Abstract
The majority of Korean patients with pandemic influenza A (H1N1) during the 2009 epidemic were under 20 yr of age. The limited data on the clinical characteristics of these children led us to conduct a case note-based investigation of children admitted to 6 university hospitals with 2009 H1N1 influenza. A total of 804 children was enrolled. The median age was 5 yr; 63.8% were males; and 22.4% had at least one chronic underlying disease. Ninety-five of the patients (11.8%) were critically ill and they suffered more from shortness of breath, dyspnea and lymphopenia than the other patients. Among all the patients, 98.8% were treated with antivirals and 73% received treatment within 48 hr of illness onset. All the enrolled patients are alive and appear to have had good outcomes, probably due to the early intervention and antiviral treatment. This study deals with hospitalized children whose diagnoses of influenza A (H1N1) were confirmed, and therefore provides important new information about the clinical patterns of children with influenza A (H1N1) in Korea.
Keywords: Pandemic Influenza A (H1N1), Children, Korea, Epidemiology, Clinical Characteristics
INTRODUCTION
In the middle of April 2009, influenza caused by the novel A (H1N1) strain of influenza virus of swine origin was reported in Mexico and California. On April 24 the World Health Organization (WHO) announced the emergence of a novel influenza strain, and the infection was declared a pandemic in June (1). The first reported pandemic influenza A (H1N1) case in Korea, occurred in an adult who had visited Mexico in May 2009, and the infection spread into the community as an epidemic thereafter (2).
Clinical aspects of the children hospitalized with the novel A (H1N1) influenza have been reported in many countries, but patient' ages, rates of admission to intensive care units (ICU), and mortality, differed markedly depending on the study period, subjects and geographic region (3-10). Thus, for example, there was a distinct difference between Korea and USA. In Korea, the highest incidence of H1N1 virus infection occurred in young people aged 0 to 19 yr, but mortality was greatest among those over 60 yr; in the USA, incidence and mortality were highest in individuals aged 17 to 64 (2, 11). Moreover data on the impact of influenza A (H1N1) on Korean children are limited; the existing reports concern only patients with severe symptoms or deal with small numbers of cases in single institutions, or cover only the beginning of the outbreak. No study has yet appeared of children hospitalized with A (H1N1) influenza over the entire outbreak from 2009 to 2010 (12-14).
In this study, our objective was to describe the demographic characteristics, epidemiology, clinical manifestations, treatment and clinical course of children admitted to six Korean university hospitals with pandemic A (H1N1) influenza. This study allows us to identify the clinical features of infected children in a country where the rate of 2009 influenza A (H1N1) was high but mortality was low.
MATERIALS AND METHODS
We studied patients under 18 yr of age who were admitted to five capital region university hospitals, and one university hospital in Jeju-do, with influenza A (H1N1) virus infection during the 6 months between August 20, 2009 and January 30, 2010. Demographic and epidemiologic data, clinical manifestations, laboratory and radiologic findings, treatment and clinical course, were abstracted retrospectively from medical records on standardized case report forms. Cases of influenza A (H1N1) were confirmed by testing specimens collected from the oropharynx or nasopharynx with sterile swabs or aspirates using the influenza A (H1N1) real time reverse transcriptase-polymerase chain reaction (RT-PCR). Each hospital performed RT-PCR using AccuPower New Inf A (H1N1) & Inf A Real-Time RT-PCR (Bioneer Inc., Daejeon, Korea), Real-QTM H1N1 Novel One-step kits (Biosewoom., Inc., Seoul, Korea) and AdvanSure Influenza A/Influenza A (H1N1) (2009) real-time RT-PCR (LG Life Sciences Inc., Seoul, Korea) kits.
Children above 2 yr were defined as obese when a formula placed their body mass index at or above the 95th percentile on government charts specifying age and gender (15). Patients who required ventilator care or admission to an intensive care unit (ICU) were defined as critically ill patients. Full recovery meant that patients became free from symptoms and complications associated with influenza A (H1N1). We used laboratory values in the normal range for patients' ages (16). The study protocol was approved by the institutional review board (IRB) of Hanyang University Hospital. Informed consent was waived by the board. Data were entered on Microsoft Excel spreadsheets and transferred to SPSS 17.0 (SPSS Inc, Chicago, IL, USA) and SAS 9.1 for further analysis. Data were analyzed using the chi-square test, Fisher's exact test, the independent t-test or multiple logistic regression, and statistical significance was set at P < 0.05.
