Abstract
The concept of magnetic resonance perfusion-diffusion mismatch (PDM) provides a practical and approximate measure of the tissue at risk and has been increasingly applied for the evaluation of hyperacute and acute stroke in animals and patients. Recent studies demonstrated that PDM does not optimally define the ischemic penumbra; because early abnormality on diffusion-weighted imaging overestimates the infarct core by including part of the penumbra, and the abnormality on perfusion weighted imaging overestimates the penumbra by including regions of benign oligemia. To overcome these limitations, many efforts have been made to optimize conventional PDM. Various alternatives beyond the PDM concept are under investigation in order to better define the penumbra. The PDM theory has been applied in ischemic stroke for at least three purposes: to be used as a practical selection tool for stroke treatment; to test the hypothesis that patients with PDM pattern will benefit from treatment, while those without mismatch pattern will not; to be a surrogate measure for stroke outcome. The main patterns of PDM and its relation with clinical outcomes were also briefly reviewed. The conclusion was that patients with PDM documented more reperfusion, reduced infarct growth and better clinical outcomes compared to patients without PDM, but it was not yet clear that thrombolytic therapy is beneficial when patients were selected on PDM. Studies based on a larger cohort are currently under investigation to further validate the PDM hypothesis.
Keywords: Diffusion, Ischemic, Magnetic resonance imaging, Mismatch, Penumbra, Perfusion, Stroke
INTRODUCTION
The concept of ischemic penumbra was originally introduced by Astrup et al[1] in 1981, and was defined as an area of reduced cerebral blood flow (CBF) with electrical failure but preserved ion homeostasis and transmembrane electrical potentials. Since then, some other definitions for the ischemic penumbra have been proposed based on energy metabolism, CBF thresholds and protein synthesis[2]. Because the target of thrombolytic therapy in acute stroke is the brain tissue at risk of infarction, the most clinically relevant definition of the penumbra indicates the ischemic tissue but still viable and salvageable if local perfusion is efficiently restored[3,4]. It is widely acknowledged that the mismatch of abnormality volume between perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI) of magnetic resonance imaging (MRI), has previously and frequently been applied as an imaging equivalent of the ischemic penumbra[5,6]. The concept of perfusion-diffusion mismatch (PDM) provides a practical and approximate measure of the tissue at risk and has been increasingly applied for the evaluation of acute stroke in animals[7-10] and patients[3,4,6,11].
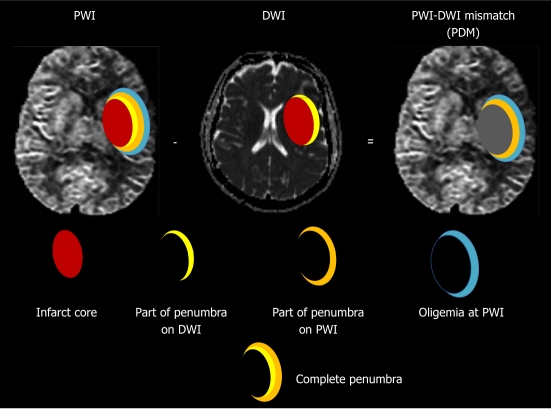
However, this conventional PDM has been challenged[3,12] by recent studies. It has been evolved into the conviction that PDM does not optimally define the ischemic penumbra. Sufficient data have demonstrated that early abnormality on DWI overestimates the infarct core by including part of the penumbra; and the visible lesion on PWI overestimates the penumbra by including regions of benign oligemia[4,12-14], in which the mild reductions in tissue perfusion do not actually place the tissue at risk[4] (Figure 1). This is reflected in clinical results, where the area of final infarction is normally smaller than the maximum perfusion deficit from PWI[15].
Figure 1.
Modern concept of ischemic penumbra. Early abnormality on diffusion weighted imaging (DWI) equals the infarct core plus a part of the tissue at risk (penumbra); and the perfusion deficiency on perfusion weighted imaging (PWI) includes the infarct core plus penumbra and region of benign oligemia. PWI-DWI mismatch (PDM) does not optimally define the ischemic penumbra.
OPTIMAL DEFINITION OF PERFUSION-DIFFUSION MISMATCH
Identification of the PDM is believed to be of considerable therapeutic importance and provides a guideline in patient triage for thrombolytic therapy[16,17]. However, there exist fundamental controversies in defining a PDM. For instance, there is a lack of consensus regarding what constitute the pairs of mismatch[18,19] and which PWI-derived parameter best defines the hypoperfused region or predicts lesion growth[11,19-23].
Some authors suggested that cerebral blood volume (CBV) or CBF is the most reliable parameter to predict final infarct size[20,24-26]. However, a recent meta-analysis reported varied CBF thresholds for discrimination between infarct core, penumbra and oligemia[27]. Although it has been accepted by most imaging groups that a parameter from the time domain is the most accurate, there is still no agreement as to which is superior, i.e., time to peak (TTP)[21,22,28] or mean transit time (MTT)[11,18,19,29-31]. However, both semiquantitative TTP and MTT tend to overestimate the ischemic lesion volume because of collateral flow[32,33].
Technically, there are two methods to measure the PDM. One method is to visually rate the PWI-derived parametric maps and DWI as generated by commercial MRI console software. Another method is to quantify the PWI maps and DWI by offline postprocessing based on region-of-interest volumetric calculation. In this quantitative approach, PDM is defined as a ratio of perfusion:diffusion lesion volume of > 1.2, or as a difference of > 10-50 mL volumes of abnormality between PWI and DWI[34-36]. Some researchers considered that qualitative visual evaluation of PDM performed equally with the quantitative PDM measurements. But Campbell et al[36] reported that the visual assessment of PDM at console is insufficiently reliable for use in clinical trials due to the great interobserver variability. In addition, Butcher et al[37,38] found that variability in planimetric PDM measurements arises primarily from differences in PWI volume assessment; because the perfusion-derived readouts may vary greatly due to different examiners and kynatic models applied[38]. Ma et al[39] also found that volumetric analysis consistently underestimates the PDM volume. All those mentioned reasons combine to make visual PDM substantially different to quantitative PDM. Better ways are sought to improve the measurement of PDM. These include the semi-automated[40] or fully automated processing of PDM with dedicated software[41-43] and the more precise co-registration method for DWI and PWI[35,39,44].
Although the new concept of PDM has been proposed, currently there is a lack of reliable measures to separate the penumbra part from infarct core on DWI and the benign oligemia from real penumbra on PWI in clinical practice. Many efforts have been made to optimize conventional PDM as described below in order to better define the penumbra.
Serial measurements of perfusion-diffusion mismatch
Since the PDM is strictly time dependent, the moment to acquire PWI is particularly critical in the clinic[45,46]. It has been shown that the mismatch may exist up to 3 d or even later in patients after symptom onset[47-51] and most cases of PDM (75%) occurred within the first 6 h after stroke onset in patients[47]. Recent clinical studies indicated that the presence and extent of reperfusion and collaterals were key factors affecting the evolution of PDM patterns and outcomes of patient with acute stroke[47,52-56]. Therefore, serial measurements of PDM have been proved to be useful in real-time monitoring of PDM evolution, and might be beneficial for rescuing more stroke patients[48-50,57,58].
Threshold method for defining perfusion-diffusion mismatch
Recent studies suggested that using a threshold derived from PWI or DWI appeared to provide more accurate discrimination between benign oligemia and penumbra or reversible lesion and infarct core[27,59-61]. Rohi et al[62] reported that cutoff values of relative CBF < 0.59 and MTT > 1.63 were optimal in distinguishing the benign oligemia and real penumbra. Oppenheim et al[63] suggested that the apparent diffusion coefficient (ADC) values best excluded penumbra (7.82 ± 0.82×10-4 mm2/s) from benign oligemia (8.23 ± 0.41×10-4 mm2/s). Prospective investigations are currently undergoing to validate these thresholding techniques with automated software programs[59].
Other approaches for defining perfusion-diffusion mismatch
Many other approaches have been tried to optimize the definition of PDM. Chen et al[64] presented initial experiences of utilizing arterial spin labeling PWI in pediatric ischemic stroke patients. Tsang et al[65] reported that sodium intensity remains unchanged in PDM tissue, indicating preservation of ionic homeostasis. Based on intravoxel incoherent motion MRI, Suzuki et al[66] used an independent component analysis, a higher-order statistical signal processing technique, to obtain a perfusion map from a set of diffusion-weighted images based on assumed difference in ADC values. In this way, the PDM can be identified without the PWI data.
