Abstract
DNA polymerase ɛ (Polɛ) is thought to be involved in DNA replication, repair, and cell-cycle checkpoint control in eukaryotic cells. Although the requirement of other replicative DNA polymerases, DNA polymerases α and δ (Polα and δ), for chromosomal DNA replication has been well documented by genetic and biochemical studies, the precise role, if any, of Polɛ in chromosomal DNA replication is still obscure. Here we show, with the use of a cell-free replication system with Xenopus egg extracts, that Xenopus Polɛ is indeed required for chromosomal DNA replication. In Polɛ-depleted extracts, the elongation step of chromosomal DNA replication is markedly impaired, resulting in significant reduction of the overall DNA synthesis as well as accumulation of small replication intermediates. Moreover, despite the decreased DNA synthesis, excess amounts of Polα are loaded onto the chromatin template in Polɛ-depleted extracts, indicative of the failure of proper assembly of DNA synthesis machinery at the fork. These findings strongly suggest that Polɛ, along with Polα and Polδ, is necessary for coordinated chromosomal DNA replication in eukaryotic cells.
The duplication of genetic information encoded by chromosomal DNA is performed by several distinct DNA polymerases in eukaryotic cells. Among them, DNA polymerases α, δ, and ɛ (Polα, -δ, and -ɛ) are thought to be the major replicative DNA polymerases (1, 2). Polα is tightly associated with primase, so that it can start de novo DNA synthesis, and is thought to participate in the initiation of both leading and lagging strand synthesis (1, 2). However, Polα/primase synthesizes only a short RNA-DNA primer, which is then extended by a processive DNA polymerase(s). The previous biochemical studies on simian virus 40 (SV40) DNA replication, which has been extensively used as one of the model systems for eukaryotic DNA replication, reveals that the primer synthesized by Polα is elongated by Polδ, a processive DNA polymerase, and that these two DNA polymerases, Polα and Polδ, are sufficient for the completion of SV40 DNA replication in vitro (3, 4).
Polɛ is another highly processive DNA polymerase, and it has a 3′-5′ proofreading exonuclease activity (5, 6). It has been shown that Polɛ is essential for cell viability and is required for chromosomal DNA replication in budding yeast (7, 8). In addition to DNA replication, Polɛ is thought to be involved in DNA repair and cell-cycle checkpoint control in eukaryotic cells (5, 9). However, recent studies showed that its DNA polymerase domains are dispensable for cell viability; thus its function in DNA synthesis is in question (10, 11). Furthermore, biochemical studies of in vitro SV40 DNA replication have failed to prove the involvement of Polɛ in DNA replication (3, 4). Thus, the requirement of Polɛ for chromosomal DNA replication in other eukaryotic cells remains enigmatic.
To understand the role of Polɛ in DNA replication, we attempted to determine whether Polɛ is required for cell-free DNA replication in Xenopus egg extracts, in which chromosomal DNA replication can be carried out faithfully in vitro (12). The data presented here suggest that Polɛ is required for the efficient elongation of nascent DNA and the appropriate assembly of replication proteins at the fork.
Materials and Methods
cDNA Cloning.
The cDNA for the p60 subunit of Xenopus Polɛ (GenBank accession no. AB048257) was isolated by screening a Xenopus ovary cDNA library (Stratagene) with the cDNA for the p59 subunit of HeLa Polɛ (13). Both strands of its cDNA insert were sequenced with the use of an Applied Biosystems Prism dye terminator cycle sequencing kit and a DNA sequencer (ABI377). The initiation methionine was postulated on the basis of a comparison with the amino acid sequence of HeLa Polɛ p59 (13).
Antibodies.
Rabbit anti-Xenopus Polɛ p60 antibodies were raised against bacterially expressed 10 histidine-tagged p60 or glutathione S-transferase-fused, amino-terminal polypeptide (from amino acid 1 to 105) of p60. The p60-specific antibodies were affinity-purified with the use of antigen-immobilized Affi-Gel 15 (Bio-Rad). The purified p60 antibodies or whole rabbit IgG (Pierce) as a control was crosslinked to Affi-Prep Protein A matrix (Bio-Rad) (1 μg of IgG per μl of matrix) and used for immunoprecipitation and immunodepletion. The antibodies for the catalytic subunit of Xenopus Polα or Polɛ, replication protein A (RPA), proliferating cell nuclear antigen, Mcm2 and -3, and Cdc45 are described elsewhere (14). The antibody for Xenopus Polδ is a generous gift from Masahiro Akiyama (Nara Institute of Science and Technology, Ikoma, Nara, Japan). The antibody for the second subunit (p70) of Xenopus Polα was raised against 10 histidine-tagged recombinant protein (T. Fukui and S.W., unpublished observations).