RESULTS
Demographic and epidemiologic characteristics
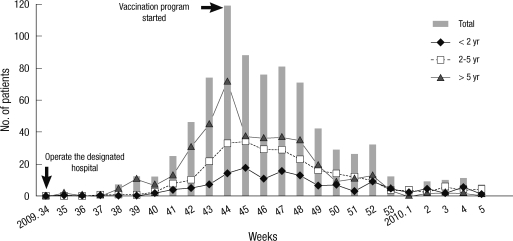
Between August 20, 2009 and January 30, 2010, 827 patients were admitted with influenza A (H1N1) to the 6 hospitals. Of these, 23 were positive in the influenza A (H1N1) rapid antigen test but negative for influenza A (H1N1) RT-PCR. Consequently, 804 patients were enrolled in the study. The first hospitalizations for influenza A (H1N1) occurred in week 34, 2009, when the government started to operate the designated hospital system which included the six study hospitals. Cases began to increase in week 38 and peaked in week 44 at a total of 119 cases; at this point the government initiated its vaccination strategy. Thereafter new cases declined steadily (Fig. 1).
Fig. 1.
Weekly distribution of children hospitalized with influenza A (H1N1) in 6 university hospitals from August 2009 to January 2010 in Korea. The first day of 34th week of year 2009 is August 16, 2009, and the first study patient developed the illness on August 20, 2009.
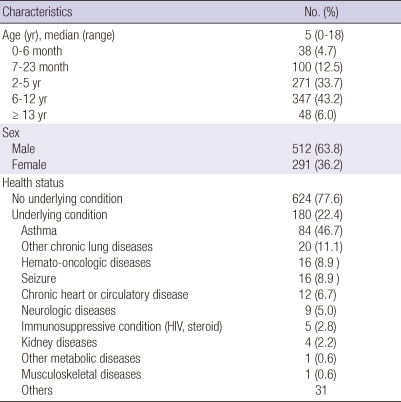
The mean age of the patients was 5 yr (range 0-18 yr). Thirty-eight (4.7%) were less than 6 months old, 100 (12.5%) were 7-23 months old, 271 (33.7%) were 2-5 yr old, 347 (43.2%) were 6-12 yr old and 48 (6.0%) were over 13 yr old (Table 1).
Table 1.
Epidemiology and underlying diseases in patients hospitalized with influenza A (H1N1) in 6 university hospitals from August 2009 to January 2010 in Korea
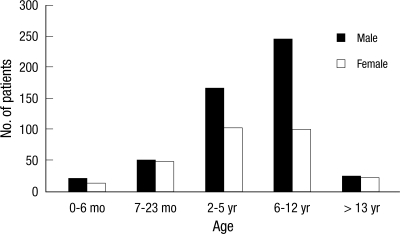
Of the 804 patients, 512 (63.8%) were boys and 291 (36.2%) girls. There were more boys than girls at all ages, particularly in the 2-5 yr group and the 6 yr and over groups, and this difference was statistically significant (Fig. 2). Of the patients, 7.7% (45/582) had been vaccinated against influenza A (H1N1), and 22.5% (91/403) against Streptococcus pneumoniae. Eighty seven (11%) of 785 patients had received oseltamivir less than 14 days prior to the study period. The proportion of the children with at least one underlying health condition was 22.4% (180/804). Respiratory disorders (57.8%) including asthma (n = 84, 46.7%) and other chronic lung diseases (n = 20, 11.1%) were the most common; neurologic disorders (n = 25, 13.9%) including seizure were next, followed by hemato-oncologic disease (n = 16, 8.9%), chronic heart or circulatory disease (n = 12, 6.7%) and kidney disease (n = 4, 2.2%) (Table 1).
Fig. 2.
Age and sex distribution of hospitalized children.
