Abstract
This study was designed to compare the hospital-related costs of elective abdominal aortic aneurysm (AAA) treatment and cost structure between endovascular aneurysm repair (EVAR) and open surgical repair (OSR) in Korean health care system. One hundred five primary elective AAA repairs (79 OSRs and 26 EVARs) performed in the Seoul National University Hospital from 2005 to 2009 were included. Patient characteristics were similar between two groups except for older age (P = 0.004) and more frequent history of malignancy (P = 0.031) in EVAR group. Thirty-day mortality rate was similar between two groups and there was no AAA-related mortality in both groups for 5 yr after repair. The total in-hospital costs for the index admission were significantly higher in EVAR patients (mean, KRW19,857,119) than OSR patients (mean KRW12,395,507) (P < 0.001). The reimbursement was also significantly higher in EVAR patients than OSR patients (mean, KRW14,071,081 vs KRW6,238,895, P < 0.001) while patients payments was comparable between two groups. EVAR patients showed higher follow-up cost up to 2 yr due to more frequent imaging studies and reinterventions for type II endoleaks (15.4%). In the perspective of cost-effectiveness, this study suggests that the determination of which method to be used in AAA treatment be more finely trimmed and be individualized.
Keywords: Aortic Aneurysm, Abdominal; Endovascular Repair; Open Surgical Repair; Cost Effectiveness
INTRODUCTION
Abdominal aortic aneurysm (AAA) is a life-threatening disease when rupture occurs. Elective open surgical repair (OSR) has been the optimal therapy to prevent AAA rupture. However, after the first introduction by Parodi et al. (1), endovascular aneurysm repair (EVAR) has increased dramatically and is the most popular therapy nowadays (2). A number of studies reported that EVAR has significant benefit over OSR in terms of rapid postoperative recovery, fewer intensive care unit administration and reduction in hospital stay (3, 4). These benefits might result in reduced hospital costs for AAA repair. Early studies reported that EVAR was associated with lower hospital cost (5). However, this initial advantage in cost is gradually disappearing in the long-term after EVAR due to more frequent life-long follow-up imaging and higher reintervention rates. Some recent studies have reported even similar or higher hospital costs for EVAR (4, 6-10).
The cost-effectiveness of a new medical therapy can vary according to the medical insurance system in each nation. Although some studies from western countries have dealt with cost-effectiveness of EVAR in the management of AAA, these results cannot be applied directly to Korea. To justify the routine use of EVAR for AAA treatment in Korea, it is needed to prove the cost-effectiveness under the current situation of reimbursement and patient payment of the National Health Insurance (NHI) Corporation. Health care costs can affect clinical decision making but also the policy of the National Health Insurance program. Until recently, however, no studies regarding cost-effectiveness of EVAR is conducted in Korea. Therefore the aim of this study was to compare the hospital-related cost of elective AAA treatment including peri- and post-operative costs and to study cost structure between those selected for open or endovascular repair.
MATERIALS AND METHODS
Between January 2005 and December 2009, 120 infrarenal AAA repairs were performed in Seoul National University Hospital. Among them, 15 operations were excluded from the study due to various reasons, including 9 ruptured aneurysms, 2 stent graft migrations, 1 foreign patient, and 3 complicated AAAs such as infected aneurysm. The remaining 105 primary elective AAA repairs (79 OSRs and 26 EVARs) were included in this study.
Patients were generally admitted 3 or 4 days before OSR or EVAR. The decision to choose the repair method was based on the anatomic configuration of the aneurysm, patient comorbidities, and patient preference with surgeon's recommendation. OSR was performed through midline (n = 70) or retroperitoneal (n = 9) approach and a Dacron (DuPont, Wilmington, DE, UAS) graft was implanted. EVAR was performed using bilateral femoral cutdown under general anesthesia. Zenith (Cook, Bloomington, IN, USA; n = 22) or Excluder (W.L. Gore & Associates, Flagstaff, AZ, USA; n = 4) were placed. For follow-up, patients treated with EVAR were routinely checked with computed tomography (CT) angiography at 1, 6, and 12 months after surgery and annually thereafter with clinical examination at each hospital visit. The standard follow-up program of OSR patients consisted of clinical examination at 1, 6, and 12 months after surgery and annually thereafter
Medical records were reviewed to obtain patients characteristics, preoperative investigations, operative details, early and late complications, the need for additional procedures, the length of stay in the intensive care unit (ICU) and hospital, and outcome parameters as defined by the Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery (11). Additional data on survival for patients with loss to follow-up were obtained from the Electronic Data for Resident Registration of the Ministry of Public Administration and Security, Korean Government and by telephone survey.