Egg Extracts and DNA Replication Assay.
Xenopus egg extracts (low-speed supernatant) were prepared as described previously (15). Immunodepletion was performed by mixing egg extracts three times with the antibody-crosslinked matrix at 4°C. DNA replication with membrane-removed sperm nuclei (2,000 sperm heads per μl of extract) was carried out at 23°C in the presence of [α-32P]dATP as described elsewhere (15). The reaction products were purified by RNase A digestion, proteinase K digestion, and phenol/chloroform extraction followed by ethanol precipitation and then separated by 0.8% agarose gel electrophoresis under neutral (Tris/borate/EDTA buffer) or alkaline (30 mM NaOH/1 mM EDTA) conditions as described before (4). After electrophoresis, the gel was fixed, dried, and subjected to autoradiography. The quantification of replication products was carried out with a Fuji image analyzer (BAS1500).
For digestion of replication products with nuclease P1, purified products (equivalent to products from a replication reaction containing 2.5 ng of sperm DNA) were incubated with 0.02 unit of nuclease P1 (United States Biochemical) in the buffer containing 25 mM Tris⋅HCl (pH 7.5), 10 mM MgCl2, and 100 mM NaCl at 37°C for 10 min. Under these conditions, a supercoiled form of pBluescript plasmid was converted to a nicked circular or linear form.
Chromatin Isolation.
Isolation of sperm chromatin after the incubation with the depleted egg extracts and analysis of chromatin-bound proteins were as described previously (14).
Purification of Xenopus Polɛ Complex.
Polɛ was purified from Xenopus egg extracts by column chromatography as described below. Throughout the purification, column fractions were assayed for DNA polymerase activity with the use of [α-32P]dTTP and oligo(dT)⋅poly(dA) (1:19; 0.04 mM nucleotides) as a primer/template and were analyzed by Western blotting. The egg extracts, after centrifugation at 220,000 × g for 90 min at 4°C, were loaded onto a phosphocellulose (P11; Amersham Pharmacia) column equilibrated with buffer A (25 mM Tris⋅HCl, pH 7.5/10% glycerol/1 mM EDTA/0.01% Nonidet P-40/1 mM DTT) containing 0.1 M NaCl, and Polɛ was eluted stepwise with 0.2–0.33 M NaCl in buffer A. Note that most of Polδ was separated from Polɛ in this phosphocellulose step. The Polɛ fractions were dialyzed against buffer B (the same as buffer A, except for a pH of 8.0) containing 50 mM NaCl and then applied to a Q Sepharose column (Amersham Pharmacia), and Polɛ was eluted with a linear gradient of 50–700 mM NaCl in buffer B (peaking at 280 mM NaCl). The Q fractions containing Polɛ were loaded onto a hydroxyapatite (Bio-Rad HTP) column, and Polɛ was eluted with a linear gradient of 20–500 mM potassium phosphate (pH 7.5) (peaking at about 150 mM potassium phosphate). The active fractions were then applied to an SP Sepharose (Amersham Pharmacia) column, and Polɛ was eluted with a linear gradient of 25–600 mM NaCl in buffer containing 25 mM potassium phosphate (pH 7.5), 10% glycerol, 1 mM EDTA, 0.05% Triton X-100, and 1 mM DTT (peaking at about 180 mM). Finally, the SP Sepharose fractions were applied to a Mono Q column and eluted with a 0.1–0.5 M NaCl linear gradient in buffer B with a SMART chromatography system (Amersham Pharmacia).