POTENTIAL ALTERNATIVES FOR PERFUSION-DIFFUSION MISMATCH
pH-weighted imaging-perfusion-weighted imaging or diffusion-weighted imaging mismatch
According to Astrup’s classic definition of penumbra, the anaerobic metabolism and the formation of lactate lead to a decrease in pH in the area of the penumbra. Sun et al[67,68] detected the pH-dependent amide proton transfer between endogenous mobile proteins/peptides and tissue water, and obtained pH-weighted imaging (pHWI) during acute ischemia. This modality allowed them to subdivide the PDM into regions with and without tissue acidosis. It was found that the pHWI-deficit area at 3 h was constant during the hyperacute phase of stroke onset; the outer boundary of the hypoperfused area that shows a decrease in pH without DWI abnormality may better correspond to the classic ischemic penumbra area than the PWI deficit. The PWI-pHWI mismatch would then match to benign oligemia, and the pHWI-DWI mismatch to the minimal penumbral area.
Magnetic resonance thermometry-diffusion-weighted imaging mismatch
Elevated temperatures, or pyrexia, in the body or even in brain tissues are common in acute cerebral ischemic stroke[69,70]. Pyrexia is associated with a worse outcome after stroke, e.g., increased infarct size than normothermia in animal models. In contrast, hypothermia reduced ischemic lesion volume on DWI and may improve functional outcome[71]. Brain temperature (T) can be measured noninvasively with magnetic resonance spectroscopy imaging (MRSI). For each voxel, cerebral temperature can be calculated from the apparent chemical shift of the N-acetylaspartate (NAA) peak, using the following formula: T = 37 °C + 100 (NAApeak - 2.035), where a chemical shift of 2.035 ppm was found in healthy control subjects with an assumed brain temperature of 37 °C. Using this approach, Karaszewski B et al[71] found that the tissues were hotter in “potential penumbra” (marginally abnormal on DWI or just outside the edge of the DWI lesion) than the “likely infarct core” (definitely abnormal on DWI). The “likely infarct core”, in turn, was hotter than normal brain tissues. Therefore, MRSI provides a promising approach in the study of temperature after stroke and to monitor interventions[69-71].
Based blood oxygen level-dependent magnetic resonance imaging-based penumbra
This is based on the finding that oxygen extraction fraction (OEF) is significantly increased in the ischemic penumbra[72]. Deoxyhemoglobin (deoxy-Hb) can be used as an indicator of OEF that can be visualized by T2*-based blood oxygen level-dependent (BOLD) imaging. Geisler et al[73] applied quantitative T2*-based BOLD imaging (T2’) in patients with acute stroke. They found that a signal reduction in the T2’ images presumably corresponding with an increase of deoxy-Hb was attributable to an increase of OEF. They also detected shortened T2’ values adjacent to the ADC lesion in the region later evolving into infarction, which represents the essential penumbra, and a significant T2’ signal loss in the region of a benign oligemia. Therefore, this negative BOLD MRI technique provided an additional metabolic parameter in the better description of the ischemic penumbra[74,75].
Positron emission tomography-based estimation of penumbra
For positron emission tomography (PET), penumbra was defined as the region with increased OEF and termed “misery perfusion” for this purpose. Normally, cerebral perfusion was assessed by H2(15)O-PET and tissue damage was estimated by 11C-flumazenil. The determination of absolute values of thresholds for penumbra in patients, however, is difficult since the necessary calculation requires arterial blood sampling. The reported values for the threshold of morphological damage and of the upper limit of penumbra vary considerably (14~22 mL/100 g per minute) by different authors[72]. Studies demonstrated that PET and PDM were related to the tissue with increased OEF as an indicator of penumbra. PWI was limited in estimating flow and yielded values comparable to H2(15)O-PET only in the range between 20 and 30 mL/100 g per minute[76]. In a coregistered PET and DWI study, the characteristic changes of both infarction and penumbra were defined with PET in areas of abnormal DWI[77,78], with a value of OEF > 150% suggesting the real penumbra[60].
PET is still considered the gold standard and is the first line technique employed for detection of penumbra and irreversibly damaged tissue, but it is not a readily available methodology[78,79].
Magnetic resonance angiography-diffusion-weighted imaging mismatch
The magnetic resonance angiography (MRA)-DWI mismatch was defined as an MRA score of 3 (for the intracranial internal carotid artery (ICA) and M1 segment of the MCA, 1 = normal; 2 = reduced flow; and 3 = occlusion) and a DWI lesion volume < 25 mL, or an MRA score of 2 and a DWI lesion volume < 15 mL[80]. Kim et al[80] found that the MRA-DWI mismatch was more prevalent in the intracranial large artery atherosclerotic stroke group than in other stroke subtypes. Ma et al[81] reported that baseline MRA was helpful in categorizing acute ischemic stroke patients into subgroups and should be used in advance of PDM acquisition, because patients may not need thrombolytic therapy if they do not have initial vessel occlusion found by MRA. Another advantage of baseline MRA is the favorable response in patients with arterial occlusion visualized by MRA to increasing doses of thrombolytic agents such as desmoteplase[82].
Diffusion-weighted imaging - T2W mismatch
It is well accepted that a DWI lesion does not represent the real infarct core because it includes part of the tissue at risk or penumbra. Since the DWI may show initial reduction with no abnormal change on T2W or fluid-attenuated inversion-recovery (FLAIR) images in hyperacute stroke, a DWI-T2W mismatch was proposed to represent the ischemic penumbra. The DWI-T2W mismatch was defined as a hyperintense lesion on DWI (low ADC) with no hyperintense lesion on T2WI or FLAIR, and no hypointense lesion on T1WI. This method may be particularly beneficial to patients with undefined time windows or beyond 3 h after symptom onset and without PWI data[83,84].
However, recent progress in DWI suggested that the reversal of the tissue at risk included in DWI abnormality was uncommon in ischemic stroke patients. The volume of reversed lesion on DWI was small and would rarely affect therapy decisions based on PDM theory[85,86]. Therefore, DWI is generally considered a reliable indicator for predicting ultimate infarction with or without reperfusion[81,85-89].
Clinical-diffusion mismatch
Since MR-perfusion has limited utilities in many hospitals due to technical reasons[90], the clinical-diffusion mismatch (CDM) model was proposed as an alternative method for PDM. The CDM is technically less challenging because it does not require PWI[91]. In order to measure the CDM, Alberta Stroke Programme Early Computed Tomography Score (ASPECTS) methodology was applied to the MRI sequences in an analogous topographical technique as used for computed tomography (CT)[92]. The CDM was defined as a score of National Institute of Health Stroke Score (NIHSS) ≥ 8 and DWI-ASPECTS ≥ 8 or DWI (lesion volume) ≤ 25 mL. It has been suggested that NIHSS ≥ 8 is a clinical indicator of a large volume of ischemic brain tissue, and had a high rate of early neurological deterioration and lesion growth[93]. However, there was a discrepancy between CDM and PDM[94]. Iwanaga et al[95] found no increased benefit from tissue plasminogen activator (tPA) in patients with CDM, because the positive effects of reperfusion were similar in patients with and without CDM.
Computed tomography-derived mismatch
Infarct core measured on CT was usually segmented based on a CBV threshold of 56% relative to the opposite side. A previous study compared DWI and CBV lesion volumes using thresholds of 46%, 56% and 66% of the contralateral normal side as well as a 2 mL/100 g absolute threshold. The results indicated that the DWI and CBV correlation was optimal using the 56% threshold[96]. Another study[97] reported that ischemic penumbra was determined based on a MTT threshold of 150% relative to the contralateral side. Percent volume mismatch was defined as [(MTT - CBV)/CBV] × 100%. In addition to CBV and MTT, some researchers proposed the use of rCBF to calculate the CT mismatch[98].
Instead of using computed tomography perfusion maps (CTP), Wang et al[99] suggested the use of CTP source images (CTP-SI) to define a CTP mismatch. Here, the CTP mismatch was defined as a delayed perfusion between arterial phase CTP-SI ASPECTS and venous phase CTP-SI ASPECTS. The presence of such a delayed perfusion can be used as an indicator for thrombolysis.
In a retrospective study, Messé et al[100] tested a mismatch between ischemic changes on head CT and clinical examination findings (CT-NIHSS mismatch), but his results did not show any correlation between the CT-NIHSS mismatch and MRI PDM.
APPLICATIONS OF PERFUSION-DIFFUSION MISMATCH
The PDM theory was introduced in the late 1990s[85]. Despite its limitations in imaging ischemic penumbra, this paradigm has been widely used in preclinical research and clinical trials[16,17]. The PDM model combined with other MRI techniques such as T2WI, FLAIR and MRA have been employed for various purposes[12].
First, it was used as a practical selection tool for stroke treatment[101-106]. Only those with appropriate PDM patterns will be enrolled for therapy, like clinical trials of Desmoteplase in Acute Ischemic Stroke Trial (DIAS)[107], Dose Escalation of Desmoteplase for Acute Ischemic Stroke[101], and DIAS 2[104]. However, the results were not repeatable[82,108-110] in those trials, although better outcomes were found with higher doses of desmoteplase corresponding to the frequency of reperfusion.
