Abstract
Background
Glutathione S-transferase P1 1 (GSTP1) belongs to the multigene isozyme family involved in cellular response to oxidative stress and apoptosis. Our initial retrospective proteomic analysis suggested that GSTP1 is associated with heart failure (HF). Although pro–B-type natriuretic peptide (proBNP) serves currently as a surrogate diagnostic and prognostic parameter in HF patients, its specificity remains uncertain. We hypothesized that GSTP1 might be a useful serum marker in the monitoring of HF patients.
Methods and Results
Serum GSTP1 and proBNP were prospectively measured in 193 patients subdivided based on their ejection fraction (EF) either in equal-sized quintiles or predefined EF groups >52%, 43%–52%, 33%–42%, 23%–32% and ≤22%. At a cutoff of ≥231 ng/mL, GSTP1 identified HF patients with EF ≤22% with 81% sensitivity and 83% specificity, and at a cutoff of ≥655 pg/mL, proBNP identified the same patient group with 84% sensitivity and 22% specificity. GSTP1 at a ≥126 ng/mL cutoff identified EF ≤42% with 90% sensitivity and 95% specificity, or proBNP at a ≥396 pg/mL cutoff had 97% sensitivity and 20% specificity. In regression analyses, GSTP1, but not proBNP, discriminated between EF ≤42% and EF >42% in HF patients.
Conclusions
These results suggest that GSTP1 is strongly associated with HF and could serve as a sensitive and specific marker to predict the ventricular function in HF patients.
Key Words: Heart failure, ventricular function, marker, serum, GSTP1
Heart failure (HF) results in recurrent exacerbation leading to hospitalization or death.1 Therefore, close surveillance, with tremendous cost, is required to achieve the optimal HF treatment in these patients. A biomarker that could predict ejection fraction (EF) in HF patients and help to optimize treatment monitoring and outcome is therefore highly desirable. Ideally, such a test should be sensitive, specific, noninvasive, quick, and cheap. Although a number of factors2–4 have been reported as HF biomarkers, B-type natriuretic peptide (BNP) and its inactive N-terminal fragment (proBNP) have gained the broadest acceptance in diagnosis and monitoring of patients with suspected or established HF.3,5,6 However, circulating BNP and proBNP levels are affected by renal function and are age and sex dependent.7,8
Glutathione S-transferase P1 1 (GSTP1) is the most prevalent mammalian isozyme of the glutathione S-transferase family. GSTP1 is an important regulator of inflammation and performs a key role in cellular homeostasis, including inhibition of apoptosis, detoxification of reactive oxygen species, and maintenance of the cellular redox state.9–11 Whether serum GSTP1 could monitor cardiac function in HF patients, particularly regarding their EF, remains unknown. We therefore addressed this issue.
Patients and Methods
Patients
The Ethics Committee of the Medical University of Vienna approved this study, and each of the individuals gave informed consent to be enrolled. The study comprised two parts: first, an initial retrospective protemic analysis of serum and myocardial biopsy samples obtained from HF patients undergoing heart transplantation or heart transplant donors who served as control subjects (Fig. 1; Table 1); and second, prospective serum analyses of 193 HF patients. Furthermore, 20 healthy individuals who had no history of cardiovascular diseases and had a normal EF in echocardiography served as control subjects (Fig. 1, Table 1).
Fig. 1.
Study design and patient groups. EF, ejection fraction; ELISA, enzyme-linked immunosorbent assay; GSTP1, glutathione S-transferase P1 1; HF, heart failure.
Table 1.
