Abstract
There is strong evidence that vascular risk factors play a role in the development of Alzheimer's disease (AD) or vascular dementia (vaD). Ethanol (EtOH) and cholesterol are such vascular risk factors, and we recently showed that hypercholesterolemia causes pathologies similar to AD [Ullrich et al. (2010) Mol Cell Neurosci 45, 408–417]. The aim of this study was to investigate the effects of long-term (12 months) EtOH treatment (20% v/v in drinking water) alone or long-term 5% cholesterol diet alone or a combination (mix) in adult Sprague–Dawley rats. Long-term EtOH treatment (plasma EtOH levels 58±23 mg/dl) caused significant impairment of spatial memory, reduced the number of choline acetyltransferase- and p75 neurotrophin receptor-positive nucleus basalis of Meynert neurons, decreased cortical acetylcholine, elevated cortical monocyte chemoattractant protein-1 and tissue-type plasminogen activator, enhanced microglia, and markedly induced anti-rat immunoglobulin G-positive blood–brain barrier leakage. The effect of long-term hypercholesterolemia was similar. Combined long-term treatment of rats with 20% EtOH and 5% cholesterol (mix) did not potentiate treatment with EtOH alone, but instead counteracted some of the EtOH-associated effects. In conclusion, our data show that vascular risk factors EtOH and cholesterol play a role in cognitive impairment and possibly vaD.
Key words: cholinergic dysfunction, ethanol, blood–brain barrier leakage, cognitive decline
Abbreviations: ACh, acetylcholine; AD, Alzheimer's disease; ANOVA, analysis of variance; APP, amyloid precursor protein; Aβ, beta-amyloid; BBB, blood–brain barrier; ChAT, choline-acetyltransferase; ELISA, enzyme-linked immunosorbent assay; EtOH, ethanol; HPLC, high-performance liquid chromatography; IgG, immunoglobulin G; IL-1β, interleukin 1-β; MIP-2, macrophage inflammatory protein-2; nbM, basal nucleus of Meynert; NGF, nerve growth factor; PBS, phosphate-buffered saline; RET, retention; RME, reference memory error; TNF-α, tumor necrosis factor-alpha; tPA, tissue-plasminogen activator; T-PBS, triton/PBS; WME, working memory errors; WRME, working-reference memory error
Highlights
▶Long-term chronic ethanol causes cognitive impairment in rats in vivo. ▶Chronic ethanol causes cholinergic neurodegeneration. ▶Chronic ethanol induces cortical rat IgG-influx and MCP-1 upregulation. ▶Chronic mild hypercholesterolemia causes similar impairments. ▶Hypercholesterolemia partly counteracts some of the ethanol-induced effects.
Alzheimer's disease (AD) is the most common cause of dementia in the elderly people. The hallmark pathologies include beta-amyloid (Aβ) depositions in brain (plaques) and vessels (amyloid angiopathy), tau pathology, cerebrovascular dysfunction, cholinergic impairment, microglia activation, and inflammation. The causes of AD are unknown, but cerebrovascular damage may play a key role in its development (Bell and Zlokovic, 2009; Deane and Zlokovic, 2007; Iadecola, 2004; Humpel, 2011). Ethanol (EtOH) is such a vascular risk factor (Monforte et al., 1990; Hillbom and Kaste, 1981) and heavy EtOH consumption leads to neurodegenerative impairment as indicated by the loss of cortical and subcortical brain structures, as well as increased ventricular volumes (Crews and Nixon, 2009; Charness, 1993). These findings may be caused by cerebrovascular abnormalities, accompanied by the influx of toxic substances into the brain and an impaired clearance at the blood–brain barrier (BBB). To date there is no evidence that EtOH could be involved in the development of AD. Although some studies reported that EtOH may alter amyloid precursor protein (APP) and APP-processing enzymes (Kim et al., 2011), may cause neuritic plaques in rats (Paula-Barbosa and Tavares, 1984), may increase levels of hyperphosphorylated tau (Sun et al., 2005), and may favor Aβ production (Lahiri et al., 2002), a direct correlation between AD and EtOH consumption has not been found (Graves et al., 1990; Rosen et al., 1993; Tanaka et al., 2002; Aho et al., 2009). Several studies suggest a protective effect of moderate chronic EtOH on AD development (Anstey et al., 2009; Tyas, 1996). In fact, EtOH has a cholesterol-reducing effect on the cardiovascular system and a protective effect against AD, whose pathology has been limited to altered cholesterol hemostasis in the brain (Guizzetti and Costa, 2007).
However, there is clear evidence that EtOH consumption causes cognitive decline and cholinergic dysfunction, symptoms also found in AD and vascular dementia (vaD) (Floyd et al., 1997; Arendt, 1994; Arendt et al., 1995). In fact, the cholinergic system seems to be particularly sensitive to EtOH-induced degeneration (Floyd et al., 1997; Arendt, 1994; Arendt et al., 1995), and chronic EtOH resulted in a degeneration of cholinergic neurons in the basal forebrain (Arendt, 1994), decreased levels of acetylcholine (ACh), choline acetyltransferase (ChAT), and acetylcholine esterase (AChE) in the basal nucleus of Meynert (nbM) in rats (Arendt et al., 1988), accompanied by increased cortical nerve growth factor (NGF) levels (Arendt et al., 1995). NGF supports the survival of cholinergic neurons (Humpel and Weis, 2002), and accumulation in cortical cholinergic projection areas may be caused by the impairment of retrograde transport mechanisms (Salehi et al., 2007; Scott et al., 1995; Mufson et al., 1995).
The aim of the present study was to investigate the effects of long-term (12 months) moderate EtOH treatment in male Sprague–Dawley rats on (1) spatial memory, (2) cholinergic neurons, (3) inflammation, and (4) the vascular system. As we previously showed that the vascular risk factor cholesterol markedly affected all four mentioned functions after short-term (5 months) treatment (Ullrich et al., 2010), we now wanted to compare the effects of long-term (12 months) EtOH and cholesterol treatment. Finally, we were interested in investigating whether some of these markers induced by hypercholesterolemia are affected by EtOH co-treatments (mix). These experiments may bring some insights into the mechanisms how long-term vascular risk factors may contribute to cognitive impairment.
Experimental procedures
Animals
Adult male Sprague–Dawley rats were housed at the Animal Department of Innsbruck Medical University with free access to food and water and a 12/12 h light–dark cycle. Animals were randomly divided into four groups: (1) control group: tap water and normal food (n=12), (2) EtOH group: 20% EtOH (Gatt-Koller, Austria) as the sole drinking fluid and normal food (n=12), (3) cholesterol group: tap water and a diet supplemented with 5% cholesterol (n=12) (see Ullrich et al., 2010), (4) mixed group: 20% EtOH as the sole drinking fluid and a diet supplemented with 5% cholesterol (n=12) (see Ullrich et al., 2010). The EtOH concentration was initially 5% and was progressively increased by 5% per week until a final concentration of 20% (v/v) was achieved 4 weeks later. All animals were treated for 12 months as well as during the testing period and were weighed before and at the end of the experiment. All animal experiments were approved by the Austrian Ministry of Science. All attempts were done to minimize the number of animals and their suffering.
Spatial memory testing in the eight-arm radial maze
Spatial memory was assessed using the eight-arm radial maze (PanLab, Spain), as described in detail (Pirchl et al., 2010; Ullrich et al., 2010). Eleven months after starting the experiment spatial learning and long-term memory performance were assessed. The maze consists of eight identical open, dark Plexiglas® arms with side panels and recessed cups at the outer end radiating from a circular platform. To facilitate spatial navigation, small high-contrast visual cues (triangle, vertical bars, cross and square) were placed above the doors of four arms and in a higher magnification on the corresponding walls. Two days before the experiment food deprivation commenced (2 g food pellets administered per animal per day), however, animals had free access to water and ethanol exposure was continued. Animals were then habituated to the maze for 1 day (four trials of 10 min each). Three days after habituation the actual spatial learning test commenced, which consisted of five trials per day (session) on five consecutive days. For each trial four arms were baited with food pellets (chocolate cereals), and the trial ended when all baits were found or after 10 min had elapsed. To exclude any olfactory effects additional baits were placed under the food cup in each arm and the maze was cleaned with 70% ethanol after every trial. Thereafter, the sessions and food deprivation were discontinued for 19 days, namely 2 days before the retention session, when food deprivation was resumed. The animals were again tested for one session (consisting of five trials) to assess spatial long-term memory performance (retention). Memory errors were quantified according to Jarrard's definition (Jarrard et al., 1984). Working memory errors (WME) were defined as repeat visits to baited arms. The first visit to an unbaited arm was defined as a reference memory error (RME), and the combination of both errors, namely repeat visits to unbaited arms, was specified as a working-reference memory error (WRME). The whole experiment was automatically controlled and monitored by a computer with Mazesoft Software (version 8.1.9).