Second, to test the hypothesis that patients with PDM pattern will benefit from treatment, while those without mismatch pattern will not, all patients were treated no matter their phases and pretreatment PDM patterns[12,111]. Clinical trials like The Diffusion and Perfusion Imaging Evaluation For Understanding Stroke Evolution (DEFUSE) and Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) were conducted for this purpose. However, the results were not very straightforward. In DEFUSE, no clear pattern of response was found to reperfusion treatment with tPA between patients with PDM and patient without. In EPITHET, although the infarct growth was smaller in patients treated with tPA, the difference was not statistically significant compared to the controls. Furthermore, the outcomes were better in all patients and PDM patients treated with tPA, but the difference was also not statistically significant[82,85,101,112].
Third, PDM was applied as a surrogate measure for stroke outcome. Some studies suggested that the abnormality from PWI can be used to predict the lesion growth or final infarct volume[113-115]. However, this has been challenged due to the evolving PDM concept[89,116]; because the regions of benign oligemia included in the PWI abnormality did not finally develop into infarction[4,12,68], and patients without PDM were equally likely to have lesion growth as those with PDM and should also be enrolled in acute stroke treatment[89,117]. A reduced volume of PDM can be used as an indicator of improvement of stroke severity in a combined anti-stroke study[89] and of potential for recovery of functions[118]. Since an acute ischemic lesion was detected with PDM in about 60% of patients with transient ischemic attack (TIA), PDM may play a role in the triage of acute TIA and brain infarction[118]. PDM can also be applied as an indication for performing interventional procedures like clot removal therapy by aspiration and extraction[110], urgent carotid artery stenting for acute stroke patients[108], and transluminal balloon angioplasty for patients with cerebral vasospasm[109]. In a report by Heidenreich et al[119] the presumptive treatment plan was changed after PDM-based evaluation in 26% (25/97) of patients with hyperacute stroke. Studies also revealed that the relation between the anatomic location and PDM may have some indications for the progression or prognosis in patients with hyperacute and acute stroke. For instance, the ischemia stroke was found to progress and have more severe TTP abnormalities in the central part of the MCA territory including the inferior frontal gyrus, superior temporal gyrus, insula, and underlying hemispheric white matter[120]. The loss of PDM volume (evolving into infarction) was increased in the insula area[120], and target mismatch was more frequent in the cortex with better outcomes if reperfusion was timely established[121]. In addition, the severity of leukoaraiosis was associated with the loss of PDM and seems to be a predictor of infarct growth[122].
MAIN PATTERNS OF PDM AND THEIR CLINICAL RELEVANCE
As the target of thrombolytic therapy of acute stroke, the PDM is normally defined as a mismatch ratio of PWI/DWI ≥ 1.2, reflecting the presence of clinically significant mismatch[106,123,124]. However, recent studies suggested the use of a larger ratio of 1.8 to 2.6, because a larger mismatch was associated with a higher response rate in the condition of reperfusion therapy[125,126].
Although the PWI/DWI mismatch ≥ 1.2 is the predominant pattern in PDM discrepancies[47], many other PDM patterns have been recently reported and their relevance to subtypes of stroke has not been fully elucidated[20,47,52,127,128]. Discrepancies between the extent of abnormality on PWI and DWI are supposed to depend predominantly on time from stroke onset to MRI scanning[45,46,52]. The topographic profiles of these PDM patterns were summarized as follows and in Table 1.
Table 1.
Patterns of discrepancy between perfusion-weighted imaging and diffusion-weighted imaging
| Pattern | Incidence % | Main etiology | Indications | Potential interventions | ||
| < 6 h | Total | |||||
| I | PWI > DWI[20,47,51-53,114,121,125,127-130,135], target mismatch | 57~86 | 49~70 | Large-artery atherosclerosis, cardioembolism, cryptogenic | Larger lesion on PWI and DWI. Part of tissue at risk and oligemia. Infarct may growth without effective therapy. More common in white patients | Reperfusion therapy: Thrombolytic therapy or angioplasty, stenting |
| II | PWI = DWI[20,52,125,131] | ~17 | ~28 | Cryptogenic, large-artery atherosclerosis, cardioembolism, lacunar infarction | No additional tissue at risk. Collateral flow limits the infarct volume to that depicted at DWI. Most common in patients with diabetes | Neuroprotection |
| III | PWI < DWI[51,52,114,123,132], inverse mismatch | ~29 | 6~34 | Cryptogenic, cardioembolism, large-artery atherosclerosis, lacunar infarction | Smaller lesion on PWI and DWI. Partial reperfusion may occur at the time of MR scan | Neuroprotection |
| IV | PWI (-), DWI (+)[47,114,125] | ~8 | ~24 | Single small MCA branch occlusion, small subclinical infarct | Full reperfusion may occur or due to collaterals at the time of MR scan. More common in Asian patients | Neuroprotection |
| V | PWI (+), DWI (-)[47,80,128,133,134], total mismatch | ~8 | ~3 | Migraine, TIA | Pure perfusion deficit (tissue at risk but not committed to infarction) | Reperfusion therapy |
| VI | PWI (-), DWI (-)[20,135] | ~14 | ~18 | Migraine, TIA | No abnormality on both PWI and DWI. Normal or hypoperfusion on PET | No interventional therapy |
| VII | PWI or DWI > 100 mL[82,112], malignant mismatch | Large-artery atherosclerosis | Poor outcome, strongly associated with reperfusion-related brain hemorrhage | Exclusion of therapy | ||
(+): There is abnormality; (-): No abnormality; PWI: Perfusion weighted imaging; DWI: Diffusion weighted imaging; MCA: Middle cerebral artery; TIA: Transient ischemic attack; PET: Positron emission tomography.
Type I, perfusion-weighted imaging > diffusion-weighted imaging
This pattern is the main type (49%-70%) of PDM and defined as the target mismatch[125] or positive mismatch[51] for reperfusion therapy (Figure 2). Bang et al[125] reported that this pattern is more common in white patients compared with Asian ethnicity with medium-sized lesions on DWI. This pattern was also defined as the classical type of PDM by Ma et al[121,129], i.e., a DWI lesion within a hypoperfused territory on PWI. In their study, a larger mismatch volume in a cortical location was considered an important factor relevant to the classical pattern, and the presence of distal hyperintense vessels on FLAIR (a potential MRI marker for collaterals) was found to be associated with a large PDM[130]. But this classic pattern may evolve into a fragmented non-classical PDM pattern over time (up to 48 h), i.e., the dissociation of PWI and DWI lesions. They even found that the types of PDM patterns have little effect on infarct growth, clinical outcomes and the benefit of thrombolytic agent; this suggests that mismatch topography is less important at least during the hyperacute phase of stroke. An animal study also found that the PDM pattern may have evolved over time from the so-called classic pattern (PWI > DWI) during initial hours through to a match pattern (PWI = DWI) around 6-12 h to a reverse mismatch (PWI < DWI) at a later time up to 3 d after stroke onset[51].
Figure 2.
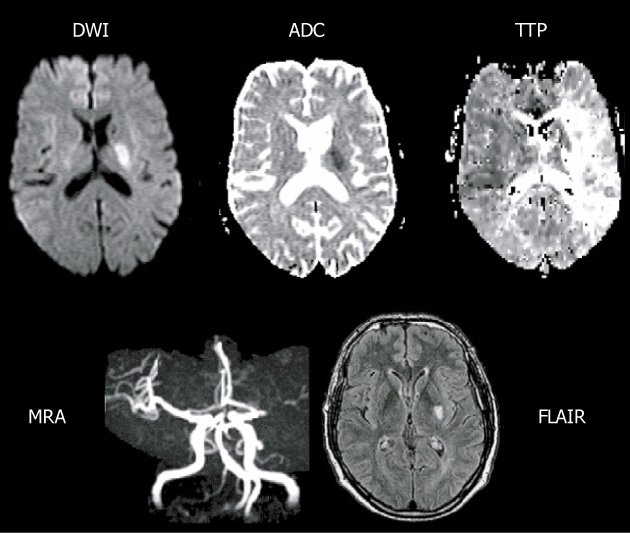
Main pattern of perfusion-diffusion mismatch, perfusion-weighted imaging > diffusion-weighted imaging, in a patient with acute stroke. Extensive area of prolonged time to peak (TTP) and small diffusion-weighted imaging (DWI) lesion in deep middle cerebral artery (MCA) territory, with a complete proximal MCA occlusion on magnetic resonance angiography (MRA) (reprint from Muir KW et al Lancet Neurol 2006; 5: 755-68 with permission). ADC: Apparent diffusion coefficient; FLAIR: Fluid-attenuated inversion-recovery.