All hospital-related costs linked to AAA treatment were included in the cost analysis. The cost for each patient was determined by accessing the hospital cost accounting system which includes detailed data on operations, anesthesia, pre- and postoperative investigations, intensive care, and postoperative hospitalization. The total hospital cost for the index admission was divided into preoperative costs, operative costs, and postoperative costs including costs for ICU stay and hospital stay. Follow-up costs linked to AAA treatment were obtained from discharge until July 2010.
Preoperative cost was determined as costs incurred by all investigations and hospitalization performed preoperatively after the decision for aneurysm repair was made. Therefore, preoperative costs included cost of CT angiography, cardiorespiratory investigations, consultations, medications, clinical laboratory costs, bed costs and interventions performed during hospital admission. Operative costs included graft costs, operation charges, anesthesia charges, medications, and additional costs for guidewires, catheters, sheath, and contrast used during EVAR. Postoperative cost was defined as the sum of costs incurred by postoperative ICU stay and all cost after returning to the general ward until discharge such as bed and nursing costs, medical imaging, consultations, medications, clinical laboratory costs, and additional interventions. Follow-up costs included all AAA treatment-related costs after discharge such as routine follow-up costs, CT angiography, additional interventions required to treat endoleaks and rehospitalization.
Statistical outliers (OSR, n = 2; EVAR, n = 1) of more than 3 standard deviations from the mean for total hospital costs during the main admission were excluded from the cost analysis (12).
In Korea, total hospital costs are divided into benefit-service charges of which 80% are reimbursed by the NHI Corporation, and nonbenefit-service charges. Patients pay 20% of the benefit services and all nonbenefit-service charges. Thus, total hospital cost, reimbursement by NHI Corporation and patient payment were calculated for each patient and compared between OSR and EVAR patients.
Statistical analyses were conducted using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as means ± standard deviation values or as frequencies (percentages), according to the data type. For data which did not fit normal distribution, medians were reported with interquartile range and compared with Wilcoxon rank sum test. Mean values were compared by Mann-Whitney U test for continuous variables. Noncontinuous variables were compared by chi-squared test. Patient survival rates were determined by Kaplan-Meier analysis and log-rank method. A P value < 0.05 was considered statistically significant. All costs and charges were expressed in Korean won (KRW).
Ethics statement
This study was performed after obtaining approval from the institutional review board of Seoul National University Hospital (IRB No. H-1006-005-319). This study was waived of informed consent from the board.
RESULTS
Patient characteristics
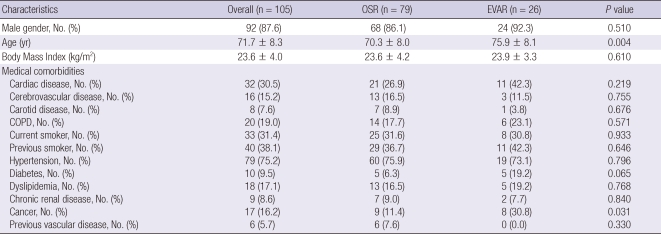
During the study period, 79 patients underwent OSR and 26 patients underwent EVAR as primary elective treatment for AAA. The baseline clinical characteristics of the patients in the two groups are shown in Table 1. The mean age of the patients in the EVAR group was higher than the OSR group (75.0 ± 8.1 vs 70.3 ± 8.0, P = 0.004), and combined malignant disease was more frequent in the EVAR group compared to the OSR group (30.8% vs 11.4%, P = 0.031). There was no significant difference between the groups with regard to other clinical characteristics examined.
Table 1.
Demographics and clinical characteristics of patients undergoing OSR and EVAR
OSR, open surgical repair; EVAR, endovascular aneurysm repair; COPD, chronic obstructive pulmonary disease.
