Highlights
► Chromium VI severely affects cell growth, ultrastructure and photosynthesis. ► Micrasterias deposits Cr in bag like structures as Cr–iron–oxygen compound. ► Increase in Cr content leads to a depletion of intracellular iron levels. ► Cr detoxification possibly involves glutathione.
Abbreviations: CAT, catalase; EELS, electron energy loss spectroscopy; ESI, electron spectroscopic imaging; ROS, reactive oxygen species; SOD, superoxide dismutase
Keywords: Chromium, Electron energy loss spectroscopy, Glutathione, Iron, Micrasterias denticulata, Ultrastructure
Abstract
Various contaminants like metals and heavy metals are constantly released into the environment by anthropogenic activities. The heavy metal chromium has a wide industrial use and exists in two stable oxidation states: trivalent and hexavalent. Chromium can cause harm to cell metabolism and development, when it is taken up by plants instead of necessary micronutrients such as for example iron. The uptake of Cr VI into plant cells has been reported to be an active process via carriers of essential anions, while the cation Cr III seems to be taken up inactively. Micrasterias denticulata, an unicellular green alga of the family Desmidiaceae is a well-studied cell biological model organism. Cr III and VI had inhibiting effects on its cell development, while cell division rates were only impaired by Cr VI. Transmission electron microscopy (TEM) revealed ultrastructural changes such as increased vacuolization, condensed cytoplasm and dark precipitations in the cell wall after 3 weeks of Cr VI treatment. Electron energy loss spectroscopy (EELS) and electron spectroscopic imaging (ESI) were applied to measure intracellular chromium distribution. Chromium was only detected after 3 weeks of 10 μM Cr VI treatment in electron dense precipitations found in bag-like structures along the inner side of the cell walls together with iron and elevated levels of oxygen, pointing toward an accumulation respectively extrusion of chromium in form of an iron–oxygen compound. Atomic emission spectroscopy (EMS) revealed that Micrasterias cells are able to accumulate considerable amounts of chromium and iron. During chromium treatment the Cr:Fe ratio shifted in favor of chromium, which implied that chromium may be taken up instead of iron. Significant and rapid increase of ROS production within the first 5 min of treatment confirms an active Cr VI uptake. SOD and CAT activity after Cr VI treatment did not show a response, while the glutathione pool determined by immuno-TEM decreased significantly in chromium treated cells, showing that glutathione is playing a major role in intracellular ROS and chromium detoxification.
1. Introduction
Chromium is the seventh most abundant metal in the earth's crust (Panda and Choudhury, 2005) and is naturally occurring in soil, but can be found in all phases of the environment. In fresh water concentrations range from 0.1 to 117 μg l−1, while concentrations in serpentine soils can reach up to 125 g kg−1 (Shanker et al., 2005). Chromium has a wide industrial use and is released into the environment by processes such as electroplating, tanning, polishing, painting, pigment manufacture and wood preservation (Peralta-Videa et al., 2009). These anthropogenic activities have led to a widespread contamination of the environment.
Chromium is not an essential element for plant nutrition, but may nevertheless be taken up by plants (Liu et al., 2008). Only two oxidative forms Cr III and Cr VI are stable enough to occur naturally, but they are drastically different in charge, physiochemical properties as well as chemical and biochemical reactivity (Kotas and Stasicka, 2000). Overall, Cr VI is considered to be the more toxic than Cr III. As an anion it is negatively charged and highly soluble in water and thus has a better bioavailability and is more mobile than the cationic form Cr III. Like other heavy metals chromium is phytotoxic and can result in growth inhibition, degrade photosynthetic pigments, lead to nutrient and water imbalance and induce oxidative stress (Panda and Choudhury, 2005). Terrestrial plants take up essential and non-essential elements from the soil, while aquatic plants take up ions from all their surroundings. There are many studies of the effects of chromium on higher plants (Liu and Kottke, 2003; Rai and Mehrotra, 2008; Upadhyay and Panda, 2010), but in respect to algae most research focuses on biosorption abilities of certain species for phytoremidiation to remove chromium from contaminated water (Sheng et al., 2004; Rai et al., 2005; Gupta and Rastogi, 2008,). Only few studies investigate the effects of chromium on physiological processes in the algal cells (e.g. Hörcsik et al., 2007; Vignati et al., 2010) and none seem to determine where chromium is located intracellular. Nevertheless it is of relevance to study not only how much metal can be accumulated, but also to understand how the contaminant is entering a plant cell, what effects it causes on cell physiology, as well as development, whether it is compartmentalized and which detoxification mechanisms exist. This is particularly important since plants are an essential source of food to animals and humans and they are also used as resource for medical drugs and other commonly used products. When plants are cultivated in contaminated areas there is a risk of heavy metal accumulation, allowing contaminants such as chromium to enter the food chain (Gorbi et al., 2002; Rai et al., 2004). Cr VI is not only considered highly toxic to plants but also to mammals and humans, due to its detrimental effects on several organs and tissues, it is a potential carcinogen (Peralta-Videa et al., 2009).
The unicellular, fresh water, green algae Micrasterias denticulata has been employed as a sensitive model organism and may be representative for the bottom of the food chain. Micrasterias has been shown to respond in similar ways as higher plants in experiments and has been used for many years as a model system in cell biology (e.g. Kiermayer, 1981; Meindl, 1993; Holzinger and Lütz-Meindl, 2002; Eder and Lütz-Meindl, 2008; Darehshouri et al., 2008).
This study is intended to analyze the impact of chromium on the unicellular model system Micrasterias at different levels. The effects of Cr III and Cr VI on cell development, division rates, vitality and photosynthesis are compared. Further ultrastructure, ROS levels, antioxidative enzyme activities and glutathione levels were investigated in Cr VI treated cells. The uptake of chromium was not only analyzed quantitatively by atomic emission spectroscopy, but also qualitatively by TEM-coupled electron energy loss spectroscopy (EELS) and electron spectroscopic imaging (ESI) allowing the determination of chromium accumulation sites at a high spatial resolution. The results give insight on chromium uptake, accumulation and detoxification in algal cells as a basis for future investigations and for a better understanding of the ongoing intracellular mechanisms.
2. Materials and methods
2.1. Chemicals
All chemicals were purchased from Sigma–Aldrich (Vienna, Austria) and Fluka (Steinheim, Germany) unless stated differently.
2.2. Cell cultures
Erlenmeyer flasks containing Desmidiaceaen nutrient solution (Schlösser, 1982) were used to grow the unicellular green alga M. denticulata in mono-algal, non-axenic, clone cultures. The cells were kept at a 14:10 h light to dark regime at a constant temperature of 20 °C. Sub-culturing was done every 3–4 weeks.
2.3. Light microscopy
Dividing cells between 20 and 75 min after mitosis, were collected from cell cultures and the following chromium nutrient solutions were tested to determine the effective concentration range: 1 mM to 10 nM CrKO8S2 × 12 H2O (Cr III) and 1 mM to 10 nM K2Cr2O7 (Cr VI). The cells were incubated for 4 h and the effects were observed after 2 and 4 h with a Univar light microscope (Reichert, Vienna, Austria). Pictures were acquired with a Canon Power Shot G5 camera (Tokyo, Japan).
Purposely a wide range of chromium concentrations was tested to find the highest non-lethal concentrations, which had the strongest effect on the alga, intending to determine suitable concentrations for electron microscopical investigations and long term treatments of Micrasterias.