Demographic Data, Clinical Characteristics, and the Most Heart Failure (HF)–Relevant Medication of the Study Individuals
| Characteristic | Retrospective Study |
Prospective Study (ELISA) |
||||
|---|---|---|---|---|---|---|
| Control Subjects |
HF Patients |
Control Subjects | HF Patients |
|||
| Serum Array | Tissue Array | Serum Array | EF >42% | EF ≤42% | ||
| n | 40 | 20 | 40 | 20 | 40 | 153 |
| Age (y) | 47 ± 11 | 45 ± 11 | 54 ± 8 | 52 ± 4 | 55 ± 11 | 52 ± 12 |
| Male (%) | 48% | 60% | 55% | 50% | 70% | 70% |
| DM, n (%) | – | – | 7 (18%) | – | – | 47 (21%) |
| IDDM, n (%) | – | – | 4 (10%) | – | – | 31 (14%) |
| NIDDM, n (%) | – | – | 3 (8%) | – | – | 16 (7%) |
| NYHA functional classification, n (%) | ||||||
| I | – | – | – | – | 19 (48%) | |
| II | – | – | – | – | 18 (45%) | |
| III | – | – | 15 (38%) | – | 3 (7%) | 99 (45%) |
| IV | – | – | 25 (62%) | – | 54 (24%) | |
| Diagnosis, n (%) | ||||||
| ICM | – | – | 17 (42%) | – | 20 (50%) | 54 (24%) |
| DCM | – | – | 20 (50%) | – | 20 (50%) | 87 (39%) |
| Other | – | – | 3 (8%) | – | – | 12 (6%) |
| Hemodynamic parameters | ||||||
| EF (%) | >60 | >60 | 22 ± 6 | >60 | 45 ± 5 | 23 ± 10 |
| PAP (mm Hg) | – | – | 30 ± 15 | – | 42 ± 5 | 32 ± 10 |
| PCWP (mm Hg) | – | – | 21 ± 11 | – | – | 22 ± 14 |
| PVR (Wood units) | – | – | 2.86 ± 1.72 | – | – | 2.56 ± 1.52 |
| CI (L min−1 m−2) | – | – | 2.36 ± 0.81 | – | – | 2.18 ± 0.72 |
| Laboratory findings | ||||||
| Creatinine (mg/dL) | – | – | 1.48 ± 0.64 | – | 1.69 ± 1.46 | 1.41 ± 0.53 |
| GSTP1 (ng/mL) | 21 ± 10 | 22 ± 8 | 395 ± 73 | 20 ± 5 | 89 ± 40 | 269 ± 160 |
| proBNP (pg/mL) | 84 ± 7 | 88 ± 5 | 1,356 ± 587 | 90 ± 7 | 841 ± 594 | 1,265 ± 609 |
| Medication, n (%) | ||||||
| ACE inhibitor | – | – | 15 (38%) | – | 152 (68%) | |
| ARB | – | – | 8 (20%) | – | 49 (22%) | |
| Beta-blocker | – | – | 29 (73%) | – | 181 (82%) | |
| Levosimendan | – | – | 7 (18%) | – | 33 (15%) | |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CI, cardiac index; DCM, dilated cardiomyopathy; DM, diabetes mellitus; EF, ejection fraction; ICM, ischemic cardiomyopathy; IDDM, insulin-dependent diabetes mellitus; NIDDM, noninsulin-dependent diabetes mellitus; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance.
All HF patients underwent echocardiography, coronary artery angiography, and right-side heart catheterization for evaluation of ventricular function and standard hemodynamic parameters by two independent cardiologists. Patients with malignancies (n = 2) and acute endocarditis (n = 17) were excluded.
Echocardiography
All patients and control subjects underwent echocardiographic examination including M-Mode, two-dimensional echocardiography, and conventional and color Doppler. Left ventricular EF was determined using the biplane Simpson method.12 Patients were subdivided into 5 predefined groups (Fig. 1) based on their left ventricular (LV) EF as follows: EF >52% (n = 10), EF 43%–52% (n = 30); EF 33%–42% (n = 20), EF 23%–32% (n = 40), and EF ≤22% (n = 93), according to the recently reported risk stratification criteria.13,14 To avoid sample size–related bias, the study patients were also classified based on their EF in equal-sized quintiles. The LV mass index (LVMI) was calculated as described previously.15
Serum and Cardiac Tissue Collection
Peripheral venous blood was collected from end-stage HF patients during their routine surveillance or shortly before transplantation, and myocardial biopsies were obtained from the anterior LV wall of their explanted hearts as previously reported.16 Serum and myocardial biopsies obtained from the anterior LV wall were collected from heart donors during cardiac allograft implantation. Serum samples were collected from HF patients with preserved EF during their routine surveillance. All samples were coded and snap frozen in liquid nitrogen until further use.
Serum and Cardiac Tissue Protein Array
Randomly selected serum samples from end-stage HF patients and donors were pooled for each group. For protein array, the tissue lysates from LV myocardial biopsies of explanted hearts in transplant patients and donor control hearts were used. The protein array was performed on an antibody Microarray 500 (Clontech, Mountain View, California) as described elsewhere.17 The fluorescence was detected by a GenePix 4000B scanner (Molecular Dynamics, Sunnyvale, California) and quantified by the Microarray Analysis Workbook (http://bioinfo.clontech.com).
GSTP1 and proBNP Assays
Enzyme-linked immunosorbent assay (ELISA) for GSTP1 (Hepkit-Pi; Biotrin International, Dublin, Ireland) was performed and quantified spectrophotometrically by an automated microplate reader (Anthos, Salzburg, Austria). N-Terminal proBNP was measured in undiluted serum automatically by a chemiluminescent noncompetitive ELISA (Roche Diagnostics, Mannheim, Germany) on a Roche Elecsys 2010 analyzer.
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was isolated from LV biopsies using Trizol (Invitrogen, Carlsbad, California) with a Magna Lyser system (Roche). Real-time reverse-transcription polymerase chain reaction (average value of 3 measurements) was performed on a Lightcycler instrument (Roche).16 The primer sequences were (sense/antisense): GSTP1: 5′-CCAAAGGTGGTGAGCTTCAT-3′/5′-TCTACCCAGCATGGAGGAAC-3′; and β2-microglobulin: 5′-GATGAGTATGCCTGCCGTGTG-3′/5′-CAATCCAAATGCGGCATCT-3′. mRNA expression levels of GSTP1 were normalized to those of β2-microglobulin as a housekeeping gene.16
Western Blotting
GSTP1 expression was analyzed in tissue lysates by Western blotting as described previously16 using primary monoclonal antihuman GSTP1 antibody (Bethyl, Montgomery, Texas). Protein bands were quantified by Imagequant software, and specific protein signals (average value of 3 measurements) were normalized to loading control samples.