Results and Discussion
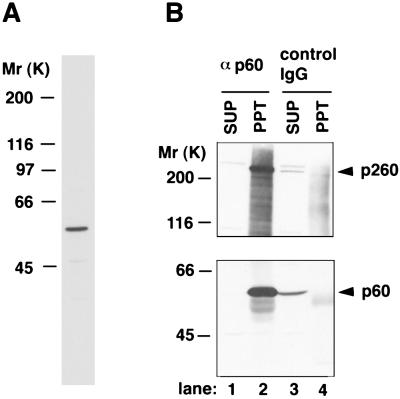
First, we isolated a full-length cDNA for the p60 subunit of Xenopus Polɛ. The isolated cDNA encodes a polypeptide with a Mr of 60,000, and its predicted amino acid sequence showed an 81% identity to that of HeLa Polɛ p59 (13) (data not shown). As shown in Fig. 1A, antibodies raised against the recombinant Polɛ p60 specifically recognized a single polypeptide with Mr of about 60,000 in Xenopus egg extracts. In addition, the antibodies quantitatively coprecipitated the catalytic subunit (p260) of Polɛ from egg extracts (Fig. 1B), indicating that p60 indeed forms a complex with Polɛ catalytic subunit.
Figure 1.
Characterization of the antibodies against the p60 subunit of Polɛ. (A) Western blotting of Xenopus egg extracts (0.5 μl) with the Polɛ p60 antibodies. (B) Coimmunoprecipitation of the catalytic subunit of Polɛ with the p60 antibodies. Immunoprecipitation from egg extracts with the p60 antibodies (αp60) (lanes 1 and 2) or with whole rabbit immunoglobulin G (control IgG) (lanes 3 and 4) was performed. The resultant supernatants (SUP, lanes 1 and 3) and precipitates (PPT, lanes 2 and 4) were analyzed by Western blotting with the p60 antibodies (Lower) or the antibodies against the catalytic subunit of Polɛ (Upper). Arrowheads indicate the catalytic subunit (p260) and p60 of Polɛ, respectively.
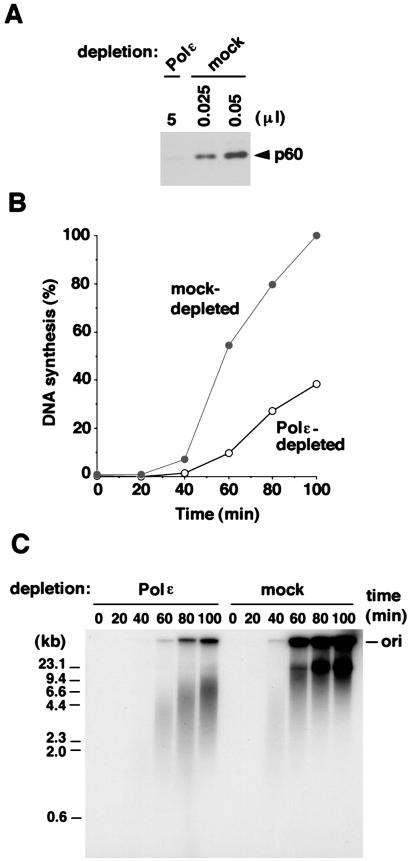
Using the p60 antibody-conjugated matrix, we depleted Polɛ complex from egg extracts. It was possible to remove more than 99.5% of p60 from egg extracts through the antibody matrix (Fig. 2A). It was also confirmed that more than 96% of the catalytic subunit of Polɛ was removed from the extracts (data not shown). We tested the ability of this Polɛ-depleted extract to replicate Xenopus sperm chromatin in vitro. As shown in Fig. 2B, the amount of DNA synthesis observed in Polɛ-depleted extracts was significantly lower than that in mock-depleted extracts. Moreover, the gel analysis of the replication products revealed an even more remarkable difference between Polɛ-depleted and mock-depleted egg extracts (Fig. 2C). In mock-depleted extracts, replication products mainly consisted of high-molecular-weight DNA as shown previously (12). On the other hand, relatively small DNA replication intermediates (about 2–10 kb in length) clearly accumulated in Polɛ-depleted extracts (Fig. 2C). However, no significant defect in DNA synthesis with a single-stranded M13 DNA template was detected in Polɛ-depleted extracts, in which a full-length, closed circular M13 DNA was produced with the same kinetics as seen in mock-depleted extracts (data not shown). This observation suggests that the DNA replication defect seen above is likely to result from events after the bona fide initiation of chromosomal DNA replication.