2.4. Cell vitality-assay
The percentage of living cells was determined by analyzing the ability of the cells to perform plasmolysis. After chromium treatment 50 cells were collected and the nutrient solution was substituted with 500 mM sorbitol. Cells not undergoing plasmolysis within 15 min sorbitol exposure were assumed dead and counted. Cell vitality-assays were carried out in triplets with 1 mM Cr III and Cr VI after 4 h treatment and with 5 μM and 10 μM Cr III and Cr VI after 3 weeks treatment.
2.5. Cell division rates
To determine interphase cells, dividing Micrasterias cells were selected from the cultures after mitosis and grown at culture conditions for 2 days before Cr treatment. The cell division rates of alga cells treated with 5 μM and 10 μM Cr III and Cr VI were examined over the course of 21 days and compared to rates of untreated control cells. Additionally dividing cells were treated with 1 mM Cr VI for 4 h, then transferred into nutrient solution for recovery, before their cell division rates were also observed for 21 days. All experiments were carried out three times starting with 10 cells at a time.
2.6. Photosynthesis and respiration
Micrasterias cultures were exposed to 5 μM and 10 μM Cr III and Cr VI for 3 weeks. To determine the physiological status by means of photosynthesis and respiration, approximately 2000 cells were used for each run with 3–4 light/dark cycles, which were repeated 3 times each. Oxygen turnover as indicator for photosynthetic activity, was measured by means of a Hansatech (King's Lynn, UK) polarographic oxygen electrode as μM oxygen/h/mg chlorophyll. Illumination was set to 200 μM photons m2 s−1 and the temperature was kept constant at 21 °C. After each measurement an aliquot of the suspension was removed and total chlorophyll was determined after extraction of the pigments into dimethylformamide (Porra et al., 1989). Photosynthetic efficiency was measured as described by Affenzeller et al. (2009) using the Handy Pea (Hansatech, King's Lynn, UK) with a minimum of 7 assays per treatment.
2.7. TEM-analysis
Dividing Micrasterias cells were treated with 1 mM Cr VI for 4 h. Interphase cells were grown in 5 μM and 10 μM Cr VI nutrient solution for 3 weeks and in 5 μM Cr VI for 6 weeks as long term treatments.
2.7.1. High-pressure freeze fixation, cryo-substitution and TEM
Treated cells and controls were cryofixed in a Leica EMPACT high-pressure freezer, underwent freeze substitution in a Leica EM AFS (Leica Microsysteme GmbH, Vienna, Austria) as described by Meindl et al. (1992) and Lütz-Meindl and Aichinger (2004), and were then embedded in Agar low viscosity resin. Ultrathin sections were placed on formvar coated copper grids for ultrastructural investigation. The sections were viewed in a LEO 912 AB transmission electron microscope with in-column energy filter (Zeiss, Oberkochen, Germany), operated with a LaB6 cathode at 80 kV. Images were filtered at zero-loss energy. For image acquisition, EELS and ESI, a slow scan dual speed CCD camera “Sharpeye” (Tröndle, Moorenweis, Germany) was used, operated by iTEM software (Soft Image System, Münster, Germany).
2.7.2. Intracellular chromium localization by EELS and ESI
Ultrathin sections of approx. 50 nm were placed on hexagonal copper grids for EELS measurements. EELS spectra were acquired at an acceleration voltage of 120 kV and magnifications between 25,000× and 40,000× were chosen. The measurement area was defined by a 100 μm spectrometer entrance aperture. When EELS were acquired 5–7 integration cycles were taken per measurement. Chromium was measured at the L2,3 edge at 570 eV, with illumination angles between 1 and 1.6 mrad and exposure times between 2 and 5 s. The spectrum magnification was 200×. In order to determine metal complexation O—K (532.0 eV), N—K (397.0 eV), S—L1 (189.0) and Fe—L2,3 (708.0 eV) edges were additionally measured on sites of metal accumulation and element maps (ESI) were taken with the two-window power-law method.
2.8. Quantitative analysis of algal chromium content by atomic emission spectroscopy
Micrasterias cultures were grown for 1 and 3 weeks in 10 μM Cr VI and for 6 weeks in 5 μM Cr VI nutrient solution. Cells were harvested by centrifugation and then transferred to quartz tubes in which they were dried for approximately 24 h until a constant weight was reached. The biological material was digested in HNO3 (170 °C, 8 bar, 12 h) in a Seif-Apperatus (Seif, Unterschleissheim) as described by Schramel et al. (1980) and analyzed by an ICP-Optical Emission Spectrometer Optima 7300 DV (PerkinElmer, Waltham, USA) equipped with Win LAB 32 IPC.
2.9. Determination of glutathione levels via immunogold-labeling
For cytohistochemical investigations Micrasterias cells were cryofixed as described above and afterwards freeze substituted in 1% glutaraldehyde and 0.05% uranylacetate in acetone, before they were embedded in LR-White resin (London Resin Company Ltd., Berkshire, UK). Immunogold labeling was done on ultrathin sections on gold grids after a modified protocol by Zechmann et al. (2008). Samples were first blocked with 2% BSA in 0.05 M Trizma-buffer for 20 min at room temperature. The excess blocking solution was removed with filter paper. Then grids were transferred to the primary antibody (anti-gluthatione rabbit polyclonal IgG; Millipore, California, USA) diluted 1:25 in 0.05 M Trizma-buffer containing 1% goat serum and incubated over night at 4 °C. Sections were rinsed in 1% BSA in 0.05 M Trizma-buffer, then treated with 10 nm gold-conjugated secondary antibody (goat anti rabbit IgG) diluted 1:25 in 1% BSA in 0.05 M Trizma-buffer for 60 min at room temperature and finally sprayed with 1% BSA in 0.05 M Trizma-buffer and double distilled water. Access liquid was removed with filter paper from the grids, which were dried before observed in a LEO 912 AB TEM (see above) at 80 kV. A negative control was carried out to confirm the specificity of the procedure, by only using the gold conjugated secondary antibody without prior incubation of the sections with the primary antibody. Quantitative analysis was carried out by taking random micrographs of mitochondria, chloroplasts, cytoplasm, vacuoles and cell walls at a magnification of 5000×. In each cell 50 regions of interest (ROI = 131,065.74 nm2) were then randomly placed in each of the selected cell compartments, within which the amount of gold particles was counted. For statistics 5 cells of each treatment, both 5 μM and 10 μM Cr VI, and controls were evaluated this way.
2.10. ROS determination
Dichlorofluorescein diacetate (H2DCFDA, Invitrogen, Eugene, Oregon, USA) is a dye, which becomes fluorescent after oxidation and is used to determine intracellular ROS levels. Controls and Micrasterias interphase cells were treated with 1 mM Cr VI for 5, 10, 15, 30, 45 and 60 min, before they were stained with 100 μM H2DCFDA for approximately 20 min at room temperature. The cells were then analyzed in a confocal laser scanning microscope (CLSM). For visualization a Leica TCS SP5 AOBS confocal laser scanning system (Leica Microsystems, Wetzlar, Germany) was used, coupled with an inverted Leica DMI 6000 Cs light microscope, equipped with differential interference contrast optics (DIC). An argon laser at 496 nm was applied for excitation and emission wavelengths between 514 and 549 nm were band-pass filtered. An additional detection of wavelengths between 662 and 721 nm was acquired to simultaneously detect the chlorophyll autoflourescence. For all images a 40× dry planapochromatic objective was used. For each treatment time 25 cells were observed and the procedure was carried out three times. To estimate mean fluorescence the Leica application suite advanced fluorescence software was employed.