Clinical manifestations and laboratory findings
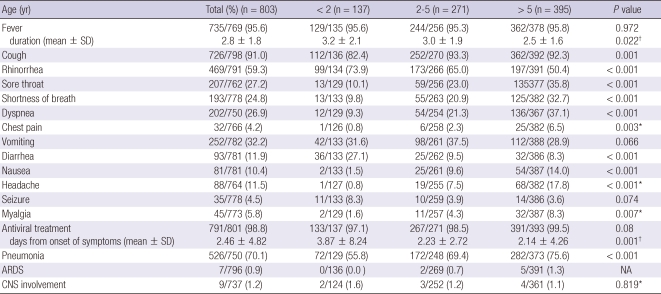
Seven hundred and thirty-five (95.6%) of the 769 patients admitted with influenza A (H1N1) complained of fever, and in fact, 556 patients (72.3%) had fevers when their temperatures were taken. The next most common symptoms were cough (n = 726/798, 91%), rhinorrhea (n = 469/791, 59.3%), vomiting (n = 252/782, 32.2%), sore throat (n = 207/762, 27.2%), dyspnea (n = 202/750, 26.9%), shortness of breath (n = 193/778, 24.8%), sneezing (n = 130/773, 16.8%), diarrhea (n = 93/781, 11.9%), myalgia (n = 45/773, 5.8%), seizure (n = 35/778, 4.5%), chest pain (n = 32/766, 4.2%), mental drowsy or change (n = 15/775, 1.9%), arthralgia (n = 7/772, 0.9%), conjunctivitis (n = 6/777, 0.8%) and epistasix (n = 4/771, 0.5%). Five of the patients with seizure, had febrile seizure and nine patients had both seizure and mental change. Fever and cough were the most common symptoms in all age groups. The patients with rhinorrhea and diarrhea were on average younger, and those with shortness of breath, dyspnea, chest pain, sore throat, headache, myalgia and nausea were older, while the proportion of patients with fever did not differ significant according to age, but the fevers lasted longer in the younger age groups. Sneezing, conjunctivitis, epistaxis, mental drowsiness or change and arthralgia did not differ significantly between age groups (Table 2).
Table 2.
Symptoms and clinical course according to age in Korean patients hospitalized with influenza A (H1N1)
Chi-square test. *Fisher's exact test; †Independent t-test. ARDS, acute respiratory distress syndrome; NA, not available; CNS, central nervous system.
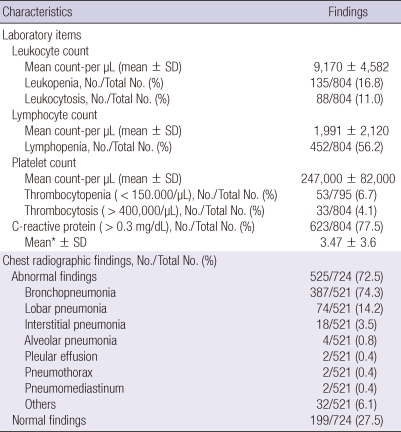
Mean white-cell count was 9,170 ± 4,582/µL (range 30-28,610), with leukopenia in 135/804 (16.8%) and leukocytosis in 88/804 (11%), and mean lymphocyte count was 1,991 ± 2,120/µL (range 0-25,050). Lymphopenia was observed in 452/804 (56.2%). Mean platelet count was 247,000 ± 82,000/µL (range 5,000-828,000/µL), with thrombocytopenia in 53/795 (6.7%) and thrombocytosis in 33/804 (4.1%). Six hundred and twenty-three (77.5%) of the 804 patients were positive for C-reactive protein (CRP), and mean CRP was 3.47 ± 3.6 mg/dL. Abnormalities in chest radiographs were detected in 525 (72.5%) of 724 patients. The most common abnormalities were bronchopneumonia (n = 387, 74.3%), followed by lobar pneumonia (n = 74, 14.2%), interstitial pneumonia (n = 18, 3.5%) and alveolar pneumonia (n = 4, 0.8%) (Table 3).
Table 3.
Laboratory findings in patients hospitalized with influenza A (H1N1)
*CRP greater than 0.3 mg/dL was considered positive.
