Abstract
Rationale
Previously, we demonstrated that a deoxycorticosterone acetate (DOCA)-salt hypertensive mouse model produces cardiac oxidative stress and diastolic dysfunction with preserved systolic function. Oxidative stress has been shown to increase late inward sodium current (INa), reducing the net cytosolic Ca2+ efflux.
Objective
Oxidative stress in the DOCA-salt model may increase late INa resulting in diastolic dysfunction amenable to treatment with ranolazine.
Methods and Results
Echocardiography detected evidence of diastolic dysfunction in hypertensive mice that improved after treatment with ranolazine (E/E′, sham 31.9 ± 2.8, sham+ranolazine 30.2 ± 1.9, DOCA-salt 41.8 ± 2.6, and DOCA-salt+ranolazine 31.9 ± 2.6, p = 0.018). The end diastolic pressure volume relationship slope was elevated in DOCA-salt mice, improving to sham levels with treatment (sham 0.16 ± 0.01 vs. sham+ranolazine 0.18 ± 0.01 vs. DOCA-salt 0.23 ± 0.2 vs. DOCA-salt+ranolazine 0.17 ± 0.01 mm Hg/L, p < 0.005). DOCA-salt myocytes demonstrated impaired relaxation, τ, improving with ranolazine (DOCA-salt 0.18 ± 0.02, DOCA-salt + ranolazine 0.13 ± 0.01, Sham 0.11 ± 0.01, Sham + ranolazine 0.09 ± 0.02 s, p = 0.0004). Neither late INa nor the Ca2+ transients were different from sham myocytes. Detergent extracted fiber bundles from DOCA-salt hearts demonstrated increased myofilament response to Ca2+ with glutathionylation of myosin binding protein C. Treatment with ranolazine ameliorated the Ca2+ response and cross-bridge kinetics.
Conclusions
Therefore, diastolic dysfunction could be reversed by ranolazine, likely resulting from a direct effect on myofilaments, indicating that cardiac oxidative stress may mediate diastolic dysfunction through altering the contractile apparatus.
Keywords: Diastole, ranolazine, oxidative stress, myofilaments
Introduction
Diastolic dysfunction is characterized by prolonged relaxation of the myocardium and untreated can lead to the clinical syndrome, heart failure with preserved ejection fraction (HFpEF). HFpEF is an increasingly prevalent health burden accounting for significant morbidity, mortality, and healthcare expenditures.1–4 The underlying mechanisms in diastolic dysfunction are not clearly understood, limiting treatment options.5 Recent large clinical trials using the standard therapies for systolic heart failure have failed to demonstrate improvement, further emphasizing differences in the underlying pathophysiology of diastolic dysfunction.6–8
There are several potential mechanisms for diastolic dysfunction. One is increased diastolic Ca2+ resulting in a slowed ventricular relaxation and diastolic dysfunction. Ca2+ is removed from the cytosol during diastole by the sarcoplasmic reticular Ca2+-ATPase (SERCA) and the Na+/Ca2+ exchanger (NCX). The NCX couples Ca2+ extrusion to the transmembrane Na+ gradient.9 In the failing heart, a small number of the Na+ channels fail to inactivate creating a late Na+ current (INa).10–13 The late INa increases Na+ entry into the cell, reducing Ca2+ extrusion by NCX.14 Since late INa is increased with oxidative stress15, this represents a possible mechanism underlying diastolic dysfunction. Alternatively, myofilament cross-bridge kinetics and response to Ca2+ are regulated16–19 and, if altered, could contribute to diastolic dysfunction.
Previously, we have reported a hypertensive mouse model (DOCA-salt) of diastolic dysfunction in which oxidative stress is central to the pathology.20 Ranolazine, a novel anti-ischemic medication, is used clinically to treat angina without lowering blood pressure or heart rate.21, 22 ROS induced increases in cardiomyocyte late INa have been shown to be attenuated in vitro with ranolazine treatment.15 Therefore, we tested if, in the DOCA-salt model of isolated diastolic dysfunction, treatment with the late INa blocker, ranolazine, would improve cardiac relaxation, negating the deleterious effects of increased cardiac oxidative stress.
Methods
Generation of DOCA-salt mouse model
Previously, we have shown that this model leads to mild hypertension, myocardial oxidative stress, and diastolic dysfunction.20 A gradual and mild elevation in blood pressure was induced by unilateral nephrectomy, subcutaneous implantation of a controlled release deoxycorticosterone acetate (DOCA) pellet (0.7 mg/d; Innovative Research of America, Sarasota, FL), and substituting drinking water with 1.05% saline. Control animals underwent a sham operation, had placebo pellet implantation, and received water without salt.
Invasive hemodynamic studies, noninvasive echocardiography, and myocyte isolation were done on postoperative day 14–18 for DOCA-salt and control mice. All experiments were approved by the University of Illinois at Chicago Animal Care and Use Committee.
Chronic Administration of Ranolazine
A special diet contained 5 mg ranolazine, 0.3 mg P450 inhibitor, and 0.25 mg red food color was pressed into a 1-g nutritionally complete grain-based tablet (Harlan, Madison, WI). The control diet contained 0.3mg P450 inhibitor and 0.25 mg yellow food color pressed into the same type of 1-g tablets. DOCA-salt and sham mice consumed daily ~1g of the special diet from postoperative day 11 to 18.
Noninvasive assessment of diastolic dysfunction
Mice were anesthetized, maintained at 37°C, and studied by echocardiography (Vevo 770, VisualSonics Inc, Toronto, Canada). M-mode images in the parasternal long axis and the left ventricle (LV) short-axis views at the mid-papillary level were taken. Measurements were averaged from three consecutive beats during expiration. LV inflow velocities (E and A waves) were interrogated by conventional pulsed-wave Doppler from the apical four-chamber view. The mitral annulus longitudinal velocities (Sm, E′, and A′) were determined by pulsed-wave tissue Doppler from the apical four-chamber view. Interpretation was done by a two investigators blinded to the treatment groups. First, baseline images were acquired. Subsequently to determine the acute effect, the mice were injected with 30 mg/kg ranolazine by intraperitoneal route, followed by a second echocardiogram 30 min later.
Invasive assessment of diastolic dysfunction
Mice were anesthetized with 1–1.5% isoflurane and maintained at 37°C. The pressure-volume (PV) catheter was inserted into the right common carotidartery and advanced into the LV. Inferior vena cava occlusion was performed via a diaphragm incision. Volume and parallel conductance calibration were performed as previously described.20 Baseline hemodynamic measurements were obtained, and subsequently to determine the acute effect, the mice received an intravenous injection of ranolazine (5 mg/kg) followed by an infusion at 4.8 mg/kg/h, while additional hemodynamic measurements were recorded. Blood samples were obtained during the last five minutes of the procedure to determine the plasma ranolazine concentration.
