Abstract
The purpose of this essay was to illustrate the radiological and pathological findings in a wide spectrum of dural lesions mimicking meningiomas. Familiarity with and knowledge of these findings will narrow the differential diagnosis and provide guidance for patient management. In this pictorial review, we describe the following entities: Solitary fibrous tumors, hemangiopericytoma, gliosarcoma, leiomyosarcoma, dural metastases, Hodgkin’s disease, plasmocytoma, Rosai-Dorfman disease, neurosarcoidosis, melanocytic neoplasms and plasma cell granuloma.
Keywords: Differential diagnosis, Dural lesions, Imaging, Meningiomas
INTRODUCTION
There are multiple neoplastic and non-neoplastic entities that clinically and radiographically mimic meningiomas. This pictorial review considers some of the lesions that can mimic meningiomas, including solitary fibrous tumors, hemangiopericytoma, gliosarcoma, leiomyosarcoma, dural metastases, Hodgkin’s disease, plasmocytoma, Rosai-Dorfman disease, neurosarcoidosis, melanocytic neoplasms and plasma cell granuloma.
SOLITARY FIBROUS TUMORS
Central nervous system (CNS) solitary fibrous tumors are rare, dural-based mesenchymal neoplasms. Although initially described as primary neoplasms of the pleura, solitary fibrous tumors have subsequently been reported in virtually every body site[1]. The typical histological features of solitary fibrous tumors are spindle cells arranged in a patternless architecture or interlacing fascicles, prominent collagenous bands, and branching vascular channels with thin walls; these tumors exhibit diffuse CD34 reactivity[2]. The tumors present at any age and have been reported to occur in both supra and infratentorial locations.
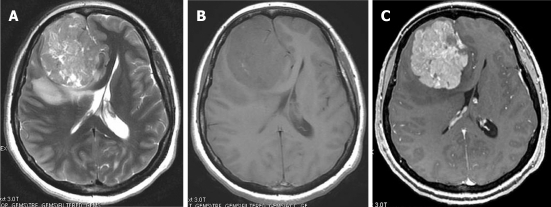
On computed tomography (CT), the mass is most often hyperdense and contains some calcifications. The appearance on magnetic resonance imaging (MRI) is that of an extra-axial, dural-based mass predominantly isointense on T1- and T2-weighted images. Intra-lesional inhomogeneity with areas of hypointensity and hyperintensity, best described as having a patchy appearance, has been reported. In most cases, homogenous enhancement on post-contrast T1-weighted images is seen (Figure 1). Surgery is the treatment of choice. Stereotactic and external beam radiation therapy may be indicated for postsurgical tumor remnants and for unresectable recurrences[3].
Figure 1.
Solitary fibrous tumor. Post-contrast computed tomography. A: A dural-based lobulated mass with dural tail enhancement (arrow); B: Axial T1-weighted magnetic resonance (MR) image shows an extra-axial mass, isointense to the gray matter compressing the adjacent cortical convolutions (arrow); C: Axial T2-weighted MR image shows that the mass has intermediate signal intensity. Note the peritumoral cysts with high signal intensity (arrow); D: On the post-contrast, T1-weighted MR image, the mass shows intense enhancement, intra-tumoral cysts (arrowheads) and dural tail sign (arrow).
HEMANGIOPERICYTOMA
Intracranial hemangiopericytomas are neoplasms of the pericytes that originate in the meninges, represent less than 1% of all CNS tumors, are aggressive and tend to occur at an earlier age than other meningeal tumors, recur with high frequency, and metastasize extra-cranially. The location of intracranial hemangiopericytomas is similar to that of meningiomas. Histologically, hemangiopericytomas are highly cellular, vascular tumors composed of angular pericytes surrounding often ill-defined capillaries in a branching pattern (staghorn vascularity).
On CT, hemangiopericytomas are heterogeneous, hyperdense, dural-based lesions that, unlike meningiomas, are not associated with calcifications or hyperostosis, and they typically show heterogeneous enhancement. Bone erosion is a feature seen in more than half of hemangiopericytoma cases[4,5]. On MRI, hemangiopericytomas are heterogeneous, predominantly isointense masses on T1- and T2-weighted images, show prominent internal vessel voids, and heterogeneously enhance on contrast-enhanced images (Figure 2). Approximately one-third of hemangiopericytomas exhibit a narrow base of dural attachment, with the remaining two-thirds showing broad-based attachment with a dural tail sign[6]. Surgical resection is the primary treatment for this tumor. Preoperative embolization can aid in reducing operative blood loss. Postoperative radiation therapy is recommended[7].