2.11. SOD activity
Native polyacrylamide gel electrophoresis (PAGE) analysis, after a modified protocol by Lee et al. (2007) was employed to determine SOD activity. Micrasterias cultures were treated for 5, 10, 15, 30 and 45 min with 1 mM Cr VI nutrient solution. After harvesting the algae cells by centrifugation they were homogenized in a chilled mortar with a pestle. A 100 mM potassium phosphate buffer (pH 7.8) containing 3 mM EDTA, 2% polyvinylpyrrolidone (PVPP) and 3 mM magnesiumsulfate was added to the homogenate, while samples were kept on ice and then centrifuged at 18,000 × g for 20 min at 4 °C. The supernatant was used for analysis. Protein content in the extracts was determined colorimetrically (Bradford, 1976). For the in-gel analysis of SOD isozyme activity by native PAGE the procedure of Beauchamp and Fridovich (1971) was carried out. To identify the different isoforms of SOD, 2 mM potassium cyanide (KCN) (inhibitor of CuZnSOD), or 5 mM H2O2 (inhibitor of CuZnSOD and FeSOD) were used. Enzyme activity was measured at room temperature.
2.12. CAT activity
For sample preparation Micrasterias cells were treated for 5, 10, 15, 30 and 45 min with 1 mM Cr VI nutrient solution before they were harvested by centrifugation. Cells were homogenized in approximately 1.2 ml of a 50 mM potassium phosphate buffer (pH 7.8) containing 0.5 M EDTA, 2% polyvinylpyrrolidone and 0.5% Triton (Benavides et al., 2010). The homogenates were centrifuged at 4 °C with 13,000 × g for 30 min twice and the supernatant fraction was used for the enzyme assay. Enzyme activity was again determined by measuring the decrease of absorbance at 240 nm in a Lambda XLS photometer (PerkinElmer, Waltham, USA) after an amount of supernatant containing 10 μg protein was added to a reaction mixture which consisting of 50 mM potassium phosphate buffer (pH 7.8) and 2 mM H2O2. The decrease in H2O2 absorption was determined and enzyme activity was calculated with the extinction coefficient (ɛ = 0.0396 mM/l cm−1) and expressed as n kat mg−1.
3. Results
3.1. Light microscopy, cell vitality and cell division
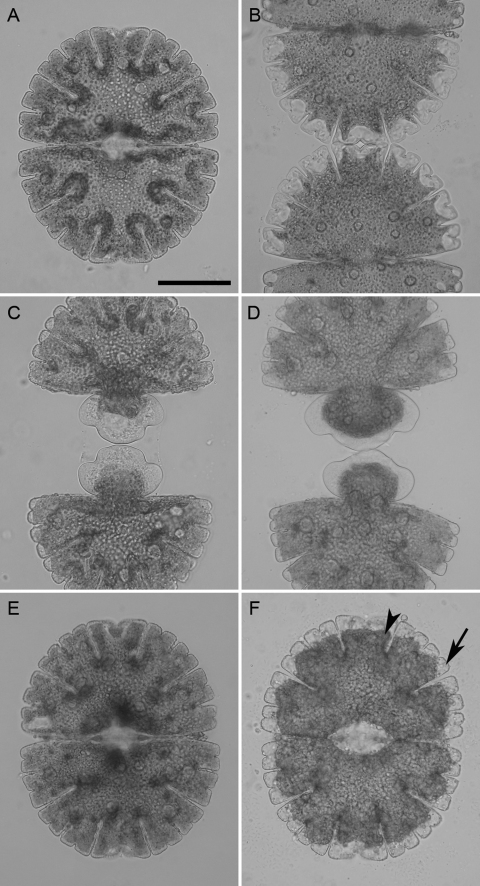
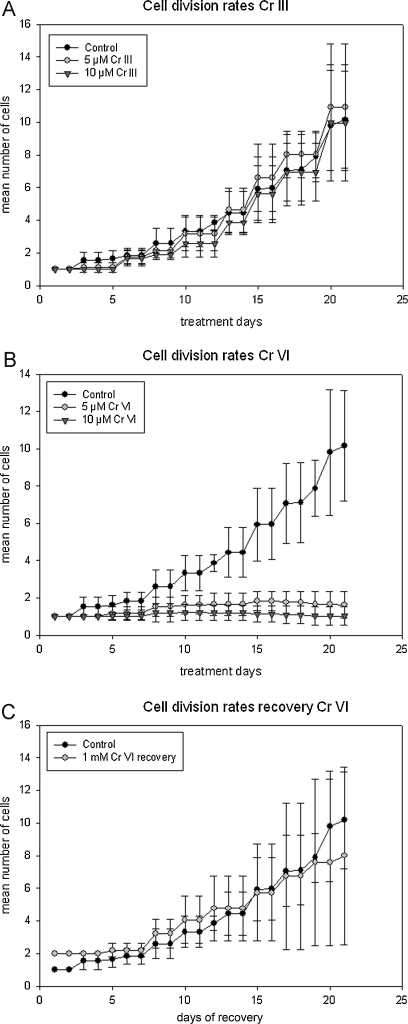
Micrasterias responded differently to different oxidation states of chromium and to different treatment times as well as concentrations. After 4 h under the influence of 1 mM Cr III and Cr VI dividing algae cells were unable to develop their younger half-cells (Fig. 1C and D) when compared to controls (Fig. 1B). Both oxidation states of chromium led to an almost complete inhibition of cell development and to morphological changes. Cell vitality tests revealed that 100% of the Cr III treated cells were still alive after 4 h treatment time, while only 95.33% of the cells treated with 1 mM Cr VI were alive (Table 1). Recovery experiments where Cr VI treated cells (1 mM, 4 h) were transferred back into nutrient solution, revealed that despite the strong effects cells were able to recover and their cell division rates did not significantly differ from controls (Fig. 2C). Cells growing in 5 (Fig. 1E) and 10 μM Cr III (data not shown) for 3 weeks did not seem to be affected at all when compared to control cells (Fig. 1A). The morphology of Micrasterias cells cultured in 5 and 10 μM Cr VI for 3 weeks was also not affected, only the chloroplast appeared abnormally dark and condensed and lobe tips seemed vacuolated (Fig. 1F, arrows). The latter effects were even more pronounced after 3 weeks 10 μM Cr VI treatment (data not shown). These light microscopic observations correlate well with the cell division rates showing that 5 and 10 μM of Cr VI led to a complete inhibition of cell division over the course of 3 weeks (Fig. 2B), whereas the same concentrations and treatment time of Cr III had no significant effect on cell division rates of the algae (Fig. 2A). Further the cell vitality tests are also in line with these findings, where the percentage of living cells after 3 weeks Cr III treatment are higher than after 3 weeks of Cr VI treatment (Table 1).
Fig. 1.
Micrasterias control cell in interphase (A) and fully grown cell about 5 h after mitosis (B). Micrasterias cell after short term treatment (4 h) with 1 mM Cr III (C) and 1 mM Cr VI (D). Cell growth inhibited and cell shape of growing semicells malformed. Interphase cells after long term treatment (3 weeks) with 5 μM Cr III (E) and 5 μM Cr VI (F). Chloroplast contracted (arrowhead) and vacuoles (arrow) visible after Cr VI exposure. Scale bar 100 μm.
Table 1.
Percentage of living cells after Cr III and VI short and long time treatments determined after sorbitol plasmolysis.
| Control | 1 mM Cr 4 h | 5 μM Cr 3 W | 10 μM Cr 3 W | |
|---|---|---|---|---|
| Cr III | 100% | 100% | 95.33% | 94% |
| Cr VI | 100% | 95.33% | 90.66% | 78% |
Fig. 2.