Cell shortening and calcium transient measurements
The mechanical properties of the cardiomyocytes were assessed using an IonOptix Myocam System (Ionoptix Inc., Milton, MA). Unloaded cardiomyocytes were placed on a glass slide and allowed to adhere for 10 min at 37°C. Cardiomyocytes were then imaged with an inverted microscope and perfused with a Tyrode’s buffer containing 1.2 mmol/L calcium at room temperature. Cardiomyocytes were paced at 0.5, 1, or 2 Hz for 10 ms duration, and sarcomere shortening and relengthening were assessed using the following indices: peak fractional shortening (FS), time to 90% peak shortening, and τ, the relaxation time constant (a0+a1et/τ, t = time). Cardiomyocytes were treated with 10 μmol/L ranolazine for 10 min prior to evaluation. Initial experiments were done at room temperature and subsequent studies at 37°C showed no change in the effects of DOCA-salt or ranolazine. For calcium transient measurements, cardiomyocytes were loaded with 1 μmol/L fura 2-AM for 10 min at 37°C, and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (IonOptix). After loading, the cells were washed and resuspended in normal Tyrode solution. The cardiomyocytes were placed then in the cell chamber, stimulated at 0.5, 1, or 2 Hz for 10 ms duration, and imaged through a Fluor x 40 objective lens. Cells were exposed to light emitted by a 75-W Xenon lamp and passed though either a 340- or 380-nm wavelength filter. The emitted fluorescence was detected at 510 nm. To take into account any interference, the background fluorescence for each cardiomyocyte was determined by moving the cardiomyocyte out of the view and recording the fluorescence from the bath solution alone.
Studies with detergent extracted (skinned) fiber bundles
We measured myofilament response to Ca2+ under two experimental conditions as described in detail in the supplementary materials. In a first series of experiments the skinned fiber bundles were mounted in a force measuring apparatus and sarcomere length (SL) was adjusted to 2.2 μm using a laser diffraction pattern and width and thickness were determined for calculation of cross-sectional area. Force was measured over a range of pCa values. Skinned fiber bundles were treated with 10 μmol/L ranolazine for 15 min prior to evaluation. In a second series of experiments under different experimental conditions, we determined steady-state Mg-ATPase activity, while simultaneously measuring isometric tension in skinned fiber bundles as a function of pCa. In all experiments, skinned fibers were treated with ranolazine or DMSO vehicle for 15 min prior to beginning either tension or tension/ATPase rate measurements. Tension generated was computed from the force/cross-sectional area and data were analyzed in the GraphPad Prism software and fit with a sigmoidal modified Hill equation in order to generate tension-pCa curves, Hill coefficients, and pCa50 (pCa value at half-maximum tension). We also determined cross-bridge kinetics employing a quick release/restretch protocol. The rate constant for force redevelopment, Ktr, provides a measure of the rate of cross-bridge entry into the force generating state. In all experiments, only fiber bundles retaining more than 80% of their initial maximum tension were included in the analysis. We treated the fibers with ranolazine from a DMSO stock solution or DMSO alone added to relaxing solution for 15 min prior to the experimental run. Methods for gel electrophoresis analysis of myofilament protein phosphorylation and oxidative modifications are provided in the supplementary file.
Results
Ranolazine attenuated diastolic dysfunction in vivo
As previously described, DOCA-salt mice had evidence of diastolic dysfunction with preserved systolic function by transthoracic echocardiography at postoperative days 14–18 (table 1).20 Intraperitoneal injection of ranolazine improved diastolic dysfunction without affecting systolic function. DOCA-salt mice had significant reductions in tissue mitral annulus early longitudinal (E′) velocities and the ratio of early annulus to late annulus (E′/A′) velocities, which improved to sham levels with ranolazine treatment (Online Figure I). The ratio of early diastolic filling velocity to the early diastolic mitral annulus velocity (E/E′) has been reported to have the highest correlation with invasive hemodynamic measures of diastolic dysfunction. 20, 23 Hypertensive mice had a higher E/E′ compared to controls, and ranolazine returned this ratio toward normal in hypertensive mice. The mitral inflow velocities, E and A, were similar among the groups, a pseudonormal pattern, as reported before.20 The changes in relaxation parameters occurred in the absence of valvular regurgitation, LV wall motion abnormalities, or hypertrophy. Despite a slight reduction in fractional shortening (FS, %) when comparing sham to DOCA-salt + ranolazine groups, LVEF (%) and septal annulus systolic velocity (Sm) were statistically indistinguishable among the groups, suggesting the treatments had little effect on systolic function (Table 1A).
Table 1A.
Effect of ranolazine on echocardiographic parameters
| Sham | Sham + ranolazine | DOCA-salt | DOCA-salt + ranolazine | |
|---|---|---|---|---|
| EF (%) | 52.6 ± 1.5† | 52.8 ± 0.8† | 49.6 ± 1.3 | 46.9 ± 1.6 |
| Sm (cm/s) | 2.45 ± 0.08 | 2.31 ± 0.09 | 2.18 ± 0.15 | 2.15 ± 0.14 |
| E/A | 1.47 ± 0.05 | 1.63 ± 0.07* | 1.21 ± 0.10 | 1.62 ± 0.11* |
| E′ (cm/s) | 2.77 ± 0.15* | 2.67 ± 0.11* | 2.15 ± 0.11 | 2.47 ± 0.18 |
| E′/A′ | 1.22 ± 0.06* | 1.16 ± 0.02* | 0.82 ± 0.06 | 1.31 ± 0.11* |
| E/E′ | 31.9 ± 2.8* | 30.2 ± 1.9* | 41.8 ± 2.6 | 31.9 ± 2.6* |
Data are means ±SEM. EF, ejection fraction; Sm, systolic septal mitral annulus velocity measured by tissue doppler imaging (TDI); E, early diastolic filling velocity and A, late diastolic filling velocity measured by conventional doppler; E′, early septal mitral annulus velocity (TDI); A′, late diastolic septal mitral annulus velocity (TDI). n=8–10,
p < 0.05 vs. DOCA-salt.
p < 0.05 vs. DOCA-salt + ran.
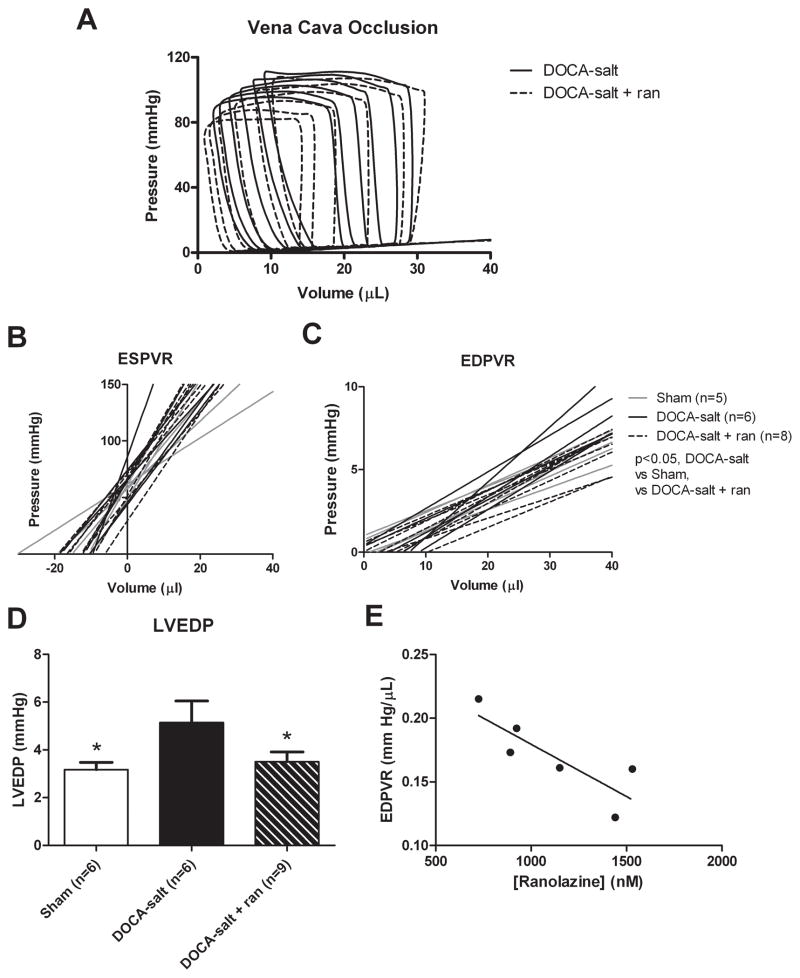
Invasive hemodynamic evaluation confirmed the echocardiographic findings (Table 1B). As expected, systolic blood pressure (SBP), diastolic blood pressure (DBP), and left ventricle (LV) end-systolic pressure were mildly elevated in DOCA-salt mice compared with sham and sham treated mice, although DOCA-salt mice treated with ranolazine did not differ significantly from DOCA-salt mice in any of these parameters. As described before, the best fit for the end-diastolic pressure-volume relation (EDPVR) was by the following linear function: pressureend diastole = EDPVR × volumeend diastole + intercept.20 Hypertensive DOCA-salt mice had a steeper EDPVR compared with DOCA-salt treated and control groups (p< 0.005; Figure 1B and 1C). The slopes were 0.23 ± 0.026, 0.17 ± 0.01,and 0.16 ± 0.01 mm Hg/μL; for DOCA-salt, DOCA-salt + ranolazine and sham, respectively. Additionally, the EDPVR in DOCA-salt mice demonstrated a linear response to serum ranolazine levels (correlation coefficient = 0.70, p < 0.05; Figure 1E).