The anatomical characteristics of AAA were quite similar between the two groups. The mean diameter of the aneurysms were 58.5 ± 10.6 mm in the OSR group and 57.2 ± 8.6 mm in the EVAR group (P = 0.563). There was no difference in the length of proximal neck (26.8 ± 14.9 mm in the OSR group vs 27.9 ± 9.8 mm in the EVAR group, P = 0.727) between the two groups and more than two third of the patients showed aneurysmal involvement of common iliac artery in both groups (P = 0.607).
Postoperative complications and clinical outcome
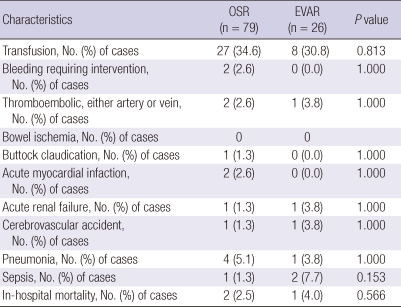
Both OSR and EVAR groups had a low morbidity. The perioperative and postoperative complications are listed in Table 2. EVAR resulted in a tendency for a reduced length of hospital stay as well as length of ICU stay. The median length of postoperative hospital stay was 8 days in the EVAR group (range, 7-14.5 days) and was 10 days in the OSR group (range, 8-14 days; P = 0.078). All patients except one in the EVAR group went to the ICU postoperatively but there appeared to be a shorter length of ICU stay in the EVAR group, with a median ICU stay of 1 day (range, 1-2 days) in the EVAR group compared with 2 days (range, 1-2 days) in the OSR group (P = 0.110).
Table 2.
Peri- and post-operative morbidities
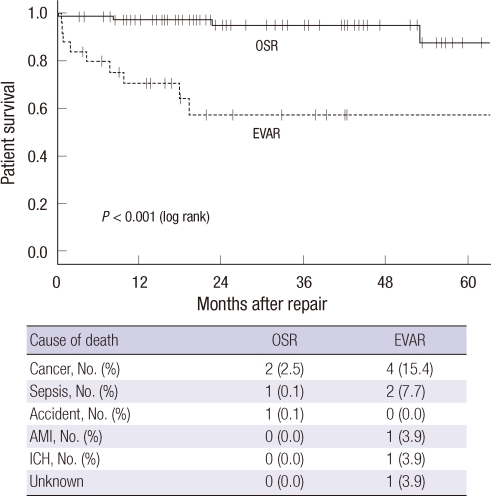
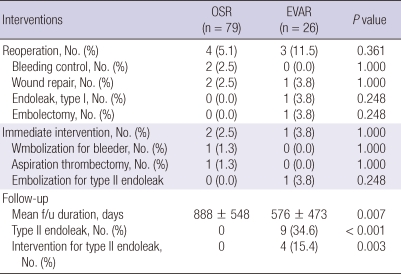
There was no significant difference in 30-day mortality between the groups (2.5% for OSR vs 4.0% for EVAR, P = 0.566). Causes of 3 inhospital mortalities were sepsis in 2 patients and subdural hemorrhage in an other patient. Although 5-yr patient survival was significantly inferior in the EVAR group compared to OSR group (54.5% vs 91.9%, P < 0.001), there was no AAA-related mortality in both groups. The causes of patient death were malignant disease, sepsis, acute myocardiac infarction, fall down accident, and intracranial hemorrhage (Fig. 1). With regard to the frequencies of postoperative interventions during the index admission, there were no differences between the two groups (Table 3). Four patients (5.1%) in the OSR group and 3 patients (11.5%) in the EVAR group required reoperation (P = 0.361). One patient from the EVAR group underwent OSR because of persistent type I endoleak at 7 days after EVAR. The EVAR group showed significantly higher rate of type II endoleak (34.6%) and need for intervention of type II endoleak (15.4%, P < 0.001), with a mean follow up duration of 888 ± 548 days in the OSR group and 576 ± 473 days in the EVAR group.
Fig. 1.
Patient survival and the causes of death in both groups. OSR, open surgical repair; EVAR, endovascular repair; AMI, acute myocardial infarction; ICH, intracranial hemorrhage.
Table 3.