Figure 2.
DNA replication is impaired in Polɛ-depleted extracts. (A) The Polɛ complex can be efficiently removed from egg extracts by the p60 antibodies. Five microliters of Polɛ-depleted extracts and various amounts of mock-depleted extracts were analyzed by Western blotting with the p60 antibodies. (B) Time course of DNA replication in Polɛ-depleted extracts. The amount of DNA synthesis (%) relative to that of a 100-min incubation with mock-depleted extracts is shown. (C) Replication products made with Polɛ-depleted extracts or mock-depleted extracts. The replication products of the same reactions in B were separated by neutral agarose gel electrophoresis followed by autoradiography. The position of the origin for gel electrophoresis (ori) is indicated on the right. The sizes of marker DNA are on the left. Note that the materials remaining in the gel wells are likely to be the replication intermediates because those disappeared from the wells after digestion with a single-strand DNA-specific endonuclease P1 (see Fig. 4B).
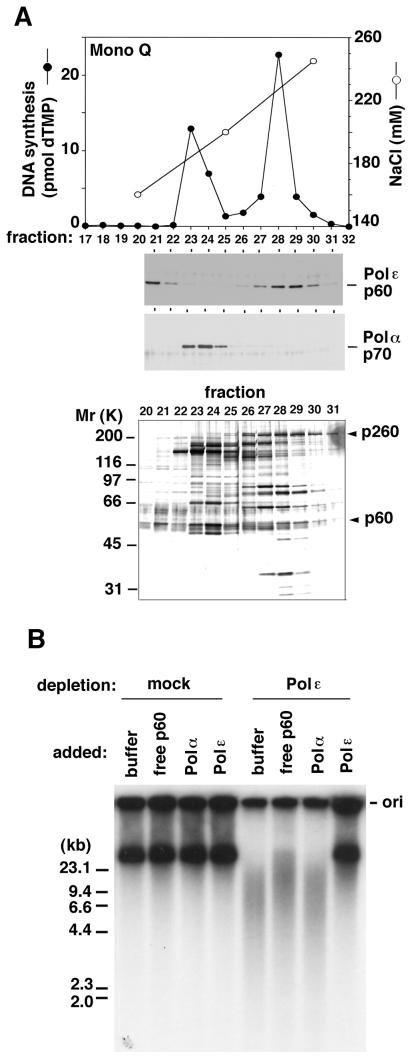
We next attempted to determine whether the DNA replication defect observed above could be due to a lack of Polɛ. To date, the exact subunit composition of Polɛ in higher eukaryotes is not known, except for that of human Polɛ (16). Thus, we extensively purified the Polɛ complex from Xenopus egg extracts. Polɛ was clearly separated from other replicative DNA polymerases, Polα and Polδ (Fig. 3A and data not shown). When the most purified and active Polɛ was added to Polɛ-depleted extracts, DNA replication was almost fully restored to the levels observed in mock-depleted extracts, whereas the addition of either Polα or free p60 failed to restore normal levels of DNA synthesis (data not shown). More importantly, the replication products made from the rescued reactions were almost the same as those in mock-depleted extracts (Fig. 3B). Although many bands other than Polɛ p260 and p60 polypeptides were detected in the purified Polɛ fraction (fraction 28 in Fig. 3A), none of them, except for Polɛ subunits, were found to correspond to the proteins specifically immunoprecipitated with the Polɛ p60 antibodies; Polɛ subunits were the major polypeptides in the p60 antibody-specific immunoprecipitates (data not shown). Furthermore, a comparative immunoblotting showed that the amount of p60 added back in the depleted extracts (Fig. 3B) was equivalent to about 30% of that in mock-depleted extracts (data not shown), suggesting that any minor component in the purified fraction is unlikely to contribute to the restoration of DNA replication. Judging from these observations and the fact that neither Polδ nor Polα was coprecipitated with the p60 antibodies (data not shown), it is highly likely that the DNA replication defect seen above is due to a lack of Polɛ in the egg extracts. We conclude, therefore, that Polɛ plays an important function during chromosomal DNA replication in Xenopus egg extracts.
