%, and 21.8% in 1995, 2000, and 2006, respectively (3, 4).
The effects of environmental change on respiratory allergic diseases are not yet completely understood. There have been a few epidemiological and experimental studies on the relationship between asthma and environmental and climate changes (12, 13). Some studies have demonstrated that urbanization, high levels of vehicle emissions and a westernized lifestyle correlate with a higher frequency of pollen-induced respiratory allergies. Singer et al. (18) demonstrated that Amb a1 concentration in ragweed pollen increases as a function of CO2 concentration, although a well demonstrated difference in allergen content between individual ragweed populations must be considered.
Among weed plants, ragweed is known as the most important airborne allergen in autumn. Ragweed grows largely in vacant or waste lands around urban areas (18). Because the amount of ragweed pollen has increased in Korea during the last few years, the Ministry of Environment has carried out the identification and surveillance of ragweed foci. Major accumulation of ragweed foci have been detected in the Seoul metropolitan area and other northern provinces of Korea (19). However, there was no information on ragweed in reports on pollen distribution during the 1960s (20).
In the present study, ragweed pollen was commonly collected the middle of August and the end of September. There may be several possible reasons why the presence of ragweed has increased rapidly in Seoul through to 2005. First, with economic growth, many parts of Korea have been changed. In particular, Seoul, the largest and most quickly-changing city in Korea, and farmlands around Seoul have been transformed into industrial and residential areas. Due to potential increase in unused land, ragweed, which exhibit reproduction have proliferated spectacularly. Studies also suggest that in developing land, ragweed seeds in soil were contaminated onto clothes and other products and were carried nationwide via vehicles (5). Second, it was reported that when CO2 concentration increases two-fold due to climate change or the greenhouse effect, the production of ragweed increases by 61%. CO2 concentration increased by more than 29% in the 1990s than before the industrialization period (21, 22). This tendency was also observed in the 2000s, suggesting that environmental changes such as greenhouse gas amounts influenced the proliferation of ragweed in Korea. However, results of this study indicate that ragweed pollen amount plateaued in 2006 and then decreased in 2007, with ragweed pollen counts plateauing until 2009. This phenomenon may be due to the decline of ragweed plants around Seoul due to systematic monitoring of the colonization of ragweed plants by the Ministry of Environment from 1990. These initiatives may have contributed to the plateau curve of ragweed pollen in Seoul (19). Similarly, the Swiss annual report of 2009 indicates that European countries may also be experiencing the effects of removing ragweed plants from 2007 (23). The study reports a plateau pattern of ragweed pollen amounts since 2006 followed by a decrease in 2007.
Although counts of ragweed pollen decreased since 2007, sensitization rates to ragweed pollen kept rising even after that point. This phenomenon might imply that even though counts of pollen decreased, sensitization to pollen lingered for a long time after children were sensitized to pollen. In addition, sensitization rates to weed allergens have increased every year in young children less than 9 yr of age. This might be due to several factors. First, as stated earlier, environmental changes may have an increasing impact on the distribution and quantity of pollen, followed by increased sensitization to pollen even in early childhood. Second, there have been recent increases in the number of children who live in urban areas of Korea and attend day care centers from infancy. Consequently, exposure to outdoor allergens associated with outdoor activity as well as indoor allergens may have increased.
Japanese hop (Humulus japonicus) grows rapidly and spreads out as an annual vine with double- and single-hooked climbing hair (24). Its rapid growth causes serious problems in riverine wetlands as Japanese hop covers neighboring weeds in the growing season and hinders the growth of the other plants. Such plants that cause significant changes in the structure and function of an ecosystem are known as invasive plants (25). Japanese hop allocates energy to the stem rather than the leaf as a survival strategy to get more light and a larger habitat. Most nutrients in Japanese hop correlate with growth rate. Therefore, preferentially the eradication program must evaluate its situation under the given circumstances to find the best methods and consider life history and physiological characteristics of species (26). We believe that pollen of the Japanese hop should be considered as a relevant allergen during the autumn season and thus be included in skin test batteries in Korea.
This study concludes that sensitization rates for weed pollens increase in Korean children with increasing pollen counts of ragweed and Japanese hop. The results of this study should provide important contributions to the discussion on the relationship between counts of pollen and sensitization rates in symptomatic allergic patients over an extended period of time.
Footnotes
This study was supported by the grant of National Institute of Meteorological Research, 2007-2009 and the fund of pollen study committee of Korean Academy of Pediatric Allergy and Respiratory Diseases (KAPARD).