Figure 2.
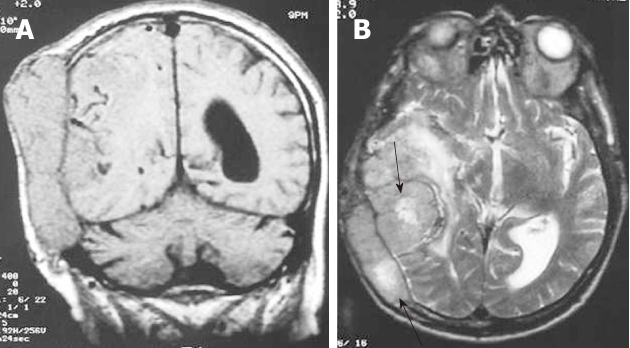
Hemangiopericytoma. A: The coronal T1-weighted magnetic resonance (MR) image shows an extra-axial isointense mass with internal flow voids and calvarial destruction; B: On the axial, T2-weighted MR image the mass remains isointense with internal areas of hyperintensity and moderate peritumoral edema.
GLIOSARCOMA
Gliosarcoma is a rare cerebral tumor closely related to glioblastoma in terms of both its clinical and therapeutic characteristics. Gliosarcoma consists of gliomatous and sarcomatous elements (Figure 3A)[8].
Figure 3.
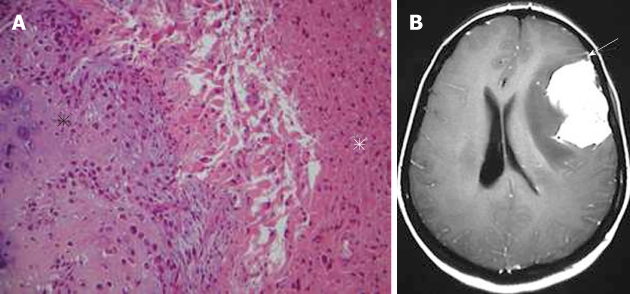
Gliosarcoma. A: Pathological image showing an area with features of osteogenic sarcoma (black asterisk) adjacent to the glial component of the tumor (white asterisk) (hematoxylin-eosin, original magnification × 100); B: On the axial, post-contrast T1-weighted magnetic resonance image, the tumor shows intense homogeneous enhancement. Note the close relation of the mass with the dura, which is enhanced in a way mimicking meningioma (arrow).
Gliosarcoma usually occurs in middle aged adults. The ability of gliosarcoma to metastasize to other locations in the neuroaxis is well known[9].
Radiation-induced gliosarcoma may appear at the site of a treated intracranial neoplasm and should be considered in the differential diagnosis of a recurrent mass[10].
On CT, gliosarcoma tends to present as a peripherally located, sharply defined, round or lobulated, isodense or hyperdense solid mass with heterogeneous or ring enhancement due to a fibrous component. On MRI, the tumors tend to be well-defined, demonstrating either an inhomogeneous or cystic appearance with surrounding vasogenic edema. On T2-weighted images, intermediate signal intensity with surrounding edema is visible. The signal intensity of the tumor is similar to gray matter but is hypointense relative to other glial neoplasms. Post-contrast T1-weighted images show intense tumor enhancement, often with a ring-like appearance (Figure 3B)[11]. Postsurgical treatment of gliosarcoma includes adjuvant radiotherapy with concurrent administration of temozolomide[12].
LEIOMYOSARCOMAS
Leiomyosarcomas of the CNS are extremely rare; however, they are becoming more prevalent in immunocompromised patients. Patients with acquired immunodeficiency syndrome are known to exhibit an increased incidence of smooth muscle tumors associated with Epstein virus. Intracranial leiomyosarcoma reveals dural masses or a skull-base location. Leiomyosarcoma should be included in the differential diagnosis of extra-axial CNS lesions in human immunodeficiency virus (HIV)-infected patients[13].