Table 1B.
Effect of ranolazine on hemodynamics
| Sham | Sham + ranolazine | DOCA-salt | DOCA-salt + ranolazine | |
|---|---|---|---|---|
| SBP(mmHg) | 88 ±3*† | 93 ±3* | 109 ±7 | 102 ±3 |
| DBP(mm Hg) | 52 ±5*† | 58 ±2*† | 78 ±4 | 73 ±2 |
| HR(bpm) | 618 ± 24 | 620 ±5 | 589 ± 16 | 588 ± 16 |
| LVESP (mm Hg) | 83 ±2* | 85 ±3* | 108 ±7 | 98 ±3 |
| LVEDP(mmHg) | 3.2 ±0.3* | 4.2 ±0.5 | 5.1 ±0.9 | 3.5 ±0.4* |
| EF (%) | 71 ±6 | 73 ±5 | 67 ±6 | 67 ±4 |
| EDPVR (mm Hg/μL) | 0.16 ±0.01* | 0.18 ±0.01* | 0.23 ± 0.02 | 0.17 ±0.01* |
| ESPVR (mm Hg/μL) | 3 ±0.6 | 3.9 ±0.5 | 4.7 ±0.8 | 4.1 ±0.2 |
| dP/dt min (mm Hg/sec) | −10,420 ± 594 | −10,083 ± 685 | −12,181 ±641 | −12,211 ±792 |
| dP/dt max (mm Hg/sec) | 12,818 ± 490 | 12,032± 787 | 13,585 ± 832 | 12,495 ± 555 |
Data are means ± SEM. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LVESP, left ventricular end systolic pressure; LVEDP, left ventricular end diastolic pressure; EF, ejection fraction; EDPVR, end diastolic pressure volume relationship. ESPVR, end systolic pressure volume relationship. n=8,
p < 0.05 vs. DOCA-salt.
p < 0.05 vs. DOCA-salt + ranolazine.
Figure 1.
Invasive hemodynamic assessment of LV diastolic dysfunction. A: Representative pressure-volume loops with vena cava occlusion for DOCA-salt and DOCA-salt + ranolazine groups. B: The EDPVR slope is greater in DOCA-salt vs. sham mice (P<0.05) and is improved with ranolazine treatment (P<0.05, vs. DOCA-salt). The mean EDPVR is significantly greater in the DOCA-salt mice compared to sham and ranolazine-treated mice. Additionally, treatment with ranolazine reduces the EDPVR in DOCA-salt mice to that of controls (n= 8, *p < 0.05 vs. all groups). C: LV contractility assessed by the end-systolic pressure-volume relation (ESPVR) slope (P=NS) and the volume axis intercept (Vo, P=NS) are similar in sham, DOCA-salt and DOCA-salt + ranolazine groups. D: Comparisions of LV end-diastolic pressure (LVEDP) for sham (3.2 ± 0.3 mmHg, P<0.05 vs. DOCA-salt), DOCA-salt (5.1 ± 0.9 mmHg), and DOCA-salt + ranolazine (3.5 ± 0.4 mmHg, P<0.05 vs. DOCA-salt) groups. E: Ranolazine shows an inverse concentration dependent effect on the EDPVR in DOCA-salt mice with a Pearson correlation coefficient of 0.70 (n=6, p < 0.05).
In an additional set of experiments, we treated DOCA-salt mice with ranolazine for 7 days and measured diastolic function using echocardiography to evaluate the effect of chronic ranolazine therapy on myocardial function (Online Table I). Prolonged treatment of DOCA-salt mice with ranolazine significantly improved the E′/A′ ratio in DOCA-salt mice (DOCA-salt 0.74 ± 0.05 vs. DOCA-salt + Ran 1.10 ± 0.08, p < 0.05). Moreover, ranolazine decreased E/E′ in DOCA-salt mice when treated chronically (DOCA-salt 43.69 ± 2.73 vs. DOCA-salt + Ran 29.27 ± 5.07, p < 0.05). Prolonged ranolazine treatment did not affect the systolic function (Sm) in any of the groups.