Immunohistochemistry
Paraffin sections of cardiac biopsies obtained from the explanted hearts of HF patients (n = 6) undergoing cardiac transplantation or those obtained from donated hearts during allograft implantation (n = 4) were blocked with phosphate-buffered saline solution supplemented with 5% goat serum and stained with a monoclonal antihuman GSTP1 antibody (Bethyl). The primary antibody was detected by sequential incubation with Alexa Fluor 488–conjugated secondary antibody (Invitrogen). Slides were then counterstained with 0.1 μg/mL 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, Oregon) and analyzed under a fluorescence microscope.
Statistical Analysis
GSTP1 and proBNP serum concentrations were compared between patient groups by 1-way analysis of variance (ANOVA) and post hoc Tukey test according to the scale of the variable. In case of skewed data, a nonparametric test (Mann-Whitney U test) or 1-way ANOVA with Tukey test accomplished with log transformation was used. To investigate the relationship between GSTP1 and proBNP, as well as the relationship of GSTP1 to age, pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), pulmonary vascular resistance (PVR), cardiac index (CI), and creatinine, Spearman rank correlation coefficients (rS) were computed. The differences between the correlations were tested with t test for GSTP1 and proBNP with EF, or Fischer transformationfor GSTP1 with proBNP. The corresponding receiver operating characteristic curve (ROC) analyses were used to find optimal cutoff levels for GSTP and proBNP as the values that minimize the distance between the ROC curve and the upper left corner of the panel. The area under the ROC curve (AUC) of GSTP1 and proBNP for cutoff points were compared by the DeLong and DeLong method and by the Pencina method expressed as net reclassification improvement (NRI) and integrated discrimination improvement (IDI). Sensitivity and specificity of GSTP1 and proBNP cutoffs were calculated by table analysis and compared by McNemar test. Univariate and multivariate logistic regression was used to assess the ability of GSTP1, proBNP, LVMI, age, PCWP, PVR, CI, and creatinine to predict LV function assessed by EF. For logistic regression analysis, EF was considered as a dichotomous variable at a cutoff level of 42%. All statistical analyses were performed using SAS system for Windows, version 9.1.3, and Enterprise Guide, version 4.1 (SAS Institute, Cary, North Carolina). Statistical significance was set at P < .05. Results are expressed as mean ± SD.
Results
GSTP1 Is Associated with HF
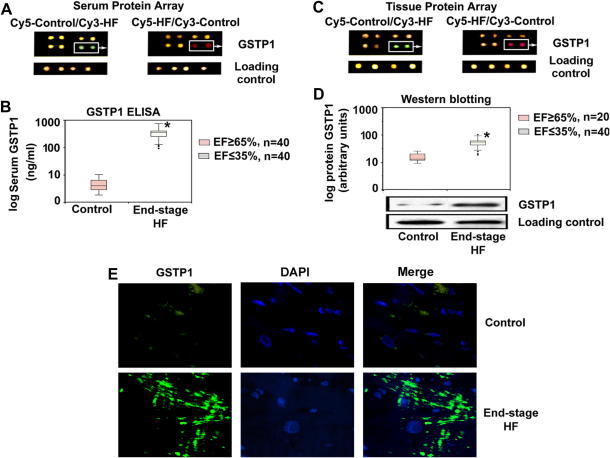
The screening array identified GSTP1 to be a protein associated with HF and that its serum levels are increased in HF patients compared with control donors (Fig. 2A). ELISA analyses verified the array findings and indicated that serum GSTP1 concentrations were significantly increased in end-stage HF patients compared to controls (P < .001; Fig. 2B). The tissue protein array in the same patient cohort indicated elevated GSTP1 expression levels in myocardium of end-stage HF patients compared with control subjects (Fig. 2C). These findings were confirmed by immunohistochemistry and Western blot analyses (P < .001; Fig. 2D and 2E).
Fig. 2.
Serum and cardiac glutathione S-transferase P1 1 (GSTP1) association with heart failure (HF). (A) Representative serum protein array images demonstrate enhanced staining for serum GSTP1 protein in end-stage HF patients compared with control subjects. (B) Enzyme-linked immunosorbent assay (ELISA) indicates elevation of serum GSTP1 in HF patients. ∗P < .0001. (C) Array images demonstrate enhanced cardiac tissue GSTP1 signals in HF patients. (D) Western blotting indicates elevation of GSTP1 protein in HF patients. ∗P < .001. (E) Immunohistochemistry indicates higher expression of GSTP1 in cardiac tissue of HF patients.