DURAL METASTASES
Metastases to the dura constitute one of the least frequent patterns of neoplastic spread to the craniospinal axis. The neoplasms arise either by direct extension from skull metastases or by hematogeneous spread. Breast cancer, prostate cancer, adenocarcinoma of the lung, and renal cell carcinoma are the most common. Meningeal metastasis from medulloblastoma is also a well known entity[14]. Typically, these tumors produce MR images with increased signals on T2-weighted images, often with an enhancing dural tail mimicking a meningioma (Figure 4). Patients with a single resectable symptomatic dural metastasis, controlled systemic cancer, and acceptable surgical risk should be considered for resection as first-line therapy.
Figure 4.
Dural metastasis in a 36-year-old woman with breast carcinoma. A: Axial T2-weighted image shows a large extra-axial frontal heterogeneous mass; B: On T1-weighted magnetic resonance (MR) image the mass is isointense with the gray matter; C: Axial post-contrast T1-weighted MR image shows an extra-axial strongly enhanced lesion broad based to the falx mimicking meningioma.
HODGKIN’S DISEASE
Intracranial Hodgkin’s disease occurs by direct spread from the bones of the skull or in continuity with meningeal deposits that gained access to the skull via the cranial nerves[15,16]. The involvement of bone in Hodgkin’s lymphomas usually occurs in the late stage of the disease. Extension of the scalp mass to both the epidural space and subcutaneous soft tissue is usually seen.
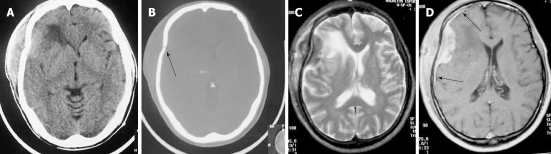
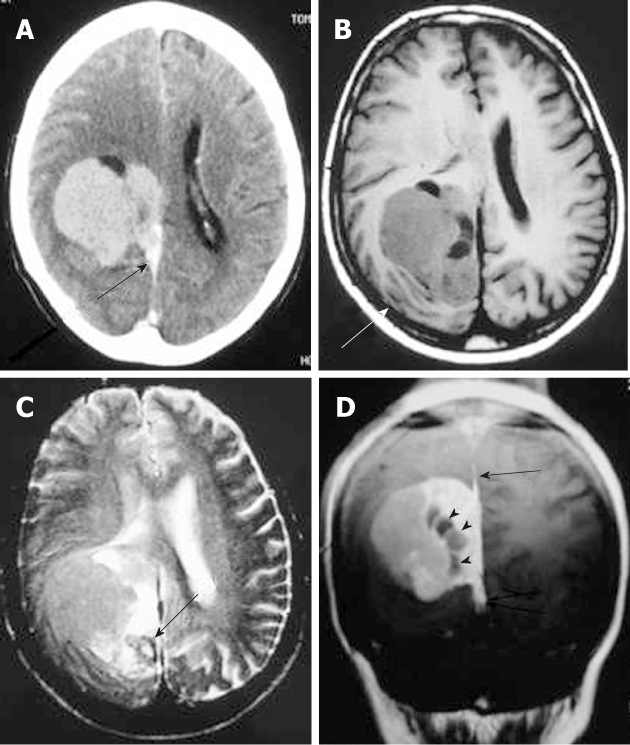
Regarding the histology of Hodgkin’s disease with intracranial relapse, both a nodular sclerosis and mixed cellularity variety have been described. In the majority of reported skull lymphomas, the mass is hyperdense on unenhanced CT and shows marked enhancement on post-contrast studies. Destruction of the bone may not be seen in some cases because the characteristic permeating growth pattern of lymphoma is a large soft tissue component and very little bone destruction. Bone window setting is useful for depicting tiny lytic lesions of the bony calvarium (Figure 5A and B).
Figure 5.
Non-Hodgkin Lymphoma. The axial non-contrast computed tomography. A: A hyperdense extra-axial mass extending to both sides of the frontoparietal bone; B: Bone window setting depict lytic lesion of the calvarium (arrow); C: On axial T2-weighted magnetic resonance images, the mass appears isointense with peritumoral edema; D: After the administration of contrast media, a strong homogeneous enhancement is seen with a dural tail (arrows).