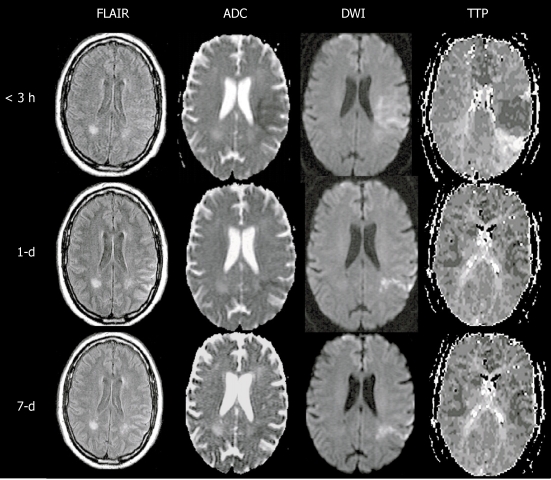
Type II, perfusion-weighted imaging = diffusion-weighted imaging
This match pattern means that no additional tissue at risk or penumbra was found, because collateral flow may limit the infarct core evolving into the hypoperfused area on PWI[131] (Figure 3). Contrary to that, Bang et al[125] reported that this no target mismatch was the most common type in patients with diabetes. The possible mechanism may be attributed to the early and longstanding hyperglycemia and increased lactate production. All of these may lead to occlusion of small perforators, collateral failure, impaired autoregulation, and consequent loss of penumbra.
Figure 3.
Main pattern of perfusion-diffusion mismatch, perfusion-weighted imaging = diffusion-weighted imaging, in a patient with acute stroke. < 3 h: Fuzzy diffusion-weighted imaging (DWI) lesion in left middle cerebral artery territory matching an area of diminished time to peak, indicating local hyperperfusion and spontaneous recanalization had occurred prior to imaging at 3 h after onset (note the prolonged time to peak at the posterior edge of the DWI lesion, suggesting distal branch occlusion); 1-d: The next day, perfusion has essentially normalized as well as the DWI lesion, save for a narrow posterior streak, suggesting the spontaneous recanalization saved the at-risk tissue from progressing to infarction; 7-d: At day 7, there has been no return of the DWI lesion, indicating the tissue was effectively salvaged (reprint from Muir KW et al Lancet Neurol 2006; 5: 755-768 with permission). PWI: Perfusion-weighted imaging; ADC: Apparent diffusion coefficient; TTP: Time to peak; FLAIR: Fluid-attenuated inversion-recovery.
Type III, perfusion-weighted imaging < diffusion-weighted imaging
Since the abnormality volume on PWI is smaller than that on DWI, it is considered an inverse or negative mismatch[51]. The mechanism for this pattern may be attributed to the partial reperfusion which occurred at the time of the MRI scan (Figure 4). Fiebach et al[123] found that this inverse mismatch was frequent in small subcortical ischemic stroke and infarct core may develop beyond the initial hypoperfusion area. In another study, Ma et al[132] found that in 11 of 34 (32%) patients with negative mismatch (PWI < DWI) assessed by the volumetric subtraction technique, all had positive mismatch (PWI > DWI) identified when the more precise coregistration analysis was performed. They named it “hidden mismatch”, which provided an explanation for the previous illogical finding that infarct expansion seems to occur even in the presence of inverse mismatch (PWI < DWI). The “hidden mismatch” observation indicated the possible benefit of treatment for patients even with inverse mismatch[51,123,132].
Figure 4.
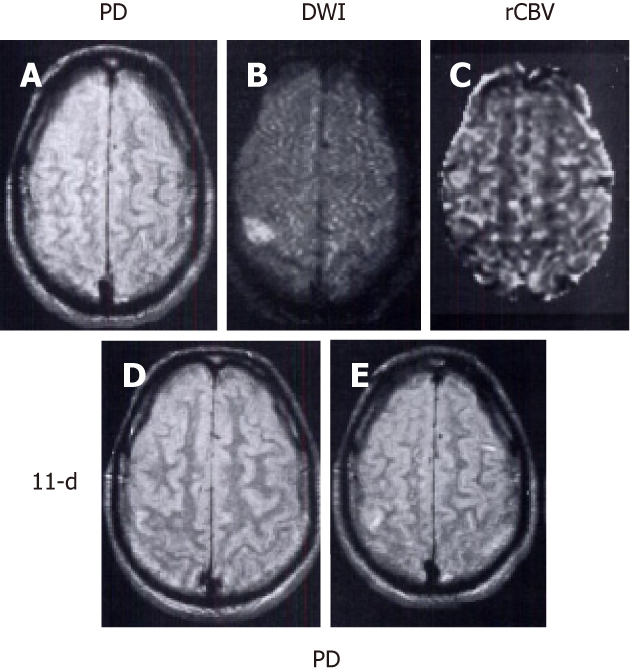
Main pattern of perfusion-diffusion mismatch: perfusion-weighted imaging < diffusion-weighted imaging, in a patient with acute stroke. Patient’s left-sided weakness was partially resolved 3 h after the onset of symptoms. A: Proton density (PD) weighted fast spin-echo image shows no abnormality; B: Diffusion-weighted imaging (DWI) shows a focal cortical ischemic abnormality; C: Relative cerebral blood volume (rCBV) map demonstrates a smaller lesion with decreased rCBV compared to the same abnormality as depicted in B; D, E: Follow-up PD images acquired 11 d later show a new tiny hyperintense infarct in the area of initially observed lesion on B and C. Note that the initial DWI lesion is larger than the final infarct volume. The patient’s symptoms resolved completely after 2 d (reprint from Sorensen et al Radiology 1996; 199: 391-401 with permission).
Type IV, perfusion-weighted imaging (-), diffusion-weighted imaging (+)
This pattern shows only an infarct lesion on DWI with absence of perfusion deficiency on PWI due to single small artery occlusion or presence of small subclinical infarct. This type is more common in Asian patients, because intracranial atherosclerosis and small arterial occlusions were common causes of strokes in Asians and Blacks. Consequently, very small or even absence of the hypoperfusion region was seen in this pattern[125].
Type V, perfusion-weighted imaging (+), diffusion-weighted imaging (-)
This pattern is also termed total mismatch, i.e. negative DWI and extensive perfusion defects. Recent studies seem to suggest that total mismatch does not necessarily progress to infarction, but may suggest stroke pathogenesis and site of current arterial occlusion. Patients with total mismatch usually have a favorable outcome after recanalization with or without thrombolysis. It also provided evidence of brain ischemia in patients with a clinical diagnosis of migraine or TIA[80,133,134].
Type VI, perfusion-weighted imaging (-), diffusion-weighted imaging (-)
In this type, there is no abnormality on both DWI and PWI. This pattern was normally found in patients with migraine headaches. Patients’ symptoms were associated with localized or “spreading hypoperfusion” along the cerebral cortex as confirmed by PET, and may be resolved completely within 1-48 h[20,135]. Therefore, the observed hypoperfusion most likely belongs to a type of mild oligemia that cannot even be detected with PWI.
Type VII, perfusion-weighted imaging or diffusion-weighted imaging > 100 mL
The DEFUSE clinical trial defined PWI or DWI volume ≥ 100 mL as a malignant mismatch. A number of studies indicated that patients with the malignant mismatch pattern were more likely to have a poor outcome (modified Rankin Scale score 5 to 6) with reperfusion than without and were strongly associated with reperfusion-related brain hemorrhage due to the severe ischemic brain and microvessel injury. Exclusion of patients with malignant mismatch could improve the safety and efficacy of reperfusion therapies[82,112].
CONCLUSION
Although the PDM is not yet perfectly matched with the ischemic penumbra, it has been widely recognized as a crude but practical approximation of an imaging equivalent of the pathological ischemic penumbra[5,6,51]. There is still a lack of consensus regarding the best definition and optimal measurement for PDM. Conclusions draw from recent clinical trials including DEFUSE and EPITHET suggested that patients with PDM documented more reperfusion, reduced infarct growth and better clinical outcomes compared to patients without PDM[82,85,125,136,137], but it is not yet clear that thrombolytic therapy is beneficial when patients are selected on PDM[82]. Studies based on a larger cohort are currently under investigation to further validate the PDM hypothesis[82]. Given the limitation of current PDM theory, studies in other potential alternatives or imaging biomarkers beyond the PDM concept may help in the management of ischemic stroke patients[82,85,138].