Ranolazine improved relaxation in DOCA-salt cardiomyocytes
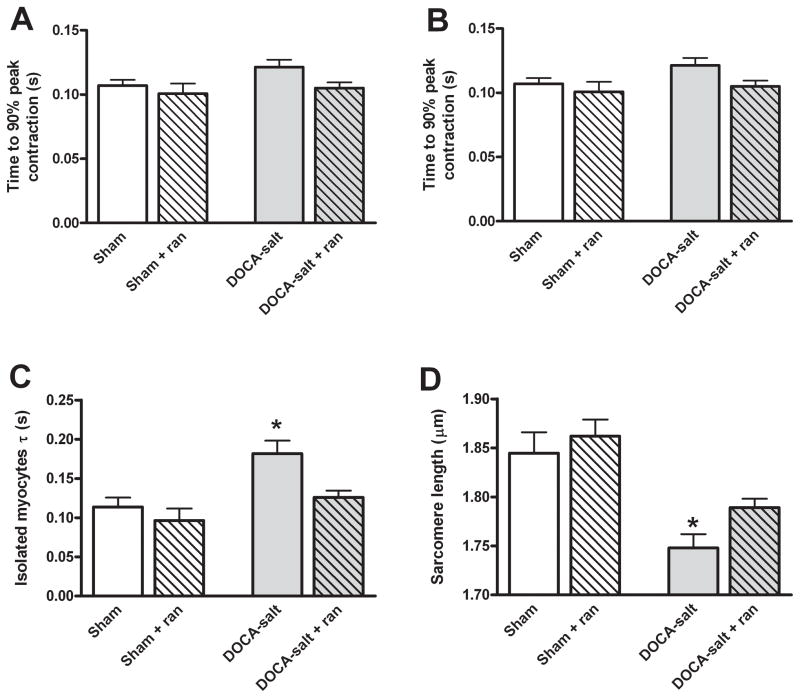
Silberman et al. showed that impaired relaxation of cardiomyocytes was the main contributor to diastolic dysfunction in the DOCA-salt hypertensive model, finding no increase in cardiac fibrosis or inflammation.20 To confirm ranolazine was working directly on DOCA-salt cardiomyocytes to improve relaxation, freshly isolated ventricular cardiomyocytes were stimulated at 0.5, 1, and 2 Hz at 37°C and assessed. DOCA-salt cardiomyocytes had preserved contractile function, as previously reported20 (Figures 2A and 2B). Additionally, treatment with ranolazine did not affect contraction in sham and DOCA-salt mice. In contrast, relaxation τ was significantly impaired in DOCA-salt mice and improved to normal levels with ranolazine treatment (DOCA-salt 0.18 ± 0.02, DOCA-salt + ranolazine 0.13 ± 0.01, Sham 0.11 ± 0.01, Sham + ranolazine ± 0.09 ± 0.02 s, p = 0.0004; Figure 2C). The baseline sarcomere length of cardiac myocytes was significantly reduced in DOCA-salt mice compared to sham mice (Figure 2D). Treatment with ranolazine improved resting sarcomere length in the DOCA-salt mice to levels similar to sham, and ranolazine did not affect sham sarcomere length (DOCA-salt 1.75 ± 0.01, DOCA-salt + ranolazine 1.81 ± 0.01, sham 1.85 ± 0.02, sham + ranolazine 1.86 ± 0.02 μm, p< 0.0001). In the absence of changes in resting Ca2+ (see below), this indicated that myofilament response to Ca2+ may be altered in diastolic dysfunction. Comparable effects were seen at 1 and 2 Hz stimulation rates and physiological temperature (Online Figure II). The effect of ranolazine in these experiments was reversible upon washout out (data not shown).
Figure 2.
Functional analysis of isolated cardiomyocytes. A: Fractional shortening of isolated cardiomyocytes paced at 0.5 Hz at 37°C represented as the peak shortening divided by the baseline sarcomere length (n=12, p=NS). B: Time to 90% peak contraction in isolated cardiomyocytes (n=12, p=NS). C: Isolated cardiomyocytes from DOCA-salt mice have a prolonged relaxation constant (τ) compared to control animals. The addition of ranolazine to isolated DOCA-salt cardiomyocytes normalizes relaxation kinetics (n=12, *p < 0.0001 vs. all groups). D: The mean diastolic sarcomere length was significantly shorter in the DOCA-salt cardiomyocytes compared to the sham. The addition of ranolazine to the DOCA-salt cardiomyocytes significantly lengthened resting sarcomeres, but had no effect on sham cardiomyocytes (n=12, *p < 0.0001 vs. all groups).
Diastolic dysfunction was independent of intracellular calcium cycling
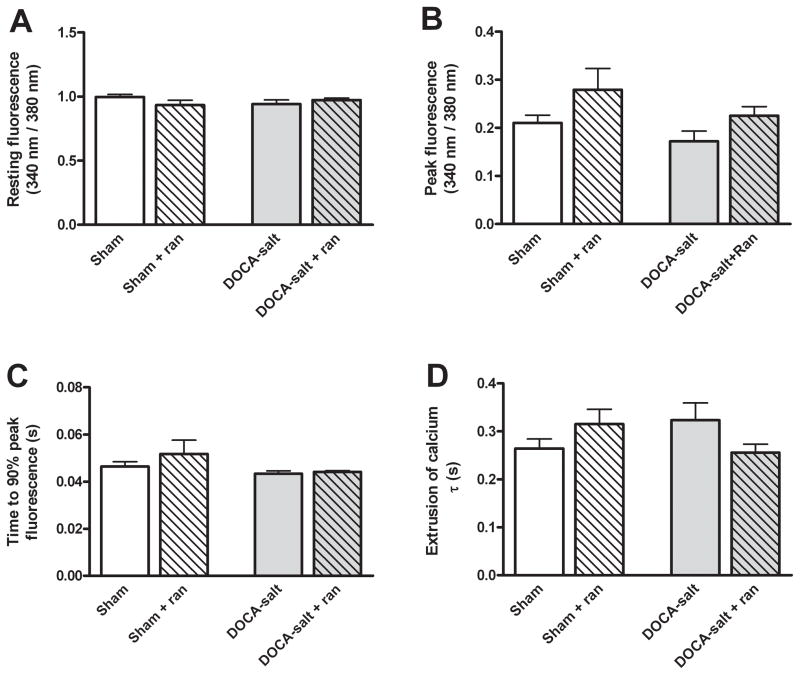
To elucidate the mechanism underlying the impaired diastolic function in hypertensive mice, Ca2+ transients were measured in freshly isolated ventricular myocytes. Ca2+ transients did not differ significantly between sham and DOCA-salt mice, and the addition of 10 μM ranolazine did not affect either group (Figure 3). Baseline intracellular Ca2+ was similar in all groups (Figure 3A). Additionally, peak intracellular Ca2+ and the rate of Ca2+ release was similar between all four groups (Figure 3B and 3C). Surprisingly, there was no difference among the rates of intracellular Ca2+ egress among the four groups measured as the time constant τ (Figure 3D). This held true at pacing rates of 1 and 2 Hz as well (Online Figure III). We cannot rule out regional variations in myocyte properties may obscure changes in Ca2+ handling, however. In Online Figure 9, we show phase-plane plots that of the averaged Fura-2 fluorescence dual excitation ratio (340/380) vs. sarcomere length during entire contraction-relaxation cycle in sham, DOCA-salt, and ranolazine treated groups.
Figure 3.
DOCA-salt mice have no difference in intracellular calcium cycling when compared to sham mice. B: The peak Ca2+ fluorescence in isolated cardiomyocytes loaded with the ratiometric fluorescent dye, Fura-2AM, and paced at 0.5 Hz (n=12, p=NS). C: The time to 90% peak Ca2+ fluorescence representing the rate of calcium entry into the cytosol (n=12, p=NS). D: The rate of relaxation measured as the time constant τ did not differ among groups (n=12, p=NS).