On MRI, the densely cellular deposits of lymphoma most often do not show high signal intensity on T2-weighted images and typically remain hypo- to isointense on all pulse sequences. Post-contrast T1-weighted images show either homogeneous or inhomogeneous dense enhancement. Linear dural enhancement is usually seen on both sides of the dural mass (dural tail) and, although it is an extra-axial mass, white matter edema is usually present (Figure 5C-D)[17]. Radiotherapy and chemotherapy are the treatment of choice.
PLASMOCYTOMA
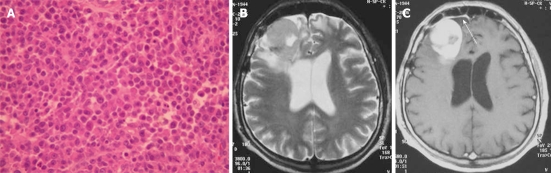
Plasmocytoma rarely involve the CNS as dural-based lesions[18]. Careful systemic evaluation should be made in such a presentation to rule out multiple myeloma which would require different management and prognosis. Some patients develop bone marrow biopsy-proven myeloma within months of the initial diagnosis of CNS plasmocytoma[19]. Histopathological examination of the mass after excision shows multiple myeloma immunopositive for IgG, kappa light chain, and CD38 (Figure 6A). On CT, plasmacytomas are extra-axial masses without calcification or bone involvement, and they are usually high-density lesions. An isointense lesion is revealed on both T1- and T2-weighted images. On post-contrast T1-weighted images, intense homogeneous contrast enhancement is demonstrated with a dural tail sign mimicking meningioma (Figure 6B and C)[20]. Systemic chemotherapy and radiotherapy are recommended after surgical excision of the mass.
Figure 6.
Plasmocytoma in a 62-year-old patient. A: Typical plasma cell morphology hematoxylin-eosin stain; B: Axial T2-weighted magnetic resonance images show an isointense frontal mass with peritumoral edema compressing the right ventricle; C: The axial post-contrast image shows intense homogeneous enhancement of the lesion with a dural tail (arrow).
ROSAI-DORFMAN DISEASE
Rosai-Dorfman disease is also known as sinus histiocytosis with massive lymphadenopathy. The disease commonly presents as massive, painless, bilateral lymph node enlargement in the neck with fever. Rarely, sites other than the lymph nodes are involved, including the CNS, eyes, upper respiratory tract, skin, and head and neck region[21]. CNS Rosai-Dorfman most commonly involves patients between 20-40 years.
Symptoms of intracranial disease include seizures, headache and weakness. Over 90% of CNS Rosai-Dorfman cases involve the leptomeninges. Histopathologically, the dura is thickened, fibrotic, and contains a variable density of chronic inflammatory cells, dominated by lymphocytes and plasma cells. The finding of emperipolesis is characteristic of Rosai-Dorfman disease of the leptomeninges, but in 30% of cases this feature is not identified[22].
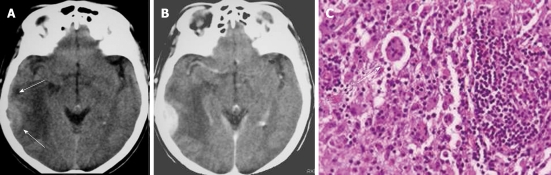
On CT and MRI, Rosai-Dorfman disease presents as dural-based, epidural or subdural, contrast-enhancing masses that often elicit vasogenic edema in the underlying cerebral cortex and white matter (Figure 7A and B). The dural mass shows low signal intensity on T2-weighted images. Thus, clinically and radiologically, the disease is thought to represent meningioma[23]. The definitive diagnosis of Rosai-Dorfman disease involving the leptomeninges is established by biopsy (Figure 7C) and pathological examination. Treatment should be based on clinical manifestations. Many lesions are asymptomatic, heal spontaneously, and do not require treatment[24].
Figure 7.
Rosai-Dorfman disease. A: The non-contrast computed tomography (CT) shows a peripheral, poorly marginated, hyperdense mass with peritumoral edema (arrows); B: Post-contrast CT shows a convex, homogeneously enhanced extra-axial mass; C: Pathologic image of Rosai-Dorfman disease with a characteristic finding of emperipolesis (arrow).