Footnotes
Peer reviewer: Rivka R Colen, Dr., Department of Radiology, Brigham and Women’s Hospital/Harvard Medical School, 150 Brookline Ave Apt 703, Boston, MA 02215, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Xiong L
References
- 1.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M. The ischemic penumbra: identification, evolution and treatment concepts. Cerebrovasc Dis. 2004;17 Suppl 1:1–6. doi: 10.1159/000074790. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M, Ginsberg M. Current concepts of the ischemic penumbra: introduction. Stroke. 2004;35(Suppl 1):2657–2658. [Google Scholar]
- 4.Kidwell CS, Alger JR, Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2004;35:2662–2665. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 5.Hoehn M, Nicolay K, Franke C, van der Sanden B. Application of magnetic resonance to animal models of cerebral ischemia. J Magn Reson Imaging. 2001;14:491–509. doi: 10.1002/jmri.1213. [DOI] [PubMed] [Google Scholar]
- 6.Warach S. Measurement of the ischemic penumbra with MRI: it’s about time. Stroke. 2003;34:2533–2534. doi: 10.1161/01.STR.0000092395.19554.9A. [DOI] [PubMed] [Google Scholar]
- 7.Kohno K, Hoehn-Berlage M, Mies G. Relationship between diffusion-weighted MR images, cerebral blood flow, and energy state in experimental brain infarction. Magn Reson Imaging. 1995;13:73–80. doi: 10.1016/0730-725x(94)00080-m. [DOI] [PubMed] [Google Scholar]
- 8.Müller TB, Haraldseth O, Jones RA, Sebastiani G, Godtliebsen F, Lindboe CF, Unsgård G. Combined perfusion and diffusion-weighted magnetic resonance imaging in a rat model of reversible middle cerebral artery occlusion. Stroke. 1995;26:451–457; discussion 451-457. doi: 10.1161/01.str.26.3.451. [DOI] [PubMed] [Google Scholar]
- 9.Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab. 1995;15:1002–1011. doi: 10.1038/jcbfm.1995.126. [DOI] [PubMed] [Google Scholar]
- 10.Lo EH, Pierce AR, Mandeville JB, Rosen BR. Neuroprotection with NBQX in rat focal cerebral ischemia. Effects on ADC probability distribution functions and diffusion-perfusion relationships. Stroke. 1997;28:439–446; discussion 446-447. doi: 10.1161/01.str.28.2.439. [DOI] [PubMed] [Google Scholar]
- 11.Schlaug G, Benfield A, Baird AE, Siewert B, Lövblad KO, Parker RA, Edelman RR, Warach S. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 12.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 13.Hussain MS, Bhagat YA, Liu S, Scozzafava J, Khan KA, Dillon WP, Shuaib A. DWI lesion volume reduction following acute stroke treatment with transient partial aortic obstruction. J Neuroimaging. 2010;20:379–381. doi: 10.1111/j.1552-6569.2009.00407.x. [DOI] [PubMed] [Google Scholar]
- 14.Heiss WD. The concept of the penumbra: can it be translated to stroke management? Int J Stroke. 2010;5:290–295. doi: 10.1111/j.1747-4949.2010.00444.x. [DOI] [PubMed] [Google Scholar]
- 15.Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27:1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- 16.Provenzale JM, Jahan R, Naidich TP, Fox AJ. Assessment of the patient with hyperacute stroke: imaging and therapy. Radiology. 2003;229:347–359. doi: 10.1148/radiol.2292020402. [DOI] [PubMed] [Google Scholar]
- 17.Sunshine JL, Tarr RW, Lanzieri CF, Landis DM, Selman WR, Lewin JS. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology. 1999;212:325–332. doi: 10.1148/radiology.212.2.r99au52325. [DOI] [PubMed] [Google Scholar]
- 18.Butcher KS, Parsons MW, Davis S, Donnan G. PWI/DWI mismatch: better definition required. Stroke. 2003;34:e215–e216; author reply e215-e216. doi: 10.1161/01.STR.0000099066.23627.24. [DOI] [PubMed] [Google Scholar]
- 19.Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, Levi C, Kimber T, Schultz D, Fink J, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, Reese TG, Weisskoff RM, Davis TL, Suwanwela N, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199:391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 21.Neumann-Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Mödder U, Freund HJ. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- 22.Grandin CB, Duprez TP, Smith AM, Oppenheim C, Peeters A, Robert AR, Cosnard G. Which MR-derived perfusion parameters are the best predictors of infarct growth in hyperacute stroke? Comparative study between relative and quantitative measurements. Radiology. 2002;223:361–370. doi: 10.1148/radiol.2232010673. [DOI] [PubMed] [Google Scholar]
- 23.Kane I, Carpenter T, Chappell F, Rivers C, Armitage P, Sandercock P, Wardlaw J. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007;38:3158–3164. doi: 10.1161/STROKEAHA.107.483842. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer PW, Hunter GJ, He J, Hamberg LM, Sorensen AG, Schwamm LH, Koroshetz WJ, Gonzalez RG. Predicting cerebral ischemic infarct volume with diffusion and perfusion MR imaging. AJNR Am J Neuroradiol. 2002;23:1785–1794. [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Kim D, Jeong E, Yoon P, Cha S, Lee J. Temporal changes in reversible cerebral ischemia on perfusion- and diffusion-weighted magnetic resonance imaging: the value of relative cerebral blood volume maps. Neuroradiology. 2002:44; 103–108. doi: 10.1007/s002340100705. [DOI] [PubMed] [Google Scholar]
- 26.Simonsen CZ, Røhl L, Vestergaard-Poulsen P, Gyldensted C, Andersen G, Østergaard L. Final infarct size after acute stroke: prediction with flow heterogeneity. Radiology. 2002;225:269–275. doi: 10.1148/radiol.2251011249. [DOI] [PubMed] [Google Scholar]
- 27.Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37:1334–1339. doi: 10.1161/01.STR.0000217418.29609.22. [DOI] [PubMed] [Google Scholar]
- 28.Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, Jacobs A, Neveling M, Heiss WD. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35:2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- 29.Koga M, Reutens DC, Wright P, Phan T, Markus R, Pedreira B, Fitt G, Lim I, Donnan GA. The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke. 2005;36:2132–2137. doi: 10.1161/01.STR.0000181066.23213.8f. [DOI] [PubMed] [Google Scholar]
- 30.Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- 31.Kane I, Hand PJ, Rivers C, Armitage P, Bastin ME, Lindley R, Dennis M, Wardlaw JM. A practical assessment of magnetic resonance diffusion-perfusion mismatch in acute stroke: observer variation and outcome. J Neurol. 2009;256:1832–1838. doi: 10.1007/s00415-009-5202-7. [DOI] [PubMed] [Google Scholar]
- 32.Hamberg LM, Hunter GJ, Maynard KI, Owen C, Morris PP, Putman CM, Ogilvy C, González RG. Functional CT perfusion imaging in predicting the extent of cerebral infarction from a 3-hour middle cerebral arterial occlusion in a primate stroke model. AJNR Am J Neuroradiol. 2002;23:1013–1021. [PMC free article] [PubMed] [Google Scholar]
- 33.Fiehler J, von Bezold M, Kucinski T, Knab R, Eckert B, Wittkugel O, Zeumer H, Röther J. Cerebral blood flow predicts lesion growth in acute stroke patients. Stroke. 2002;33:2421–2425. doi: 10.1161/01.str.0000032554.19600.60. [DOI] [PubMed] [Google Scholar]
- 34.Luby M, Ku KD, Latour LL, Merino JG, Hsia AW, Lynch JK, Warach S. Visual perfusion-diffusion mismatch is equivalent to quantitative mismatch. Stroke. 2011;42:1010–1014. doi: 10.1161/STROKEAHA.110.603290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagakane Y, Christensen S, Brekenfeld C, Ma H, Churilov L, Parsons MW, Levi CR, Butcher KS, Peeters A, Barber PA, et al. EPITHET: Positive Result After Reanalysis Using Baseline Diffusion-Weighted Imaging/Perfusion-Weighted Imaging Co-Registration. Stroke. 2011;42:59–64. doi: 10.1161/STROKEAHA.110.580464. [DOI] [PubMed] [Google Scholar]
- 36.Campbell BC, Christensen S, Foster SJ, Desmond PM, Parsons MW, Butcher KS, Barber PA, Levi CR, Bladin CF, Donnan GA, et al. Visual assessment of perfusion-diffusion mismatch is inadequate to select patients for thrombolysis. Cerebrovasc Dis. 2010;29:592–596. doi: 10.1159/000311080. [DOI] [PubMed] [Google Scholar]
- 37.Butcher K, Parsons M, Allport L, Lee SB, Barber PA, Tress B, Donnan GA, Davis SM. Rapid assessment of perfusion-diffusion mismatch. Stroke. 2008;39:75–81. doi: 10.1161/STROKEAHA.107.490524. [DOI] [PubMed] [Google Scholar]