Collection of blood and brains
One day after the last retention, animals were anesthetized with a subcutaneous injection of sodium thiopental (12.5 mg/ml; Sandoz, Austria). Blood was drawn directly from the heart using a 21-gauge butterfly blood collection system (BD Valu-Set, BD, UK) and collected in ethylenediaminetetraacetic acid tubes (S-monovettes, Sarstedt, Germany). Blood was centrifuged at 250×g for 15 min to obtain plasma, further centrifuged twice at 2300×g for 10 min to remove remaining platelets, and finally stored at −80 °C. For brain extracts, the brains (n=6 per group) were removed, the frontal cortex dissected (left and right hemispheres separate in a tube) and immediately frozen in CO2 snow and stored at −80 °C until further use. For immunohistochemistry, rats were perfused with 4% paraformaldehyde (PFA, Merck, Germany) in phosphate-buffered saline (PBS), brains were removed and postfixed for 30 min in 4% PFA and stored in 20% sucrose/sodium azide. Brains were frozen under CO2 snow and sliced into 60-μm sections with a cryostate (Leica Jung CM3000, Germany).
Measurement of EtOH in plasma and cortex
Cortex tissue (left hemisphere) was homogenized in 100 μl ice-cold PBS with a protease-inhibitor cocktail (Sigma, Germany) using an ultrasonic device (Branson Sonifier 250, Danbury, USA) and centrifuged at 16,000×g for 10 min at 4 °C. EtOH levels in cortex extracts and plasma were measured using an EnzyChrom™ EtOH Assay Kit Ecet-100 (BioAssay Systems, Hayward, CA, USA).
Measurement of cholesterol in plasma
Cholesterol was analyzed by high-performance liquid chromatography (HPLC) and UV detection as described by Webb et al. (1982) and reported recently by us (Ullrich et al., 2010). Briefly, 50 μl of plasma or standards (0–5 mg/ml cholesterol in 70% chloroform) were mixed with 1 ml of alcoholic potassium hydroxide and incubated for 30 min at 75 °C. After cooling to room temperature, 1 ml of de-ionized water and 2 ml of n-hexane were added to the tubes. The tubes were agitated for 15 min and centrifuged for 5 min at 200×g. One ml of the n-hexane (upper) layer was transferred to a glass tube and evaporated at 75 °C. Residues were dissolved in 500 μl mobile phase (44% acetonitrile, 54% isopropanol, 2% a.d.) and 100 μl was injected onto the column (reversed phase C18 100×3 mm, Shimadzu, Kyoto, Japan) at a flow rate of 0.6 ml/min. Cholesterol was monitored at 205 nm with a UV detector (Antec-Leyden Decade II, Zoeterwoude, Netherlands). Sample values were calculated from the standard curve in a linear range.
Measurement of ACh in the cortex
ACh levels were analyzed by HPLC and electrochemical detection as described by us (Ullrich et al., 2010; Pirchl et al., 2010). Briefly, the tissue (right hemisphere) was homogenized in 150-μl ice-cold PBS (+5 μM neostigmine, pH 5.3, Sigma, Austria) using an ultrasonic device (Branson Sonifier 250, Danbury, USA) and centrifuged at 16,000×g for 5 min at 4 °C. The supernatant (100 μl) was rapidly injected onto the analytical column (BASI MF6150, Warwickshire, UK) connected to an “immobilized enzyme reactor” (BASI MF6151, Warwickshire, UK). This reactor hydrolyzes ACh and generates hydrogen peroxide (H2O2), which is electrochemically detected through oxidation (+500 mV vs. Ag/AgCl2) using an electrochemical detector (Antec-Leyden Decade II, Zoeterwoude, Netherlands). The mobile phase included 50 mM sodium phosphate and 0.01% sodium azide, pH 8.5. The amount of ACh in the homogenates was quantified using calibration curves for external ACh standards (Sigma, Germany).
Immunohistochemistry
Immunohistochemistry using the avidin-biotin technique was performed as described previously (Pirchl et al., 2010; Ullrich et al., 2010). All incubations were performed at 4 °C over 2 days including 0.1% Triton (Merck, Germany). Fixed sections (n=6) were rinsed for 30 min with 0.1% Triton/PBS (T-PBS) at room temperature and pretreated for 20 min with 20% methanol/1% H2O2/PBS. Subsequently, the slices were washed three times for 10 min with PBS, blocked with 20% horse serum/0.2% BSA/T-PBS and incubated with the primary antibody against goat anti-choline acetyltransferase (1:750, Chemicon, USA), anti-p75 neurotrophin receptor (p75NTR, 1:1000, Promega Madison, WI, USA) mouse anti-laminin (1:500 Sigma, UK), mouse anti-RECA-1(1:100 Serotec, Kidlington, UK), mouse anti-CD11b (1:500 Millipore, Billerica, USA), rabbit anti-ionized calcium-binding adapter molecule 1 (Iba-1, 1:500, Wako, Wako, Germany) in 0.2% BSA/T-PBS for 2 days at 4 °C. Slices were washed and incubated with secondary biotinylated anti-goat, anti-mouse, or anti-rabbit antibody (1:200, Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. After rinsing three times in PBS, slices were incubated in avidin-biotin complex solution (ABC; Elite Standard PK 6100, Vector Laboratories, Burlingame, CA, USA) for 1 h and subsequently washed three times in 50 mM Tris-buffered saline (TBS). The signal was detected using 0.5 mg/ml 3,3′ diaminobenzidine (DAB) in TBS with 0.003% H2O2 as substrate. Slices were then rinsed in PBS and mounted on glass slides.
Evaluation of BBB disruption
Impairment of BBB was assessed using immunohistochemistry for rat immunoglobulin G (IgG) as described by us (Pirchl et al., 2010; Ullrich et al., 2010). Sections were incubated in biotinylated rabbit anti-rat IgG (1:400 Vector Laboratories, USA) for 2 h. After rinsing they were incubated in an avidin-biotin complex solution (ABC-Elite Vectastain reagent, Vector Laboratories, USA) for 1 h. Visualization was performed identically as described for immunohistochemistry.
Enzyme-linked immunosorbent assay (ELISA) measurements for NGF, inflammatory markers, tPA, and beta-amyloid
Cortical extracts from both hemispheres were used. NGF analysis was performed with a commercial ELISA kit (Promega, Madison, WI, USA) as previously described in detail (Pirchl et al., 2010; Ullrich et al., 2010). Inflammatory markers were analyzed using a Multiplex rat ELISA (SearchLight®, Aushon Biosystems, USA) as previously described in detail (Pirchl et al., 2010; Ullrich et al., 2010). Tissue-plasminogen activator (tPA) was measured with a commercial ELISA kit (Oxford Biochemical Research, PA 94, USA). ELISA measurements of rat Aβ(1-40) and Aβ(1–42) were performed with commercial highly sensitive ELISA kits (294-64701 and 292-64501, Wako, Germany) as previously described (Marksteiner and Humpel, 2008).
Quantitative analysis and statistics
Behavioral testing was statistically analyzed within groups using a one-way repeated measures analysis of variance (ANOVA) (S1–S5 and S5-Ret) and between control and EtOH- and cholesterol-fed animals using Student's t-test and the F-test to compare for equal variance. The number of ChAT- or p75NTR-positive neurons was counted in the nbM between bregma −0.7 mm and −3.1 mm in control and EtOH-treated as well as in EtOH- and cholesterol-fed rats visualized under a 20× objective, according to the rat brain atlas (Paxinos and Watson, 1986), by an investigator blinded to the treatment code. Rat anti-IgG-positive spots (as a marker for BBB leakage) were counted in the cortex in six fields per section and in four sections per brain under a 20× objective in all groups. Sections were photographed under the microscope and digitized pictures overlaid in NIH ImageJ software with a grid (150×150 μm2 per square). The capillary network in the cortex was assessed, as described by us in detail (Pirchl et al., 2010; Ullrich et al., 2010), by counting the number of crossings of RECA-1- or laminin-positive capillaries across the lines of the overlaid grid. Quantitative data are presented as mean values±SEM. The significance of differences between the control and the EtOH or mixed group was assessed with the one-way ANOVA, followed by the Fisher PLSD post hoc test by comparing controls with the EtOH or the mixed EtOH and cholesterol group or with Student's t-test, where P<0.05 represents statistical significance.
Results
Effect of EtOH treatment on plasma levels and weight
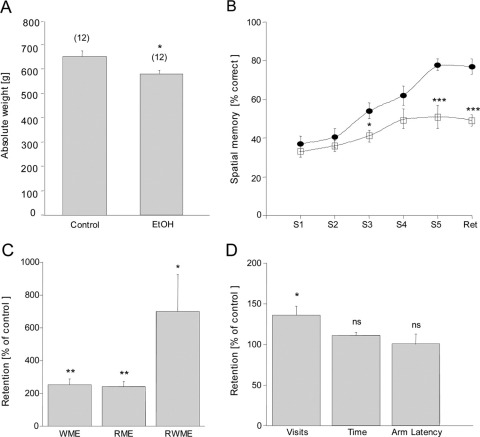
When rats were treated with 20% EtOH for 12 months, the plasma EtOH levels were seen to be significantly increased in the EtOH group as compared with controls (Table 1). No EtOH levels were detectable in the cortex of either group. The weight of the EtOH-treated rats (580±16 g, n=6) was significantly decreased after 12 months as compared with that of the control animals (652±23 g, n=6; Fig. 1A). The plasma levels of tPA, Aβ, and several inflammatory markers were not affected by EtOH (Table 1).
Table 1.