NEUROSARCOIDOSIS
Neurosarcoidosis occurs in approximately 5% of patients with sarcoidosis[25]. Diagnosis is based on the documentation of systemic sarcoidosis in the absence of other neurological disease. The histopathological hallmarks of neurosarcoidosis include epithelioid granulomas without caseation or staining for infectious agents. Imaging findings of neurosarcoidosis include pachymeningeal/dural masses, leptomeningeal involvement, enhancing brain parenchymal lesion, and cranial nerve involvement.
A dural-based mass is one of the least common manifestations of neurosarcoidosis.
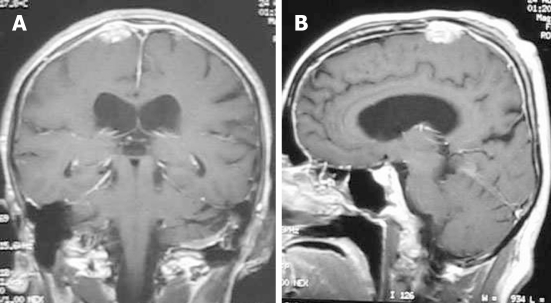
Lesions typically homogeneously enhance on contrast-enhanced T1-weighted images (Figure 8). Variable amounts of vasogenic edema can also be seen in the white matter adjacent to the mass[26,27]. Differential considerations include lymphoma and idiopathic hypertrophic cranial pachymeningitis. Steroid therapy is usually indicated. Infliximab is a valuable pharmacological agent in the management of patients with refractory and disabling neurosarcoidosis[28].
Figure 8.
Neurosarcoidosis. Coronal (A) and sagittal (B) T1-weighted magnetic resonance post-contrast images show an enhancing dural-based lesion. Also, note the enhancement of the dura along the convexity.
MELANOCYTIC NEOPLASMS
Melanocytes are normally present in the intracranial leptomeninges, usually in the posterior cerebral fossa, and can give rise to benign (meningeal melanocytoma) or malignant (malignant melanoma) tumors. Most reported cases of meningeal melanocytomas are located in the posterior fossa and spinal cord, although large supratentorial meningeal melanocytomas mimicking a convexity meningioma have been reported[29].
Histologically, most melanocytomas are spindle cell neoplasms, although epithelioid cytology may dominate in some cases. Tumor cells grow in sheets, nests, or fascicles (Figure 9A).
Figure 9.
Melanocytic tumor. A: Photomicrograph of the mass. The neoplasm contains sheets of cells, many of which contain dark brown melanin pigment (hematoxylin-eosin, × 400); B: T1-weighted magnetic resonance and post-contrast; C: T1-weighted MR images show a left intraconal mass with intermediate signal intensity that homogeneously enhances.
On CT, primary melanocytic lesions appear as well-circumscribed, isoattenuating to hyperattenuating, extra-axial tumors with homogeneous enhancement. Even if there is no hyperostosis, the neoplasms can mimic meningioma[30]. MRI demonstrates variable signal intensity on T1- and T2-weighted images, in proportion to the amount of melanin within the tumor. Therefore, melanocytic neoplasms are isointense or hyperintense to adjacent normal brain tissue on T1-weighted images and isointense or hypointense on T2-weighted images. The neoplasms enhance after intravenous administration of contrast material (Figure 9B and C)[31]. Complete surgical excision of the tumor is the treatment of choice. The role and efficacy of radiotherapy and chemotherapy remain controversial.
PLASMA CELL GRANULOMA
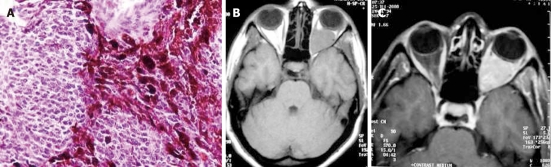
Plasma cell granuloma is a rare form of idiopathic inflammatory pseudotumor often characterized by non-neoplastic proliferation of plasma cells clinically mimicking a neoplastic process. Pseudotumors of the CNS, however, are exceptional and rare. In reported cases, these reactive lesions occurred primarily in adults, usually presenting as discrete tumors arising from the leptomeninges or dura. Histologically, the granulomas are populated by a polytypic population of mature plasma cells, plasmatoids, and small non-transformed lymphocytes in a background of variable fibrosis, fibroblasts, and entrapped leptomeningeal cells (Figure 10A)[32].
Figure 10.