Treatment and clinical course
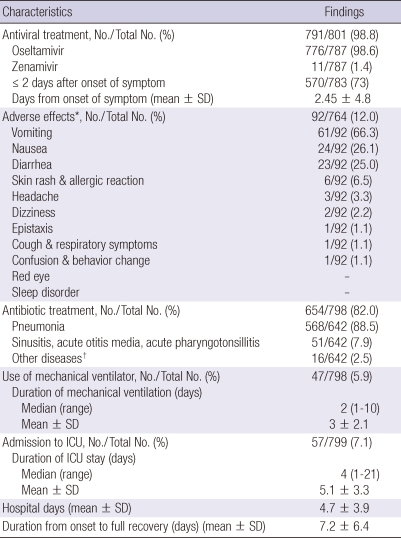
Five hundred and twenty-six (70.1%) of the 750 patients had pneumonia, 7/796 (0.9%) had acute respiratory distress syndrome (ARDS) and 9/737 (1.2%) had central nervous involvement. The proportion of patients with pneumonia and ARDS was higher among the older age groups (Table 2). The mean interval between symptom onset and hospitalization was 1.89 days (median, 1 day). Antiviral use was reported in 791/801 (98.8%) patients, and oseltamivir and zanamivir were given to 776/787 (98.6%) and 11/787 (1.4%) patients, respectively. Mean time from onset of symptoms to start of antiviral treatment was 2.5 days (median, 1 day) and 570 (73%) of the 783 patients were given an antiviral within 48 hr after onset of illness (Table 4) and this interval was shorter in older patients than in younger ones (Table 2).
Table 4.
Treatment strategies for patients hospitalized with influenza A (H1N1)
*Patients were treated with oseltamivir; †Urinary tract infection, meningitis, etc.
Ninety-two (12%) of the 764 patients taking oseltamivir reported one or more side effects. The most frequently reported symptom overall was vomiting (n = 61, 66.3%), followed by nausea (n = 24, 26.1%), diarrhea (n = 23, 25%), skin rash (n = 6, 6.5%) and headache (n = 3, 3.3%). There were no differences in side effects between the age groups.
Of the 798 patients, 654 (82%) had been treated with antibacterial agents for associated diseases: 568/642 (88.5%) for pneumonia-suspected bacterial infection, 51/642 (7.9%) for sinusitis, acute otitis media and pharyngotonsilitis, and 16/642 (2.5%) for other bacterial diseases such as urinary tract infection. Of the total patients, 40 (5.7%) were confirmed to have Mycoplasma pneumonia as well as influenza A (H1N1).
The mean time from symptom onset to full recovery was 7.2 ± 6.4 days. The mean duration of ventilator care in the 47/798 (5.9%) patients who received ventilator care was 3 ± 2.1 days, and the mean duration of ICU hospitalization in the 57/799 (7.1%) patients admitted to the ICU was 5.1 ± 3.3 days. Patients who required ventilator care or ICU admission totaled 95 (11.8%). These patients were classified as critically ill, and the others as not critically ill. All the patients recovered completely without complications (Table 4).
We undertook an analysis of the relationships between days from onset of symptoms to date of antiviral treatment, and days from symptom onset to full recovery, using Pearson's correlation coefficients. The correlation coefficient was about 0.754 and there was a strong positive correlation between the two variables (P < 0.001).
Comparison between critically ill patients and not critically ill patients
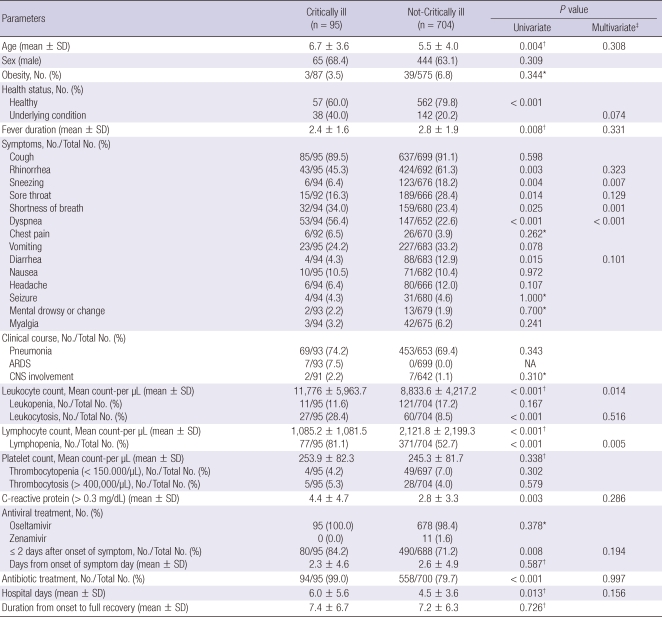
The mean ages of the critically ill patients and not critically ill patients were 6.7 and 5.5 yr, respectively (P = 0.004). More of the patients with underlying health conditions were critically ill patients (40% vs 20.2%, P < 0.001). There was no significant difference in obesity. The mean duration of fever was shorter in the critically ill patients (2.4 days vs 2.8 days, P = 0.017). More of the critically ill patients complained of shortness of breath and dyspnea, and fewer suffered from rhinorrhea, sneezing, sore throat and diarrhea (P < 0.03). There was no significant difference in frequency of pneumonia or central nervous involvement, but 7.5% of the critically ill patients suffered from acute respiratory distress syndrome (ARDS) compared to none of the not critically ill patients (Table 5).