GSTP1 Correlates with EF in HF
Serum GSTP1 concentrations were significantly higher in patients with EF ≤22% compared with all other EF groups (P < .0001), and HF patients with EF 23%–32% had significantly higher GSTP1 serum concentrations compared with those with EF 43%–52% or >52% (P < .0001; Fig. 3A). However, serum proBNP concentrations were significantly higher only in patients with EF ≤22% compared with other groups (P < .0001; Fig. 3B). When using equal-sized quintiles, serum GSTP1 concentrations were still significantly different among quintiles (P < .0001), with the higher concentrations belonging to patients with lower EF (Fig. 3C). In contrast, serum proBNP concentrations were significantly lower only in patients with EF >45% compared with EF <45% (P < .0001; Fig. 3D). Overall, serum GSTP1 concentrations correlated more significantly (rS = −0.82; P < .0001) with EF than serum proBNP did (rS = −0.37; P < .0001; Table 2; Fig. 4). Considering EF as a dichotomous variable based on a cutoff level of 42% suggested that serum GSTP1 concentrations still correlated significantly with EF in patients with EF >42% (rS = −0.20; P = .05) and in those with EF ≤42% (rS = −0.64, P < .0001); whereas proBNP serum concentrations did not (Table 2; Fig. 4).
Fig. 3.
Serum cardiac glutathione S-transferase P1 1 (GSTP1) and pro–B-type natriuretic peptide (proBNP) association with ejection fraction (EF). Patients are divided into relative arbitrary groups (A, B) and equal-sized quintiles (C, D). (A) HF patients with EF ≤22% have significantly higher serum GSTP1 concentrations compared with all other EF groups. Patients with EF 23%–32% and EF ≤22% have significantly higher serum GSTP1 concentration compared with those with EF 43%–52% and EF >52%. (B) Patients with EF ≤22% have significantly higher serum proBNP concentration compared with all other EF groups. (C) Serum GSTP1 concentrations are significantly different among EF quintiles, and higher GSTP1 concentrations are associated with low EF. (D) Serum proBNP concentrations are significantly lower in patients with EF >45% compared with other quintiles. ∗P < .0001 vs all groups; †P < .0001 vs EF 43%–52% and EF >52%; ‡P < .0001 vs all groups.
Table 2.
Spearman Correlation Analysis Between Glutathione S-Transferase P1 1 (GSTP1) and pro–B-Type Natriuretic Peptide (proBNP) and Clinical Variables of Study Patients (rS, Values)
| Covariate | All patients |
EF >42% |
EF ≤42% |
|||
|---|---|---|---|---|---|---|
| GSTP1 | proBNP | GSTP1 | proBNP | GSTP1 | proBNP | |
| GSTP1 ng/mL | – | 0.36, <.0001 | – | 0.34,=.03 | – | 0.087, .29 |
| EF | −0.82, <.0001 | −0.37, <.0001 | −0.20, .05 | −0.24, .41 | −0.64, <.0001 | −0.08, .32 |
| Age (y) | −0.26, <.001 | −0.1, .22 | −0.04, .91 | −0.14, .68 | −0.30, <.001 | −0.15, .021 |
| PCWP (mm Hg) | 0.32, .05 | 0.04, .63 | 0.18, .97 | −0.09, .63 | −0.041, .63 | 0.065, .44 |
| PVR (Wood units) | −0.091, .62 | −0.06, .82 | −0.02, .73 | −0.18, .56 | −0.1, .27 | −0.07, .4 |
| CI (L min−1 m−2) | −0.45, <.001 | −0.21, .41 | −0.03, .68 | 0.14, .81 | −0.05, .63 | 0.15, .14 |
| Creatinine (mg/dL) | −0.13, .11 | 0.12, .14 | 0.073, .83 | 0.52, .10 | 0.24,<.005 | 0.01, .93 |
| LVMI | 0.10, .43 | 0.16, .04 | 0.21, .31 | 0.57, <.0001 | 0.082, .34 | 0.41, <.0001 |
CI, cardiac index; EF, ejection fraction; LVMI, left ventricular mass index; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance.
Fig. 4.
Correlation of (A) glutathione S-transferase P1 1 (GSTP1) and (B) pro–B-type natriuretic peptide (proBNP) with ejection fraction (EF), indicating a higher significant negative correlation of EF with serum GSTP1 compared with proBNP for all study patients. Considering EF as a dichotomous variable at a cutoff level of 42%, serum GSTP1 concentrations still significantly correlated with EF in both resulting subgroups, whereas proBNP did not.
Serum GSTP1 Is an Independent Predictor of EF in HF
Univariate logistic regression analyses demonstrated that GSTP1 significantly discriminates between HF patients with EF >42% and those with EF <42% (odds ratio [OR] 0.98; P < .001; Table 3). Also, GSTP1 discriminated patients with EF >22% (OR 0.98; P < .0001; Table 3). The subsequent multivariate logistic regression analyses suggested that GSTP1 is an independent parameter that predicted LV function in HF patients with EF >42% (OR 0.98; P < .002) and EF >22% (OR 0.99; P < .0001; Table 3); pro-BNP was not associated with such predictive abilities (Table 3).