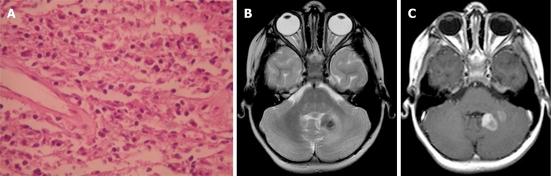
Plasma cell granuloma in a 4-year-old girl. A: Pathologic image. The fibrosis and infiltration is composed of lymphocytes, plasmocytes and histiocytes (hematoxylin-eosin stain); B: Axial T2-weighted magnetic resonance (MR) image shows a hypointense left cerebellar lesion adjacent to the 4th ventricle with moderate peritumoral edema; C: Post-contrast T1-weighted MR image shows heterogeneous enhancement of the lesion.
On CT, the mass is usually a well-circumscribed area of increased density markedly enhanced with the administration of contrast. On MRI, the mass has slightly high signal intensity on T1-weighted images and marked low signal intensity on T2-weighted images. The granuloma is usually heterogeneously enhanced with gadolinium-DTPA (Figure 10B and C). Parenchymal involvement has been reported[33].
CONCLUSION
The differential diagnosis of dural-based lesions in the brain varies from incidental and benign to symptomatic and malignant lesions. The above mentioned entities involving the dura are rare and almost always diagnosed after tissue is obtained because of their clinical and radiographic similarity to meningiomas. These lesions can closely resemble meningiomas in terms of signal characteristics, enhancement pattern, and location. Imaging characteristics alone can be misleading; neuropathological support is essential for accurate diagnosis.
A thorough clinical evaluation can reveal likely diagnostic possibilities. In the presence of a heterogeneous dural-based mass with prominent internal vessels and bone erosion, the case of hemangiopericytoma should be considered. Leiomyosarcomas should be included in the differential diagnosis of dural-based lesions in HIV-infected patients.
Careful vigilance in patients with a history of cancer, presenting with new symptoms or imaging evidence of dural-based lesions, should raise the possibility of dural metastasis.
The diagnosis of lymphoma should be considered for lesions affecting the dura in high-risk immunocompromised patients. The presence of an apparent dural tail can be seen in a lymphoma. The absence of hyperostosis helps differentiate lymphoma from meningioma.
Hypercalcemia, hyperproteinemia, and serum gamma globulin peak in a patient with an extra-axial dural mass should raise the possibility of plasmocytoma. Rosai-Dorfman disease should be considered when a young adult with painless neck lymphadenopathy, fever and anemia presents with a dural mass. The already known history of systemic sarcoidosis in a patient with a dural mass and leptomeningeal enhancement is suggestive of neurosarcoidosis. Awareness that these lesions involve the dura may facilitate radiological and intra-operative recognition and, in some cases, preclude unnecessary additional surgery [34].
Footnotes
Peer reviewers: Tetsu Niwa, MD, PhD, Department of Radiology, Kanagawa Children’s Medical Center, 2-138-4 Mutsukawa, Minamiku, Yokohama 252-8555, Japan; Farideh Nejat, MD, MPH, Department of Neurosurgery, Children’s Hospital Medical Center, Tehran University of Medical Sciences, Gharib Street, Mail Box 14155-7854, Tehran, Iran; Aaron Cohen-Gadol, MD, MSc, Clinical Assistant Professor, Indiana University Department of Neurosurgery, Clarian Neuroscience Center, 1801 North Senate Blvd, Suite 610, Indianapolis, IN 46202, United States; Tayfun Hakan, MD, Neurosurgery Clinic, Haydarpasa Numune Teaching and Research Hospital, Tibbiye Caddesi, Uskudar 34668, Istanbul, Turkey
S- Editor Cheng JX L- Editor Webster JR E- Editor Xiong L
References