Table 5.
Comparison between critically ill patients and not-critically ill patients
Chi-square test; *Fisher's exact test; †Independent t-test; ‡Factors significant (P < 0.05) in univariate analysies were submitted to multiple logistic regression analysis. ARDS, acute respiratory distress syndrome; NA, not available; CNS, central nervous system.
The critically ill patients had higher scores than the others in the following laboratory tests; leukocytosis 28.4% vs 8.5% (P < 0.001), lymphopenia 81.1% vs 52.7% (P < 0.001), and mean CRP 4.4 mg/dL vs 2.8 mg/dL (P = 0.003). There were no significant differences between the two groups in frequency of antiviral use and interval from symptom onset to initiation of antiviral treatment, but more of the critically ill patients received antiviral treatment in less than 2 days (84.2% vs 71.2%, P = 0.008). Also more of the patients receiving antibacterial agents were critically ill patients (99% vs 79.7%, P < 0.001) and their average length of hospital stay was greater (6 days vs 4.5 days, P = 0.013), but times from onset to full recovery did not differ significantly.
When we compared rates of lymphopenia in the critically ill patients and the not critically ill patients by age group, we found no significant difference in the group of under 2 yr (25% vs 10.1%), but significant differences in the 2-5 yr (72.7% vs 42.5%, P = 0.006) and 6-12 yr (90.8% vs 77.1%, P = 0.013) groups .
Factors that were significantly associated (P < 0.05) with the critically ill patients in univariate analyses were put into a full model for multiple logistic regression analysis. Shortness of breath, dyspnea, leukocytosis and lymphopenia were found to be positively associated with critical illness, while sneezing was negatively associated with critical illness.
DISCUSSION
In 2009 the influenza A (H1N1) virus infection became a full-scale pandemic, and Korea was among the countries severely affected. In this study we sought to assess the clinical patterns of children who were confirmed with H1NI infection and treated in six university hospitals.
In 2009, when the H1N1 virus infection swept the country, a total of 760,000 patients diagnosed with the infection were reported to the Korea Centers for Disease Control and Prevention (KCDC), 72% of whom were aged 0 to 19 yr (2). According to a report by the US Centers for Disease Control and Prevention, of the total number of individuals infected with the H1N1 virus, 32% were children aged 0-17 yr in the USA; in the UK, 41% were aged 0-14 yr; and in Japan, 59% were aged 0-15 yr. Thus, the proportion of infections occurring among children was higher in Korea than in these other countries (11, 17, 18).
The median age of the Korean patients was 5 yr, similar to the 4.8-6.0 yr in reports on child patients in other countries. Exceptions were Argentina with a median age of 10 months, and India with a median age of 2 yr (3-10).
The first of the patients in our study conducted from August 20, 2009 to January 30, 2010 was seen in the 34th week of 2009 and the peak occurred in the 44th week. The corresponding times in the KCDC data pertaining to all influenza A (H1N1) patients throughout the country were the 18th and 44th week of 2009, respectively (2, 19, 20).
Over the period of our study, both the first reported patient and the peak of infections were earlier in the age groups above 6 yr than in those below 6 yr. In addition, according to the KCDC report, the 10-19 yr age group began to be infected earlier, followed by the 0-9 yr group and those above 20 yr of age. Accordingly, it can be assumed that Korea's 2009 influenza A (H1N1) virus pandemic affected first those who returned from overseas trips, and the contagion then spread to school children and adolescents (2, 20).
The main symptoms of our child patients were similar to those of the influenza A (H1N1)-virus-infected patients in other countries. More of the younger patients suffered diarrhea and rhinorrhea, whereas more of the older patients suffered headache, sore throat, cough, dyspnea, chest pain, myalgia, and nausea (3-10).