- 38.Ay H, Arsava EM, Vangel M, Oner B, Zhu M, Wu O, Singhal A, Koroshetz WJ, Sorensen AG. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke. 2008;39:1171–1176. doi: 10.1161/STROKEAHA.107.502104. [DOI] [PubMed] [Google Scholar]
- 39.Canal N, Frattola L, Smirne S. The metabolism of cyclic-3’-5’-adenosine monophosphate (cAMP) in diseased muscle. J Neurol. 1975;208:259–265. doi: 10.1007/BF00312801. [DOI] [PubMed] [Google Scholar]
- 40.James JR, Yoder KK, Osuntokun O, Kalnin A, Bruno A, Morris ED. A supervised method for calculating perfusion/diffusion mismatch volume in acute ischemic stroke. Comput Biol Med. 2006;36:1268–1287. doi: 10.1016/j.compbiomed.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, Bammer R, Olivot JM, Desmond P, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke. 2011;42:1608–1614. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotter B, Pittl S, Ebinger M, Oepen G, Jegzentis K, Kudo K, Rozanski M, Schmidt WU, Brunecker P, Xu C, et al. Prospective study on the mismatch concept in acute stroke patients within the first 24 h after symptom onset - 1000Plus study. BMC Neurol. 2009;9:60. doi: 10.1186/1471-2377-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luby M, Warach S. Reliability of MR perfusion-weighted and diffusion-weighted imaging mismatch measurement methods. AJNR Am J Neuroradiol. 2007;28:1674–1678. doi: 10.3174/ajnr.A0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Touzani O, Roussel S, MacKenzie ET. The ischaemic penumbra. Curr Opin Neurol. 2001;14:83–88. doi: 10.1097/00019052-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Davis SM, Donnan GA. Advances in penumbra imaging with MR. Cerebrovasc Dis. 2004;17 Suppl 3:23–27. doi: 10.1159/000075301. [DOI] [PubMed] [Google Scholar]
- 47.Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, Li T, Tress BM, Davis SM. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 48.Copen WA, Rezai Gharai L, Barak ER, Schwamm LH, Wu O, Kamalian S, Gonzalez RG, Schaefer PW. Existence of the diffusion-perfusion mismatch within 24 hours after onset of acute stroke: dependence on proximal arterial occlusion. Radiology. 2009;250:878–886. doi: 10.1148/radiol.2503080811. [DOI] [PubMed] [Google Scholar]
- 49.Baron JC, Moseley ME. For how long is brain tissue salvageable? Imaging-based evidence. J Stroke Cerebrovasc Dis. 2000;9:15–20. doi: 10.1053/jscd.2000.18910. [DOI] [PubMed] [Google Scholar]
- 50.Perez A, Restrepo L, Kleinman JT, Barker P, Beauchamp N, Wityk RJ. Patients with diffusion-perfusion mismatch on magnetic resonance imaging 48 hours or more after stroke symptom onset: clinical and imaging features. J Neuroimaging. 2006;16:329–333. doi: 10.1111/j.1552-6569.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 51.Chen F, Liu Q, Wang H, Suzuki Y, Nagai N, Yu J, Marchal G, Ni Y. Comparing two methods for assessment of perfusion-diffusion mismatch in a rodent model of ischaemic stroke: a pilot study. Br J Radiol. 2008;81:192–198. doi: 10.1259/bjr/70940134. [DOI] [PubMed] [Google Scholar]
- 52.Restrepo L, Jacobs MA, Barker PB, Beauchamp NJ, Skolasky RL, Keswani SC, Wityk RJ. Etiology of perfusion-diffusion magnetic resonance imaging mismatch patterns. J Neuroimaging. 2005;15:254–260. doi: 10.1177/1051228405275199. [DOI] [PubMed] [Google Scholar]
- 53.Paciaroni M, Medeiros E, Bogousslavsky J. Desmoteplase. Expert Opin Biol Ther. 2009;9:773–778. doi: 10.1517/14712590902991497. [DOI] [PubMed] [Google Scholar]
- 54.Marks MP, Olivot JM, Kemp S, Lansberg MG, Bammer R, Wechsler LR, Albers GW, Thijs V. Patients with acute stroke treated with intravenous tPA 3-6 hours after stroke onset: correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology. 2008;249:614–623. doi: 10.1148/radiol.2492071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakuda W, Hamilton S, Thijs VN, Lansberg MG, Kemp S, Skalabrin E, Albers GW. Optimal outcome measures for detecting clinical benefits of early reperfusion: insights from the DEFUSE Study. J Stroke Cerebrovasc Dis. 2008;17:235–240. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.González RG, Hakimelahi R, Schaefer PW, Roccatagliata L, Sorensen AG, Singhal AB. Stability of large diffusion/perfusion mismatch in anterior circulation strokes for 4 or more hours. BMC Neurol. 2010;10:13. doi: 10.1186/1471-2377-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris AD, Kosior RK, Chen HS, Andersen LB, Frayne R. Evolution of hyperacute stroke over 6 hours using serial MR perfusion and diffusion maps. J Magn Reson Imaging. 2009;29:1262–1270. doi: 10.1002/jmri.21763. [DOI] [PubMed] [Google Scholar]
- 59.Olivot JM, Albers GW. Using advanced MRI techniques for patient selection before acute stroke therapy. Curr Treat Options Cardiovasc Med. 2010;12:230–239. doi: 10.1007/s11936-010-0072-y. [DOI] [PubMed] [Google Scholar]
- 60.Heiss WD, Sobesky J, Hesselmann V. Identifying thresholds for penumbra and irreversible tissue damage. Stroke. 2004;35:2671–2674. doi: 10.1161/01.STR.0000143329.81997.8a. [DOI] [PubMed] [Google Scholar]
- 61.Carrera E, Jones PS, Iglesias S, Guadagno JV, Warburton EA, Fryer TD, Aigbirhio FI, Baron JC. The vascular mean transit time: a surrogate for the penumbra flow threshold? J Cereb Blood Flow Metab. 2010;31:1027–1035. doi: 10.1038/jcbfm.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Røhl L, Ostergaard L, Simonsen CZ, Vestergaard-Poulsen P, Andersen G, Sakoh M, Le Bihan D, Gyldensted C. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke. 2001;32:1140–1146. doi: 10.1161/01.str.32.5.1140. [DOI] [PubMed] [Google Scholar]
- 63.Oppenheim C, Grandin C, Samson Y, Smith A, Duprez T, Marsault C, Cosnard G. Is there an apparent diffusion coefficient threshold in predicting tissue viability in hyperacute stroke? Stroke. 2001;32:2486–2491. doi: 10.1161/hs1101.098331. [DOI] [PubMed] [Google Scholar]
- 64.Chen J, Licht DJ, Smith SE, Agner SC, Mason S, Wang S, Silvestre DW, Detre JA, Zimmerman RA, Ichord RN, et al. Arterial spin labeling perfusion MRI in pediatric arterial ischemic stroke: initial experiences. J Magn Reson Imaging. 2009;29:282–290. doi: 10.1002/jmri.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsang A, Stobbe RW, Asdaghi N, Hussain MS, Bhagat YA, Beaulieu C, Emery D, Butcher KS. Relationship between sodium intensity and perfusion deficits in acute ischemic stroke. J Magn Reson Imaging. 2011;33:41–47. doi: 10.1002/jmri.22299. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki K, Igarashi H, Watanabe M, Nakamura Y, Nakada T. Separation of perfusion signals from diffusion-weighted image series enabled by independent component analysis. J Neuroimaging. 2011;21:384–394. doi: 10.1111/j.1552-6569.2010.00514.x. [DOI] [PubMed] [Google Scholar]
- 67.Sun PZ, Benner T, Copen WA, Sorensen AG. Early experience of translating pH-weighted MRI to image human subjects at 3 Tesla. Stroke. 2010;41:S147–S151. doi: 10.1161/STROKEAHA.110.595777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun PZ, Zhou JY, Sun WY, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27:1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 69.Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology. 1997;48:762–767. doi: 10.1212/wnl.48.3.762. [DOI] [PubMed] [Google Scholar]
- 70.Corbett R, Laptook A, Weatherall P. Noninvasive measurements of human brain temperature using volume-localized proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1997;17:363–369. doi: 10.1097/00004647-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, Armitage PA, Bastin ME, Dennis MS. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol. 2006;60:438–446. doi: 10.1002/ana.20957. [DOI] [PubMed] [Google Scholar]
- 72.Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Geisler BS, Brandhoff F, Fiehler J, Saager C, Speck O, Röther J, Zeumer H, Kucinski T. Blood-oxygen-level-dependent MRI allows metabolic description of tissue at risk in acute stroke patients. Stroke. 2006;37:1778–1784. doi: 10.1161/01.STR.0000226738.97426.6f. [DOI] [PubMed] [Google Scholar]