Table 3.
Logistic Regression Analysis of Covariates That Could Predict EF >42% or EF >22%
| HF Patients with EF >42% |
HF Patients with EF >22% |
|||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Univariate log. regression | ||||
| GSTP1 | 0.98 (0.97–0.99) | .001 | 0.98 (0.98–0.99) | .0001 |
| proBNP | 0.99 (0.997–1) | .01 | 0.99 (0.99–1) | .03 |
| LVMI | 0.98 (0.93–1.03) | .38 | 0.99 (0.098–0.99) | .03 |
| Age | 1.02 (0.96–1.08) | .54 | 1.04 (1.01–1.08) | .006 |
| PCWP | 0.99 (0.97–1.02) | .32 | 0.98 (0.94–1.02) | .35 |
| PVR | 0.94 (0.71–1.25) | .12 | 1.08 (0.8–1.4) | .62 |
| CI | 1.1 (1.001–2.53) | .06 | 1.02 (0.5–1.9) | .96 |
| Creatinine | 1.31 (0.81–2.13) | .27 | 1.74 (0.9–3.4) | .1 |
| Multivariate log. regression | ||||
| GSTP1 | 0.98 (0.97–0.99) | .002 | 0.99 (0.98–0.99) | .0001 |
| ProBNP | 0.99 (0.99–1) | .02 | 1 (0.99–1.001) | .94 |
| LVMI | – | – | 0.99 (0.99–1.02) | .06 |
| Age | – | – | 1.04 (0.98–1.2) | .18 |
CI, confidence interval; OR, odds ratio; other abbreviations as in Tables 1 and 2.
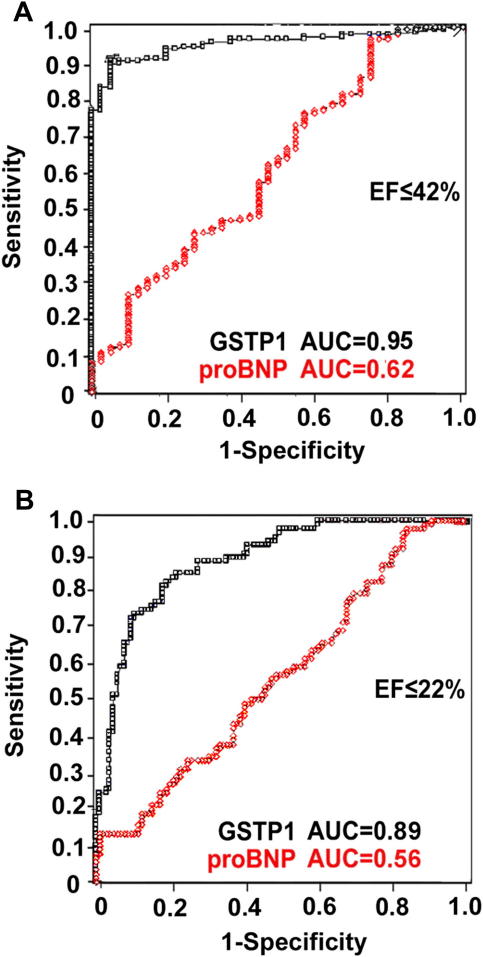
In concert with these findings, ROC curve analyses suggested that GSTP1 at an optimal cutoff level of >126 ng/mL diagnosed HF patients with EF <42% with a 90% sensitivity and a 95% specificity (AUC 0.95; P < .0001), whereas proBNP at an optimal cutoff level of >396 pg/mL (AUC 0.62; P < .01) identified EF <42% with a 97% sensitivity and a 20% specificity (Fig. 5A). Furthermore, at an optimal cutoff level of >231 ng/mL GSTP1 diagnosed EF <22% (AUC 0.89; P < .0001; Fig. 5B) with a sensitivity of 81% and a specificity of 83%. In comparison, serum proBNP at an optimal cutoff level of >655 pg/mL diagnosed EF <22% (AUC 0.56; P = .07; Fig. 5B) with a sensitivity of 84% and a specificity of 22%. In McNemar test, proBNP was more sensitive to diagnose both EF <42% and EF <22%; however, GSTP1 had a higher specificity (P < .001; Table 4). Comparison of the AUC of both GSTP1 and proBNP indicated GSTP1 to be superior in diagnosing both EF <42% (AUC difference 0.34, confidence interval [CI] 0.23–0.45; P < .0001) and EF <22% (AUC difference 0.33, CI 0.24–0.41; P < .0001; Table 4). Additionally, Pencina analysis revealed significantly different NRI and IDI for serum GSTP1 AUCs (P < .001; Table 4). These results indicated GSTP1 to be an independent, sensitive, and specific predictor of LV function in HF.
Fig. 5.