Analysis of plasma markers after long-term (12 mon) ethanol (EtOH) treatment
| Plasma | Control | EtOH |
|---|---|---|
| EtOH [mM=‰=mg/dl] | below DL | 12.5 ± 5.2=0.7 ± 0.3=57.7 ± 23.3⁎ |
| Cholesterol [mg/ml] | 0.7 ± 0.1 | 0.5 ± 0.1 ns |
| tPA [ng/ml] | 10.9 ± 1.4 | 13.7 ± 3.0 ns |
| Aβ(1–40) [pM] | 38.7 ± 5.9 | 37.6 ± 8.9 ns |
| Aβ(1–42) [pM] | 3.7 ± 0.3 | 4.8 ± 0.8 ns |
| IL-1β [pg/ml] | 9.7 ± 7.7 | 10.1 ± 4.0 ns |
| MIP-2 [pg/ml] | 3.8 ± 1.7 | 4.8 ± 2.7 ns |
| TNF-α [pg/ml] | 29.3 ± 19.5 | 19.4 ± 13.0 ns |
| MCP-1 [ng/ml] | 3.6 ± 0.5 | 3.0 ± 0.3 ns |
Sprague–Dawley rats were administered normal water (controls) or 20% EtOH-enriched water for 12 mon. Blood was collected and plasma samples were analyzed with the ethanol detection kit for EtOH levels, cholesterol by HPLC-UV detection, and ELISAs for interleukin 1-beta (IL-1β), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), tumor necrosis factor-alpha (TNF-α), tissue plasminogen activator (tPA), beta amyloid(1–40) (Aβ(1–40)), beta-amyloid(1–42) (Aβ(1–42)). Values are given as mean±SEM in [pg/ml, ng/ml, pM, mg/dl, or %]. Statistical analysis was performed with a one-way ANOVA with a Fisher LSD post hoc test (n=6 animals per group; tPA, n=3). ns, not significant; below DL, below detection limit,
P<0.001.
Fig. 1.
Effects of long-term (12 months) ethanol (EtOH) treatment in male Sprague–Dawley rats on weight and spatial memory. (A) Rat weight was significantly decreased in EtOH-treated animals as compared with controls. (B) After 12 mon EtOH-treated rats (filled cycles) exhibited a significant decline in spatial memory performance (sessions 3 and 5), which was consistently declined after 3 wk (retention, Ret) as compared with controls (open squares). (C) EtOH-treated animals showed enhanced WME, RME as well as WRME. (D) Although the number of total visits was enhanced in EtOH rats, no change was seen in the time needed to find all four baits or the length of time spent in the visited arm. Values are expressed as mean±SEM. Values in parenthesis give the number of rats. Statistical analysis was performed with Student's t-test. *** P<0.001; ** P<0.01; * P<0.5; ns, not significant.
EtOH affects spatial memory
Spatial memory was tested in an eight-arm partially baited maze. Rats treated with EtOH showed a markedly impaired performance in the learning tasks in sessions 3 and 5 (Fig. 1B) and in retention after 3 weeks (Fig. 1B). WME, RME, and WRME were markedly enhanced in EtOH-treated rats as compared with controls (Fig. 1C). EtOH-treated animals did not show any difference in the time needed to find all baits or in the length of time spent in the visited arms (Fig. 1D). However, the number of total visits was markedly increased in EtOH-treated rats (Fig. 1D).
EtOH decreases cholinergic activity in the nbM
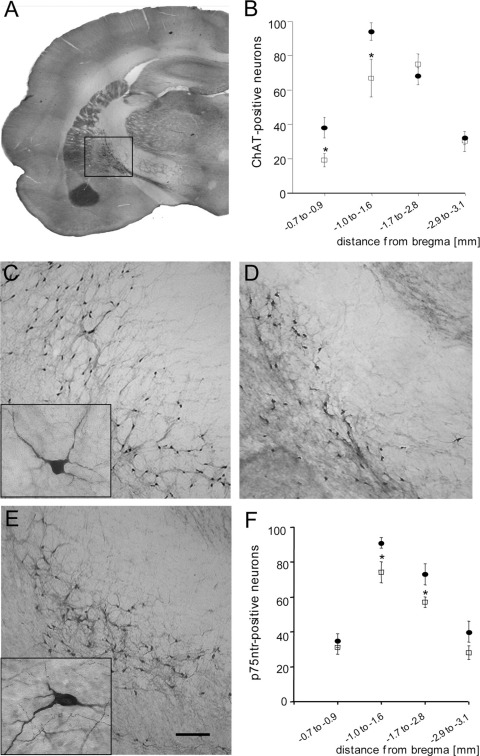
Cholinergic neurons in the nbM were immunohistochemically stained for the ACh synthesizing enzyme ChAT (Fig. 2A, C, D) and p75 neurotrophin receptor (p75NTR; Fig. 2E). ChAT- (Fig. 2B) and NGF receptor p75NTR-positive (Fig. 2F) neurons were evaluated within the bregma area −0.7 to −3.1 mm. The number of ChAT-positive neurons in EtOH-treated rats was significantly decreased as compared with controls (Fig. 2B). EtOH treatment revealed a significant decline in p75NTR-positive neurons (Fig. 2F). The neurotransmitter ACh levels in the cortex were markedly reduced in EtOH-treated rats (Table 2).
Fig. 2.
Effects of long-term (12 months) ethanol (EtOH) treatment on cholinergic neurons in the brain. (A) The number of choline-acetyltransferase (ChAT)-positive neurons in the basal nucleus of Meynert was counted between bregma −0.8 mm and −3.1 mm (B) and revealed a decreased number of ChAT-positive neurons in EtOH-fed rats (open squares, D) as compared with control animals (filled cycles, C). Small boxes show a magnification of ChAT- and p75NTR-positive neurons. EtOH-treated rats exhibited a decreased number of p75NTR-positive neurons in the nbM (F) as compared with controls (E). Statistical analysis was performed with a one-way ANOVA with a Fisher LSD post hoc test. * P<0.05. Scale bar in A=2800 μm (C, D) and =150 μm (E) and =20 μm (small boxes).
Table 2.
Analysis of cortical markers after long-term (12 mon) ethanol (EtOH) treatment
| Cortex | Control | EtOH |
|---|---|---|
| EtOH [mM] | below DL | below DL |
| ACh [ng/mg] | 10.9 ± 2.3 | 5.1 ± 1.3⁎ |
| NGF [pg/mg] | 46.9 ± 3.2 | 48.8 ± 2.8 ns |
| tPA [ng/mg] | 0.68 ± 0.06 | 1.08 ± 0.07⁎⁎ |
| Aβ(1–40) [pM] | 37.1 ± 2.6 | 39.5 ± 4.6 ns |
| Aβ(1–42) [pM] | 4.8 ± 0.1 | 4.7 ± 0.6 ns |
| IL-1β [pg/mg] | 2.5 ± 0.2 | 3.1 ± 0.4 ns |
| MIP-2 [pg/mg] | 1.2 ± 0.3 | 1.3 ± 0.3 ns |
| TNF-α [pg/mg] | 2.6 ± 0.4 | 2.1 ± 0.3 ns |
| MCP-1[pg/mg] | 16.5 ± 1.2 | 23.8 ± 3.7⁎ |
Sprague–Dawley rats were administered normal water (control) or 20% EtOH-enriched water for 12 mon. Brains were removed and cortex extracts were analyzed with ELISAs for interleukin 1-beta (IL-1β), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), tumor necrosis factor-alpha (TNFα), tissue plasminogen activator (tPA), Aβ(1–40), Aβ(1–42), and nerve growth factor (NGF). EtOH levels were measured with an ethanol detection kit. Acetylcholine (ACh) levels were assessed by means of HPLC and electrochemical detection. Values are given as mean±SEM in [pM, mM, pg/mg tissue, or ng/mg tissue]. The number of animals per group, n=6; tPA, n=3; Statistical analysis was performed with a one-way ANOVA with a Fisher LSD post hoc test.
P<0.001;
P<0.05; ns, not significant; below DL, below detection limit.
Effects of EtOH on cortical markers
Cortical levels of NGF (the most potent cholinergic neuroprotective marker) showed no difference between EtOH-treated rats and controls (Table 2). ELISA measurements of Aβ(1–40) and Aβ(1–42) (the peptides that aggregate in AD) revealed no differences between EtOH-treated rats and controls (Table 2). EtOH did not affect the inflammatory cytokines interleukin 1-β (IL-1β) and tumor necrosis factor-alpha (TNF-α) or the chemokine macrophage inflammatory protein-2 (MIP-2) in the cortex (Table 2). However, MCP-1 (another inflammatory marker) and tPA (a general marker of degeneration) levels in the cortex were significantly increased in EtOH-treated animals (Table 2).