Cell division rates of Micrasterias during Cr III treatment (A), Cr VI treatment (B) and during recovery after a 4 h 1 mM Cr VI short term treatment (C), over the course of 3 weeks.
3.2. Photosynthesis and respiration
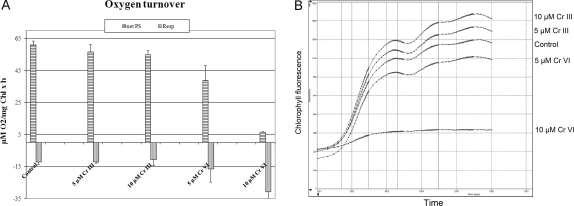
The long term treatments (3 weeks) of Cr III and Cr VI, both 5 and 10 μM were tested and compared. Net photosynthesis determined by oxygen production after Cr III treatment was only slightly reduced and respiration did not significantly differ from controls (Fig. 3A), whereas both applied Cr VI concentrations had a strong impact on net photosynthesis and respiration, showing a high level of toxicity. The strongest impact was induced by 10 μM Cr VI, reducing net photosynthesis down to 1/10 of control levels, while increasing the respiration more than double the amount of controls (Fig. 3A). Chlorophyll contents were reduced to about 65% of the controls, except for Cr VI 10 μM, where a marked reduction to 20% was observed (data not shown). This meets the ultrastructural observations of the changes in plastid structure. Further measurements of the chlorophyll fluorescence indicating photosystem II activity shows that the electron transport in photosystem II was almost completely inhibited after 10 μM Cr VI, while the transport remained active and at control levels in all other treatments (Fig. 3B). The effects of 5 μM Cr VI were less pronounced, but did still clearly reduced net photosynthesis and increase respiration (Fig. 3A).
Fig. 3.
Measurements of photosynthesis by oxygen production and consumption (respiration) in Micrasterias (A), as well as chlorophyll fluorescence (Fv/Fm) (B), after long term Cr III and Cr VI treatment. net PS – net photosynthesis, Resp. – respiration.
3.3. Chromium effects on ultrastructure and intracellular chromium localization by EELS and ESI
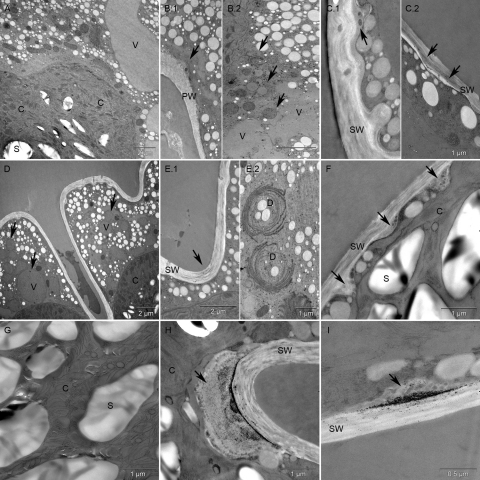
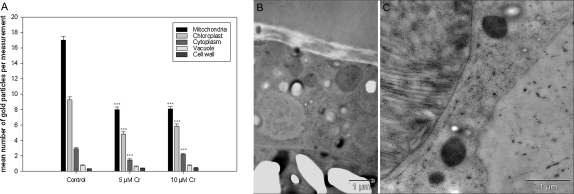
As Cr VI has been shown to induce the most pronounced effects on Micrasterias in our light microscopic approaches, only this oxidative form was used for ultrastructural analysis. Algae cells treated with 1 mM Cr VI for 4 h were not able to develop their younger half cells (see above) and ultrastructural changes were found. Many small vacuoles containing electron dense material and bloated mitochondria appeared (Fig. 4B.2). Secretory activity was decreased and dictyosomes were partly involute (data not shown). In some areas the primary wall showed thickenings with dark vesicle-like enclosures (Fig. 4B.1). Chromium could not be detected intracellular with EELS or ESI (data not shown) after short term treatment.
Fig. 4.
Ultrastructure of a Micrasterias control cell (A) and alga cells after short and long term Cr VI treatment (B–I). Vesicle like accumulation along the primary wall (B.1) after 1 mM Cr VI short term treatment, increased number of small vacuoles and slightly swollen mitochondria (B.2). Vesicle like accumulation along the secondary wall (C.1) after 5 μM Cr VI long term treatment, partly including electron dense precipitations (C.2). Further increased vacuolization (D), dark precipitation in the cell wall (E.1) and involute dictyosomes (E.2) were observed after 6 weeks of 5 μM Cr VI treatment. 3 weeks of 10 μM Cr VI treatment (F–I) lead to an increased amount of vesicle like accumulation along the inner side of the secondary wall (F) and a disruption of the chloroplast ultrastructure (G). In some areas these vesicle like accumulations were increased in size and contained considerable amounts of dark, granular precipitations (H and I). C – chloroplast, D – dictyosomes, PW – primary wall, S – starch grain, SW – secondary wall, V – vacuole.
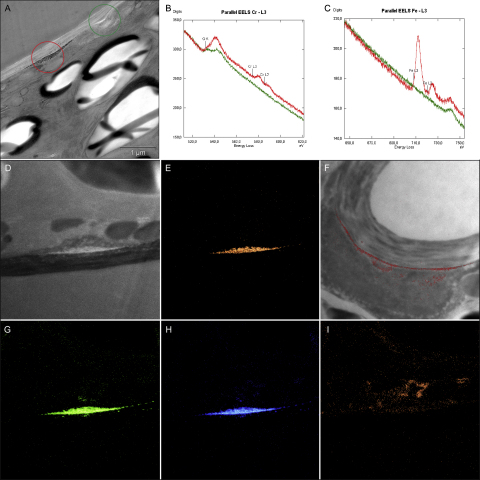
Long-term treatment with lower Cr VI concentrations (5 and 10 μM) led to similar effects and additionally showed an occasional increase in the number of starch grains in the chloroplast (data not shown) when compared to control cells (Fig. 4A). Micrasterias cells exposed to 5 μM for 3 and 6 weeks were more vacuolated (Fig. 4D) than control cells (Fig. 4A) and also revealed partly involute dictyosomes (Fig. 4E.2). Further in the 3 and 6 weeks treatments electron dense precipitations were found in parts of the cell wall (Fig. 4C.2 and E.1). Vesicle like accumulations along the inner side of the secondary wall, predominantly around pores, were found in all long term treatments (Fig. 4C.1), but the effect was most pronounced in cells treated with 10 μM Cr VI for 3 weeks (Fig. 4F), as was the vacuolization and the inhibitory effects on the secretory activity (data not shown). Moreover the ultrastructure of the chloroplast was disrupted, thylakoids were dilated and grana stacks were no longer visible (Fig. 4G). In cells of this treatment accumulations of dark, granular precipitations, varying considerably in size, were randomly found on the inner side of the cell wall. These precipitations were continuously found to be surrounded by vesicle like accumulations forming a bag like structure (Fig. 4H and I). Cr—L2.3, Fe—L2.3 and O—K edges were clearly identified by EELS and ESI in all such accumulations suggesting deposition of a chromium–iron–oxygen compound (Fig. 5A–H and A–C). Additionally an elevated N—K edge was measured locally around these accumulations, but not inside the dark precipitations (Fig. 5I). EELS and ESI did not reveal any other intracellular storage sites of chromium.
Fig. 5.