Postoperative reinterventions
Costs
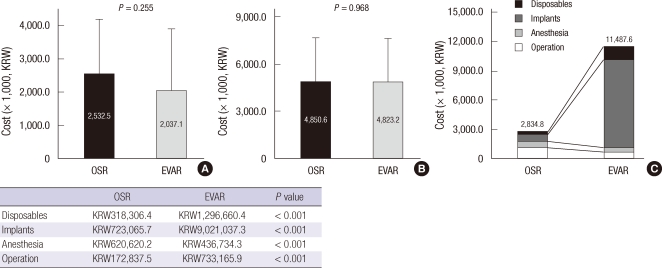
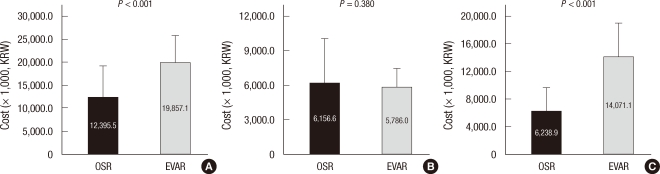
Figs. 2 and 3 display the cost structure of both treatment groups. Operative costs comprised a large proportion of total hospital costs. Operative cost was significantly higher in the EVAR group compared to the OSR group, which was mainly attributed by the high cost of endografts and disposables such as sheaths and guidewires (Fig. 2). Therefore total hospital costs (KRW19,857,119.2 ± 5,909,556.0 vs KRW12,395,507.6 ± 6,788,119.2, P < 0.001) were significantly higher in EVAR patients compared to the OSR group. Because of the reimbursement of the endo-graft by the NHI Corporation, patient payment was comparable between two groups (KRW6,156,612.5 ± 3,931,410.6 for OSR vs KRW5,786,037.8 ± 1,684,082.5 for EVAR, P = 0.380) while the reimbursement by NHI Corporation was significantly higher in EVAR group (KRW 6,238,895.1 ± 3,393,210.0 for OSR vs KRW14,071,081.4 ± 4,856,932.5 for EVAR, P < 0.001) (Fig. 3).
Fig. 2.
Cost structures for OSR and EVAR. (A) Preoperative costs, (B) Postoperative costs including costs for ICU stay, and (C) cost for procedure per se. OSR, open surgical repair; EVAR, endovascular repair.
Fig. 3.
Cost structure in OSR and EVAR group during the index admission. (A) total hospital cost, (B) patient payments and (C) NHI reimbursement. OSR, open surgical repair; EVAR, endovascular repair; NHI, National Health Insurance.
When the total hospital costs incurred during the index admission were divided into three stages, the preoperative and postoperative costs were similar in both groups. The mean preoperative costs (Fig. 2A) including consultation, cardiorespiratory investigations, imaging studies, clinical laboratory costs and bed costs (KRW2,532,484 ± 1,652,591 for OSR vs KRW2,037,121 ± 1,876,712 for EVAR, P = 0.255) were similar. Furthermore, the mean postoperative costs (Fig. 2B) including ICU care and postoperative ward admission (KRW4,823,165.8 ± 2,813,945.4 vs KRW4,850,649.2 ± 2,807,148.9, respectively for EVAR and OSR group, P = 0.968) were similar. The length of postoperative ICU and hospital stay tended to be shorter in the EVAR group, however it could not induce economic advantage nor offset the cost of endo-graft (Fig. 2).
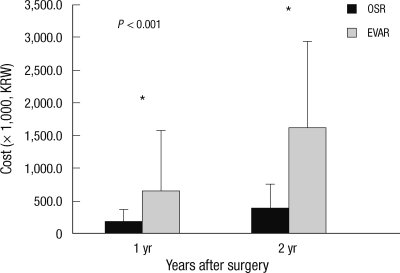
During the postoperative follow-up, patients in the EVAR group were routinely checked with CT angiogram at 1, 6, and 12 months after surgery. Four (15.4%) out of 9 (34.6%) patients with type II endoleak were treated with additional intervention. No patient required conversion to OSR. At 1 yr follow-up, the EVAR group required significantly higher cost than the OSR group (KRW648,538.1 ± 926,975.6 vs KRW180,844.8 ± 189,659.1, P < 0.001) and the discrepancy in the cumulative cost increased further till 2 yr follow-up (KRW1,612,226 ± 1,319,305.8 vs KRW 393,203.8 ± 356,785.8 respectively, P < 0.001).