EtOH induces cortical BBB leakage and microglia activation
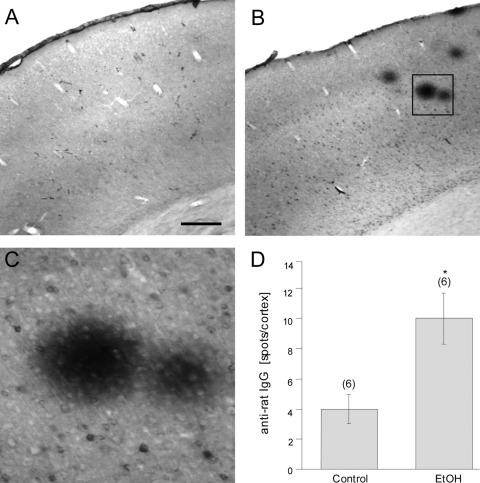
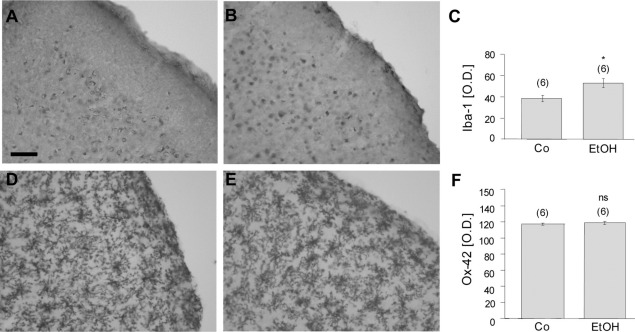
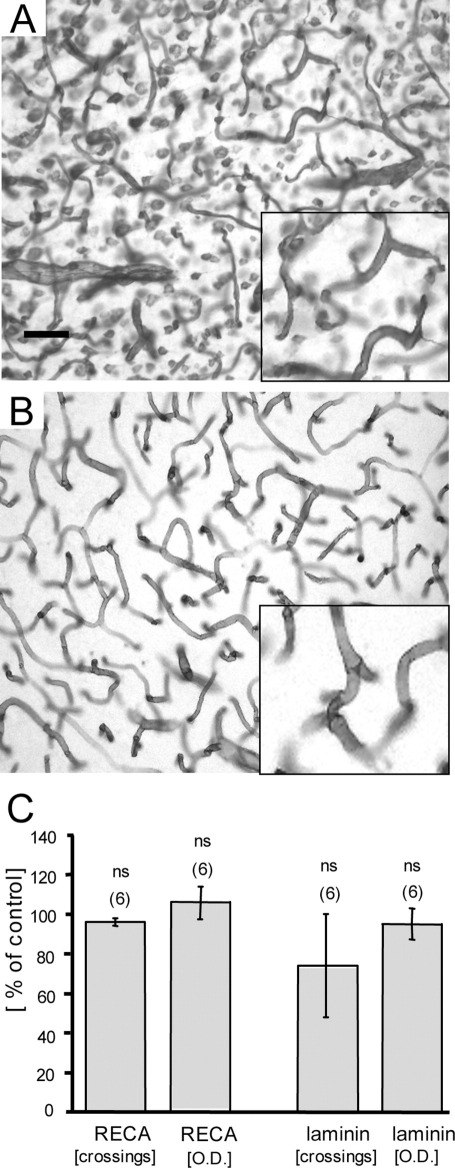
BBB leakage in the cortex was identified by immunohistochemistry against rat IgG in controls (Fig. 3A) and EtOH-treated animals (Fig. 3B, C). Quantitative analysis revealed a markedly increased number of IgG spots in the EtOH group as compared with controls (Fig. 3D). Microglia in cortex were stained for Iba-1 (Fig. 4A) and OX-42 (both of which label resting or activated microglia) (Fig. 4D) in controls (Fig. 4A, D) and EtOH-treated animals (Fig. 4B, E). Quantitative analysis showed a significantly enhanced optical density of Iba-1 immunoreactivity after EtOH treatment (Fig. 4C), whereas no changes in OX-42 density were detectable (Fig. 4F). Vascular structures in the cortex were stained for RECA-1 (Fig. 5A) and laminin (Fig. 5B), but no differences in the number of crossings per grid or in the optical density were seen after EtOH treatment (Fig. 5C).
Fig. 3.
Effect of long-term (12 months) ethanol (EtOH) treatment on anti-rat IgG staining in the cortex. Blood–brain barrier leakage in the cortex of controls (A) and EtOH-treated rats (B,C) was immunohistochemically visualized with anti-rat IgG staining. A magnification of anti-rat IgG-positive spots is shown in (C, D). The number of anti-rat IgG-positive spots in the cortex was markedly enhanced in EtOH-treated animals as compared with controls. Values are expressed as mean±SEM. Values in parenthesis give the number of experiments. Statistical analysis was performed with a one-way ANOVA with a Fisher LSD post hoc test. * P<0.05. Scale bar A=800 μm (A, B) and =360 μm (C).
Fig. 4.
Effect of long-term (12 months) ethanol (EtOH) treatment on cortical microglia Iba-1 and OX-42 stainings. Control (A) and EtOH-treated (B) animals were immunohistochemically stained for the microglial marker Iba-1. (C) Quantitative analysis revealed a significant enhancement of Iba-1-like immunoreactivity in the cortex of EtOH-treated rats. OX-42 density of controls (D) and EtOH-treated animals (E) was visualized by immunohistochemistry. Cortical OX-42 density was not affected by EtOH treatment (F). Values are expressed as mean±SEM. Values in parenthesis give the number of experiments. Statistical analysis was performed with Student's t-test, P<0.05; ns, not significant. Scale bar in A=200 μm (A, B, D, E).
Fig. 5.
Vascular structures in cortex after EtOH treatment for 12 mon. Brain sections were immunohistochemically stained for laminin (A) or RECA-1 (B). The inserts in (A, B) show a magnification. Note that in (A) laminin staining is also visible outside the blood vessels, possibly pointing to astrocytic staining. (C) Vascular structures were not seen to be affected by EtOH treatment, namely measurement of neither vascular crossings nor optical density (OD) showed an effect. Vascular density was assessed by densitometry and crossings were counted in 150×150 μm2 grids. Values are expressed as mean±SEM. Values in parenthesis give the number of experiments. Statistical analysis was performed with Student's t-test. ns, not significant. Scale bar in A=800 μm (A, B), and =1100 μm (inserts).
Effects of cholesterol (12 months)
The cholesterol group showed significantly increased weight as compared with the other groups (Table 3); plasma cholesterol levels (1.5±0.2 mg/ml) and plasma tPA levels were also elevated (Table 3). Spatial memory was impaired and performance by cholesterol-fed animals was poorer in session 5 and on retention in the maze (Table 3). Cortical IgG-positive spots and Aβ(1–40) content were significantly increased (Table 3). MCP-1, but no other inflammatory marker, was enhanced by the cholesterol treatment. The optical density of the vascular and extracellular matrix marker laminin was decreased, but the number of crossings of laminin-positive capillaries was increased (Table 3). The number of cholinergic neurons in the nbM was decreased as compared with controls (Table 3).
Table 3.
Effects of long-term (12 mon) ethanol (EtOH) and cholesterol (Chol) treatment
| Controls | EtOH | p1 | Chol | p2 | EtOH+Chol | p3 | p4 | |
|---|---|---|---|---|---|---|---|---|
| Weight | 100 ± 4 (12) | 89 ± 2 (12) | * | 112 ± 4 (12) | * | 96 ± 3 (12) | ns | ** |
| Maze | ||||||||
| Session 5 | 100 ± 4 (12) | 51 ± 6 (12) | *** | 57 ± 6 (9) | ** | 55 ± 3 (12) | ns | ns |
| Retention | 100 ± 5 (12) | 49 ± 3 (12) | *** | 51 ± 6 (9) | ** | 55 ± 4 (12) | ns | ns |
| WME | 100 ± 25 (12) | 252 ± 36 (12) | ** | 173 ± 48 (9) | ns | 267 ± 41 (12) | ns | ns |
| RME | 100 ± 25 (12) | 243 ± 32 (12) | ** | 133 ± 31 (9) | ns | 205 ± 18 (12) | ns | * |
| RWME | 100 ± 77 (12) | 700 ± 226 (12) | * | 200 ± 71 (9) | ns | 500 ± 107 (12) | ns | * |
| Visits | 100 ± 6 (12) | 136 ± 11 (12) | * | 97 ± 13 (9) | ns | 132 ± 7 (12) | ns | ** |
| Duration | 100 ± 9 (12) | 111 ± 4 (12) | ns | 113 ± 9 (9) | ns | 99 ± 8 (12) | ns | ns |
| Arm latency | 100 ± 12 (12) | 100 ± 13 (12) | ns | 181 ± 50 (9) | ns | 84 ± 16 (12) | ns | ns |
| Plasma | ||||||||
| EtOH | below DL (6) | 2500 ± 1040 (6) | *** | 89 ± 7 (3) | ns | 140 ± 20 (6) | * | ** |
| Cholesterol | 100 ± 14 (6) | 68 ± 8 (6) | ns | 149 ± 29 (3) | *** | 65 ± 16 (5) | ns | * |
| tPA | 100 ± 13 (3) | 106 ± 30 (3) | ns | 163 ± 21 (3) | * | 84 ± 40 (3) | ns | ns |
| Cortex | ||||||||
| Acetylcholine | 100 ± 21 (6) | 42 ± 21 (6) | * | 47 ± 11 (3) | * | 60 ± 19 (6) | ns | ns |
| NGF | 100 ± 7 (6) | 92 ± 17 (6) | ns | 133 ± 23 (3) | ns | 107 ± 14 (6) | ns | ns |
| tPA | 100 ± 9 (6) | 155 ± 10 (6) | *** | 110 ± 10 (3) | ns | 132 ± 10 (6) | ns | ns |
| IgG spots | 100 ± 29 (6) | 323 ± 55 (6) | * | 438 ± 85 (6) | ** | 329 ± 68 (6) | ns | ns |
| β-amyloid(1–40) | 100 ± 7 (4) | 106 ± 12 (4) | ns | 207 ± 8 (3) | * | 60 ± 4 (6) | ** | *** |
| β-amyloid(1–42) | 100 ± 2 (4) | 98 ± 13 (4) | ns | 224 ± 79 (3) | ns | 96 ± 2 (6) | ns | * |
| Interleukin-1 | 100 ± 8 (6) | 100 ± 36 (3) | ns | 124 ± 16 (6) | ns | 108 ± 12 (6) | ns | ns |
| MIP-2 | 100 ± 25 (6) | 49 ± 16 (3) | ns | 108 ± 25 (6) | ns | 108 ± 25 (6) | ns | ns |
| TNF-α | 100 ± 15 (6) | 73 ± 19 (3) | ns | 81 ± 12 (6) | ns | 69 ± 15 (6) | ns | ns |
| MCP-1 | 100 ± 7 (6) | 143 ± 21 (3) | * | 144 ± 22 (6) | * | 109 ± 7 (6) | ns | ns |
| RECA −1[OD] | 100 ± 10 (6) | 106 ± 8 (6) | ns | 114 ± 6 (6) | ns | 133 ± 6 (6) | * | * |
| RECA-1 [cross] | 100 ± 5 (6) | 96 ± 2 (6) | ns | 94 ± 2 (6) | ns | 89 ± 4 (6) | ns | ns |
| Laminin [OD] | 100 ± 3 (6) | 95 ± 8 (6) | ns | 79 ± 3 (6) | ** | 90 ± 8 (6) | ns | ns |
| Laminin [cross] | 100 ± 9 (6) | 74 ± 10 (6) | ns | 120 ± 3 (6) | * | 77 ± 11 (6) | ns | ** |
| nbM | ||||||||
| ChAT+neurons | 100 ± 6 (6) | 83 ± 8 (6) | ns | 82 ± 4 (6) | * | 98 ± 7 (6) | ns | ns |
Sprague–Dawley rats were administered normal food and drinking water (controls) or 20% v/v EtOH in drinking water (EtOH) or a 5% cholesterol-enriched diet (Chol) or a combination of 20% EtOH v/v in drinking water and a cholesterol-enriched diet (EtOH+Chol) for 12 mon. All values are given as mean±SEM% of control. Absolute raw values of controls see Tables 1 and 2. Statistical analysis was performed with a one-way ANOVA with a Fisher LSD post hoc test. p1 compares EtOH and controls; p2 cholesterol and controls; p3 mixed and EtOH; p4 mixed and cholesterol (* P<0.05; ** P<0.01; *** P<0.001). Abbreviations: WME, working memory error; RME, reference memory error; RWME, reference working memory error; DL, detection limit; NGF, nerve growth factor; tPA, tissue plasminogen activator; MIP-2, macrophage inflammatory protein-2; TNF-α, tumor necrosis factor-alpha; MIP-1, monocyte-chemotactic protein-1; OD, optical density; ChAT, choline-acetyltransferase; cross, crossings; ns, not significant; nbM, nucleus basalis of Meynert.