The power of serum glutathione S-transferase P1 1 (GSTP1) and pro–B-type natriuretic peptide (proBNP) to diagnose ejection fraction (EF). (A) At the optimal cutoff levels of ≥126 ng/mL GSTP1, with a sensitivity of 90% and specificity of 95%, and ≥396 pg/mL proBNP, with a sensitivity of 97% and specificity of 20%, identify EF ≤42%. (B) At ≥231 ng/mL cutoff level, GSTP1 identifies EF ≤22% with a sensitivity of 81% and specificity of 83%, whereas proBNP at ≥655 pg/mL cutoff level identifies EF ≤22% with a sensitivity of 84% and specificity of 22%.
Table 4.
Comparison Between Glutathione S-Transferase P1 1 (GSTP1) and pro–B-Type Natriuretic Peptide (proBNP) Sensitivity, Specificity, and Area Under the Receiver Operating Characteristic Curve (AUC) Identified for ejection fraction (EF) ≤22% and EF ≤42% HF Patients
| EF ≤22% |
EF ≤42% |
|||||
|---|---|---|---|---|---|---|
| proBNP, 655 pg/mL | GSTP1, 231 ng/mL | P Value | proBNP, 396 pg/mL | GSTP1 126 ng/mL | P Value | |
| Sensitivity, %∗ | 84 | 81 | <.001 | 97 | 90 | <.001 |
| Specificity, %∗ | 22 | 83 | <.001 | 20 | 95 | <.001 |
| AUC† | 0.56 | 0.89 | <.0001 | 0.62 | 0.95 | <.0001 |
| NRI, %‡ | Reference | 47.5 | <.001 | Reference | 57.8 | <.001 |
| IDI, %‡ | Reference | 34.3 | <.001 | Reference | 34.4 | <.001 |
IDI, integrated discrimination improvement; NRI, net reclassification improvement.
Values were compared by McNemar test.
Values were compared by de Long and de Long test.
Values were compared by Pencina test.
Association of GSTP1 and proBNP with NYHA Classification
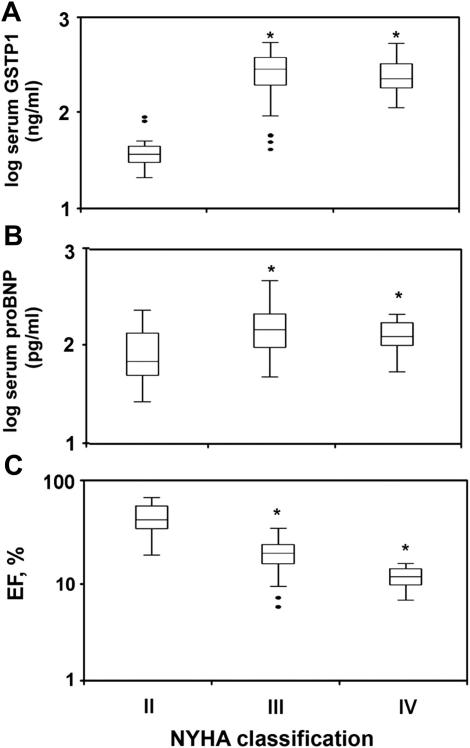
Both serum GSTP1 and proBNP concentrations were significantly increased in New York Heart Association (NYHA) functional class III and IV compared with NYHA II patients (P < .0001; Fig. 6A and 6B), although serum GSTP1 and proBNP were not significantly different when NYHA III and IV patients were compared. In addition, we found significantly higher EF values in NYHA II compared with NYHA III and IV patients (P < .0001), although EF was not significantly different between NYHA III and IV patients (Fig. 6C).
Fig. 6.
Serum (A) glutathione S-transferase P1 1 (GSTP1) and (B) pro–B-type natriuretic peptide (proBNP) and (C) ejection fraction (EF) association with NYHA classification. Serum GSTP1 and proBNP concentrations were significantly higher in patients with NYHA functional class III and IV compared with those with NYHA II. ∗P < .0001 vs NYHA II.
Correlation of GSTP1 and proBNP with Demographic and Clinical Parameters
Serum GSTP1 correlated significantly but only weakly with patient age (rS = −0.26; P < .001), PCWP (rS = 0.32; P = .05), and CI (rS = −0.45; P < .001; Table 2). In addition, LVMI showed a significant correlation with proBNP but not GSTP1 in all patients (rS = 0.16; P = .04). A significant but not strong correlation was observed between serum GSTP1 and creatinine in patients with EF <42% (rS = 0.24; P = .005; Table 2). A significant correlation was also present between GSTP1 and proBNP in the entire study cohort (rS = 0.36; P < .0001) and in patients with EF >42% (rS = 0.34; P < .03; Table 2; Fig. 7).
Fig. 7.
Correlation plot of serum glutathione S-transferase P1 1 (GSTP1) and pro–B-type natriuretic peptide (proBNP) for all study subjects. A significant positive correlation was observed between GSTP1 and proBNP for all study patients. Moreover, significant correlation was still present in patients with ejection fraction (EF) >42% but not in those with EF <42%. HF, heart failure.