As in previous studies, the fact that younger patients experienced more instances of diarrhea suggests that in young children the virus actively proliferates in the gastrointestinal tract as well as in the respiratory system. According to Yoo et al. (21), however, there was no statistically significant relationship between patient age, frequency of occurrence of gastrointestinal symptoms, and detection of H1N1 in feces. For rhinorrhea, the general finding is that the symptom is more common in younger children. In view of the higher viral load in younger patients and the longer period of shedding in patients with lowered immunity reported in previous studies, it is possible that patients' immunity, rather than virus proliferation itself, affects virus loads and symptoms (22).
According to reports from other countries, critically ill patients were older than those who were not critically ill (6, 23). We considered the possibility that younger patients were treated earlier, but actually the average time from symptom onset to initiation of antiviral agents in the younger patients was longer, and fewer of them received prescriptions within two days of the onset of illness. Probably younger children mounted weaker immune reactions, whereas school children, other adolescents, and young adults, had strong immune reactions. Moreover more of the older children developed excessive inflammation due to the virus, experienced pneumonia and serious complications such as ARDS and post-infectious encephalitis, were admitted to the ICU, and suffered death. Similarly more of the older patients in our study developed pneumonia, and suffered from ARDS and neurologic diseases, although the numbers of such cases were too small for statistical significance.
Interestingly, we found that duration of fever was significantly shorter in the critically ill patients, and that significantly more of these patients were prescribed antiviral drugs within two days of the onset of illness. In the Colorado study, fever or non-fever and duration of fever were unrelated to the probability of hospitalization of patients in wards or intensive care units, which also suggests that the extent of fever may not be directly relevant to disease severity (23).
Obesity was not found to be a risk factor in the child patients in this study, which was also the case in the Colorado research, but obesity was reported to be a risk factor in adults (23, 24).
Lymphopenia is known to occur in influenza virus infections, and we found that it was more common in the critically ill patients. This difference was not apparent in the below 2 yr group, but was clear in the 2-6 yr and above 6 yr groups. This is consistent with a report that lymphopenia develops more often in older patients (14). It also suggests that, since cytokine secretion varies according to age in influenza virus-infected patients, there is a difference in the extent of cell death with patient age, and that in children over 2 yr, with mature immune systems, differences in individual immune reactions may influence the occurrence of lymphopenia, and in turn the severity of disease (25, 26).
In our study, 11.8% of the patients were hospitalized in the ICU, but none of them died; and mortality in the KCDC's 0-19 yr group was only 0.005% (25/552,786). These figures reveal that Korea's highly contagious influenza A (H1N1) virus infection was not in fact very severe (2). This is substantially different from Argentina's reported ICU hospitalization rate of 19% and mortality of 5%, but similar to Japan's reported mortality of 0.00003% (38/1,220,000) for the below 15 yr group. These low rates are presumably due to accessibility of medical care and early administration of antiviral drugs. The proportion of cases receiving antiviral drugs within two days of the onset of illness was 73% in the present study and 88.9% in Japan, whereas in Argentina it was 12.5%, a marked difference. This suggests that mortality and critical illness was rare in young Korean children, presumably because of the early diagnosis and early administration of antiviral drugs (18, 27).
The frequency of side effects of the antiviral drugs used in this study was 12%, much lower than the 41%-53% in other studies. This could be due to preventive drug administration versus therapeutic drug administration, outpatient versus inpatient treatment, and interview-based surveys vs questionnaire-based surveys. The same frequent side effects were reported, however: mainly gastrointestinal symptoms such as nausea, vomiting, and diarrhea, followed by headache, insomnia, and psychoneurotic symptoms such as changes in emotions and behavior (28-30).
Oseltamivir, which had not been frequently used in young children, especially in those below 12 months, was widely used in this major epidemic, and this paved the way for further use of the drug. The data showed that oseltamivir has infrequent and mild side effects, and that when administered early it reduces the severity of disease, which suggest that it can be used to treat young children in a serious outbreak of influenza.
One limitation of this study was the small number of research institutions that participated; this led to inconsistent treatment and process observation. Hence, since Korea offers good access to medical care and Koreans prefer hospital treatment, the severity of the symptoms of the hospitalized patients may have been lower than in the public as a whole. Another limitation was the inability of our study to target all young Korean children infected with the influenza A (H1N1) virus. It did, however, target hospitalized child patients who had been confirmed as diagnosed with the disease, and thus it has been able to provide important information about the clinical patterns of Korean children infected with influenza A (H1N1).