Effects of combined EtOH and hypercholesterolemia
When comparing combined EtOH and cholesterol treatment (mix) with EtOH-only treatment plasma EtOH levels were significantly decreased, Aβ(1–40) content markedly declined, and RECA-1 optical density significantly increased, whereas all other measured parameters were not affected (Table 3). When comparing combined EtOH and cholesterol treatment (mix) with cholesterol-only treatment the animal weight was significantly decreased, the RME and RWME were significantly increased, and visits to the maze were significantly increased, plasma EtOH levels were enhanced, plasma cholesterol levels were decreased, cortical Aβ(1–40) and Aβ(1–42) content was decreased and RECA-1 optical density was increased. Although laminin-positive crossings were decreased, no other measured parameters were affected (Table 3).
Discussion
The present study shows that long-term (12 months) EtOH treatment (similar to hypercholesterolemia) induced spatial learning impairment, a decline in cholinergic nbM neurons, cortical BBB leakage, and partly increased inflammation. Combined treatment with EtOH and cholesterol did not potentiate the EtOH-induced effects, but instead EtOH partly counteracted the cholesterol-associated effects.
EtOH consumption has emerged as an important risk factor for cerebrovascular diseases, such as stroke (Monforte et al., 1990; Hillbom and Kaste, 1981) or vascular dementia (Mostofsky et al., 2010), whereas there is increasing evidence that chronic EtOH does not affect development of AD, but instead is protective. EtOH readily passes the BBB. Thus, brain and blood levels rapidly equilibrate after EtOH consumption (Diamond and Messing, 1994). Intoxication in non-alcoholics starts at 50–150 mg/dl (Goldstein et al., 1983) and levels of 500 mg/dl can be fatal for non-drinkers (Diamond and Messing, 1994). In the present study we administered 20% EtOH in drinking water to rats, resulting in plasma EtOH levels of 57.7±23.3 mg/dl, which is in line with others (Arendt et al., 1995) and correlates with low to moderate EtOH consumption in humans. Body weight was markedly decreased in EtOH-treated animals, which most likely results from the malnutritive effect of EtOH, which may also play a role in neurodegeneration and cognitive decline (Mendenhall et al., 1993; Zahr et al., 2011). In EtOH research isocaloric diet or pair feeding is commonly used to eliminate effects owing to reduced food intake during EtOH treatment. This treatment has not been performed in the present study due to long-term treatment over 12 months, thus we cannot exclude that some of the effects (e.g. weight reduction) may occur because of reduced food or liquid uptake rather than to the EtOH-exposure.
Evaluation of spatial learning and memory testing was performed in the partially baited eight-arm maze. Our study revealed markedly impaired spatial learning skills and memory deficits in rats after EtOH treatment, which is fully in line with others (Irle and Markowitsch, 1983; Arendt et al., 1988; Beracochea et al., 1992; Lukoyanov et al., 2003). Three weeks after a 5-day training period, long-term memory was assessed in the retention test, and performance by EtOH-treated animals was significantly poorer than in controls. It needs to be noted that animals were not withdrawn from ethanol during the whole testing, but had still access to ethanol ad libitum. Thus, as rats were habituated for 12 months for EtOH exposure, we can exclude any acute effects of EtOH on the spatial memory testing during the maze.
Our data obtained during the retention test show a significant increase in working memory errors but also in reference memory errors, as well as in the number of visits in EtOH-treated rats, thus pointing to a decreased long-term memory. The mechanism of EtOH-induced impairment of cognitive function is not well understood. However, it has been reported that EtOH interacts with various signal transduction cascades (Ku et al., 2007; Aroor and Shukla, 2004), ion channels (Allgaier, 2002; Crews et al., 1996), second messengers (Deng and Deitrich, 2007), neurotransmitters (Foddai et al., 2004; Jamal et al., 2007), and their receptors (Diamond and Messing, 1994).
It is well established that EtOH impairs the cholinergic system, which is closely associated with learning and memory (McKinney, 2005; Olton, 1983; Kentroti and Vernadakis, 1996; Costa and Guizzetti, 1999; Jamal et al., 2009; Casamenti et al., 1982; Arendt et al., 1988; Arendt, 1994). Cholinergic neurons of the basal forebrain nbM are most vulnerable to age-related neurodegeneration (Sassin et al., 2000), and a significant reduction in cholinergic basal forebrain neurons has been reported for AD (Mesulam, 2010). In order to test the effect of EtOH treatment on the cholinergic system, we investigated the ACh-synthesizing enzyme ChAT and the NGF receptor p75NTR on cholinergic neurons in the nbM; both serve as well-known markers for cholinergic neurons (Oda, 1999). Our data reveal a markedly decreased number of ChAT- and p75 NTR-positive cholinergic neurons in the nbM. This was accompanied by decreased ACh levels in the cortex, and it is well established that a loss of the neurotransmitter ACh directly correlates with cognitive decline in AD (Mufson et al., 2005; Mesulam, 2010; Bowen et al., 1976). It has been reported that approximately 95% of p75NTR-positive neurons co-localize with ChAT-positive neurons in the nbM (Mufson et al., 2003). Thus, our data clearly show that a small population of ChAT- or p75NTR-positive nbM neurons was reduced after chronic EtOH treatment, and it is likely that these neurons degenerated because both markers declined at the same time.
NGF and its high-(trkA) and low-affinity (p75NTR) receptors play an important role in survival of cholinergic basal forebrain neurons. In AD a loss of NGF in the basal forebrain and high NGF levels in the cortex have been reported (Mufson et al., 2008). NGF accumulates in target regions, such as the cortex and hippocampus, because of damage of the retrograde transport mechanism (Schindowski et al., 2008). In addition, it has been reported that NGF is able to counteract EtOH-induced deficits in the cholinergic system (Lukoyanov et al., 2003; Aloe and Tirassa, 1992; Paula-Barbosa et al., 2001) as well as in cultured cerebral cortical (Mooney and Miller, 2007), hippocampal (Webb et al., 1997), and cerebellar neurons (Luo et al., 1997). Our data show that after 12 months of EtOH treatment NGF levels in the cortex did not differ from those of controls, which is in line with others (Baek et al., 1994). It seems possible that NGF may be increased at earlier time points of EtOH treatment to counteract cholinergic cell loss, as suggested by Nakano et al. (1996).