DISCUSSION
The number of patients with AAA is rapidly increasing in Korea and, after the inception of reimbursement for endo-grafts by the Health Insurance Reimbursement Association (HIRA), EVAR has become the preferred method for AAA treatment, replacing OSR (13). Health economic evaluation for a new treatment strategy should be mandatory before it is reimbursed within the health service system and a number of reports on cost-effectiveness of EVAR have been published in western countries (5-10, 14-23). However, this issue of cost-effectiveness of EVAR has never been evaluated in the Korean health care system.
The principal finding of this study is that the total in-hospital costs for EVAR (KRW19,857,119.2) were significantly higher than those for OSR (KRW12,395,507.6), which contrasted with several reports from Western countries that showed EVAR was less costly or equal in cost to OSR (10, 19). The most important cause for the difference in total in-hospital costs between EVAR and OSR was the cost of the procedures themselves. Although there were reductions in anesthesia and operation fee in the EVAR group, this could not recoup the high costs of the endovascular graft (KRW9,021,037.3) and disposables (KRW1,296,660.4) such as guidewires, sheaths and radiocontrast materials (Fig. 2). This is mainly due to the lower costs of operation-related fees in Korea, while higher operation fees in western countries recoup the endograft costs. In addition, almost every patient in the EVAR group in this study was admitted to the ICU and the length of hospital stay was also comparable between the two study groups. This might be caused by the treatment policy of the vascular surgery team in the study hospital. However, this reduction in hospital and ICU stay cannot offset the costs of the endovascular grafts, because the mean cost of the endografts accounted for 45.4% of the total in-hospital cost in the EVAR group and the mean cost of postoperative ICU and hospital stay accounted for only 24.3%. Furthermore, this study showed that 2-yr cumulative follow-up costs were also higher for EVAR than OSR (Fig. 4), which is similar to the western studies. Because CT was regularly checked to identify endoleaks, graft migration, graft kinking or other complications related to EVAR and secondary intervention was additionally required in some cases, follow-up costs increase cost disparity after EVAR (4, 8, 17).
Fig. 4.
Aneurysm-related costs for follow-up after discharge. OSR, open surgical repair; EVAR, endovascular repair.
Another interesting finding in this study was that patient payments were not different between EVAR (KRW5,786,037.8) and OSR (KRW6,156,612.5) patients. This was caused by the reimbursement off higher cost materials including endovascular grafts and disposables by the HIRA which accounted for the largest portion of the costs in EVAR, the amount of reimbursement by NHI Corporation reaching KRW14,071,081.4. Therefore, as the number of patients undergoing EVAR increases, the financial burden of the NHI Corporation is becoming more considerable. This issue should be discussed for the proper distribution of the national health resources.
Three randomized trials comparing EVAR and OSR have all shown a markedly reduced 30-day operative mortality for EVAR and this benefit has made EVAR a more prevalent treatment option (3, 4, 24). This study also revealed the safety of EVAR with limited follow-up period. No AAA-related deaths occurred in both groups (Fig. 1). However, considering the recent EVAR1 trial by Greenhalgh et al. (8), longer-term follow-up analysis showed increased rates of endograft-related complications including type II endoleaks and additional interventions in the EVAR group. In contrast to EVAR, OSR has strong evidence regarding durability (25, 26) and no graft-related complications occurred in this study population. Overall, current evidence including this study show that EVAR has early advantage in terms of operation-related mortality and morbidity but is associated with significantly higher graft cost which is not offset by short stay in ICU or hospital, higher graft-related complications and reintervention resulting in higher follow-up costs.