Figure 3.
DNA replication can be restored by adding purified Polɛ back to the depleted extracts. (A) Purification of Xenopus Polɛ from egg extracts. An elution profile of DNA polymerase activity from Mono Q column (Top), immunoblots with Polɛ p60 or Polα p70 antibodies (Middle), and a silver-stained protein gel (Bottom) of the corresponding fractions are shown. Arrowheads indicate the positions of the catalytic subunit (p260) and p60 of Polɛ, respectively. (B) Restoration of DNA replication by the addition of purified Polɛ back to Polɛ-depleted extracts. The products from the reactions (70-min incubation) with mock- or Polɛ-depleted extracts (14 μl each) supplemented with 1 μl each of control buffer or each Mono Q fraction containing Polɛ (fraction 28 in A), Polα (fraction 23 in A), or free p60 (fraction 21 in A) were analyzed as in Fig. 2C. The position of the origin for gel electrophoresis (ori) is indicated on the right. The sizes of marker DNA are on the left.
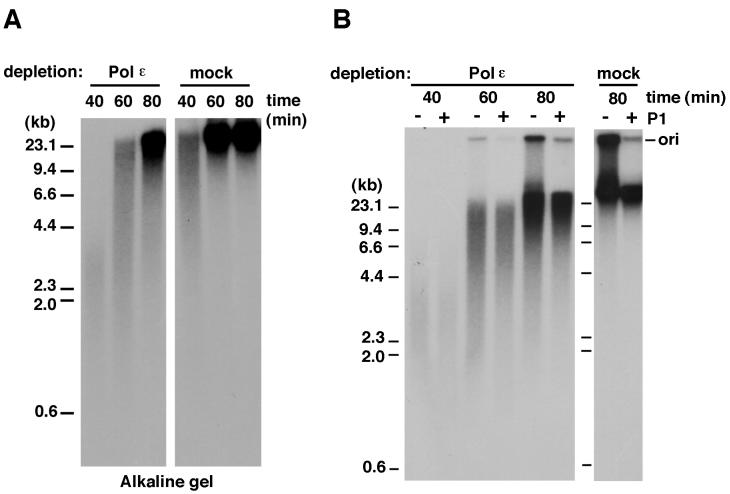
In the absence of Polɛ, the remaining DNA synthesis that results in the accumulation of small replication intermediates is likely to be performed by Polα and Polδ, both of which seem to be present in Polɛ-depleted extracts in large quantities. The analysis of the reaction products by alkaline agarose gel electrophoresis showed that the elongation of nascent DNA was indeed retarded in Polɛ-depleted extracts (Fig. 4A). This observation suggests that Polɛ is required for efficient elongation of nascent DNA during replication. The slowed elongation in the absence of Polɛ might result simply from a lack of a polymerase activity responsible for efficient elongation and/or from failure of proper assembly of DNA synthesis machinery at the fork (see also below). Furthermore, a majority of the short DNA products made with Polɛ-depleted extracts were double-stranded DNA, inasmuch as those were resistant to digestion with a single-stranded DNA-specific nuclease P1, whereas most of the materials remaining in the wells were digested under the same conditions (Fig. 4B). Thus it is possible that in Polɛ-depleted extracts a stalling or lowering of the rate of DNA synthesis may cause the accumulation of structurally unstable replication intermediates or double-strand breaks, which may result in the production of small DNA fragments harboring a nascent DNA strand.
Figure 4.
Elongation of nascent DNA is retarded in Polɛ-depleted extracts. (A) Analysis of replication products by alkaline agarose gel electrophoresis. The replication reactions with Polɛ- or mock-depleted extracts were carried out for various times as indicated, and the purified products were analyzed by alkaline agarose gel electrophoresis followed by autoradiography. (B) The small DNA fragments produced in Polɛ-depleted extracts are double-stranded DNA. The replication reactions with the depleted extracts were carried out for various times as indicated. Purified products were divided in half and incubated with nuclease P1 (+) or control buffer (−). The digested products were then analyzed by neutral agarose gel electrophoresis followed by autoradiography.