Secondary wall of Micrasterias after 10 μM Cr VI long term treatment. Measurement areas indicated (A). Cr L-3 edge and O—K edge (B) and Fe L-3 edge (C) measured via EELS in electron dense precipitations (red) and in the cell wall (green). High contrast image of electron dense precipitations on the inner side of the cell wall (D). Element distribution of Cr in this area (E). Overlay image showing the distribution of Cr in another area where electron dense precipitation occurred (F). Element distribution of Fe (G), O (H) and N (I) in the area shown in D.
3.4. Quantitative analysis of cellular chromium content
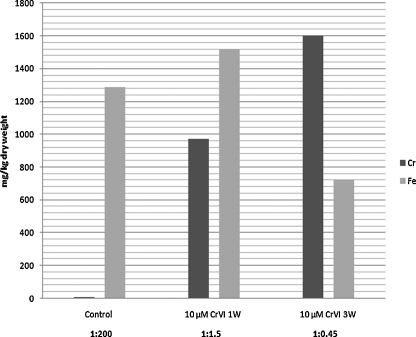
Micrasterias cells are able to accumulate high amounts of iron and chromium. The iron content of untreated control cultures was high, with a mean value of 1288.33 mg/kg dry weight whereas the chromium content was negligible amounting only 5.8 mg/kg dry weight when determined by AES (atomic emission spectroscopy). In cultures treated with Cr VI the Cr:Fe ratio shifted in favor of the chromium content from 1:200 to 1:1.5 after only 1 week of treatment. After long-term treatment of 3 weeks with 10 μM Cr VI intracellular chromium levels markedly exceeded the iron content (Fig. 6).
Fig. 6.
Amounts of Cr and Fe (mg/kg dry weight) in Micrasterias controls and after 1 and 3 weeks of Cr VI treatment measured by AES. (CI Contol: 0.0050 ± 0.0040/CI 1 week: 0.6839 ± 2.3378/CI 3 weeks: 2.2261 ± 0.9183; α = 10%.)
3.5. Glutathione levels determined via immunogold-labeling
In Micrasterias treated with 5 μM and 10 μM Cr VI for 3 weeks not only sites of glutathione accumulation were determined, but were also evaluated quantitatively via immunogold-labeling. Glutathione in controls (Fig. 7C) as well as treated cells (Fig. 7B) was mainly detected in mitochondria, chloroplasts and cytoplasm (Fig. 7A). Only very few gold particles were localized in vacuoles and cell walls, where no difference was found between controls and Cr VI treated cells. However, both concentrations of Cr VI reduced the labeling highly significantly in mitochondria, chloroplasts and cytoplasm (Fig. 7A and B).
Fig. 7.
Number of gold particles counted in different cell compartments of Micrasterias before and after long term treatment with 5 and 10 μM Cr VI after immunogold labeling with antibody against glutathione (A). Micrograph with immunogold labeling after 5 μM Cr VI long term treatment (B) and micrograph of a labeled control (C).
3.6. ROS-levels
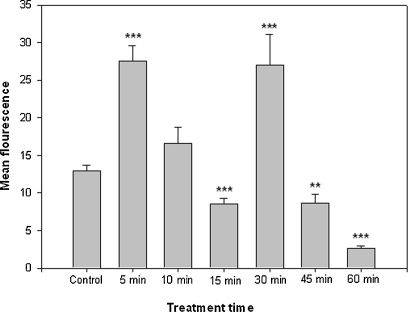
ROS-levels in the cells changed markedly within 1 h of 1 mM Cr VI treatment. Intracellular levels rose and fell rather fast resulting in a double peak pattern. After 5 min treatment ROS production increased significantly but decreased to about control level after 10 min and reached a significant low after 15 min. After 30 min ROS levels increased again and then fell continuously after 45 and 60 min of chromium treatment (Fig. 8).
Fig. 8.
Fluorescence intensity of the dye H2DCFDA observed in the CLSM after varying treatment times with 1 mM Cr VI indicating the amount of ROS in Micrasterias.
3.7. SOD and CAT activity
The SOD activity in Micrasterias cells was determined after 5, 10, 15, 30 and 45 min of 1 mM Cr VI incubation and compared to untreated controls. No significant differences between treatments and controls could be found (data not shown).
In correlation with ROS and SOD treatment times, CAT activity was tested after 5, 15, 30 and 60 min of 1 mM Cr VI treatment. Differences in enzyme activity were small and insignificant (data not shown).
4. Discussion
Exposure of M. denticulata to trivalent and hexavalent chromium resulted in an almost complete inhibition of cell development after mitosis, indicating that both naturally stable oxidation forms of chromium were taken up into the cell. How Cr is entering the plant cells is not fully understood, but Cr III seems to be taken up passively while Cr VI is supposed to be taken up actively (Liu et al., 2008), possibly involving carriers of essential anions (Shanker et al., 2005). Despite the strong inhibiting effects of Cr on cell growth more than 95% of the Cr VI short term treated Micrasterias cells were alive and further also proved to be able to recover and almost reach cell division rates of control cells over the course of 3 weeks. Cell division rates of Micrasterias treated with 5 and 10 μM Cr III did also not differ significantly from control cell levels, whereas division rates of algae cells treated with 5 and 10 μM Cr VI were stagnant for 3 weeks, showing that Cr VI has a stronger impact on plant cells and thus seems to be more toxic than Cr III (Shanker et al., 2005; Henriques, 2010; Upadhyay and Panda, 2010). The increased percentage of dead cells in long term Cr VI treated Micrasterias cell cultures affirm this finding and so do the many studies reporting that especially Cr VI leads to a decrease in biomass (Rai et al., 2004; Choudhury and Panda, 2005; Panda, 2007), due to an inhibition of cell division (Zou et al., 2006a,b). Rai and Mehrotra (2008) found that increasing concentrations of Cr VI led to a decreasing biomass in Phyllantus amarus and further induced a degradation of photosynthetic pigments, especially chlorophyll and carotenoids. Cr treatment also reduced the chlorophyll content in Micrasterias down to 20%. Shukla et al. (2007) reported that in plant cells exposed to elevated concentrations of heavy metals photosynthetic activity has been routinely observed to decline, due to the degradation of the photosynthetic pigments. In the unicellular alga Micrasterias net photosynthesis decreased after 3 weeks Cr treatment. Cr III treatment only led to an insignificant decline, while net photosynthesis in cells treated with Cr VI rapidly decreased down to 1/10 of control levels, confirming again our finding that Cr VI is more toxic to Micrasterias than Cr III. Further, respiration only increased in Cr VI treated cells as a general sign of stress (Larcher, 2001; Masarovicova et al., 2004), whereas respiration in Cr III treated cells remained at control levels. The electron transport of PS II was affected in Cr VI treated Micrasterias cells and almost completely ceased after a 3 weeks 10 μM Cr VI treatment. Also in the green alga Ulva lactuca chlorophyll fluorescence measurements revealed a severe inhibition of the photosynthetic process after Cr VI treatment (Unal et al., 2010). The Cr VI induced effects on photosynthesis in Micrasterias are correlating with the disrupted ultrastructure of the chloroplast. This is additionally affirming the suggestion that Cr VI is a highly toxic contaminant for plants and alga (Liu et al., 2008; Peralta-Videa et al., 2009; Perreault et al., 2009).