- 1.Caroli E, Salvati M, Orlando ER, Lenzi J, Santoro A, Giangaspero F. Solitary fibrous tumors of the meninges: report of four cases and literature review. Neurosurg Rev. 2004;27:246–251. doi: 10.1007/s10143-004-0331-z. [DOI] [PubMed] [Google Scholar]
- 2.Westra WH, Gerald WL, Rosai J. Solitary fibrous tumor. Consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol. 1994;18:992–998. doi: 10.1097/00000478-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bisceglia M, Galliani C, Giannatempo G, Lauriola W, Bianco M, D’angelo V, Pizzolitto S, Vita G, Pasquinelli G, Magro G, et al. Solitary fibrous tumor of the central nervous system: a 15-year literature survey of 220 cases (August 1996-July 2011) Adv Anat Pathol. 2011;18:356–392. doi: 10.1097/PAP.0b013e318229c004. [DOI] [PubMed] [Google Scholar]
- 4.Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996;17:1365–1371. [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama M, Sakai H, Onoue H, Miyazaki Y, Abe T. Imaging intracranial haemangiopericytomas: study of seven cases. Neuroradiology. 2004;46:194–197. doi: 10.1007/s00234-003-1157-z. [DOI] [PubMed] [Google Scholar]
- 6.Fountas KN, Kapsalaki E, Kassam M, Feltes CH, Dimopoulos VG, Robinson JS, Smith JR. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006;29:145–153. doi: 10.1007/s10143-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan A, Demonte F. Tumors of the meninges. Handb Clin Neurol. 2012;105:641–656. doi: 10.1016/B978-0-444-53502-3.00014-8. [DOI] [PubMed] [Google Scholar]
- 8.Meis JM, Martz KL, Nelson JS. Mixed glioblastoma multiforme and sarcoma: a clinicopathologic study of 26 Radiation Therapy Oncology Group cases. Cancer. 1991;67:2342–2349. doi: 10.1002/1097-0142(19910501)67:9<2342::aid-cncr2820670922>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Witwer BP, Salamat MS, Resnick DK. Gliosarcoma metastatic to the cervical spinal cord: case report and review of the literature. Surg Neurol. 2000;54:373–378. doi: 10.1016/s0090-3019(00)00315-3. [DOI] [PubMed] [Google Scholar]
- 10.Lach M, Wallace CJ, Krcek J, Curry B. Radiation-associated gliosarcoma. Can Assoc Radiol J. 1996;47:209–212. [PubMed] [Google Scholar]
- 11.Dwyer KW, Naul LG, Hise JH. Gliosarcoma: MR features. J Comput Assist Tomogr. 1996;20:719–723. doi: 10.1097/00004728-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Han SJ, Yang I, Ahn BJ, Otero JJ, Tihan T, McDermott MW, Berger MS, Prados MD, Parsa AT. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer. 2010;116:1358–1366. doi: 10.1002/cncr.24857. [DOI] [PubMed] [Google Scholar]
- 13.Lerdlum S, Lalitanantpong S, Numkarunarunrote N, Chaowanapanja P, Suankratay C, Shuangshoti S. MR imaging of CNS leiomyosarcoma in AIDS patients. J Med Assoc Thai. 2004;87 Suppl 2:S152–S160. [PubMed] [Google Scholar]
- 14.Maroldi R, Ambrosi C, Farina D. Metastatic disease of the brain: extra-axial metastases (skull, dura, leptomeningeal) and tumour spread. Eur Radiol. 2005;15:617–626. doi: 10.1007/s00330-004-2617-5. [DOI] [PubMed] [Google Scholar]
- 15.Hirmiz K, Foyle A, Wilke D, Burrell S, Brownstone R, Ago C, Pahil R, Couban S. Intracranial presentation of systemic Hodgkin’s disease. Leuk Lymphoma. 2004;45:1667–1671. doi: 10.1080/10428190410001673409. [DOI] [PubMed] [Google Scholar]
- 16.Guermazi A, Brice P, de Kerviler E E, Fermé C, Hennequin C, Meignin V, Frija J. Extranodal Hodgkin disease: spectrum of disease. Radiographics. 2001;21:161–179. doi: 10.1148/radiographics.21.1.g01ja02161. [DOI] [PubMed] [Google Scholar]
- 17.Thurnher MM, Rieger A, Kleibl-Popov C, Schindler E. Malignant lymphoma of the cranial vault in an HIV-positive patient: imaging findings. Eur Radiol. 2001;11:1506–1509. doi: 10.1007/s003300000754. [DOI] [PubMed] [Google Scholar]