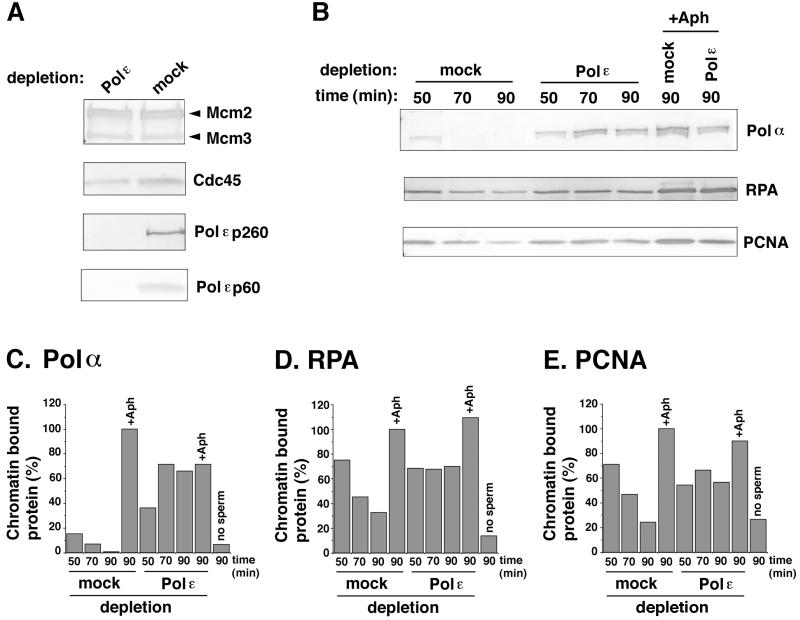
We also investigated the loading of other replication proteins onto chromatin during DNA synthesis in the absence of Polɛ. As shown in Fig. 5A, the loading of Mcm2 and -3 or Cdc45, all of which are required for the initiation of DNA replication, was not significantly inhibited by the depletion of Polɛ. The loading of Cdc45 onto chromatin has been shown to occur in a cyclin-dependent kinase (CDK)-dependent manner after the activation process involving Dbf4-Cdc7 and before the start of DNA synthesis and to be required for the loading of Polα onto chromatin (17–19). Thus, Polɛ seems not to be required for the formation of prereplicative complex, Cdc7-dependent activation of prereplicative complex, and the subsequent loading of Cdc45 onto chromatin. However, we cannot exclude the possibility that the lack of Polɛ may decrease the efficiency of origin firing but increase the stability of the initiation complex including Cdc45 at the origins.
Figure 5.
The loading of replication proteins onto chromatin is increased in Polɛ-depleted extracts. (A and B) Western blot analysis of replication proteins bound to sperm chromatin during the incubation with Polɛ- or mock-depleted extracts. In A, sperm chromatin was incubated in the depleted extracts for 50 min in the presence of 10 μg⋅ml−1 aphidicolin. In B, sperm chromatin was incubated with the depleted extracts for various times as indicated, in the presence (+Aph) or absence of aphidicolin. (C–E) The quantification of chromatin-bound Polα, RPA, and proliferating cell nuclear antigen (PCNA), respectively, in B. The amounts (%) of chromatin-bound proteins relative to those in mock-depleted extracts with aphidicolin (90-min incubation) are shown.
Interestingly, the amount of chromatin-bound Polα markedly increased in Polɛ-depleted extracts, and this high level of binding was maintained during the incubation (Fig. 5 B and C). In contrast, the amount of chromatin-bound Polα was rapidly decreased as chromosomal DNA replication proceeded in mock-depleted extracts (Fig. 5 B and C). In addition, we found that the amounts of chromatin-bound RPA and proliferating cell nuclear antigen were kept constant throughout incubation in Polɛ-depleted extracts, whereas they gradually decreased in mock-depleted extracts (Fig. 5 B, D, and E). There are several possible explanations of these changes upon the depletion of Polɛ. One possibility is that, in Polɛ-depleted extracts, DNA unwinding may proceed without concomitant DNA synthesis, as previously seen in the presence of aphidicolin, an inhibitor of DNA synthesis (14, 20). The resulting, extensively unwound DNA region may serve as binding sites for RPA as well as Polα. In fact, the high levels of chromatin-bound Polα were comparable to those seen in the presence of aphidicolin (Fig. 5). Second, without Polɛ, displacement of Polα from DNA primer may not take place efficiently, so that many Polα molecules may be left on the unwound DNA region. Third, assembly of replication factors at the fork might be deregulated without Polɛ. Last, it might be possible that DNA repair machinery contributes to some extent to the production of small DNA fragments as well as relatively high levels of chromatin binding of RPA and proliferating cell nuclear antigen in Polɛ-depleted extracts. Although these possibilities remain to be tested, these results suggest that Polɛ is required for the coordinated assembly and function of replication proteins involved in the elongation of nascent DNA.