The effects of Cr VI on the algae ultrastructure were more or less pronounced depending on concentration and treatment time. A high Cr concentration of 1 mM used for short term treatments, led to several ultrastructural changes, as did the long term treatments with 5 μM Cr VI for 3 and 6 weeks and the 10 μM Cr VI for 3 weeks. As an overall stress reaction dictyosomes were party involute and the secretory activity was decreased as observed during salt stress or H2O2 incubation (Darehshouri et al., 2008; Affenzeller et al., 2009). After short term treatment the cytoplasm was condensed, mitochondria were swollen and displayed a loss of cristae. These latter effects were recorded as hallmarks for programmed cell death in Micrasterias during other types of abiotic stress (Darehshouri et al., 2008; Affenzeller et al., 2009). The swelling of mitochondria was also observed in kiwifruit pollen, where additionally increased vacuolization occurred (Speranza et al., 2007), which was also found in all Cr VI treated Micrasterias cells. As a result of chromium treatment increased vacuolization occurred in higher plants and green algae (Liu and Kottke, 2003; Panda, 2007; D’Ors et al., 2010).
In short term treatments of Micrasterias dark vesicle like enclosures were found in parts of the primary wall and in long term treatments vesicle like accumulations were visible on the inner side of the secondary wall, which additionally contained dark, granular precipitations after 3 weeks of 10 μM Cr VI treatments. Only in these, randomly placed, bag like structures, which varied considerably in size, it was possible to measure Cr and O via EELS and ESI. After Cr treatment in Allium cepa electron dense precipitations lining the inner side of the cell wall were observed and Cr was detected via EELS, indicating a local elimination of metal from the cytoplasm. High Cr peaks in Allium were also accompanied by elevated levels of O, showing that chromium preferably complexes with O atoms as ligands (Liu and Kottke, 2003). In M. denticulata additionally to elevated O levels, significant amounts of Fe were measured via EELS and ESI at sites of Cr accumulation, suggesting that Cr is deposited as a complex with Fe and O. Compounds such as chromite (FeCr2O4) are also occurring naturally mostly on serpentine soils (Samantaray et al., 2001; Prabhakar et al., 2010). When the overall Cr content of Micrasterias cell cultures was determined via AES after 10 μM Cr VI treatment, it was found that with rapidly increasing Cr content the Fe content of the cells depleted after 3 weeks. Cr was found to be able to disrupt the nutrient balance in plants in particular Fe levels (Zou et al., 2006a,b). It is also supposed to compete with Fe, S and P for carrier uptake in plant cells (Shanker et al., 2005), this may explain the reduced Fe content in Cr treated Micrasterias cultures. On the other hand Cr is able to displace other metals from physiologically important centers, especially Fe (Pandey and Sharma, 2003) causing the toxic symptoms of Cr VI to appear superficially similar to iron deficiency (Liu et al., 2008). In contrast, the Fe levels in Micrasterias measured via EELS in electron dense deposits exceed the detected amounts of Cr at these particular sites. This may indicate that the cells cannot distinguish between Fe and Cr and deposit “excess” Fe in cell walls in the attempt to eliminate Cr from the cytosol. Desmids such as Micrasterias are naturally adapted to habitats with high concentrations of Fe and are thus able to deposit dispensable Fe in the cell walls (Brook, 1981). Other green alga, such as Chlorella vulgaris have also been found to be able to accumulate high amounts of Cr exceeding control levels up to a 100 times (Regaldo et al., 2009).
Plants have different mechanisms to defend themselves against heavy metal stress, one of them is glutathione, a tripeptide, with multiple functions in plant metabolism. Its biosynthesis is restricted to cytosol and plastids, from where it is transported to various organelles (Moran et al., 2000; Zechmann et al., 2008). The main sites of glutathione accumulation in Micrasterias were determined via immuno-gold-labeling to be mitochondria, chloroplast and cytoplasm in controls as well as alga cells treated with 5 and 10 μM Cr VI for 3 weeks. Even though plastids were previously thought to be the organelle with the highest glutathione content, even higher levels were found not only in mitochondria of Micrasterias, but also in Arabidopsis thaliana and Cucurbita pepo (Zechmann et al., 2008; Kolb et al., 2009). In Cr treated Micrasterias cells the number of gold-particles declined highly significantly in mitochondria, chloroplast and cytoplasm, while the labeling maintained at the negligibly small control levels in vacuoles and cell walls. This drastic decrease in the glutathione pool is in line with studies also reporting a decline of glutathione levels as a response to Cr (Shanker et al., 2005; Yadav et al., 2010) or heavy metal stress (Schutzendubel et al., 2002; Kolb et al., 2009) in other plants. The reason for the glutathione depletion may be due to the fact that glutathione plays an important role in heavy metal detoxification (Zechmann et al., 2008) and is able to react and form complexes with Cr (Brauer and Wetterhahn, 1991; Brauer et al., 1996), which most likely leads to an inhibition of antibody binding. However, glutathione is also an essential antioxidant and redox buffer in the plant cell and is able to scavenge potentially harmful ROS, which are induced under Cr stress (Grill et al., 2004; Panda, 2007; Pandey et al., 2009; Upadhyay and Panda, 2010). 1 mM Cr VI treatment triggered a rapid ROS production in M. denticulata, which appeared as a double peak. Similar ROS dynamics were reported to indicate oxidative burst and were found as a widespread defense mechanism of higher plants to pathogen attack frequently leading to programmed cell death (Schraudner et al., 1998; Tudzynski et al., 2004; Mandal et al., 2011). Affenzeller et al. (2009) found that ROS levels in Micrasterias also increased quickly as a response to salt stress, but were then linearly decreasing over treatment time. Again this shows the similarity between Micrasterias and higher plants, as determined in previous studies (Lütz-Meindl and Brosch-Salomon, 2000; Eder et al., 2008; Eder and Lütz-Meindl, 2008; Volland et al., 2011). Elevated levels of ROS can lead to cell death in the worst case and thus plants have developed antioxidant defense systems such as for example SOD, CAT or glutathione (Choudhury and Panda, 2005; Pandey et al., 2009). Interestingly Cr was reported to increase SOD activity in higher plant cells while CAT activity decreased (Liu et al., 2008; Upadhyay and Panda, 2010). The SOD activity in Micrasterias remained at control levels after all Cr treatments. This either indicates that O2− was not part of the ROS measured as a reaction to Cr induced stress, or that there are different ways of scavenging this radical in Micrasterias. Nevertheless the SOD-test clearly shows that the enzyme is generally present in our model organism. CAT activity was tested in Micrasterias and was also found to play no significant role in Cr detoxification. This suggests that neither of these main enzymes are essential for ROS scavenging in M. denticulata, pointing toward other mechanisms preventing oxidative damage.
Overall we found that Cr of different oxidative states is readily taken up into the alga Micrasterias, despite the fact that Cr is an unessential nutrient to plants. The strong impact of Cr VI on the development of cell morphology, cell division rates, photosynthesis and ultrastructure shows that the anion Cr VI is not only more toxic than the cation Cr III, but is highly toxic to plants and algae in general. It causes severe damage to the cell by inducing rapid ROS production and disruption of the cells Fe homeostasis. Cr cannot be compartmentalized well by the alga cells leading to disintegration of cell cultures over time. As the heavy metal was determined in particular bag-like structures at the cell wall and in the cell wall itself a local elimination of the metal by the cell is strongly indicated as detoxification mechanism.
Acknowledgements
We gratefully acknowledge the financial support by the Austrian Science Fund (FWF, Project 21035-B16 to U. L.-M.). We want to thank Matthias Affenzeller for many helpful conversations and advices. Our gratitude goes to Bernd Zechmann for providing us with a glutathione antibody and suitable protocols and to Patric Schmölzer for his help with the CLSM experiments. Further we thank Peter Grill and Bärbel Benker for their instructions and assistance during the measurements conducted at the Helmholtz Research Centre for Environment and Health.