- 74.Siemonsen S, Fitting T, Thomalla G, Horn P, Finsterbusch J, Summers P, Saager C, Kucinski T, Fiehler J. T2’ imaging predicts infarct growth beyond the acute diffusion-weighted imaging lesion in acute stroke. Radiology. 2008;248:979–986. doi: 10.1148/radiol.2483071602. [DOI] [PubMed] [Google Scholar]
- 75.Santosh C, Brennan D, McCabe C, Macrae IM, Holmes WM, Graham DI, Gallagher L, Condon B, Hadley DM, Muir KW, et al. Potential use of oxygen as a metabolic biosensor in combination with T2*-weighted MRI to define the ischemic penumbra. J Cereb Blood Flow Metab. 2008;28:1742–1753. doi: 10.1038/jcbfm.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heiss WD, Sobesky J. Comparison of PET and DW/PW-MRI in acute ischemic stroke. Keio J Med. 2008;57:125–131. doi: 10.2302/kjm.57.125. [DOI] [PubMed] [Google Scholar]
- 77.Guadagno JV, Warburton EA, Aigbirhio FI, Smielewski P, Fryer TD, Harding S, Price CJ, GillardJH , Carpenter TA, Baron JC. Does the acute diffusion-weighted imaging lesion represent penumbra as well as core? A combined quantitative PET/MRI voxel-based study. J Cereb Blood Flow Metab. 2004;24:1249–1254. doi: 10.1097/01.WCB.0000141557.32867.6B. [DOI] [PubMed] [Google Scholar]
- 78.Guadagno JV, Warburton EA, Jones PS, Fryer TD, Day DJ, Gillard JH, Carpenter TA, Aigbirhio FI, Price CJ, Baron JC. The diffusion-weighted lesion in acute stroke: heterogeneous patterns of flow/metabolism uncoupling as assessed by quantitative positron emission tomography. Cerebrovasc Dis. 2005;19:239–246. doi: 10.1159/000084087. [DOI] [PubMed] [Google Scholar]
- 79.Heiss WD, Graf R, Wienhard K. Relevance of experimental ischemia in cats for stroke management: a comparative reevaluation. Cerebrovasc Dis. 2001;11:73–81. doi: 10.1159/000047616. [DOI] [PubMed] [Google Scholar]
- 80.Kim SJ, Seok JM, Bang OY, Kim GM, Kim KH, Jeon P, Chung CS, Lee KJ, Alger JR, Liebeskind DS. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29:1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma L, Gao PY, Lin Y, Xue J, Wang XC, Wang YJ, Wang YL, Liao XL, Liu ML, Cui SM, et al. Can baseline magnetic resonance angiography (MRA) status become a foremost factor in selecting optimal acute stroke patients for recombinant tissue plasminogen activator (rt-PA) thrombolysis beyond 3 hours? Neurol Res. 2009;31:355–361. doi: 10.1179/174313209X444044. [DOI] [PubMed] [Google Scholar]
- 82.Wechsler LR. Imaging evaluation of acute ischemic stroke. Stroke. 2011;42:S12–S15. doi: 10.1161/STROKEAHA.110.599555. [DOI] [PubMed] [Google Scholar]
- 83.Bai Q, Zhao Z, Li Y, Sui H, Xie X, Gong Y, Zhao X, Wang L, Xia W, Shen J, et al. The application of fast multiparametric protocol MRI-based thrombolysis with rt-PA hyperacute cerebral infarction. Neurol Res. 2008;30:344–347. doi: 10.1179/174313208X300314. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Z, Bai Q, Sui H, Xie X, Wen F. Fast multimode MRI based emergency assessment of hyperacute stroke thrombolysis. Neurol Res. 2009;31:346–350. doi: 10.1179/174313209X444053. [DOI] [PubMed] [Google Scholar]
- 85.Harrer JU. Clinical applicability and the perfusion-diffusion mismatch theory: not yet a perfect match. Neurology. 2010;75:1034–1035. doi: 10.1212/WNL.0b013e3181f6bcb3. [DOI] [PubMed] [Google Scholar]
- 86.Chemmanam T, Campbell BC, Christensen S, Nagakane Y, Desmond PM, Bladin CF, Parsons MW, Levi CR, Barber PA, Donnan GA, et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology. 2010;75:1040–1047. doi: 10.1212/WNL.0b013e3181f39ab6. [DOI] [PubMed] [Google Scholar]
- 87.Engelter ST, Wetzel SG, Bonati LH, Fluri F, Lyrer PA. The clinical significance of diffusion-weighted MR imaging in stroke and TIA patients. Swiss Med Wkly. 2008;138:729–740. doi: 10.4414/smw.2008.12249. [DOI] [PubMed] [Google Scholar]
- 88.Montiel NH, Rosso C, Chupin N, Deltour S, Bardinet E, Dormont D, Samson Y, Baillet S. Automatic prediction of infarct growth in acute ischemic stroke from MR apparent diffusion coefficient maps. Acad Radiol. 2008;15:77–83. doi: 10.1016/j.acra.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Rivers CS, Wardlaw JM, Armitage PA, Bastin ME, Carpenter TK, Cvoro V, Hand PJ, Dennis MS. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke. 2006;37:98–104. doi: 10.1161/01.STR.0000195197.66606.bb. [DOI] [PubMed] [Google Scholar]
- 90.Bates S, Read SJ, Harrison DC, Topp S, Morrow R, Gale D, Murdock P, Barone FC, Parsons AA, Gloger IS. Characterisation of gene expression changes following permanent MCAO in the rat using subtractive hybridisation. Brain Res Mol Brain Res. 2001;93:70–80. doi: 10.1016/s0169-328x(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 91.Lansberg MG, Thijs VN, Hamilton S, Schlaug G, Bammer R, Kemp S, Albers GW. Evaluation of the clinical-diffusion and perfusion-diffusion mismatch models in DEFUSE. Stroke. 2007;38:1826–1830. doi: 10.1161/STROKEAHA.106.480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barber PA, Hill MD, Eliasziw M, Demchuk AM, Pexman JH, Hudon ME, Tomanek A, Frayne R, Buchan AM. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76:1528–1533. doi: 10.1136/jnnp.2004.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tei H, Uchiyama S, Usui T. Clinical-diffusion mismatch defined by NIHSS and ASPECTS in non-lacunar anterior circulation infarction. J Neurol. 2007;254:340–346. doi: 10.1007/s00415-006-0368-8. [DOI] [PubMed] [Google Scholar]
- 94.Deguchi I, Takeda H, Furuya D, Hattori K, Dembo T, Nagoya H, Kato Y, Fukuoka T, Maruyama H, Tanahashi N. Significance of clinical-diffusion mismatch in hyperacute cerebral infarction. J Stroke Cerebrovasc Dis. 2010;20:62–67. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Ebinger M, Iwanaga T, Prosser JF, De Silva DA, Christensen S, Collins M, Parsons MW, Levi CR, Bladin CF, Barber PA, et al. Clinical-diffusion mismatch and benefit from thrombolysis 3 to 6 hours after acute stroke. Stroke. 2009;40:2572–2574. doi: 10.1161/STROKEAHA.109.548073. [DOI] [PubMed] [Google Scholar]
- 96.Schaefer PW, Barak ER, Kamalian S, Gharai LR, Schwamm L, Gonzalez RG, Lev MH. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke. 2008;39:2986–2992. doi: 10.1161/STROKEAHA.107.513358. [DOI] [PubMed] [Google Scholar]
- 97.Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, Pineda C, Serena J, van der Schaaf I, Waaijer A, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 98.Sun Z, Zhang X, Zhang Y, Guo H, Zhang J, Yu C. Estimation of the ischemic penumbra based on CT perfusion a pilot study. Acad Radiol. 2010;17:1535–1542. doi: 10.1016/j.acra.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 99.Wang XC, Gao PL, Lin Y, Xue J, Ma L, Wang CJ, Liao XL, Liu GR, Sui BB, Wang C. Diagnostic valne of CT perfusion source hnages in snperacute stroke. Zhongguo Fangshexue Zazhi. 2009;43:235–238. [Google Scholar]
- 100.Messé SR, Kasner SE, Chalela JA, Cucchiara B, Demchuk AM, Hill MD, Warach S. CT-NIHSS mismatch does not correlate with MRI diffusion-perfusion mismatch. Stroke. 2007;38:2079–2084. doi: 10.1161/STROKEAHA.106.480731. [DOI] [PubMed] [Google Scholar]
- 101.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, Sachara C, Soehngen M, Warach S, Hacke W. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 102.Chen F, Suzuki Y, Nagai N, Sun X, Wang H, Yu J, Marchal G, Ni Y. Microplasmin and tissue plasminogen activator: comparison of therapeutic effects in rat stroke model at multiparametric MR imaging. Radiology. 2007;244:429–438. doi: 10.1148/radiol.2442061316. [DOI] [PubMed] [Google Scholar]
- 103.Köhrmann M, Sauer R, Huttner HB, Engelhorn T, Doerfler A, Schellinger PD. MRI mismatch-based intravenous thrombolysis for isolated cerebellar infarction. Stroke. 2009;40:1897–1899. doi: 10.1161/STROKEAHA.108.532838. [DOI] [PubMed] [Google Scholar]
- 104.Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, Kaste M, Lipka LJ Pedraza S, Ringleb PA, Rowley HA, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cho AH, Sohn SI, Han MK, Lee DH, Kim JS, Choi CG, Sohn CH, Kwon SU, Suh DC, Kim SJ, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovasc Dis. 2008;25:572–579. doi: 10.1159/000132204. [DOI] [PubMed] [Google Scholar]