Late INa was not elevated in DOCA-salt mice
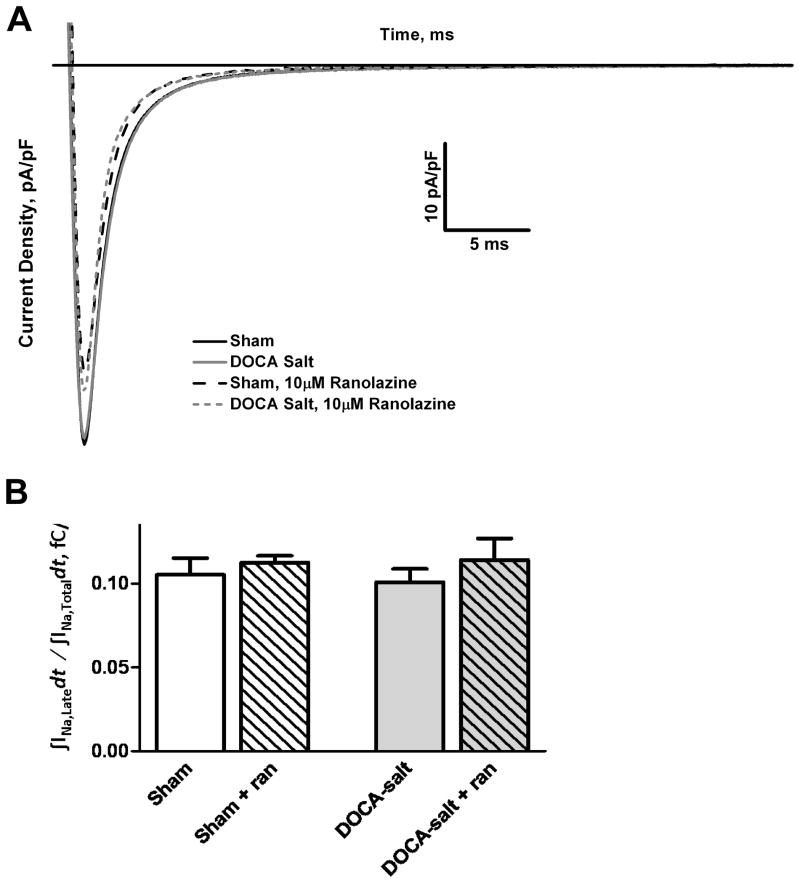
Oxidative stress is known to induce late INa that can be blocked by the anti-anginal drug, ranolazine, and the DOCA-salt model is associated with increased cardiac oxidative stress.20, 24 Nevertheless, voltage-clamp studies show no increase in late INa in DOCA-salt cardiomyocytes compared to sham (Sham 0.105 ± 0.01, Sham + ranolazine 0.113 ± 0.004, DOCA-salt 0.101 ± 0.008, DOCA-salt + ranolazine 0.114 ± 0.013, p=NS) (Figure 4A). Integrated late INa was measured starting at 5% of peak current and ending 40 ms after depolarization. DOCA-salt myocytes accumulated a similar amount of charge as sham. Extracellular addition of 10 μmol/L ranolazine did not affect the late accumulated charge in DOCA-salt cardiomyocytes, which was similar to that seen for sham and sham treated myocytes (Figure 4B).
Figure 4.
DOCA-salt mice show no difference in late INa when compared to sham mice. A: Voltage-clamp studies show no increase in late INa in DOCA-salt myocytes with respect to sham (n=7, p=NS). Extracellular addition of ranolazine had little effect on late accumulated charge in both the DOCA-salt and sham groups (n=4, p=NS). B: Graph of the ratio of the mean accumulated late Na+ charge to the mean accumulated total Na+ charge during an activating voltage step.
Skinned fibers from DOCA-salt treated hearts demonstrated increased response to Ca2+ and depressed cross-bridge kinetics
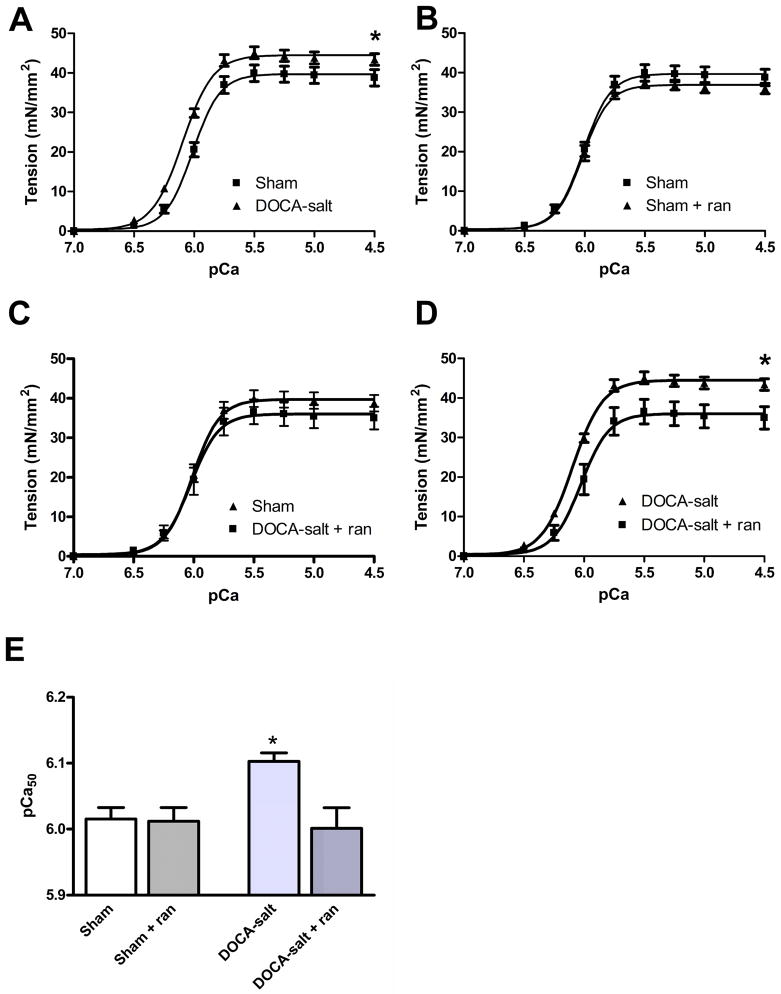
To examine directly myofilament function, the Ca-sensitivity of steady-state isometric tension development was measured in a first series of experiments with skinned fiber bundles from sham and DOCA-salt heart preparations. With DOCA-salt treatment, the mean steady-state isometric tension demonstrated an increase in maximum tension and a small but significant leftward shift of the pCa-tension relation (Figure 5, left panel). Myofilament Ca2+ sensitivity, indexed by pCa50, was significantly (p< 0.02) greater in DOCA-salt than in sham myofilaments (Figure 5). As indicated in Figure 5, ranolazine treatment reduced maximum tension and returned the DOCA-salt myofilament sensitivity to levels similar to sham fibers with little effect on fiber bundles from sham hearts. The changes in Ca2+ sensitivity are statistically significant and in a direction that explain the decreased diastolic function. Nevertheless, we cannot completely rule out other possible mechanisms for decreased diastolic relaxation. These results imply that resting tension in Ca2+-free conditions should be increased, although this was not tested here.
Figure 5.
Myofilament Ca2+ responsiveness in skinned fiber bundles isolated from DOCA-salt and sham hearts with and without ranolazine. A-D: The mean steady-state isometric tension of skinned fiber bundles is plotted as a function of pCa. DOCA-salt fibers demonstrate a significant (p<0.05) increase in maximum tension (44.51 ± 0.55 mN/mm2; n = 6) compared to shams (39.7 ± 0.79 mN/mm2; n = 6) and DOCA-salt fibers treated with ranolazine (36.03 ± 1.50 mN/mm2). DOCA-salt fibers demonstrate a significant (p<0.05) increase in mean Ca2+ sensitivity (pCa50 = 6.09 ± 0.01; n = 6) compared to shams (pCa50 = 6.02 ± 0.01; n = 6). Treatment with ranolazine normalized these changes. E: pCa-tension relations normalized to maximum tension. DOCA-salt fibers (pCa50 = 6.1 ± 0.02; Hill n = 3.42 ± 0.30) demonstrate a significant increase in mean Ca2+ sensitivity (n=6, *p < 0.05) as compared to shams without DOCA treatment (pCa50 = 6.0 ± 0.01; Hill n = 3.80 ± 0.61) shams with DOCA treatment (pCa50 = 6.0 ± 0.01; Hill n = 3.91 ± 0.50) and fibers from DOCA mice and treated with ranalozine (pCa50 = 6.0 ± 0.03; Hill n = 3.71 ± 1.0).