Discussion
ProBNP is currently accepted to be a surrogate parameter in HF monitoring, yet existing data indicate divergent results.2–8 Our purpose was therefore to find a serum marker that allows monitoring of ventricular function in HF patients. Our screening suggested that GSTP1 is associated with end-stage HF. GSTP1 belongs to the multigene family of isozymes important for detoxification and xenobiotic mechanisms9 and determination of the cellular response to oxidative stress.10 Serum GSTP1 concentrations diagnosed HF with significant association with EF independently from demographic and clinical characteristics in our patient cohort. Of note, serum GSTP1 showed better diagnostic power in HF patients with EF ≤42% and EF ≤22% compared with proBNP, although elevated serum GSTP1 and proBNP both were associated with NYHA functional class III and IV.
It is well established that EF is a determinant of cardiac risk in HF patients. The hazard ratio for all-cause mortality increases by 39% for every 10% reduction in the EF below 45%.13,14 However, HF clinical features can occur in patients with EF >45%.18 Therefore, our finding that GSTP1 is able to discriminate HF patients with EF >42% might be of important diagnostic and prognostic significance. Although, proBNP is an established tool for HF diagnosis,19 the greatest clinical utility of proBNP is the monitoring of HF-related hemodynamic decompensation and disease progression rather than LV function and EF.20,21 On the other hand, proBNP is recommended to be mainly used for exclusion of HF with normal EF in patients with symptoms attributed to HF.22 However, given that gender and older age are associated with higher proBNP levels,7 the proposed diagnostic properties of proBNP18 might be too unspecific to serve as a predictor for LV function.
Reportedly, proBNP can predict EF <30% with a sensitivity and specificity of 90% and 71%, respectively in an HF population with EF <45%.20 Plasma proBNP detected EF <28% with 77% sensitivity and 69% specificity in a cohort of patients with EF <50%,22 and others23 found that proBNP can predict EF <40% with an AUC of 0.69. In the present study, proBNP had 97% sensitivity and 20% specificity to diagnose EF >42% and 84% sensitivity and 22% specificity to diagnose EF >22%. We had no exclusion criteria for EF, which could have influenced the test characteristics regarding proBNP, which has previously been shown to vary in HF patients with higher EF.18 This fact could also be considered as an explanation for overall lower specificity of proBNP compared with GSTP1 to predict LV function in HF patients.
The reason for serum GSTP1 rising in HF patients is unclear. However, evidence is accumulating that GSTP1 participates in regulation of stress signaling and protects cells against apoptosis via its noncatalytic ligand-binding activity. Specifically, GSTP1 can act as an endogenous inhibitor of c-Jun N-terminal kinase by interaction with its C-terminal end.24 Additionally, GSTP1 expression rises in response to oxidative stress or proinflammatory stimuli.10 Dramatic elevations of reactive oxygen species are reported in HF by hypertrophy and proinflammatory stimuli, indicating multiple cellular responses, such as DNA synthesis, transcription factor activation, and gene and protein expression alteration, in HF.25 These reasons may also explain why GSTP1 was an overall better predictor of LV function in HF than proBNP, because inflammatory processes in HF (associated with GSTP1) are likely to involve the entire myocardium independently of LVMI and atrial dilation, processes that evidently affect proBNP production. Based on our findings, the likely mechanism that accounts for both cardiac tissue and serum GSTP1 elevation could be prompted by oxidative stress and inflammatory stimuli26 activated in HF.
Study Limitations and Clinical Implications
The single-center nature of this study, although prospective, is a limitation. In addition, although patients with acute endocarditis and malignancies were excluded, this study can not account for the impact of potential inflammatory stimuli on GSTP1 in patients before HF diagnosis, particularly in idiopathic dilated cardiomyopathy. Also, serial measurement in an HF patient cohort might be necessary to comparatively analyze the power of GSTP1 versus proBNP in assessing or predicting LV function. In contrast to these limitations, the noninvasive nature and ease of the suggested GSTP1 serum quantification protocol, along with the availability and low costs of ELISA, and finally the high sensitivity and specificity of GSTP1, make it an attractive serum marker to predict LV function in HF patients.
Disclosures
S. Aharinejad holds inventor rights to European patent application no. EP09174692.5, “Use of GSTP1,” but there are no financial interests to be disclosed. All other authors report no potential conflicts of interest.
Footnotes
Funding: Grant no. P22371-B19 from the Austrian Science Foundation and a grant from the Austrian Heart Foundation to S.A.
See page 260 for disclosure information.