Contributor Information
Stefanie Volland, Email: Stefanie.Volland@stud.sbg.ac.at.
Cornelius Lütz, Email: cornelius.luetz@uibk.ac.at.
Bernhard Michalke, Email: bernhard.michalke@helmholtz-muenchen.de.
Ursula Lütz-Meindl, Email: ursula.luetz-meindl@sbg.ac.at.
References
- Affenzeller M.J., Darehshouri A., Andosch A., Lütz C., Lütz-Meindl U. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata. J. Exp. Bot. 2009;60:939–954. doi: 10.1093/jxb/ern348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Benavides M.P., Iannone M.F., Rosales E.P., Groppa M.D. Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf disks exposed to cadmium. Protoplasma. 2010;245:15–27. doi: 10.1007/s00709-009-0097-9. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brauer S.L., Hneihen A.S., McBride J.S., Wetterhahn K.E. Chromium(VI) forms thiolate complexes with gamma-glutamylcysteine N-acetylcysteine, cysteine, and the methyl ester of N-acetylcysteine. Inorg. Chem. 1996;35:373–381. doi: 10.1021/ic941452d. [DOI] [PubMed] [Google Scholar]
- Brauer S.L., Wetterhahn K.E. Chromium(VI) forms a thiolate complex with glutathione. J. Am. Chem. Soc. 1991;113:3001–3007. [Google Scholar]
- Brook A.J. first ed. Blackwell; Oxford: 1981. The Biology of Desmids. [Google Scholar]
- Choudhury S., Panda S.K. Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Pollut. 2005;167:73–90. [Google Scholar]
- D’Ors A., Pereira M., Bartolome M.C., Lopez-Rodas V., Costas E., Sanchez-Fortun S. Toxic effects and specific chromium acquired resistance in selected strains of Dyctiosphaerium chlorelloides. Chemosphere. 2010;81:282–287. doi: 10.1016/j.chemosphere.2010.05.051. [DOI] [PubMed] [Google Scholar]
- Darehshouri A., Affenzeller M., Lütz-Meindl U. Cell death upon H2O2 induction in the unicellular green alga Micrasterias. Plant Biol. 2008;10:732–745. doi: 10.1111/j.1438-8677.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder M., Lütz-Meindl U. Pectin-like carbohydrates in the green alga Micrasterias characterized by cytochemical analysis and energy filtering TEM. J. Microsc. 2008;231:201–214. doi: 10.1111/j.1365-2818.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- Eder M., Tenhaken R., Driouich A., Lütz-Meindl U. Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (Streptophyta) J. Phycol. 2008;44:1221–1234. doi: 10.1111/j.1529-8817.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- Gorbi G., Corradi M.G., Invidia M., Rivara L., Bassi M. Is Cr(VI) toxicity to Daphnia magna modified by food availability or algal exudates? The hypothesis of a specific chromium/algae/exudates interaction. Water Res. 2002;36:1917–1926. doi: 10.1016/s0043-1354(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Grill D., Tausz M., Sircelj H. The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J. Exp. Bot. 2004;55:1955–1962. doi: 10.1093/jxb/erh194. [DOI] [PubMed] [Google Scholar]
- Gupta V.K., Rastogi A. Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J. Hazard. Mater. 2008;154:347–354. doi: 10.1016/j.jhazmat.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Henriques F.S. Changes in biomass and photosynthetic parameters of tomato plants exposed to trivalent and hexavalent chromium. Biol. Plant. 2010;54:583–586. [Google Scholar]
- Holzinger A., Lütz-Meindl U. Kinesin-like proteins are involved in postmitotic nuclear migration of the unicellular green alga Micrasterias denticulata. Cell. Biol. Int. Rep. 2002;26:689–697. doi: 10.1006/cbir.2002.0920. [DOI] [PubMed] [Google Scholar]
- Hörcsik Z.T., Kovàcs L., Láposi R., Mészáros I., Lakatos G., Garab G. Effect of chromium on photosystem 2 in the unicellular green alga, Chlorella pyrenoidosa. Photosynthetica. 2007;45:65–69. [Google Scholar]
- Kiermayer O. Cytoplasmic basis of morphogenesis in Micrasterias. In: Kiermayer O., editor. Cytomorphognesis in Plants. Cell Biology Monographs. Springer-Verlag; Wien, New York: 1981. pp. 147–191. [Google Scholar]
- Kolb D., Müller M., Zellnig G., Zechmann B. Cadmium induced changes in subcellular glutathione contents within glandular trichomes of Cucurbita pepo L. Protoplasma. 2009;243:87–94. doi: 10.1007/s00709-009-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas J., Stasicka Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000;107:263–283. doi: 10.1016/s0269-7491(99)00168-2. [DOI] [PubMed] [Google Scholar]
- Larcher W. sixth ed. Ulmer; Stuttgart: 2001. Ökophysiologie der Pflanzen: Leben Leistung und Stressbewältigung der Pflanzen in ihrer Umwelt. [Google Scholar]
- Lee S.H., Ahsan N., Lee K.W., Kim D.H., Lee D.G., Kwak S.S., Kwon S.Y., Kim T.H., Lee B.H. Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J. Plant Physiol. 2007;164:1626–1638. doi: 10.1016/j.jplph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Liu D., Kottke I. Subcellular localization of chromium and nickel in root cells of Allium cepa by EELS and ESI. Cell. Biol. Toxicol. 2003;19:299–311. doi: 10.1023/b:cbto.0000004984.87619.15. [DOI] [PubMed] [Google Scholar]
- Liu D., Zou J., Wang M., Jiang W. Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defence system and photosynthesis in Amaranthus viridis L. Bioresour. Technol. 2008;99:2628–2636. doi: 10.1016/j.biortech.2007.04.045. [DOI] [PubMed] [Google Scholar]
- Lütz-Meindl U., Aichinger N. Use of energy-filtering transmission electron microscopy for routine ultrastructural analysis of high-pressure-frozen or chemically fixed plant cells. Protoplasma. 2004;223:155–162. doi: 10.1007/s00709-003-0033-3. [DOI] [PubMed] [Google Scholar]
- Lütz-Meindl U., Brosch-Salomon S. Cell wall secretion in the green alga Micrasterias. J. Microsc. 2000;198:208–217. doi: 10.1046/j.1365-2818.2000.00699.x. [DOI] [PubMed] [Google Scholar]
- Mandal S., Das R.K., Mishra S. Differential occurrence of oxidative burst and antioxidative mechanism in compatible and incompatible interactions of Solanum lycopersicum and Ralstonia solanacearum. Plant Physiol. Biochem. 2011;49:117–123. doi: 10.1016/j.plaphy.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Masarovicova E., Kral’ova K., Kummerova M., Kmentova E. The effect of cadmium on root growth and respiration rate of two medicinal plant species. Biologia. 2004;59:211–214. [Google Scholar]
- Meindl U. Micrasterias cells as a model system for research on morphogenesis. Microbiol. Rev. 1993;57:415–433. doi: 10.1128/mr.57.2.415-433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl U., Lancelle S., Hepler P. Vesicle production and fusion during lobe formation in Micrasterias visualized by high-pressure freeze fixation. Protoplasma. 1992;170:104–114. [Google Scholar]