It is well known that EtOH is a cerebrovascular risk factor and may also contribute to impairment of the cholinergic system by inducing oxidative stress (Deng and Deitrich, 2007; Toda and Ayajiki, 2010; Das and Vasudevan, 2007; Haorah et al., 2005; Nordmann et al., 1990). Indeed, it has been reported that EtOH causes BBB disruption with enhanced monocyte migration into the brain (Haorah et al., 2005). In the present study macrovascular structures in the cortex were stained for the well-established vessel markers laminin and RECA-1, but no differences were seen between EtOH-treated rats and controls. However, when brains were stained for anti-rat IgG a large number of IgG-positive spots was found in EtOH-treated rats as compared with controls. These results show increased influx of rat IgG into the brain, indicating BBB breakdown, similar to our report on results following hyperhomocysteinemia (Pirchl et al., 2010) or hypercholesterolemia (Ullrich et al., 2010) in vivo in rats.
As an additional indication for a BBB breakdown we measured tPA, which is a serin protease that is predominantly found in blood and plays a role in thrombolysis. We found that tPA levels were markedly increased in the cortex, suggesting influx from blood after BBB breakdown. This agrees with others showing an increase in tPA levels after chronic EtOH treatment followed by EtOH withdrawal (Skrzypiec et al., 2009). Interestingly, tPA is also implicated in the development of fetal alcohol syndrome in mice, and tPA-deficient mice were protected from EtOH-induced neurotoxicity (Noel et al., 2011). However, tPA is also expressed by neurons and astrocytes and changes may also reflect changes in the expression/activity of tPA in these cells and not in BBB permeability. tPA activates plasminogen into plasmin, which in turn degrades laminin (Benarroch, 2007). Indeed, EtOH-induced tPA upregulation increases laminin degradation and reduces levels of laminin in the hippocampus (Skrzypiec et al., 2009). Such a tPA-induced degradation of laminin may be involved in neuronal plasticity including learning and memory (Laifenfeld et al., 2005). In our study we measured laminin as a vascular marker, but could not detect any changes in its vascular structure. However, laminin is also expressed by astrocytes, and we cannot exclude that the increase in tPA is accompanied by a decrease in astrocyte-released laminin.
Inflammation and microglia activation is a well-known pathology in AD as well as in EtOH-induced neurodegeneration (Kaur et al., 1995), prompting secretion of different pro-inflammatory mediators, such as TNF-α or MCP-1 (Qin et al., 2007, 2008). Our data demonstrate that long-term EtOH treatment significantly enhanced cortical MCP-1 levels, whereas levels of other inflammatory markers, such as IL-1β, MIP-2, or TNF-α, were not affected by EtOH treatment. The cytokine MCP-1 has been linked to inflammation, where it mediates migration and activation of microglia (McManus et al., 2000), most likely by raising the permeability of the BBB (Yamamoto et al., 2005; Stamatovic et al., 2003, 2005). In addition, it has been suggested that MCP-1 takes part in EtOH drinking behavior (Blednov et al., 2005). In fact, MCP-1 knockout mice demonstrate markedly reduced EtOH consumption and preference (Blednov et al., 2005). Furthermore, the level of MCP-1 in brains of EtOH-dependent individuals is significantly higher than in non-drinkers, which was investigated in postmortem human brain studies (He and Crews, 2008). Enhancement of MCP-1 without changes in other cytokines such as IL-1β, MIP-2, or TNF-α was also reported by others (Little et al., 2002), suggesting that MCP-1 may take part in a signaling cascade induced by EtOH treatment that differs from its role as a pro-inflammatory cytokine. MCP-1 in the brain is secreted by activated microglia (Kaul et al., 2001), and indeed our data show microglial activation in the cortex of EtOH-treated animals. Microglia are activated in inflamed brains (Kaur et al., 1995) and in the present study microglia reactivity was investigated by the well-established surface markers Iba-1 and OX-42, which label resting as well as activated microglia. The antibody OX-42 recognizes CD11b that serves as a receptor for complement (Wagner et al., 2011). We were able to show upregulation of Iba-1, but not OX-42, which indicates that the activation stages of microglia may reflect a distinct expression pattern of the surface markers (Ji et al., 2007).
We recently well established and characterized the effects of 5 months of hypercholesterolemia in rats in vivo (Ullrich et al., 2010). We showed that a 5% cholesterol diet markedly reduced spatial learning, cholinergic neurons, induced inflammation, and BBB leakage in the cortex (Ullrich et al., 2010). Interestingly, chronic EtOH or cholesterol treatment for 12 months had similar effects in vivo, although not as pronounced as the 5-month cholesterol effects. Interestingly, long-term treatment of cholesterol did not result in dramatic inflammation and changes were not as pronounced, possibly because of adaptive and compensatory mechanisms. This is in agreement with Pirchl et al. (2010), who reported that short-term (5 months) treatment with the vascular risk factor homocysteine had more severe effects than did long-term (15 months) homocysteine treatment. In future studies it will be interesting to explore whether short-term EtOH treatment induces stronger damage than long-term treatment.
The present study also aimed to observe a combined effect of EtOH and cholesterol on cognition, inflammation, cholinergic neurons, and vascular structures. It was interesting to see that the combination of EtOH and cholesterol did not dramatically affect the changes in spatial memory, cholinergic neurons, inflammation, and vascularity. In fact, our data show that EtOH rather counteracted some of the cholesterol-induced effects: it reduced weight, plasma cholesterol levels, and cortical Aβ(1–40) and Aβ(1–42) content. The Aβ-peptides are processed by secretases from APP and are deposited in the brain (neuritic plaques) or in vessels (angiopathy) of AD patients (Hardy and Selkoe, 2002). The longer Aβ(1–42) oligomer is the predominant form found in amyloid brain plaques (Querfurth and LaFerla, 2010). Accumulation of Aβ leads to damage of synapses and an abnormal phosphorylation of tau (Lacor et al., 2007). Interestingly, it has recently been reported that EtOH protected against Aβ-induced synapse damage in cultured neurons (Bate and Williams, 2011). This is in line with our present study where a protective effect of EtOH-induced beta-amyloid has been suggested.
In the maze EtOH enhanced the number of visits and thus increased RME and WRME, suggesting increased activity as compared with the cholesterol group, possibly because of reduced weight. The effect of EtOH on cholesterol-induced vascular changes is divergent, because it enhanced RECA-1 optical density but decreased laminin-positive crossings. This may indicate a loss of vessel integrity and enhanced vascular shaping. Some of the effects of EtOH may also be modulated by its antiproliferative effect on astrocytes (Costa et al., 2004) or maintenance of calcium homeostasis (Catlin et al., 1999). Our data also show that cholesterol did not markedly affect the EtOH-induced changes. There was a prominent reduction in plasma EtOH induced by cholesterol. This may indicate that cholesterol modulates the absorption of EtOH into the blood. Indeed, retention of EtOH in the stomach is prolonged after cholesterol-rich nutrition (Gentry, 2000). In addition, cholesterol decreased the cortical Aβ(1–40), but not Aβ(1–42) levels, and enhanced RECA-1 optical density, which may point to an effect on the vascular system and a possibly altered Aβ(1–40) clearance at the BBB.
In summary, long-term EtOH or cholesterol treatment (12 months) markedly impaired the cholinergic nbM neurons, reduced spatial learning and induced cortical BBB leakage accompanied by partly increased inflammation. The combined treatment of rats with EtOH and cholesterol for 12 months did not potentiate treatment with either EtOH or cholesterol alone, but instead counteracted some of the cholesterol- or EtOH-associated effects. In conclusion, the vascular risk factor EtOH may markedly contribute to some pathologies that are seen in, for example, vascular dementia but not in AD.
Acknowledgments
This study was supported by the Austrian Science Fund (P191220-B05 and L429-B05). We thank Ursula Kirzenberger-Winkler for her excellent technical assistance.