References
- 1.Centers for Disease Control and Prevention. Hospitalized patients with novel influenza A (H1N1) virus infection: California, April-May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–541. [PubMed] [Google Scholar]
- 2.Korea Centers for Disease Control and Prevention. Analysis of reported pandemic influenza (A/H1N1 2009) virus infections in Korea: From April, 2009 through August, 2010. Public Health Wkly Rep. 2010;3:638–642. [Google Scholar]
- 3.Bettinger JA, Sauvé LJ, Scheifele DW, Moore D, Vaudry W, Tran D, Halperin SA, Pelletier L. Pandemic influenza in Canadian children: a summary of hospitalized pediatric cases. Vaccine. 2010;28:3180–3184. doi: 10.1016/j.vaccine.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 4.Calitri C, Gabiano C, Garazzino S, Pinon M, Zoppo M, Cuozzo M, Scolfaro C, Tovo PA. Clinical features of hospitalised children with 2009 H1N1 influenza virus infection. Eur J Pediatr. 2010;169:1511–1515. doi: 10.1007/s00431-010-1255-y. [DOI] [PubMed] [Google Scholar]
- 5.O'Riordan S, Barton M, Yau Y, Read SE, Allen U, Tran D. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ. 2009;182:39–44. doi: 10.1503/cmaj.091724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein M, Tasher D, Glikman D, Shachor-Meyouhas Y, Barkai G, Yochai AB, Leibovitz E, Hausman-Kedem M, Hess A, Megged O, Kassis I, Gresario G, Somekh E. Hospitalization of children with influenza A (H1N1) virus in Israel during the 2009 outbreak in Israel: a multicenter survey. Arch Pediatr Adolesc Med. 2010;164:1015–1022. doi: 10.1001/archpediatrics.2010.195. [DOI] [PubMed] [Google Scholar]
- 7.Parakh A, Kumar A, Kumar V, Dutta AK, Khare S. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1): an experience from a tertiary care center in north India. Indian J Pediatr. 2010;77:981–985. doi: 10.1007/s12098-010-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie JK, Gavali S, Acosta M, Samuel MC, Winter K, Jean C, Glaser CA, Matyas BT, Schechter R California Pandemic (H1N1) Working Group. Children hospitalized with 2009 novel influenza A (H1N1) in California. Arch Pediatr Adolesc Med. 2010;164:1023–1031. doi: 10.1001/archpediatrics.2010.203. [DOI] [PubMed] [Google Scholar]
- 9.Miroballi Y, Baird JS, Zackai S, Cannon JM, Messina M, Ravindranath T, Green R, Della-Latta P, Jenkins S, Greenwald BM, Fyruya EY, Graham PL, 3rd, Sonnett FM, Platt S, Delamora P, Saiman L. Novel influenza A (H1N1) in a pediatric health care facility in New York City during the first wave of the 2009 pandemic. Arch Pediatr Adolesc Med. 2010;164:24–30. doi: 10.1001/archpediatrics.2009.259. [DOI] [PubMed] [Google Scholar]
- 10.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, Cavalieri ML, Guglielmo MC, Areso MS, Gilligan T, Santucho F, Cabral G, Gregorio GL, Moreno R, Lutz MI, Panigasi AL, Saligari L, Caballero MT, Egues Almeida RM, Gutierrez Meyer ME, Neder MD, Davenport MC, Del Valle MP, Santidrian VS, Mosca G, Garcia Dominguez M, Alvarez L, Landa P, Pota A, Bolonati N, Dalamon R, Sanchez Mercol VI, Espinoza M, Peuchot JC, Karolinski A, Bruno M, Borsa A, Ferrero F, Bonina A, Ramonet M, Albano LC, Luedicke N, Alterman E, Savy V, Baumeister E, Chappell JD, Edwards KM, Melendi GA, Polack FP. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2009;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 11.Updated CDC Estimates of 2009 H1N1 Cases and Related Hospitalizations and Deaths from April 2009 - April 10, 2010. Centers for Disease Control and Prevention. [accessed on 25 October 2011]. Available at: http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm.