References
- 1.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008;51:343–350. [Google Scholar]
- 4.Jee HM, Kim KW, Kim CS, Sohn MH, Shin DC, Kim KE. Prevalence of asthma, rhinitis and eczema in Korean children using the International Study Of Asthma and Allergies In Childhood (ISAAC) questionnaires. Pediatr Allergy Respir Dis. 2009;19:165–172. [Google Scholar]
- 5.Beggs PJ. Impacts of climate change on aeroallergens: past and future. Clin Exp Allergy. 2004;34:1507–1513. doi: 10.1111/j.1365-2222.2004.02061.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernard SM, Samet JM, Grambsch A, Ebi KL, Romieu I. The potential impacts of climate variability and change on air pollution-related health effects in the United States. Environ Health Perspect. 2001;109:199–209. doi: 10.1289/ehp.109-1240667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Amato G, Liccardi G, D'Amato M, Holgate S. Environmental risk factors and allergic bronchial asthma. Clin Exp Allergy. 2005;35:1113–1124. doi: 10.1111/j.1365-2222.2005.02328.x. [DOI] [PubMed] [Google Scholar]
- 8.Frenguelli G. Interactions between climatic changes and allergenic plants. Monaldi Arch Chest Dis. 2002;57:141–143. [PubMed] [Google Scholar]
- 9.Namork E, Johansen BV, Løvik M. Detection of allergens adsorbed to ambient air particles collected in four European cities. Toxicol Lett. 2006;165:71–78. doi: 10.1016/j.toxlet.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Oh JW, Lee HB, Kang IJ, Kim SW, Park KS, Kook MH, Kim BS, Baek HS, Kim JH, Kim JK, Lee DJ, Kim KR, Choi YJ. The revised edition of Korean calendar for allergenic pollens. Allergy Asthma Immunol Res. 2012;4:5–11. doi: 10.4168/aair.2012.4.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knox RB, Suphioglu C, Taylor P, Desai R, Watson HC, Peng JL, Bursill LA. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin Exp Allergy. 1997;27:246–251. [PubMed] [Google Scholar]
- 12.D'Amato G, Cecchi L. Effects of climate change on environmental factors in respiratory allergic diseases. Clin Exp Allergy. 2008;38:1264–1274. doi: 10.1111/j.1365-2222.2008.03033.x. [DOI] [PubMed] [Google Scholar]
- 13.Ayres JG, Forsberg B, Annesi-Maesano I, Dey R, Ebi KL, Helms PJ, Medina-Ramón M, Windt M, Forastiere F Environment and Health Committee of the European Respiratory Society. Climate change and respiratory disease: European Respiratory Society position statement. Eur Respir J. 2009;34:295–302. doi: 10.1183/09031936.00003409. [DOI] [PubMed] [Google Scholar]
- 14.Park HS, Nahm DH, Suh CH, Lee SM, Choi SY, Jung KS, Lee SY, Park K. Evidence of Hop Japanese pollinosis in Korea: IgE sensitization and identification of allergenic components. J Allergy Clin Immunol. 1997;100:475–479. doi: 10.1016/s0091-6749(97)70138-6. [DOI] [PubMed] [Google Scholar]
- 15.Hirschwehr R, Heppner C, Spitzauer S, Sperr WR, Valent P, Berger U, Horak F, Jäger S, Kraft D, Valenta R. Identification of common allergenic structures in mugwort and ragweed pollen. J Allergy Clin Immunol. 1998;101:196–206. doi: 10.1016/s0091-6749(98)70384-7. [DOI] [PubMed] [Google Scholar]
- 16.Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: common ragweed, urbanization, and public health. J Allergy Clin Immunol. 2003;111:290–295. doi: 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- 17.Fitter AH, Fitter RS. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 18.Singer BD, Ziska LH, Frenz DA, Gebhard DE, Straka JG. Increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO2 concentration. Funct Plant Biol. 2005;32:667–670. doi: 10.1071/FP05039. [DOI] [PubMed] [Google Scholar]
- 19.Environment RoKMo. List of environmentally harmful animals and plants in Korea. ME Publishing (Korean); 2009. pp. 95–102. ISBN 11-1480000-001000-01. [Google Scholar]
- 20.Oh JW, Lee HR, Kim JS, Lee KI, Kang YJ, Kim SW, Kook MH, Kang HY, Kim JS, Lee MH, Lee HB, Kim KE, Pyun BY, Lee SI, Han MJ. Aerobiological study of pollen and mold in the 10 states of Korea. Pediatr Allergy Respir Dis. 2000;10:22–33. [Google Scholar]
- 21.Ziska LH, Caulfield FA. Rising CO2 and pollen production of common ragweed (Ambrosia artemisiifolia L.), a known allergy-inducing species: implications for public health. Aust J Plant Physiol. 2000;27:893–898. [Google Scholar]
- 22.Ackermann-Liebrich U, Schindler C, Frei P, Probst-Hensch NM, Imboden M, Gemperli A, Rochat T, Schmid-Grendemeier P, Bircher AJ. Sensitisation to Ambrosia in Switzerland: a public health threat on the wait. Swiss Med Wkly. 2009;139:70–75. doi: 10.4414/smw.2009.12489. [DOI] [PubMed] [Google Scholar]
- 23.Frenz DA. Interpreting atmospheric pollen counts for use in clinical allergy: allergic symptomology. Ann Allergy Asthma Immunol. 2001;86:150–157. doi: 10.1016/S1081-1206(10)62683-X. [DOI] [PubMed] [Google Scholar]
- 24.Ehara K. Comparative morphological studies of the hop (Humulus lupulus L.) and the Japanese hop (H. japonicus Sieb. et Zucc.) Part I. J Fac Agric Kyushu Univ. 1955;10:209–232. [Google Scholar]
- 25.Cronk QC, Fuller JL. Plant invaders: the threat to natural ecosystems. London: Chapman & Hall; 1995. p. 241. [Google Scholar]
- 26.Wittenberg R, Cock MJW. Invasive alien species. How to address one of the greatest threats to biodiversity: a toolkit of best prevention and management practices. Oxon: CAB International; 2001. [Google Scholar]