In order to assess whether the fiber bundles from DOCA-salt treated hearts demonstrated altered cross-bridge kinetics, we measured tension development and simultaneously the rate of ATP hydrolysis (Online Figure IV). As summarized in Table 1C, there was a significant increase in maximum tension of fiber bundles from DOCA-salt treated hearts compared to shams. Treatment of the fiber bundles from DOCA-salt treated hearts with ranolazine restored tension to the control levels. Nevertheless, measurement of ATPase rates during the development of steady-state tension showed responses different from the tension measurements. There was no significant difference of maximum ATPase rate between fibers from sham controls compared to fibers from DOCA-salt treated hearts. Treatment of the fibers from DOCA-salt hearts with ranolazine induced a significant increase in ATPase rate. Online Figure IV shows the ATPase rate plotted as a function of tension. The slope of this relation, which provides a measure of tension cost, was significantly depressed in fibers from DOCA-salt treated hearts compared to shams, implying a slowing of myofilament exit from the cross-bridge cycle (Table 1C).25 Moreover, although ranolazine had no significant effect on the skinned fibers from shams, treatment of the DOCA-salt fibers with ranolazine significantly increased the maximum ATPase rate over that of the fibers from DOCA-salt treated hearts. We also determined Ktr, which is a measure of the rate of re-entry of cross-bridges into force generating states. No change in Ktr was observed under any of the conditions studied. In a separate set of experiments, we measured myofilament calcium sensitivity of fibers from hearts of DOCA-salt mice and DOCA-salt mice chronically treated with ranolazine and confirmed that ranolazine treatment significantly reduces maximal tension in response to Ca2+ in vivo. While undetected changes in Na+ and Ca2+ currents under stretch cannot be completely eliminated as a cause of diastolic dysfunction, because these experiments were carried out in skinned fibers, our data suggest that a major cause is alterations in the myofilaments that are ameliorated by ranolazine.
Table 1C.
Effect of Ranolazine on tension and ATPase rate of skinned fiber bundles
| Sham | Sham + ranolazine | DOCA-salt | DOCA-salt + ranolazine | |
|---|---|---|---|---|
| Maximum Tension (mN/mm2) | 26.84 ± 0.35*# | 36.80 ± 0.16 | 33.46 ± 0.38 | 27.19 ± 0.54 |
| Maximum ATPase (pmol* s−l* mg−l) | 233.2 ± 2.9†# | 302.0 ± 9.0* | 234.6 ± 3.7 | 326.9 ± 8.7* |
| Tension Cost ΔATPase/ΔTension | 8.3 ± 0.4* | 7.1 ± 0.4 | 6.4 ± 0.5 | 10.8 ± 0.8* |
| Passive Tension (mN/mm2) | 0.45 ± 0.26 | 0.55 ± 0.33 | 0.20 ± 0.32 | 0.68 ± 0.40 |
| Ktr | 10.9 ± 0.7 | 11.2 ± 0.5 | 11.2 ± 0.9 | 11.0 ± 1.0 |
Data are means ± standard error. Ktr, the rate constant for force redevelopment. N = 5–7,
p < 0.05 vs. DOCA-salt.
p < 0.05 vs. DOCA-salt + ranolazine.
p < 0.05 vs. sham + ranolazine.
Myofilament protein phosphorylation and oxidation analysis
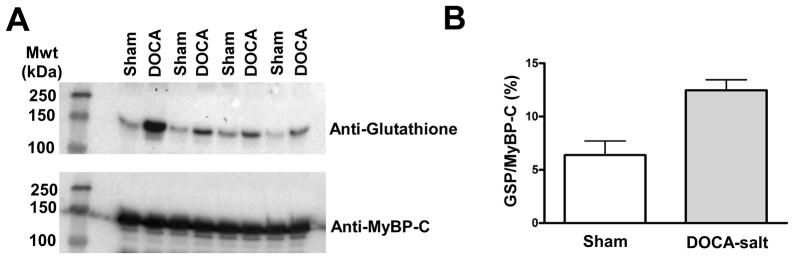
To understand the mechanism whereby hypertension generated changes in myofilament contractile properties, we performed an analysis of myofilament protein abundance and post-translational modifications. Representative ProQ and Coomassie gels can be seen in Online Figures V and VII. There were no changes in the abundances of myofilaments or the levels of phosphorylation of major myofilament proteins such as myosin binding protein C, troponin I, and myosin light chain 2 (Online Figure VI). Similar findings were noted with chronic ranolazine administration. Additionally, there was an increase in S-glutathionylation of myosin binding protein C in DOCA-salt mice (Figure 6A and 6B). We determined S-glutathionylation levels in DOCA-salt and sham hearts from mice after or without chronic administration of ranolazine, which confirmed our previous results (Online Figure VIII). These data indicate that there are DOCA-salt induced changes in the state of critical myofilament proteins, which may explain the changes in myofilament activity.
Figure 6.
A comparison in the post-translational modifications of the myofilaments from sham and DOCA-salt hearts. A: Western blot analysis demonstrating detection of glutathionylated proteins when comparing myofilament samples from sham and DOCA-salt treated hearts. The blots were also analyzed for myosin binding protein C, indicating that this protein was the major modification. Note that one pair of comparisons was removed from the gel. B: Quantification of the difference between glutathionylated proteins (GSH) normalized to myosin binding protein C (MyBP-C) in sham and DOCA-salt myofilaments. (*p < 0.05, n=3).
Discussion
Based on the idea that cardiac oxidative stress is involved in the pathophysiologies of diastolic dysfunction and the late INa 15, 20, we investigated the effects of ranolazine, a late INa inhibitor, on the mechanical derangements induced in the DOCA-salt hypertensive model of diastolic dysfunction.24, 26 Mild hypertension in this model resulted in impaired relaxation that improved acutely after ranolazine treatment. Without changes in heart rate or blood pressure, ranolazine rapidly improved relaxation when measured both noninvasively and invasively. EDPVR, the most widely accepted measurement of relaxation, significantly improved in the DOCA-salt mice confirming our noninvasive echocardiographic results. In our study, the EDPVR relationship but not dP/dt min were different between controls and DOCA-salt mice. This discrepancy has been noted in other studies and, given our results, may imply that actin-myosin crossbridge cycling affects a latter phase of cardiac diastole.27, 28 Additionally, ranolazine did not significantly affect hemodynamics in the sham mouse. At the cellular level, isolated DOCA-salt cardiomyocytes demonstrated impaired relaxation that improved with ranolazine.