It is noteworthy that new graft-related complications can develop and the need for reintervention persists even at 8 yr after EVAR (8). Considering the continued evaluation with CT for graft-related complications, EVAR may prove to be cost-effective only in very elderly patients. It is clear that, although EVAR showed lower operative mortality, careful follow-up after EVAR is also mandatory. Therefore costs for EVAR procedure and for follow-up are significantly higher. In addition, EVAR patients are potentially exposed to a significant cumulative amount of iatrogenic radiation from pre-operative assessment to death by life-long follow-up of CT angiography, with the associated carcinogenic risks (27). In this regard it is doubtful whether EVAR routinely offered even to relatively young patients with low operative risks. The eventual impact of the radiation dose by EVAR and life-long check up of CT is more pertinent in younger patients requiring aneurysm repair, potentially exposing these patients to carcinogenic risk with a latency period of between 10 and 20 yr. Therefore, the decision of which method to be used in AAA treatment should be individualized (28). All patients should be informed of the advantages and disadvantages of EVAR and OSR. Medical practitioners should balance the patient's operative risks and disadvantages of EVAR.
Summarizing, the total in-hospital costs for EVAR are significantly higher than those for OSR. A lower number of days of hospital admission and ICU care cannot offset higher procedural costs for EVAR. This study suggests "EVAR only" policy cannot be accepted in perspective of cost-effectiveness in Korea and which strategy to choose, open or endovascular, in AAA treatment should be individualized. Further research is required to determine the ideal indication of EVAR in terms of patient's age, expected follow-up period after EVAR, costs for EVAR per se and follow-up with reintervention.
References
- 1.Parodi JC. Endovascular repair of abdominal aortic aneurysms and other arterial lesions. Surg Technol Int. 1994;3:431–436. [PubMed] [Google Scholar]
- 2.Park YJ, Kim N, Kim YW. Investigation of current trend of AAA treatment in Korea. J Korean Surg Soc. 2011;80:125–130. [Google Scholar]
- 3.Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, Buskens E, Grobbee DE, Blankensteijn JD Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 4.EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 5.Hölzenbein J, Kretschmer G, Glanzl R, Schön A, Thurnher S, Winkelbauer F, Trubel W, Minar E, Ahmadi A, Huk I, Ingruber H, Ehringer H, Lammer J, Polterauer P. Endovascular AAA treatment: expensive prestige or economic alternative? Eur J Vasc Endovasc Surg. 1997;14:265–272. doi: 10.1016/s1078-5884(97)80238-9. [DOI] [PubMed] [Google Scholar]
- 6.Sternbergh WC, 3rd, Money SR. Hospital cost of endovascular versus open repair of abdominal aortic aneurysms: a multicenter study. J Vasc Surg. 2000;31:237–244. doi: 10.1016/s0741-5214(00)90154-x. [DOI] [PubMed] [Google Scholar]
- 7.Dryjski M, O'Brien-Irr MS, Hassett J. Hospital costs for endovascular and open repair of abdominal aortic aneurysm. J Am Coll Surg. 2003;197:64–70. doi: 10.1016/S1072-7515(03)00341-7. [DOI] [PubMed] [Google Scholar]
- 8.Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ United Kingdom EVAR Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863–1871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 9.Prinssen M, Buskens E, de Jong SE, Buth J, Mackaay AJ, van Sambeek MR, Blankensteijn JD DREAM Trial participants. Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. 2007;46:883–890. doi: 10.1016/j.jvs.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Mani K, Björck M, Lundkvist J, Wanhainen A. Similar cost for elective open and endovascular AAA repair in a population-based setting. J Endovasc Ther. 2008;15:1–11. doi: 10.1583/07-2258.1. [DOI] [PubMed] [Google Scholar]