References
- 1.Lee D.S., Austin P.C., Rouleau J.L., Liu P.P., Naimark D., Tu J.V. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 2.Troughton R.W., Frampton C.M., Yandle T.G., Espiner E.A., Nicholls M.G., Richards A.M. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 3.Anand I.S., Florea V.G., Fisher L. Surrogate end points in heart failure. J Am Coll Cardiol. 2002;39:1414–1421. doi: 10.1016/s0735-1097(02)01773-4. [DOI] [PubMed] [Google Scholar]
- 4.Dibbs Z., Thornby J., White B.G., Mann D.L. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J Am Coll Cardiol. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 5.Daniels L.B., Maisel A.S. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Anwaruddin S., Lloyd-Jones D.M., Baggish A., Chen A., Krauser D., Tung R. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. J Am Coll Cardiol. 2006;47:91–97. doi: 10.1016/j.jacc.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 7.Raymond I., Groenning B.A., Hildebrandt P.R., Nilsson J.C., Baumann M., Trawinski J. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89:745–751. doi: 10.1136/heart.89.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costello-Boerrigter L.C., Boerrigter G., Redfield M.M., Rodeheffer R.J., Urban L.H., Mahoney D.W. Amino-terminal pro–B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscow J.A., Fairchild C.R., Madden M.J., Ranson D.T., Wieand H.S., O’Brien E.E. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989;9:1422–1428. [PubMed] [Google Scholar]
- 10.Cumming R.C., Lightfoot J., Beard K., Youssoufian H., O’Brien P., Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med. 2001;7:814–820. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 11.Shea T.C., Kelley S.L., Henner W.D. Identification of an anionic form of glutathione transferase present in many human tumors and human tumor cell lines. Cancer Res. 1988;48:527–533. [PubMed] [Google Scholar]
- 12.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellika P.A. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Lee D.S., Gona P., Vasan R.S., Larson M.G., Benjamin E.J., Wang T.J. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon S.D., Anavekar N., Skali H., Mc Murray J.J., Swedberg K., Yusuf S. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 15.Fuster R.G., Argudo J.M., Albarova O.G., Hornero Sos F., Cánovas López S., Bueno Codoñer M. Left ventricular mass index as a prognostic factor in patients with severe aortic stenosis and ventricular dysfunction. Interact CardioVasc Thorac Surg. 2005;4:260–266. doi: 10.1510/icvts.2004.098194. [DOI] [PubMed] [Google Scholar]
- 16.Aharinejad S., Krenn K., Zuckermann A., Schäfer R., Gmeiner M., Thomas A. Serum matrix metalloprotease-1 and vascular endothelial growth factor-a predict cardiac allograft rejection. Am J Transplant. 2009;9:149–159. doi: 10.1111/j.1600-6143.2008.02470.x. [DOI] [PubMed] [Google Scholar]
- 17.Aharinejad S., Andrukhova O., Gmeiner M., Thomas A., Aliabadi A., Zuckermann A. Donor serum SMARCAL1 concentrations predict primary graft dysfunction in cardiac transplantation. Circulation. 2009;120:198–205. doi: 10.1161/CIRCULATIONAHA.108.842971. [DOI] [PubMed] [Google Scholar]
- 18.Maeder M., Kaye D. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Gardner R.S., Ozalp F., Murday A.J., Robb S.D., McDonagh T.A. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Koç M., Bozkurt A., Yildiray-Sahin D., Unal I., Acartürk E. Cutoff values of NT-proBNP for the prediction of low functional capacity, decreased ejection fraction and cardiovascular events in patients with heart failure. Cardiol J. 2009;16:43–49. [PubMed] [Google Scholar]
- 21.Ho S.J., Feng A.N., Lee L.N., Chen J.W., Lin S.J. Predictive value of predischarge spectral tissue doppler echocardiography and N-terminal pro–B-type natriuretic peptide in patients hospitalized with acute heart failure. Echocardiography. 2011;28:303–310. doi: 10.1111/j.1540-8175.2010.01322.x. [DOI] [PubMed] [Google Scholar]
- 22.Kallistratos M.S., Dritsas A., Laoutaris I.D., Cokkinos D.V. Incremental value of N-terminal pro–brain natriuretic peptide over left ventricle ejection fraction and aerobic capacity for estimating prognosis in heart failure patients. J Heart Lung Transplant. 2008;27:1251–1256. doi: 10.1016/j.healun.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Kotaska K., Popelova J., Tiserova M., Telekes P., Vrzanova M., Bronsky J. NT-proBNP and BNP values in cardiac patients with different degree of left ventricular systolic dysfunction. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:125–130. doi: 10.5507/bp.2006.019. [DOI] [PubMed] [Google Scholar]
- 24.Alder A., Yin Z., Fuchs S.Y., Rosario L., Tew K.D., Pincus M.R. Regulation of JNK signalling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López Farré A., Casado S. Heart failure, redox alterations, and endothelial dysfunction. Hypertension. 2001;38:1400–1405. doi: 10.1161/hy1201.099612. [DOI] [PubMed] [Google Scholar]
- 26.Lou L., Wang Y., Feng Q., Zhang H., Xue B., Shen J. Recombinant protein glutathione S-transferases P1 attenuates inflammation in mice. Mol Immunol. 2009;46:848–857. doi: 10.1016/j.molimm.2008.09.010. [DOI] [PubMed] [Google Scholar]