Silberman et al. reported the DOCA-salt model of diastolic dysfunction was associated with cardiac oxidative stress and targeting of reactive oxygen species production improved diastolic function.20 We sought to investigate the downstream mediators of the increased oxidant load to better elucidate the mechanisms regulating diastolic function. Oxidative stress is known to modulate a number of proteins important in cardiac function including: the SR Ca2+ release channel, SR Ca2+ pump, the sarcolemmal L-type Ca2+ channel, the sodium-calcium exchanger, phospholamban, myofilaments, and the late INa.15, 29–33 Largely based on analogy to systolic dysfunction, we expected to find increased diastolic Ca2+ resulting in slowed myocyte relaxation and diastolic dysfunction, but surprisingly, we found no changes in Ca2+ cycling between DOCA-salt and sham mice. Instead, we noted changes in myofilament activity in the absence of changes in Ca2+ handling. This is consistent with other models of diastolic dysfunction including a model of diabetic cardiomyopathy in which similar changes in sarcomere length were reported with no correlative changes in Ca2+ cycling.16–18 Previously, it has been reported that myocyte relaxation can be dissociated from the decline of intracellular Ca2+,34 and myofilament Ca2+ sensitivity is a consistent functional abnormality seen in dilated cardiomyopathy.35 These results suggest that although oxidative stress is associated with both systolic and diastolic dysfunction, mediators of these dysfunctions appear to differ. This may help explain the unimpressive results treating diastolic dysfunction when using drugs proven to be beneficial in systolic heart failure.6–8
In vitro studies demonstrating the effectiveness of ranolazine to treat impaired relaxation have used isolated muscle strips, isolated cardiomyocytes, and working heart preparations.36, 37 Previous studies in both rabbit and rat models have shown that ranolazine attenuates diastolic dysfunction in ischemia/reperfusion.38, 39 Additionally, in a dog model of chronic heart failure, ranolazine reduces LVEDP.40–42 Finally, trials in humans with ischemic heart disease and type-3 long-QT syndrome have supported a role for ranolazine in the treatment of diastolic dysfunction.43, 44 The mechanism for this effect was thought to be a reduction in late INa with a subsequent reduction in diastolic Ca2+. Despite the efficacy of ranolazine in the relief of diastolic dysfunction, no increase in late INa in the DOCA-salt cardiomyocytes was noted nor were there changes in calcium cycling to indicate significant alterations in Ca2+ handling in this form of diastolic dysfunction. On the other hand, at rest, the DOCA-salt myocytes had a significant decrease in sarcomere length in the absence of changes in Ca2+ concentration, suggesting increased diastolic tension compared to sham mice. Myofilaments from diastolic dysfunction mice had increased maximum tension and sensitivity to Ca2+ as well as a slowing of cross-bridge exit kinetics compared to sham mice that normalized with ranolazine treatment. Taken together, these results suggest ranolazine improved diastolic function at the cardiomyocyte level through the modulation of myofilament cross-bridge kinetics and sensitivity to Ca2+. The decrease in sarcomere length in intact cells with no change in passive tension of the muscle strips is consistent with our hypothesis that alterations in crossbridges are responsible for the changes in diastolic function. This result is consistent with the significance of molecular motors as determinants of relaxation in ejecting hearts.45
Our investigation of the post-translational modifications indicated that there was a significant increase in glutathionylation of myosin binding protein C in animals with diastolic dysfunction. Interestingly, accumulating evidence indicates a significant role of myosin binding protein C in control of cross-bridge kinetics.46 This post-translational modification supports the hypothesis that hypertension-induced alterations in contractile parameters result from oxidative modification of myosin binding protein C. Inasmuch as acute and direct addition of ranolazine to the skinned fiber bundles enhanced cross-bridge kinetics and the effects of ranolazine were reversible with washout, it seems likely that this agent has direct effects on the myofilaments. Since the effect of ranolazine correlated with myosin binding protein C glutathionylation, the effect of ranolazine was present only in diastolic dysfunction, and glutathionylation was not altered by ranolazine, oxidative modification of myofilaments may create a binding site for ranolazine to relieve diastolic dysfunction. Future studies will determine the post-translational modifications, not necessarily limited to phosphorylation and glutathionylation, which may occur to other myofilament proteins, how these modifications influence the modification of other proteins, and what targets are best for therapeutic interventions to obtain a desirable phenotypic response.
Measurement of diastolic dysfunction is complicated and controversial. There is no agreement on the best technique. The left ventricular pressure-volume relationship generated during venous occlusion was used in this study because it allows characterization of diastolic function independent of load conditions. Nevertheless, there can be errors in estimating volume because of nonlinearities in the conductance-volume relationship that are more prominent during large volume deviations. Using only the first six pressure-volume loops during venous occlusion resulted in an identical EDPVR, so the likelihood of a sizable error is small. Moreover, in this study, we measured diastolic dysfunction in several different ways and with several different preparations. The effect of ranolazine on diastolic function was seen in vivo by conductance catheter measurements and echocardiography and in vitro using muscle strips and isolated myocytes.
In conclusion, the present study demonstrates that ranolazine treatmentimproves diastolic function through modulation of myofilament activity. Ranolazine dosing in human studies have shown the therapeutic concentration ranges between 2–6 μM. Therefore, we were able to produce an effect on diastolic function in the DOCA-salt mice with roughly the same concentrations used clinically.26 Our DOCA-salt model is one of the few animal models of diastolic dysfunction in the absence of hypertrophy or changes in ejection fraction, but these results may not be transferrable to humans. On the other hand, a human trial suggests that ranolazine may have salutary effects in diastolic dysfunction associated with myocardial injury.43
Supplementary Material
Novelty and Significance.
What Is Known?
Diastolic heart failure is increasing in incidence, and the cause is unknown.
Diastolic dysfunction is associated with cardiac oxidative stress, and oxidation can cause prolonged sodium channel opening (i.e., a late current), leading to calcium loading of cardiomyocytes, possibly causing diastolic dysfunction.
Ranolazine blocks the sodium channel late current and may treat diastolic dysfunction.
What New Information Does This Article Contribute?
Ranolazine improved hypertension-mediated diastolic dysfunction when measured in vivo, in muscle strips, or in isolated myocytes.
Ranolazine acted directly on myofilaments to improve relaxation, not through blocking of the late sodium current.
Diastolic dysfunction was correlated with oxidative modification of myosin binding protein C, suggesting cardiac oxidative stress may mediate diastolic dysfunction through altering the contractile apparatus.
Heart failure with preserved ejection fraction occurs in approximately half of all heart failure cases. This type of heart failure is caused by a failure of the myocardium to relax properly, but the mechanism of the diastolic dysfunction is unknown. Furthermore, no specific treatments are currently available. Previously, it has been shown that diastolic dysfunction is associated with cardiac oxidation and oxidative stress causes a prolonged cardiomyocyte sodium entry. This entry leads to diastolic calcium loading which may explain the impaired relaxation. Ranolazine is known to block this type of sodium entry, so we tested its ability to treat diastolic dysfunction. In a hypertension-mediated mouse model, diastolic dysfunction was correlated with oxidative modification of myosin binding protein C. Ranolazine was able to ameliorate diastolic dysfunction measured multiple different ways and in several preparations. This effect was a result of ranolazine acting directly on the myofilaments, rather than blocking the late sodium current, however. In conclusion, ranolazine may be useful in the treatment of diastolic dysfunction.
Acknowledgments
Sources of Funding
SCD: R01 HL085558, R01 HL073753, P01 HL058000, and a Veterans Affairs MERIT grant.
JDL, MM: T32 HL007692.
DS: R01 HL090851, ARRA supplement R01HL090851-02S1.
RJS: RO1 HL022231, RO1 HL064035, PO1 HL062426
We are grateful for excellent technical assistance by Chad M. Warren with gel analysis, and by Amin Rmeileh with skinned fiber tension measurements.