- 18.Sahin F, Saydam G, Ertan Y, Calli C, Dönmez A, Tombuloglu M. Dural plasmacytoma mimicking meningioma in a patient with multiple myeloma. J Clin Neurosci. 2006;13:259–261. doi: 10.1016/j.jocn.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Haegelen C, Riffaud L, Bernard M, Carsin-Nicol B, Morandi X. Dural plasmacytoma revealing multiple myeloma. Case report. J Neurosurg. 2006;104:608–610. doi: 10.3171/jns.2006.104.4.608. [DOI] [PubMed] [Google Scholar]
- 20.Gallina P, Mascalchi M, Mouchaty H, Buccoliero A, Perrini P. Misleading imaging features of intracranial dural plasmacytoma: report of two cases. Br J Neurosurg. 2004;18:643–646. doi: 10.1080/02688690400022748. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Anderson AE, Kahn LB. A report of intracranial Rosai-Dorfman disease with literature review. Ann Diagn Pathol. 2001;5:96–102. doi: 10.1053/adpa.2001.23027. [DOI] [PubMed] [Google Scholar]
- 22.Siadati A, Powell SZ, Shahab I, Valadka AB, Parker JR. Pathologic quiz case: a 48 year-old woman with a dural-based intracranial tumor. Arch Pathol Lab Med. 2001;125:1115–1116. doi: 10.5858/2001-125-1115-PQCAYO. [DOI] [PubMed] [Google Scholar]
- 23.Konishi E, Ibayashi N, Yamamoto S, Scheithauer BW. Isolated intracranial Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) AJNR Am J Neuroradiol. 2003;24:515–518. [PMC free article] [PubMed] [Google Scholar]
- 24.Hadjipanayis CG, Bejjani G, Wiley C, Hasegawa T, Maddock M, Kondziolka D. Intracranial Rosai-Dorfman disease treated with microsurgical resection and stereotactic radiosurgery. Case report. J Neurosurg. 2003;98:165–168. doi: 10.3171/jns.2003.98.1.0165. [DOI] [PubMed] [Google Scholar]
- 25.Stern BJ. Neurological complications of sarcoidosis. Curr Opin Neurol. 2004;17:311–316. doi: 10.1097/00019052-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Lexa FJ, Grossman RI. MR of sarcoidosis in the head and spine: spectrum of manifestations and radiographic response to steroid therapy. AJNR Am J Neuroradiol. 1994;15:973–982. [PMC free article] [PubMed] [Google Scholar]
- 27.Christoforidis GA, Spickler EM, Recio MV, Mehta BM. MR of CNS Sarcoidosis: Correlation of Imaging Features to Clinical Symptoms and Response to Treatment. AJNR Am J Neuroradiol. 1999;20:655–669. [PMC free article] [PubMed] [Google Scholar]
- 28.Toth C, Martin L, Morrish W, Coutts S, Parney I. Dramatic MRI improvement with refractory neurosarcoidosis treated with infliximab. Acta Neurol Scand. 2007;116:259–262. doi: 10.1111/j.1600-0404.2007.00870.x. [DOI] [PubMed] [Google Scholar]
- 29.Beseoglu K, Knobbe CB, Reifenberger G, Steiger HJ, Stummer W. Supratentorial meningeal melanocytoma mimicking a convexity meningioma. Acta Neurochir (Wien) 2006;148:485–490. doi: 10.1007/s00701-005-0705-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen CJ, Hsu YI, Ho YS, Hsu YH, Wang LJ, Wong YC. Intracranial meningeal melanocytoma: CT and MRI. Neuroradiology. 1997;39:811–814. doi: 10.1007/s002340050510. [DOI] [PubMed] [Google Scholar]
- 31.Naul LG, Hise JH, Bauserman SC, Todd FD. CT and MR of meningeal melanocytoma. AJNR Am J Neuroradiol. 1991;12:315–316. [PMC free article] [PubMed] [Google Scholar]
- 32.Häusler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B. Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. Hum Pathol. 2003;34:253–262. doi: 10.1053/hupa.2003.35. [DOI] [PubMed] [Google Scholar]
- 33.Ozüm U, Ozer H, Karadağ O, Polat N. Intracranial plasma cell-granuloma with extensive ossification. Br J Neurosurg. 2006;20:153–156. doi: 10.1080/02688690600776978. [DOI] [PubMed] [Google Scholar]
- 34.Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002;33:1211–1226. doi: 10.1053/hupa.2002.129200. [DOI] [PubMed] [Google Scholar]