References
- Aho L., Karkola K., Juusela J., Alafuzoff I. Heavy alcohol consumption and neuropathological lesions: a post-mortem human study. J Neurosci Res. 2009;87:2786–2792. doi: 10.1002/jnr.22091. [DOI] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Aloe L., Tirassa P. The effect of long-term alcohol intake on brain NGF-target cells of aged rats. Alcohol. 1992;9:299–304. doi: 10.1016/0741-8329(92)90070-q. [DOI] [PubMed] [Google Scholar]
- Anstey K.J., Mack H.A., Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17:542–555. doi: 10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–187. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- Arendt T., Brückner M.K., Krell T., Pagliusi S., Kruska L., Heumann R. Degeneration of rat cholinergic basal forebrain neurons and reactive changes in nerve growth factor expression after chronic neurotoxic injury—II: Reactive expression of the nerve growth factor gene in astrocytes. Neuroscience. 1995;65:647–659. doi: 10.1016/0306-4522(94)00523-8. [DOI] [PubMed] [Google Scholar]
- Arendt T., Henning D., Gray J.A., Marchbanks R. Loss of neurons in the rat basal forebrain cholinergic projection system after prolonged intake of ethanol. Brain Res Bull. 1988;21:563–569. doi: 10.1016/0361-9230(88)90193-1. [DOI] [PubMed] [Google Scholar]
- Aroor A.R., Shukla S.D. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;26:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Baek J.K., Heaton M.B., Walker D.W. Chronic alcohol ingestion: nerve growth factor gene expression and neurotrophic activity in rat hippocampus. Alcohol Clin Exp Res. 1994;18:1368–1376. doi: 10.1111/j.1530-0277.1994.tb01438.x. [DOI] [PubMed] [Google Scholar]
- Bate C., Williams A. Ethanol protects cultured neurons against amyloid-β and α-synuclein-induced synapse damage. Neuropharmacology. 2011;61:1406–1412. doi: 10.1016/j.neuropharm.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Bell R.D., Zlokovic B.V. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. Tissue plasminogen activator: beyond thrombolysis. Neurology. 2007;69:799–802. doi: 10.1212/01.wnl.0000269668.08747.78. [DOI] [PubMed] [Google Scholar]
- Beracochea D., Micheau J., Jaffard R. Memory deficits following chronic alcohol consumption in mice: relationships with hippocampal and cortical cholinergic activities. Pharmacol Biochem Behav. 1992;42:749–753. doi: 10.1016/0091-3057(92)90024-a. [DOI] [PubMed] [Google Scholar]
- Blednov Y.A., Bergeson S.E., Walker D., Ferreira V.M., Kuziel W.A., Harris R.A. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen D.M., Smith C.B., White P., Davison A.N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Casamenti F., Corradetti R., Löffelholz K., Mantovani P., Pepeu G. Effects of 4-aminopyridine on acetylcholine output from the cerebral cortex of the rat in vivo. Br J Pharmacol. 1982;76:439–445. doi: 10.1111/j.1476-5381.1982.tb09237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin M.C., Guizzetti M., Costa L.G. Effects of ethanol on calcium homeostasis in the nervous system: implications for astrocytes. Mol Neurobiol. 1999;19:1–24. doi: 10.1007/BF02741375. [DOI] [PubMed] [Google Scholar]
- Charness M.E. Brain lesions in alcoholics. Alcohol Clin Exp Res. 1993;17:2–11. doi: 10.1111/j.1530-0277.1993.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Costa L.G., Guizzetti M. Muscarinic cholinergic receptor signal transduction as a potential target for the developmental neurotoxicity of ethanol. Biochem Pharmacol. 1999;57:721–726. doi: 10.1016/s0006-2952(98)00278-0. [DOI] [PubMed] [Google Scholar]
- Costa L.G., Vitalone A., Guizzetti M. Signal transduction mechanisms involved in the antiproliferative effects of ethanol in glial cells. Toxicol Lett. 2004;149:67–73. doi: 10.1016/j.toxlet.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Crews F.T., Morrow A.L., Criswell H., Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Crews F.T., Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.K., Vasudevan D.M. Alcohol-induced oxidative stress. Life Sci. 2007;81:177–187. doi: 10.1016/j.lfs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Deane R., Zlokovic B.V. Role of the blood–brain barrier in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- Deng X.S., Deitrich R.A. Ethanol metabolism and effects: nitric oxide and its interaction. Curr Clin Pharmacol. 2007;2:145–153. doi: 10.2174/157488407780598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I., Messing R.O. Neurologic effects of alcoholism. West J Med. 1994;161:279–287. [PMC free article] [PubMed] [Google Scholar]
- Floyd E.A., Young-Seigler A.C., Ford B.D., Reasor J.D., Moore E.L., Townsel J.G., Rucker H.K. Chronic ethanol ingestion produces cholinergic hypofunction in rat brain. Alcohol. 1997;14:93–98. doi: 10.1016/s0741-8329(97)86147-2. [DOI] [PubMed] [Google Scholar]
- Foddai M., Dosia G., Spiga S., Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Gentry T.R. Effect of food on the pharmacokinetics of alcohol absorption. Alcohol Clin Exp Res. 2000;24:403–404. [PubMed] [Google Scholar]
- Goldstein G., Tarter R.E., Shelly C., Alterman A.I., Petrarulo E. Withdrawal seizures in black and white alcoholic patients: intellectual and neuropsychological sequelae. Drug Alcohol Depend. 1983;12:349–354. doi: 10.1016/0376-8716(83)90006-6. [DOI] [PubMed] [Google Scholar]
- Graves A.B., White E., Koepsell T.D., Reifler B.V., van Belle G., Larson E.B., Raskind M. A case-control study of Alzheimer's disease. Ann Neurol. 1990;28:766–774. doi: 10.1002/ana.410280607. [DOI] [PubMed] [Google Scholar]
- Guizzetti M., Costa L.G. Cholesterol homeostasis in the developing brain: a possible new target for ethanol. Hum Exp Toxicol. 2007;26:355–360. doi: 10.1177/0960327107078412. [DOI] [PubMed] [Google Scholar]
- Haorah J., Knipe B., Leibhart J., Ghorpade A., Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood–brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- He J., Crews F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillbom M., Kaste M. Ethanol intoxication: a risk factor for ischemic brain infarction in adolescents and young adults. Stroke. 1981;12:422–425. doi: 10.1161/01.str.12.4.422. [DOI] [PubMed] [Google Scholar]
- Humpel C. Chronic mild cerebrovascular dysfunction as a cause for Alzheimer's disease? Exp Gerontol. 2011;46:225–232. doi: 10.1016/j.exger.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C., Weis C. Nerve growth factor and cholinergic CNS neurons studied in organotypic brain slices: Implication in Alzheimer's disease? J Neural Transm Suppl. 2002;62:253–263. doi: 10.1007/978-3-7091-6139-5_23. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Irle E., Markowitsch H.J. Widespread neuroanatomical damage and learning deficits following chronic alcohol consumption or vitamin-B1 (thiamine) deficiency in rats. Behav Brain Res. 1983;9:277–294. doi: 10.1016/0166-4328(83)90133-x. [DOI] [PubMed] [Google Scholar]
- Jamal M., Ameno K., Ameno S., Morishita J., Wang W., Kumihashi M., Ikuo U., Miki T., Ijiri I. Changes in cholinergic function in the frontal cortex and hippocampus of rat exposed to ethanol and acetaldehyde. Neuroscience. 2007;144:232–238. doi: 10.1016/j.neuroscience.2006.08.066. [DOI] [PubMed] [Google Scholar]
- Jamal M., Ameno K., Miki T., Wang W., Kumihashi M., Isse T., Kawamoto T., Kitagawa K., Nakayama K., Ijiri I., Kinoshita H. Cholinergic alterations following alcohol exposure in the frontal cortex of Aldh2-deficient mice models. Brain Res. 2009;1295:37–43. doi: 10.1016/j.brainres.2009.07.099. [DOI] [PubMed] [Google Scholar]
- Jarrard L.E., Okaichi H., Steward O., Goldschmidt R.B. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98:946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Ji K.A., Yang M.S., Jeong H.K., Min K.J., Kang S.H., Jou I., Joe E.H. Resident microglia die and infiltrated neutrophils and monocytes become major inflammatory cells in lipopolysaccharide-injected brain. Glia. 2007;55:1577–1588. doi: 10.1002/glia.20571. [DOI] [PubMed] [Google Scholar]
- Kaul M., Garden G.A., Lipton S.A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaur C., Singh J., Lim M.K., Ng B.L., Yap E.P., Ling E.A. The response of neurons and microglia to blast injury in the rat brain. Neuropathol Appl Neurobiol. 1995;21:369–377. doi: 10.1111/j.1365-2990.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Kentroti S., Vernadakis A. Ethanol neurotoxicity in culture: selective loss of cholinergic neurons. J Neurosci Res. 1996;44:577–585. doi: 10.1002/(SICI)1097-4547(19960615)44:6<577::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kim S.R., Jeong H.Y., Yang S., Choi S.P., Seo M.Y., Yun Y.K., Choi Y., Baik S.H., Park J.S., Gwon A.R., Yang D.K., Lee C.H., Lee S.M., Park K.W., Jo D.G. Effects of chronic alcohol consumption on expression levels of APP and Aβ-producing enzymes. BMB Rep. 2011;44:135–139. doi: 10.5483/BMBRep.2011.44.2.135. [DOI] [PubMed] [Google Scholar]
- Ku B.M., Lee Y.K., Jeong J.Y., Mun J., Han J.Y., Roh G.S., Kim H.J., Cho G.J., Choi W.S., Yi G.S., Kang S.S. Ethanol-induced oxidative stress is mediated by p38 MAPK pathway in mouse hippocampal cells. Neurosci Lett. 2007;23:64–67. doi: 10.1016/j.neulet.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Lacor P.N., Buniel M.C., Furlow P.W., Sanz Clemente A.S., Velasco P.T., Wood M., Viola K.L., Klein W.L. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D.K., Nall C., Chen D., Zaphiriou M., Morgan C., Nurnberger J.I. Developmental expression of the beta-amyloid precursor protein and heat-shock protein 70 in the cerebral hemisphere region of the rat brain. Ann N Y Acad Sci. 2002;965:324–333. doi: 10.1111/j.1749-6632.2002.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Laifenfeld D., Karry R., Grauer E., Klein E., Ben-Shachar D. Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol Dis. 2005;20:432–441. doi: 10.1016/j.nbd.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Little A.R., Benkovic S.A., Miller D.B., O'Callaghan J.P. Chemically induced neuronal damage and gliosis: enhanced expression of the proinflammatory chemokine, monocyte chemoattractant protein (MCP)-1, without a corresponding increase in proinflammatory cytokines. Neuroscience. 2002;115:307–320. doi: 10.1016/s0306-4522(02)00359-7. [DOI] [PubMed] [Google Scholar]