- 12.Noh JY, Yim SY, Heo JY, Choi WS, Song JY, Cheong HJ, Kim WJ. Epidemiological and clinical characteristics of pandemic influenza (H1N1 2009) Infect Chemother. 2010;42:69–75. [Google Scholar]
- 13.Park SI, Kim MJ, Hwang HY, Oh CE, Lee JH, Park JS. Clinical characteristics of children with 2009 pandemic influenza A (H1N1) admitted in a single institution. Korean J Pediatr. 2010;53:886–891. doi: 10.3345/kjp.2010.53.10.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun JK, Cha BH, Uh Y, Kim HY, Kim YK, Kwon WC, Kim HM. The association of lymphopenia with the clinical severity in the Korean children admitted to the hospital with pandemic (H1N1) 2009 infection. Infect Chemother. 2011;43:36–41. [Google Scholar]
- 15.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, Kim YT, Lee CG. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51:1–25. [Google Scholar]
- 16.Stanley FL. Reference intervals for laboratory tests and children. In: Kliegman RM, Stanton BF, St. Geme JW 3rd, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders; 2011. p. 2466. [Google Scholar]
- 17.Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, Yardley IE. Mortality from pandemic A/H1N1 2009 influenza in England: Public Health Surveillance Study. BMJ. 2009;339:b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugaya N, Shinjoh M, Mitamura K, Takahashi T. Very low pandemic influenza A (H1N1) 2009 mortality associated with early neuraminidase inhibitor treatment in Japan: Analysis of 1000 hospitalized children. J Infect. 2011;63:288–294. doi: 10.1016/j.jinf.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Shin SS, Jun BY, Lee JK. National level response to pandemic (H1N1) 2009. J Prev Med Public Health. 2010;43:99–104. doi: 10.3961/jpmph.2010.43.2.99. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Kim JH, Shin SY, Kang YA, Lee HG, Kim JS, Lee JK, Cho B. Fatal cases of 2009 pandemic influenza A (H1N1) in Korea. J Korean Med Sci. 2011;26:22–27. doi: 10.3346/jkms.2011.26.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo SJ, Moon SJ, Kuak EY, Yoo HM, Kim CK, Chey MJ, Shin BM. Frequent detection of pandemic (H1N1) 2009 virus in stools of hospitalized patients. J Clin Microbiol. 2010;48:2314–2315. doi: 10.1128/JCM.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.To KK, Chan KH, Li IW, Tsang TY, Tse H, Chan JF, Hung IF, Lai ST, Leung CW, Kwan YW, Lau YL, Ng TK, Cheng VC, Peiris JS, Yuen KY. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol. 2010;82:1–7. doi: 10.1002/jmv.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagdure D, Curtis DJ, Dobyns E, Glodé MP, Dominguez SR. Hospitalized children with 2009 pandemic influenza A (H1N1): comparison to seasonal influenza and risk factors for admission to the ICU. PLoS One. 2010;5:e15173. doi: 10.1371/journal.pone.0015173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 25.Nichols JE, Niles JA, Roberts NJ., Jr Human lymphocyte apoptosis after exposure to influenza A virus. J Virol. 2001;75:5921–5929. doi: 10.1128/JVI.73.13.5921-5929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Wang Y, Gilmore X, Xu K, Chen M, Tebebi P, Mbawuike IN. Apoptosis and reduced influenza A virus specific CD8+ T cells in aging mice. Cell Death Differ. 2002;9:651–660. doi: 10.1038/sj.cdd.4401011. [DOI] [PubMed] [Google Scholar]
- 27.Libster R, Coviello S, Cavalieri ML, Morosi A, Alabart N, Alvarez L, Ferrero F, Edwards KM, Polack FP. Pediatric hospitalizations due to influenza in 2010 in Argentina. N Engl J Med. 2010;363:2472–2473. doi: 10.1056/NEJMc1008806. [DOI] [PubMed] [Google Scholar]
- 28.Giesecke J. Experiences of giving oseltamivir to school children during the 2009 influenza A (H1N1) pandemic. Euro Surveill. 2010;15:19570. [PubMed] [Google Scholar]
- 29.Strong M, Burrows J, Stedman E, Redgrave P. Adverse drug effects following oseltamivir mass treatment and prophylaxis in a school outbreak of 2009 pandemic influenza A (H1N1) in June 2009, Sheffield, United Kingdom. Euro Surveill. 2010;15:19565. [PubMed] [Google Scholar]
- 30.Wallensten A, Oliver I, Lewis D, Harrison S. Compliance and side effects of prophylactic oseltamivir treatment in a school in South West England. Euro Surveill. 2009;14:19285. doi: 10.2807/ese.14.30.19285-en. [DOI] [PubMed] [Google Scholar]