Non-standard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- DOCA
deoxycorticosterone acetate
- EDPVR
end-diastolic pressure-volume relation
- EF
ejection fraction
- ESPVR
end-systolic pressure-volume relation
- FS
fractional shortening
- HFpEF
heart failure with preserved ejection fraction
- INa
inward sodium current
- LV
left ventricular
- LVEDP
left ventricular end diastolic pressure
- NCX
Na+/Ca2+ exchanger
- PV
pressure-volume
- SBP
systolic blood pressure
- SERCA
sarcoplasmic reticular Ca2+-ATPase
Footnotes
Disclosures
S.C.D.: patents pending, Methods and Compositions for Treating Diastolic Dysfunction (60/840,368) and Sodium Current Blockade for Treatment of Diastolic Heart Failure (61/241,585 and 61/263,920).
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 4.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 5.Ouzounian M, Lee DS, Liu PP. Diastolic heart failure: mechanisms and controversies. Nat Clin Pract Cardiovasc Med. 2008;5:375–386. doi: 10.1038/ncpcardio1245. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 9.Bers DM. Excitation-contraction coupling and cardiac contractile force. 2. Dordrecht ; Boston: Kluwer Academic Publishers; 2001. [Google Scholar]
- 10.Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497:337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992;71:1231–1241. doi: 10.1161/01.res.71.5.1231. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Corr PB. Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am J Physiol. 1994;266:H1034–1046. doi: 10.1152/ajpheart.1994.266.3.H1034. [DOI] [PubMed] [Google Scholar]
- 13.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500:631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol. 2008;44:954–967. doi: 10.1016/j.yjmcc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 16.Flagg TP, Cazorla O, Remedi MS, Haim TE, Tones MA, Bahinski A, Numann RE, Kovacs A, Schaffer JE, Nichols CG, Nerbonne JM. Ca2+-independent alterations in diastolic sarcomere length and relaxation kinetics in a mouse model of lipotoxic diabetic cardiomyopathy. Circ Res. 2009;104:95–103. doi: 10.1161/CIRCRESAHA.108.186809. [DOI] [PubMed] [Google Scholar]
- 17.Wolska BM, Kitada Y, Palmiter KA, Westfall MV, Johnson MD, Solaro RJ. CGP-48506 increases contractility of ventricular myocytes and myofilaments by effects on actin-myosin reaction. Am J Physiol. 1996;270:H24–32. doi: 10.1152/ajpheart.1996.270.1.H24. [DOI] [PubMed] [Google Scholar]
- 18.Pohlmann L, Kroger I, Vignier N, Schlossarek S, Kramer E, Coirault C, Sultan KR, El-Armouche A, Winegrad S, Eschenhagen T, Carrier L. Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res. 2007;101:928–938. doi: 10.1161/CIRCRESAHA.107.158774. [DOI] [PubMed] [Google Scholar]
- 19.Davis J, Wen H, Edwards T, Metzger JM. Thin filament disinhibition by restrictive cardiomyopathy mutant R193H troponin I induces Ca2+-independent mechanical tone and acute myocyte remodeling. Circ Res. 2007;100:1494–1502. doi: 10.1161/01.RES.0000268412.34364.50. [DOI] [PubMed] [Google Scholar]
- 20.Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cocco G, Rousseau MF, Bouvy T, Cheron P, Williams G, Detry JM, Pouleur H. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta-blocker or diltiazem. J Cardiovasc Pharmacol. 1992;20:131–138. [PubMed] [Google Scholar]
- 22.Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine Study Group. Am J Cardiol. 1999;84:46–50. doi: 10.1016/s0002-9149(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 23.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 24.Maier LS. A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: inhibition of late INa using ranolazine. J Cardiovasc Pharmacol. 2009;54:279–286. doi: 10.1097/FJC.0b013e3181a1b9e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Tombe PP, Stienen GJ. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res. 1995;76:734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- 26.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kass DA. Assessment of diastolic dysfunction. Invasive modalities. Cardiol Clin. 2000;18:571–586. doi: 10.1016/s0733-8651(05)70162-4. [DOI] [PubMed] [Google Scholar]
- 28.Yellin EL, Meisner JS. Physiology of diastolic function and transmitral pressure-flow relations. Cardiol Clin. 2000;18:411–433. vii. doi: 10.1016/s0733-8651(05)70153-3. [DOI] [PubMed] [Google Scholar]
- 29.Hiranandani N, Bupha-Intr T, Janssen PM. SERCA overexpression reduces hydroxyl radical injury in murine myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H3130–3135. doi: 10.1152/ajpheart.01315.2005. [DOI] [PubMed] [Google Scholar]
- 30.Kuster GM, Lancel S, Zhang J, Communal C, Trucillo MP, Lim CC, Pfister O, Weinberg EO, Cohen RA, Liao R, Siwik DA, Colucci WS. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med. 2010;48:1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kogler H. Na+-Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res. 2003;60:404–412. doi: 10.1016/j.cardiores.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 32.MacFarlane NG, Miller DJ. Effects of the reactive oxygen species hypochlorous acid and hydrogen peroxide on force production and calcium sensitivity of rat cardiac myofilaments. Pflugers Arch. 1994;428:561–568. doi: 10.1007/BF00374578. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294:C1566–1575. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monasky MM, Varian KD, Davis JP, Janssen PM. Dissociation of force decline from calcium decline by preload in isolated rabbit myocardium. Pflugers Arch. 2008;456:267–276. doi: 10.1007/s00424-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 35.LeWinter MM. Functional consequences of sarcomeric protein abnormalities in failing myocardium. Heart Fail Rev. 2005;10:249–257. doi: 10.1007/s10741-005-5254-4. [DOI] [PubMed] [Google Scholar]
- 36.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts--role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Hwang H, Arcidi JM, Jr, Hale SL, Simkhovich BZ, Belardinelli L, Dhalla AK, Shryock JC, Kloner RA. Ranolazine as a cardioplegia additive improves recovery of diastolic function in isolated rat hearts. Circulation. 2009;120:S16–21. doi: 10.1161/CIRCULATIONAHA.108.844167. [DOI] [PubMed] [Google Scholar]
- 38.Gralinski MR, Black SC, Kilgore KS, Chou AY, McCormack JG, Lucchesi BR. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res. 1994;28:1231–1237. doi: 10.1093/cvr/28.8.1231. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama K, Hara A, Hashizume H, Ushikubi F, Abiko Y. Ranolazine attenuates palmitoyl-L-carnitine-induced mechanical and metabolic derangement in the isolated, perfused rat heart. J Pharm Pharmacol. 2000;52:709–715. doi: 10.1211/0022357001774381. [DOI] [PubMed] [Google Scholar]
- 40.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabbah HN, Chandler MP, Mishima T, Suzuki G, Chaudhry P, Nass O, Biesiadecki BJ, Blackburn B, Wolff A, Stanley WC. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail. 2002;8:416–422. doi: 10.1054/jcaf.2002.129232. [DOI] [PubMed] [Google Scholar]
- 42.Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, Stanley WC, Sabbah HN. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–2155. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994;8:741–747. doi: 10.1007/BF00877121. [DOI] [PubMed] [Google Scholar]
- 44.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinken AC, Solaro RJ. A dominant role of cardiac molecular motors in the intrinsic regulation of ventricular ejection and relaxation. Physiology (Bethesda) 2007;22:73–80. doi: 10.1152/physiol.00043.2006. [DOI] [PubMed] [Google Scholar]
- 46.Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res. 2008;103:974–982. doi: 10.1161/CIRCRESAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.