Abstract
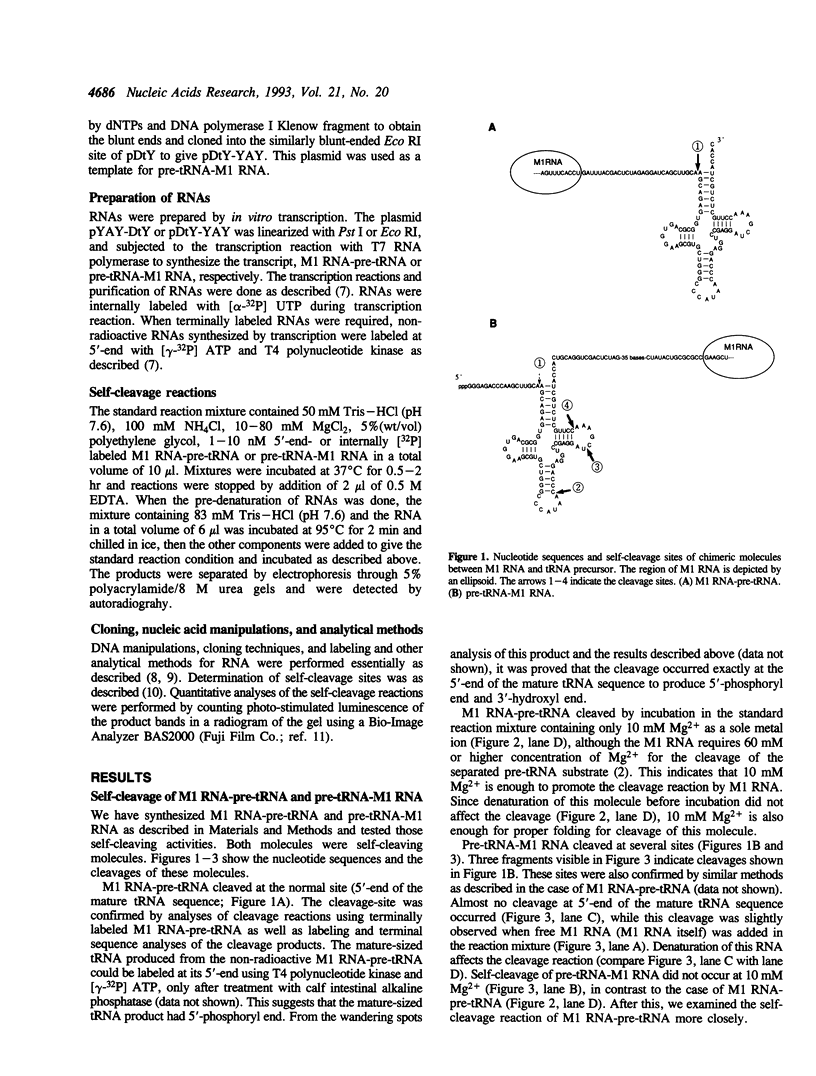
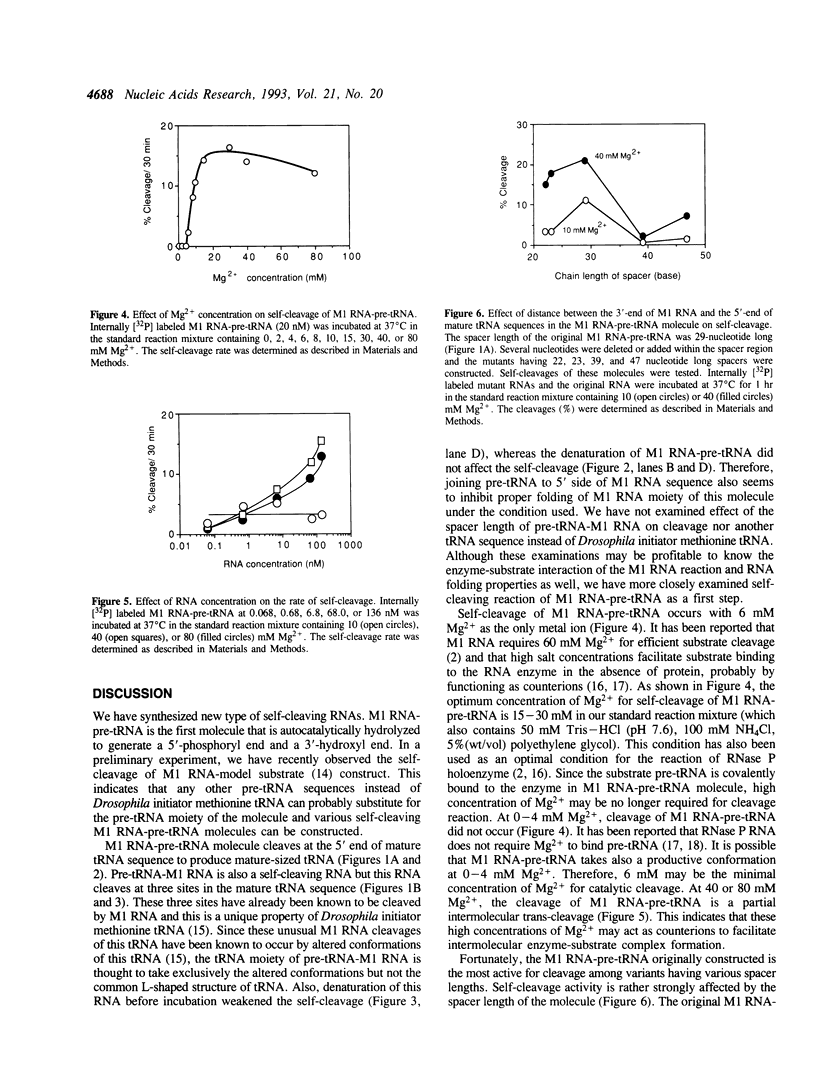
We synthesized two types of chimeric RNAs between the catalytic RNA subunit of RNase P from Escherichia coli (M1 RNA) and a tRNA precursor (pre-tRNA); one had pre-tRNA at the 3' side to the M1 RNA (M1 RNA-pre-tRNA). The second had pre-tRNA at the 5' side of the M1 RNA (pre-tRNA-M1 RNA). Both molecules were self-cleaving RNAs. The self-cleavage of M1 RNA-pre-tRNA occurred at the normal site (5'-end of mature tRNA sequence) and proceeded under the condition of 10 mM Mg2+ concentration. This reaction at 10 mM Mg2+ was an intramolecular reaction (cis-cleavage), while, at 40 mM and 80 mM Mg2+, trans-cleavage partially occurred. The self-cleavage rate was strictly affected by the distance between the M1 RNA and the pre-tRNA in the molecule. The self-cleavage of pre-tRNA-M1 RNA occurred mainly at three sites within the mature tRNA sequence. This cleavage did not occur at 10 mM Mg2+. Use of M1 RNA-pre-tRNA molecule for the in vitro evolution of M1 RNA is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Ribonuclease P: an enzyme with a catalytic RNA subunit. Adv Enzymol Relat Areas Mol Biol. 1989;62:1–36. doi: 10.1002/9780470123089.ch1. [DOI] [PubMed] [Google Scholar]
- Amemiya Y., Miyahara J. Imaging plate illuminates many fields. Nature. 1988 Nov 3;336(6194):89–90. doi: 10.1038/336089a0. [DOI] [PubMed] [Google Scholar]
- Beaudry A. A., Joyce G. F. Directed evolution of an RNA enzyme. Science. 1992 Jul 31;257(5070):635–641. doi: 10.1126/science.1496376. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Cremisi F., Scarabino D., Carluccio M. A., Salvadori P., Barsacchi G. A newt ribozyme: a catalytic activity in search of a function. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1651–1655. doi: 10.1073/pnas.89.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., Davies C., Sheldon C. C., Jeffries A. C., Symons R. H. Self-cleaving viroid and newt RNAs may only be active as dimers. Nature. 1988 Jul 21;334(6179):265–267. doi: 10.1038/334265a0. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Joyce G. F. Amplification, mutation and selection of catalytic RNA. Gene. 1989 Oct 15;82(1):83–87. doi: 10.1016/0378-1119(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Sasaki N., Ando-Yamagami Y. Cleavage of tRNA within the mature tRNA sequence by the catalytic RNA of RNase P: implication for the formation of the primer tRNA fragment for reverse transcription in copia retrovirus-like particles. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8105–8109. doi: 10.1073/pnas.87.20.8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Sasaki N. Hyperprocessing of tRNA by the catalytic RNA of RNase P. Cleavage of a natural tRNA within the mature tRNA sequence and evidence for an altered conformation of the substrate tRNA. J Biol Chem. 1992 Jun 15;267(17):11972–11976. [PubMed] [Google Scholar]
- Kikuchi Y., Sasaki N. Site-specific cleavage of natural mRNA sequences by newly designed hairpin catalytic RNAs. Nucleic Acids Res. 1991 Dec 25;19(24):6751–6755. doi: 10.1093/nar/19.24.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman N., Joyce G. F. Evolution in vitro of an RNA enzyme with altered metal dependence. Nature. 1993 Jan 14;361(6408):182–185. doi: 10.1038/361182a0. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Guerrier-Takada C., Altman S. Model substrates for an RNA enzyme. Science. 1987 Oct 23;238(4826):527–530. doi: 10.1126/science.2443980. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault J. P., Altman S. Pathway of activation by magnesium ions of substrates for the catalytic subunit of RNase P from Escherichia coli. J Mol Biol. 1993 Apr 5;230(3):750–756. doi: 10.1006/jmbi.1993.1197. [DOI] [PubMed] [Google Scholar]
- Reich C., Olsen G. J., Pace B., Pace N. R. Role of the protein moiety of ribonuclease P, a ribonucleoprotein enzyme. Science. 1988 Jan 8;239(4836):178–181. doi: 10.1126/science.3122322. [DOI] [PubMed] [Google Scholar]
- Smith D., Burgin A. B., Haas E. S., Pace N. R. Influence of metal ions on the ribonuclease P reaction. Distinguishing substrate binding from catalysis. J Biol Chem. 1992 Feb 5;267(4):2429–2436. [PubMed] [Google Scholar]
- Symons R. H. Small catalytic RNAs. Annu Rev Biochem. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]