The results in this paper demonstrate convincingly that Polɛ is required for chromosomal DNA replication in eukaryotic cells, besides budding yeast (7, 8). It is highly likely that Polɛ is also necessary for DNA replication in somatic cells of mammals as well as in Xenopus eggs. The significant decrease in DNA synthesis in Polɛ-depleted extracts indicates that the polymerase activity itself is likely to be important for chromosomal DNA replication. Polɛ appears to be a component of the replication fork in budding yeast (21, 22), in which Polɛ associates/disassociates from chromosomal DNA with kinetics similar to those of Mcm4p during the S phase of the cell cycle (21). In addition, recent biochemical studies showed cell cycle-regulated interactions among Polɛ, RPA, Cdc45p, and Mcm2p in budding yeast (22). Taking these data together, we propose that Polɛ has a crucial role in the formation of DNA synthesis machinery at the fork, so that coordinated and efficient DNA elongation can be achieved. At the fork formed at origins, Polɛ might also regulate the loading of Polα onto DNA, as suggested previously (23).
The data presented here are consistent with the previous observations that Polɛ could be photo-crosslinked to nascent DNA in mammalian cells (24) and that a neutralizing antibody against Polɛ inhibited cellular but not SV40 DNA replication (25). It might be possible that the assembly of DNA synthesis machinery including SV40 T antigen leads to the exclusion of Polɛ from the replication fork during SV40 DNA replication, so that the viral DNA might be preferentially replicated in the infected cells.
With respect to budding yeast Polɛ, the previous experiments with temperature-sensitive mutants indicated that Polɛ is required for chromosomal DNA replication (8). However, the recent observation that the polymerase domain of yeast Polɛ is dispensable for cell viability (10, 11) suggests that, because of its relatively small genome, Polδ might be able to substitute for Polɛ as long as the carboxy-terminal portion of Polɛ functions in the S/M checkpoint control (26, 27).
The results shown here suggest, however, that the polymerase activity of Polɛ may not be simply replaced by that of Polδ in higher eukaryotes. It is still possible that the carboxy-terminal portion of Polɛ in higher eukaryotes might also have an essential function, like yeast Polɛ, and that the elimination of the function of the carboxy-terminal domain rather than the lack of polymerase domain might cause the defect in DNA replication in Polɛ-depleted extracts. It will be necessary to test this possibility by adding some mutant forms of recombinant Polɛ back into Polɛ-depleted extracts. Finally, although a specific role of Polɛ in leading strand synthesis has been suggested (5), the data presented here did not provide any convincing evidence to prove such a specific role for Polɛ. Thus it remains to be determined which DNA strand is synthesized by Polɛ in normal egg extracts.
Acknowledgments
We thank H. Asahara and S. Linn for a cDNA clone of HeLa Polɛ p59, M. Akiyama for the antibody against Xenopus Polδ, and H. Mizuno and T. Fukui for production of antibodies against Polɛ p60 and Polα p70. We are also grateful to T. Hirano and S. Mimura for helpful technical advice and L. H. Johnston for critical reading of the manuscript. This work was supported in part by the Yamada Science Foundation (S.W.) and by Grants-in-Aid for Scientific Research on Priority Areas (A) from the Ministry of Education, Science, Sport and Culture of Japan (to H.T. and A.S.).
Abbreviations
- Polα
-δ, and -ɛ, DNA polymerases α, δ, and ɛ
- SV40
simian virus 40
- RPA
replication protein A
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB048257).