- Lukoyanov N.V., Pereira P.A., Paula-Barbosa M.M., Cadete-Leite A. Nerve growth factor improves spatial learning and restores hippocampal cholinergic fibers in rats withdrawn from chronic treatment with ethanol. Exp Brain Res. 2003;148:88–94. doi: 10.1007/s00221-002-1290-7. [DOI] [PubMed] [Google Scholar]
- Luo J., West J.R., Pantazis N.J. Nerve growth factor and basic fibroblast growth factor protect rat cerebellar granule cells in culture against ethanol-induced cell death. Alcohol Clin Exp Res. 1997;21:1108–1120. [PubMed] [Google Scholar]
- Marksteiner J., Humpel C. Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol Psychiatry. 2008;13:939–952. doi: 10.1038/sj.mp.4002072. [DOI] [PubMed] [Google Scholar]
- McKinney M. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem Pharmacol. 2005;70:1115–1124. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- McManus C.M., Liu J.S., Hahn M.T., Hua L.L., Brosnan C.F., Berman J.W., Lee S.C. Differential induction of chemokines in human microglia by type I and II interferons. Glia. 2000;29:273–280. [PubMed] [Google Scholar]
- Mendenhall C.L., Rouster S.D., Roselle G.A., Grossman C.J., Ghosn S., Gartside P. Impact of chronic alcoholism on the aging rat: changes in nutrition, liver composition, and mortality. Alcohol Clin Exp Res. 1993;17(4):847–853. doi: 10.1111/j.1530-0277.1993.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2010;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Monforte R., Estruch R., Graus F., Nicolas J.M., Urbano-Marquez A. High ethanol consumption as risk factor for intracerebral hemorrhage in young and middle-aged people. Stroke. 1990;21:1529–1532. doi: 10.1161/01.str.21.11.1529. [DOI] [PubMed] [Google Scholar]
- Mooney S.M., Miller M.W. Nerve growth factor neuroprotection of ethanol-induced neuronal death in rat cerebral cortex is age dependent. Neuroscience. 2007;149:372–381. doi: 10.1016/j.neuroscience.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky E., Burger M.R., Schlaug G., Mukamal K.J., Rosamond W.D., Mittleman M.A. Alcohol and acute ischemic stroke onset: the stroke onset study. Stroke. 2010;41:1845–1849. doi: 10.1161/STROKEAHA.110.580092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson E.J., Conner J.M., Kordower J.H. Nerve growth factor in Alzheimer's disease: defective retrograde transport to nucleus basalis. Neuroreport. 1995;6:1063–1066. doi: 10.1097/00001756-199505090-00028. [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Counts S.E., Perez S.E., Binder L. Galanin plasticity in the cholinergic basal forebrain in Alzheimer's disease and transgenic mice. Neuropeptides. 2005;39:233–237. doi: 10.1016/j.npep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Counts S.E., Perez S.E., Ginsberg S.D. Cholinergic system during the progression of Alzheimer's disease: therapeutic implications. Expert Rev Neurother. 2008;8:1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson E.J., Ginsberg S.D., Ikonomovic M.D., DeKosky S.T. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Nakano T., Fujimoto T., Shimooki S., Fukudome T., Uchida T., Tsuji T., Mitsuyama Y., Akimoto H., Furukawa S. Transient elevation of nerve growth factor content in the rat hippocampus and frontal cortex by chronic ethanol treatment. Psychiatry Clin Neurosci. 1996;50:157–160. doi: 10.1111/j.1440-1819.1996.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Noel M., Norris E.H., Strickland S. Tissue plasminogen activator is required for the development of fetal alcohol syndrome in mice. Proc Natl Acad Sci U S A. 2011;108:5069–5074. doi: 10.1073/pnas.1017608108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann R., Ribière C., Rouach H. Ethanol-induced lipid peroxidation and oxidative stress in extrahepatic tissues. Alcohol Alcohol. 1990;25:231–237. doi: 10.1093/oxfordjournals.alcalc.a044996. [DOI] [PubMed] [Google Scholar]
- Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- Olton D.S. The use of animal models to evaluate the effects of neurotoxins on cognitive processes. Neurobehav Toxicol Teratol. 1983;5:635–640. [PubMed] [Google Scholar]
- Paula-Barbosa M.M., Silva S.M., Andrade J.P., Cadete-Leite A., Madeira M.D. Nerve growth factor restores mRNA levels and the expression of neuropeptides in the suprachiasmatic nucleus of rats submitted to chronic ethanol treatment and withdrawal. J Neurocytol. 2001;30:195–207. doi: 10.1023/a:1012745606781. [DOI] [PubMed] [Google Scholar]
- Paula-Barbosa M.M., Tavares M.A. Neuritic plaque-like structures in the rat cerebellum following prolonged alcohol consumption. Experientia. 1984;40:110–112. doi: 10.1007/BF01959131. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. 2nd ed. Academic Press; New York: 1986. The rat brain in stereotaxic coordinates. [Google Scholar]
- Pirchl M., Ullrich C., Humpel C. Differential effects of short- and long-term hyperhomocysteinaemia on cholinergic neurons, spatial memory and microbleedings in vivo in rats. Eur J Neurosci. 2010;32:1516–1527. doi: 10.1111/j.1460-9568.2010.07434.x. [DOI] [PubMed] [Google Scholar]
- Qin L., He J., Hanes R.N., Pluzarev O., Hong J.S., Crews F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;18:5–10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;255:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth H.W., LaFerla F.M. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rosen J., Colantonio A., Becker J.T., Lopez O.L., DeKosky S.T., Moss H.B. Effects of a history of heavy alcohol consumption on Alzheimer's disease. Br J Psychiatry. 1993;163:358–363. doi: 10.1192/bjp.163.3.358. [DOI] [PubMed] [Google Scholar]
- Salehi A., Kleshevnikov A., Mobley W.C. Pharmacological Mechanisms in Alzheimer's Therapeutics. Springer; New York: 2007. Cholinergic neurodegeneration in Alzheimer's disease: basis for nerve growth factor therapy; pp. 64–104. [Google Scholar]
- Sassin I., Schultz C., Thal D.R., Rüb U., Arai K., Braak E., Braak H. Evolution of Alzheimer's disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- Schindowski K., Belarbi K., Buée L. Neurotrophic factors in Alzheimer's disease: role of axonal transport. Genes Brain Behav. 2008;1:43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S.A., Mufson E.J., Weingartner J.A., Skau K.A., Crutcher K.A. Nerve growth factor in Alzheimer's disease: increased levels throughout the brain coupled with declines in nucleus basalis. J Neurosci. 1995;9:6213–6221. doi: 10.1523/JNEUROSCI.15-09-06213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypiec A.E., Maiya R., Chen Z., Pawlak R., Strickland S. Plasmin-mediated degradation of laminin gamma-1 is critical for ethanol-induced neurodegeneration. Biol Psychiatry. 2009;66:785–794. doi: 10.1016/j.biopsych.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic S.M., Keep R.F., Kunkel S.L., Andjelkovic A.V. Potential role of MCP-1 in endothelial cell tight junction opening: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- Stamatovic S.M., Shakui P., Keep R.F., Moore B.B., Kunkel S.L., Van Rooijen N., Andjelkovic A.V. Monocyte chemoattractant protein-1 regulation of blood–brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Sun L., Wang X., Liu S., Wang Q., Wang J., Bennecib M., Gong C.X., Sengupta A., Grundke-Iqbal I., Iqbal K. Bilateral injection of isoproterenol into hippocampus induces Alzheimer-like hyperphosphorylation of tau and spatial memory deficit in rat. FEBS Lett. 2005;579:251–258. doi: 10.1016/j.febslet.2004.11.083. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Asada T., Kinoshita T., Yamashita F., Uno M. Alcohol consumption and risk of dementia. Lancet. 2002;360:491. doi: 10.1016/S0140-6736(02)09655-1. [DOI] [PubMed] [Google Scholar]
- Toda N., Ayajiki K. Vascular actions of nitric oxide as affected by exposure to alcohol. Alcohol Alcohol. 2010;45:347–355. doi: 10.1093/alcalc/agq028. [DOI] [PubMed] [Google Scholar]
- Tyas S.L. Are tobacco and alcohol use related to Alzheimer's disease?: A critical assessment of the evidence and its implications. Addict Biol. 1996;1:237–254. doi: 10.1080/1355621961000124856. [DOI] [PubMed] [Google Scholar]
- Ullrich C., Pirchl M., Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci. 2010;45:408–417. doi: 10.1016/j.mcn.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C., Hänsch G.M., Stegmaier S., Denefleh B., Hug F., Schoels M. The complement receptor 3, CR3 (CD11b/CD18), on T lymphocytes: activation-dependent up-regulation and regulatory function. Eur J Immunol. 2001;31:1173–1180. doi: 10.1002/1521-4141(200104)31:4<1173::aid-immu1173>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Webb B., Suarez S.S., Heaton M.B., Walker D.W. Cultured postnatal rat septohippocampal neurons change intracellular calcium in response to ethanol and nerve growth factor. Brain Res. 1997;778:354–366. doi: 10.1016/s0006-8993(97)01088-3. [DOI] [PubMed] [Google Scholar]
- Webb L.E., Lengle E., Izzo R., Woods E. Micromethod for lipid-chromatographic determination of cholesterol in lipemic sera. Clin Chem. 1982;28:1769–1772. [PubMed] [Google Scholar]
- Yamamoto M., Horiba M., Buescher J.L., Huang D., Gendelman H.E., Ransohoff R.M., Ikezu T. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr N.M., Kaufman K.L., Harper C.G. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7:284–294. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]