- Moran J.F., Iturbe-Ormaetxe I., Matamoros M.A., Rubio M.C., Clemente M.R., Brewin N.J., Becana M. Glutathione and homoglutathione synthetases of legume nodules. Cloning, expression, and subcellular localization. Plant Physiol. 2000;124:1381–1392. doi: 10.1104/pp.124.3.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S.K. Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. J. Plant. Physiol. 2007;164:1419–1428. doi: 10.1016/j.jplph.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Panda S.K., Choudhury S. Chromium stress in plants. Braz. J. Plant Physiol. 2005;17:95–102. [Google Scholar]
- Pandey N., Sharma C.P. Chromium interference in iron nutrition and water relations of cabbage. Environ. Exp. Bot. 2003;49:195–200. [Google Scholar]
- Pandey V., Dixit V., Shyam R. Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts. Protoplasma. 2009;236:85–95. doi: 10.1007/s00709-009-0061-8. [DOI] [PubMed] [Google Scholar]
- Peralta-Videa J.R., Lopez M.L., Narayan M., Saupe G., Gardea-Torresdey J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int. J. Biochem. Cell. Biol. 2009;41:1665–1677. doi: 10.1016/j.biocel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Perreault F., Ait Ali N., Saison C., Popovic R., Juneau P. Dichromate effect on energy dissipation of photosystem II and photosystem I in Chlamydomonas reinhardtii. J. Photochem. Photobiol. B. 2009;96:24–29. doi: 10.1016/j.jphotobiol.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Porra R.J., Thompson W.A., Kreidemann P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by absorption spectroscopy. Biochem. Biophys. Acta. 1989;975:384–394. [Google Scholar]
- Prabhakar B.C., Rashmi B.N., Gireesh R.V., Suresh B., Nijagunaiah R., Ranganath R.M. Geobotanical and biogeochemical study of chromite bearing areas of Nuggihalli schist belt, Karnataka. Curr. Sci. India. 2010;99:619–627. [Google Scholar]
- Rai U.N., Dwivedi S., Tripathi R.D., Shukla O.P., Singh N.K. Algal biomass: an economical method for removal of chromium from tannery effluent. Bull. Environ. Contam. Toxicol. 2005;75:297–303. doi: 10.1007/s00128-005-0752-6. [DOI] [PubMed] [Google Scholar]
- Rai V., Mehrotra S. Chromium-induced changes in ultramorphology and secondary metabolites of Phyllanthus amarus Schum & Thonn—an hepatoprotective plant. Environ. Monit. Assess. 2008;147:307–315. doi: 10.1007/s10661-007-0122-4. [DOI] [PubMed] [Google Scholar]
- Rai V., Vajpayee P., Singh S.N., Mehrotra S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004;167:1159–1169. [Google Scholar]
- Regaldo L., Gagneten A.M., Troiani H. Accumulation of chromium and interaction with other elements in Chlorella vulgaris (Cloroficeae) and Daphnia magna (Crustacea, Cladocera) J. Environ. Biol. 2009;30:213–216. [PubMed] [Google Scholar]
- Samantaray S., Rout G.R., Das P. Heavy metal and nutrient concentration in soil and plants growing on a metalliferous chromite minespoil. Environ. Technol. 2001;22:1147–1154. doi: 10.1080/09593332208618204. [DOI] [PubMed] [Google Scholar]
- Schlösser U.G. Sammlungen von Algenkulturen. Ber. Deutsch. Bot. Ges. 1982;95:181–276. [Google Scholar]
- Schramel P., Wolf A., Seif R., Klose B.J. New device for ashing of biological-material under pressure. Fresenius Zeitschrift für Analytische Chemie. 1980;302:62–64. [Google Scholar]
- Schraudner M., Moeder W., Wiese C., Van Camp W., Inze D., Langebartels C., Sandermann H. Ozone-induce oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16(2):235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Schutzendubel A., Nikolova P., Rudolf C., Polle A. Cadmium and H2O2-induced oxidative stress in Populus x canescens roots. Plant Physiol. Biochem. 2002;40:577–584. [Google Scholar]
- Shanker A.K., Cervantes C., Loza-Tavera H., Avudainayagam S. Chromium toxicity in plants. Environ. Int. 2005;31:739–753. doi: 10.1016/j.envint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Sheng P.X., Tan L.H., Chen J.P., Ting Y.P. Biosorption performance of two brown marine algae for removal of chromium and cadmium. J. Disp. Sci. Technol. 2004;25:679–686. [Google Scholar]
- Shukla O.P., Dubey S., Rai U.N. Preferential accumulation of cadmium and chromium: toxicity in Bacopa monnieri L. under mixed metal treatments. Bull. Environ. Contam. Toxicol. 2007;78:252–257. doi: 10.1007/s00128-007-9155-1. [DOI] [PubMed] [Google Scholar]
- Speranza A., Ferri P., Battistelli M., Falcieri E., Crinelli R., Scoccianti V. Both trivalent and hexavalent chromium strongly alter in vitro germination and ultrastructure of kiwifruit pollen. Chemosphere. 2007;66:1165–1174. doi: 10.1016/j.chemosphere.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Tudzynski P., Rolke Y., Liu S.J., Quidde T., Williamson B., Schouten A., Weltring K.M., Siewers V., Tenberge K.B., Tudzynski B. Functional analysis of H2O2-generating systems in Botrytis cinerea: the major Cu–Zn–superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol. Plant Pathol. 2004;5:17–27. doi: 10.1111/j.1364-3703.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- Unal D., Isik N.O., Sukatar A. Effects of chromium VI stress on green alga Ulva lactuca (L.) Turk. J. Biol. 2010;34:119–124. [Google Scholar]
- Upadhyay R., Panda S.K. Influence of chromium salts on increased lipid peroxidation and differential pattern in antioxidant metabolism in Pistia stratiotes L. Braz. Arch. Biol. Technol. 2010;53:1137–1144. [Google Scholar]
- Vignati D.A.L., Dominik J., Beye M.L., Pettine M., Ferrari B.J.D. Chromium(VI) is more toxic than chromium(III) to freshwater algae: a paradigm to revise? Ecotoxicol. Environ. Safety. 2010;73:743–749. doi: 10.1016/j.ecoenv.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Volland S., Andosch A., Milla M., Stöger B., Lütz C., Lütz-Meindl U. Intracellular metal compartmentalization in the green algal model system Micrasterias denticulata (Streptophyta) measured by transmission electron microscopy-coupled electron energy loss spectroscopy. J. Phycol. 2011;47:565–579. doi: 10.1111/j.1529-8817.2011.00988.x. [DOI] [PubMed] [Google Scholar]
- Yadav S.K., Dhote M., Kumar P., Sharma J., Chakrabarti T., Juwarkar A.A. Differential antioxidative enzyme responses of Jatropha curcas L. to chromium stress. J. Hazard. Mater. 2010;180:609–615. doi: 10.1016/j.jhazmat.2010.04.077. [DOI] [PubMed] [Google Scholar]
- Zechmann B., Mauch F., Sticher L., Müller M. Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. J. Exp. Bot. 2008;59:4017–4027. doi: 10.1093/jxb/ern243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J.H., Wang M., Jiang W.S., Liu D.H. Chromium accumulation and its effects on other mineral elements in Amaranthus viridis L. Acta Biol. Craco. Ser. Bot. 2006;48:7–12. [Google Scholar]
- Zou J.H., Wang M., Jiang W.S., Liu D.H. Effects of hexavalent chromium (VI) on root growth and cell division in root tip cells of Amaranthus viridis L. Pak. J. Bot. 2006;38:673–681. [Google Scholar]