- 11.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, Matsumura JS, May J, Veith FJ, Fillinger MF, Rutherford RB, Kent KC Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of the Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repai. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 12.Mani K, Lundkvist J, Holmberg L, Wanhainen A. Challenges in analysis and interpretation of cost data in vascular surgery. J Vasc Surg. 2010;51:148–154. doi: 10.1016/j.jvs.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Walker KL, Lipori P, Lee WA, Beaver TM. Cost of thoracic endovascular aortic repair versus open repair and implications for the US health care system. J Thorac Cardiovasc Surg. 2010;139:231–232. doi: 10.1016/j.jtcvs.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Patel ST, Haser PB, Bush HL, Jr, Kent KC. The cost-effectiveness of endovascular repair versus open surgical repair of abdominal aortic aneurysms: a decision analysis model. J Vasc Surg. 1999;29:958–972. doi: 10.1016/s0741-5214(99)70237-5. [DOI] [PubMed] [Google Scholar]
- 15.Quinoñes-Baldrich WJ, Garner C, Caswell D, Ahn SS, Gelabert HA, Machleder HI, Moore WS. Endovascular, transperitoneal, and retroperitoneal abdominal aortic aneurysm repair: results and costs. J Vasc Surg. 1999;30:59–67. doi: 10.1016/s0741-5214(99)70176-x. [DOI] [PubMed] [Google Scholar]
- 16.Clair DG, Gray B, O'hara PJ, Ouriel K. An evaluation of the costs to health care institutions of endovascular aortic aneurysm repair. J Vasc Surg. 2000;32:148–152. doi: 10.1067/mva.2000.105663. [DOI] [PubMed] [Google Scholar]
- 17.Hayter CL, Bradshaw SR, Allen RJ, Guduguntla M, Hardman DT. Follow-up costs increase the cost disparity between endovascular and open abdominal aortic aneurysm repair. J Vasc Surg. 2005;42:912–918. doi: 10.1016/j.jvs.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Michaels JA, Drury D, Thomas SM. Cost-effectiveness of endovascular abdominal aortic aneurysm repair. Br J Surg. 2005;92:960–967. doi: 10.1002/bjs.5119. [DOI] [PubMed] [Google Scholar]
- 19.Visser JJ, van Sambeek MR, Hunink MG, Redekop WK, van Dijk LC, Hendriks JM, Bosch JL. Acute abdominal aortic aneurysms: cost analysis of endovascular repair and open surgery in hemodynamically stable patients with 1-year follow-up. Radiology. 2006;240:681–689. doi: 10.1148/radiol.2403051005. [DOI] [PubMed] [Google Scholar]
- 20.Hynes N, Sultan S. A prospective clinical, economic, and quality-of-life analysis comparing endovascular aneurysm repair (EVAR), open repair, and best medical treatment in high-risk patients with abdominal aortic aneurysms suitable for EVAR: the Irish patient trial. J Endovasc Ther. 2007;14:763–776. doi: 10.1583/07-2194.1. [DOI] [PubMed] [Google Scholar]
- 21.Tarride JE, Blackhouse G, De Rose G, Novick T, Bowen JM, Hopkins R, O'Reilly D, Goeree R. Cost-effectiveness analysis of elective endovascular repair compared with open surgical repair of abdominal aortic aneurysms for patients at a high surgical risk: a 1-year patient-level analysis conducted in Ontario, Canada. J Vasc Surg. 2008;48:779–787. doi: 10.1016/j.jvs.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Blackhouse G, Hopkins R, Bowen JM, De Rose G, Novick T, Tarride JE, O'Reilly D, Xie F, Goeree R. A cost-effectiveness model comparing endovascular repair to open surgical repair of abdominal aortic aneurysms in Canada. Value Health. 2009;12:245–252. doi: 10.1111/j.1524-4733.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayes PD, Sadat U, Walsh SR, Noorani A, Tang TY, Bowden DJ, Gillard JH, Boyle JR. Cost-effectiveness analysis of endovascular versus open surgical repair of acute abdominal aortic aneurysms based on worldwide experience. J Endovasc Ther. 2010;17:174–182. doi: 10.1583/09-2941.1. [DOI] [PubMed] [Google Scholar]
- 24.Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Jr, Matsumura JS, Kohler TR, Lin PH, Jean-Claude JM, Cikrit DF, Swanson KM, Peduzzi PN Open Versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535–1542. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 25.Hallett JW, Jr, Marshall DM, Petterson TM, Gray DT, Bower TC, Cherry KJ, Jr, Gloviczki P, Pairolero PC. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg. 1997;25:277–284. doi: 10.1016/s0741-5214(97)70349-5. [DOI] [PubMed] [Google Scholar]
- 26.Conrad MF, Crawford RS, Pedraza JD, Brewster DC, Lamuraglia GM, Corey M, Abbara S, Cambria RP. Long-term durability of open abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:669–675. doi: 10.1016/j.jvs.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 28.Kent KC. Endovascular aneurysm repair: is it durable? N Engl J Med. 2010;362:1930–1931. doi: 10.1056/NEJMe1004299. [DOI] [PubMed] [Google Scholar]