- 106.Davis SM, Donnan GA, Parsons MW, Fracp CL, Butcher KS, Peeters A, Barber PA, Bladin C, Silva DAD, Byrnes G, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 107.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fischer M, Furlan A, Kaste M, Lees KR, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 108.Miyamoto N, Naito I, Takatama S, Shimizu T, Iwai T, Shimaguchi H. Urgent stenting for patients with acute stroke due to atherosclerotic occlusive lesions of the cervical internal carotid artery. Neurol Med Chir (Tokyo) 2008;48:49–55; discussion 55-56. doi: 10.2176/nmc.48.49. [DOI] [PubMed] [Google Scholar]
- 109.Beck J, Raabe A, Lanfermann H, Berkefeld J, De Rochemont Rdu M, Zanella F, Seifert V, Weidauer S. Effects of balloon angioplasty on perfusion- and diffusion-weighted magnetic resonance imaging results and outcome in patients with cerebral vasospasm. J Neurosurg. 2006;105:220–227. doi: 10.3171/jns.2006.105.2.220. [DOI] [PubMed] [Google Scholar]
- 110.Imai K, Mori T, Izumoto H, Takabatake N, Kunieda T, Shimizu H, Watanabe M. Clot removal therapy by aspiration and extraction for acute embolic carotid occlusion. AJNR Am J Neuroradiol. 2006;27:1521–1527. [PMC free article] [PubMed] [Google Scholar]
- 111.Davis SM, Donnan GA, Butcher KS, Parsons M. Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr Opin Neurol. 2005;18:47–52. doi: 10.1097/00019052-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 112.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 113.Chen F, Suzuki Y, Nagai N, Peeters R, Marchal G, Ni Y. Dynamic susceptibility contrast-enhanced perfusion MR imaging at 1.5 T predicts final infarct size in a rat stroke model. J Neurosci Methods. 2005;141:55–60. doi: 10.1016/j.jneumeth.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 114.Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, Donnan GA, Tress BM, Davis SM. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51:418–426. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- 115.Surikova I, Meisel S, Siebler M, Wittsack HJ, Seitz RJ. Significance of the perfusion-diffusion mismatch in chronic cerebral ischemia. J Magn Reson Imaging. 2006;24:771–778. doi: 10.1002/jmri.20686. [DOI] [PubMed] [Google Scholar]
- 116.Mezzapesa DM, Petruzzellis M, Lucivero V, Prontera M, Tinelli A, Sancilio M, Carella A, Federico F. Multimodal MR examination in acute ischemic stroke. Neuroradiology. 2006;48:238–246. doi: 10.1007/s00234-005-0045-0. [DOI] [PubMed] [Google Scholar]
- 117.Kane I, Sandercock P, Wardlaw J. Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date. J Neurol Neurosurg Psychiatry. 2007;78:485–491. doi: 10.1136/jnnp.2006.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hillis AE, Wityk RJ, Barker PB, Ulatowski JA, Jacobs MA. Change in perfusion in acute nondominant hemisphere stroke may be better estimated by tests of hemispatial neglect than by the National Institutes of Health Stroke Scale. Stroke. 2003;34:2392–2396. doi: 10.1161/01.STR.0000089681.84041.69. [DOI] [PubMed] [Google Scholar]
- 119.Heidenreich JO, Hsu D, Wang G, Jesberger JA, Tarr RW, Zaidat OO, Sunshine JL. Magnetic resonance imaging results can affect therapy decisions in hyperacute stroke care. Acta Radiol. 2008;49:550–557. doi: 10.1080/02841850801958320. [DOI] [PubMed] [Google Scholar]
- 120.Stoeckel MC, Wittsack HJ, Meisel S, Seitz RJ. Pattern of cortex and white matter involvement in severe middle cerebral artery ischemia. J Neuroimaging. 2007;17:131–140. doi: 10.1111/j.1552-6569.2007.00102.x. [DOI] [PubMed] [Google Scholar]
- 121.Ogata T, Nagakane Y, Christensen S, Ma H, Campbell BC, Churilov L, Olivot JM, Desmond PM, Albers GW, Davis SM, et al. A topographic study of the evolution of the MR DWI/PWI mismatch pattern and its clinical impact: a study by the EPITHET and DEFUSE Investigators. Stroke. 2011;42:1596–1601. doi: 10.1161/STROKEAHA.110.609016. [DOI] [PubMed] [Google Scholar]
- 122.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, Wu O, Gonzalez RG, Koroshetz WJ, Sorensen AG. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 123.Fiebach JB, Hopt A, Vucic T, Brunecker P, Nolte CH, Doege C, Villringer K, Jungehulsing GJ, Kunze C, Wegener S, et al. Inverse mismatch and lesion growth in small subcortical ischaemic stroke. Eur Radiol. 2010;20:2983–2989. doi: 10.1007/s00330-010-1858-8. [DOI] [PubMed] [Google Scholar]
- 124.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bang OY, Saver JL, Lee KH, Kim GM, Chung CS, Kim SJ, Ovbiagele B, Alger JR, Liebeskind DS. Characteristics of patients with target magnetic resonance mismatch profile: data from two geographically and racially distinct populations. Cerebrovasc Dis. 2010;29:87–94. doi: 10.1159/000256653. [DOI] [PubMed] [Google Scholar]
- 126.Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, Moseley ME, Marks MP, Albers GW. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab. 2008;28:887–891. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baird AE, Benfield A, Schlaug G, Siewert B, Lövblad KO, Edelman RR, Warach S. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 128.Restrepo L, Jacobs MA, Barker PB, Wityk RJ. Assessment of transient ischemic attack with diffusion- and perfusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25:1645–1652. [PMC free article] [PubMed] [Google Scholar]
- 129.Ma H, Zavala JA, Teoh H, Churilov L, Gunawan M, Ly J, Wright P, Phan T, Arakawa S, Davis SM, et al. Fragmentation of the classical magnetic resonance mismatch “penumbral” pattern with time. Stroke. 2009;40:3752–3757. doi: 10.1161/STROKEAHA.109.555011. [DOI] [PubMed] [Google Scholar]
- 130.Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology. 2009;72:1134–1139. doi: 10.1212/01.wnl.0000345360.80382.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen F, Suzuki Y, Nagai N, Sun X, Coudyzer W, Yu J, Marchal G, Ni Y. Delayed perfusion phenomenon in a rat stroke model at 1.5 T MR: an imaging sign parallel to spontaneous reperfusion and ischemic penumbra? Eur J Radiol. 2007;61:70–78. doi: 10.1016/j.ejrad.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 132.Ma HK, Zavala JA, Churilov L, Ly J, Wright PM, Phan TG, Arakawa S, Davis SM, Donnan GA. The hidden mismatch: an explanation for infarct growth without perfusion-weighted imaging/diffusion-weighted imaging mismatch in patients with acute ischemic stroke. Stroke. 2011;42:662–668. doi: 10.1161/STROKEAHA.110.593236. [DOI] [PubMed] [Google Scholar]
- 133.Cho TH, Hermier M, Alawneh JA, Ritzenthaler T, Desestret V, Østergaard L, Derex L, Baron JC, Nighoghossian N. Total mismatch: negative diffusion-weighted imaging but extensive perfusion defect in acute stroke. Stroke. 2009;40:3400–3402. doi: 10.1161/STROKEAHA.109.563064. [DOI] [PubMed] [Google Scholar]
- 134.Blondin D, Seitz RJ, Rusch O, Janssen H, Andersen K, Wittsack HJ, Turowski B. Clinical impact of MRI perfusion disturbances and normal diffusion in acute stroke patients. Eur J Radiol. 2009;71:1–10. doi: 10.1016/j.ejrad.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 135.Woods RP, Iacoboni M, Mazziotta JC. Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med. 1994;331:1689–1692. doi: 10.1056/NEJM199412223312505. [DOI] [PubMed] [Google Scholar]
- 136.Wohlgemuth WA, Schulte-Altedorneburg G, Becker T, Zha L, Kramer D, Kirchhof K. Evaluation of a new Spin-echo diffusion-weighted sequence on a 0.35 T open magnetic resonance imaging (MRI)-system: first experiences within 3 h after acute stroke. Neuroradiology. 2005;47:532–538. doi: 10.1007/s00234-005-1393-5. [DOI] [PubMed] [Google Scholar]
- 137.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, Schlaug G, Bammer R, Marks MP, Albers GW. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39:2257–2263. doi: 10.1161/STROKEAHA.107.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pedraza S, Puig J, Blasco G, Daunis-i-Estadella J, Boada I, Bardera A, Prats A, Castellanos M, Serena J. Magnetic resonance imaging biomarkers of ischemic stroke: criteria for the validation of primary imaging biomarkers. Drug News Perspect. 2009;22:481–486. [PubMed] [Google Scholar]