References
- 1.Waga S, Stillman B. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 2.Hübscher U, Nasheuer H-P, Syväoja J E. Trends Biochem Sci. 2000;25:143–147. doi: 10.1016/s0968-0004(99)01523-6. [DOI] [PubMed] [Google Scholar]
- 3.Waga S, Stillman B. Nature (London) 1994;369:207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- 4.Waga S, Bauer G, Stillman B. J Biol Chem. 1994;269:10923–10934. [PubMed] [Google Scholar]
- 5.Sugino A. Trends Biochem Sci. 1995;20:319–323. doi: 10.1016/s0968-0004(00)89059-3. [DOI] [PubMed] [Google Scholar]
- 6.Morrison A, Sugino A. Mol Gen Genet. 1994;242:289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 7.Morrison A, Araki H, Clark A B, Hamatake R K, Sugino A. Cell. 1990;62:1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- 8.Araki H, Ropp P A, Johnson A L, Johnston L H, Morrison A, Sugino A. EMBO J. 1992;11:733–740. doi: 10.1002/j.1460-2075.1992.tb05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgers P M. Chromosoma. 1998;107:218–227. doi: 10.1007/s004120050300. [DOI] [PubMed] [Google Scholar]
- 10.Kesti T, Flick K, Keränen S, Syväoja J E, Wittenberg C. Mol Cell. 1999;3:679–685. doi: 10.1016/s1097-2765(00)80361-5. [DOI] [PubMed] [Google Scholar]
- 11.Dua R, Levy D L, Campbell J L. J Biol Chem. 1999;274:22283–22288. doi: 10.1074/jbc.274.32.22283. [DOI] [PubMed] [Google Scholar]
- 12.Blow J J, Laskey R A. Cell. 1986;47:577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Asahara H, Patel V S, Zhou S, Linn S. J Biol Chem. 1997;272:32337–32344. doi: 10.1074/jbc.272.51.32337. [DOI] [PubMed] [Google Scholar]
- 14.Mimura S, Masuda T, Matsui T, Takisawa H. Genes Cells. 2000;5:439–452. doi: 10.1046/j.1365-2443.2000.00340.x. [DOI] [PubMed] [Google Scholar]
- 15.Chong J P, Thommes P, Rowles A, Mahbubani H M, Blow J J. Methods Enzymol. 1997;283:549–564. doi: 10.1016/s0076-6879(97)83043-1. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Pursell Z F, Linn S. J Biol Chem. 2000;275:23247–23252. doi: 10.1074/jbc.M002548200. [DOI] [PubMed] [Google Scholar]
- 17.Zou L, Stillman B. Science. 1998;280:593–596. doi: 10.1126/science.280.5363.593. [DOI] [PubMed] [Google Scholar]
- 18.Mimura S, Takisawa H. EMBO J. 1998;17:5699–5707. doi: 10.1093/emboj/17.19.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jares P, Blow J J. Genes Dev. 2000;14:1528–1540. [PMC free article] [PubMed] [Google Scholar]
- 20.Walter J, Newport J. Mol Cell. 2000;5:617–627. doi: 10.1016/s1097-2765(00)80241-5. [DOI] [PubMed] [Google Scholar]
- 21.Aparicio O M, Weinstein D M, Bell S P. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Stillman B. Mol Cell Biol. 2000;20:3086–3096. doi: 10.1128/mcb.20.9.3086-3096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masumoto H, Sugino A, Araki H. Mol Cell Biol. 2000;20:2809–2817. doi: 10.1128/mcb.20.8.2809-2817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zlotkin T, Kaufmann G, Jiang Y, Lee M Y W T, Uitto L, Syväoja J, Dornreiter I, Fanning E, Nethanel T. EMBO J. 1996;15:2298–2305. [PMC free article] [PubMed] [Google Scholar]
- 25.Pospiech H, Kursula I, Abdel-Aziz W, Malkas L, Uitto L, Kastelli M, Vihinen-Ranta M, Eskelinen S, Syväoja J E. Nucleic Acids Res. 1999;27:3799–3804. doi: 10.1093/nar/27.19.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navas T A, Zhou Z, Elledge S J. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 27.Dua R, Levy D L, Campbell J L. J Biol Chem. 1998;273:30046–30055. doi: 10.1074/jbc.273.45.30046. [DOI] [PubMed] [Google Scholar]