Abstract
AIM: To evaluate magnetic resonance imaging (MRI) features of different types of gray matter heterotopia.
METHODS: Between June 2005 and December 2009, the medical records and MRI studies of patients with gray matter heterotopia were reviewed. The MRI morphologic findings of heterotopia were recorded along with the presence and type of associated cranial malformations. Available clinical and electrophysiological data were also recorded.
RESULTS: 20 patients were included in the study. Their ages ranged from 9 mo to 39 years with a mean age of 15 years. All patients suffered from epileptic seizures. According to the location of heterotopia, patients were classified into three groups: subependymal (12), subcortical (5) and band (3) heterotopia.
CONCLUSION: MRI was useful in diagnosing and differentiating between various types of gray matter heterotopia. The severity of clinical manifestations of heterotopia was related to the location and pattern of heterotopia. Determination of heterotopia type and its extent is useful for management planning and predicting prognosis.
Keywords: Epilepsy, Gray matter heterotopias, Magnetic resonance imaging, Neuroradiology, Neuroscience
INTRODUCTION
Gray matter heterotopia is a relatively common form of neuronal migration disorder in which collections of cortical neurons are found in an abnormal location. It results from an in utero arrest of radial migration of neurons from the germinal matrix in the wall of the lateral ventricle to the developing cerebral cortex between 6 and 16 weeks of gestation[1,2]. It is usually discovered during the evaluation of children or young adults with epilepsy, children with neurodevelopmental abnormalities, or as an incidental finding[3-5].
The pathogenic mechanisms of gray matter heterotopia are not fully understood, but they lead to distinct clinicoradiological syndromes. Pathological classification of heterotopia includes nodular, laminar, and leptomeningeal, (double cortex or band heterotopia)[1,3,6]. This classification is not particularly useful from a clinical perspective[1].
Neuroradiologists, basing their ideas on magnetic resonance imaging (MRI) appearances, have classified heterotopia into subependymal, subcortical, and band heterotopia[3,7-9]. The three forms of heterotopia were classified on the basis of the location and configuration of the ectopic gray matter tissue. Subependymal heterotopia consists of small foci of gray matter that are located in a subependymal location in close proximity to the ventricular wall. Subcortical heterotopia occurs as masses of gray matter within the deep and subcortical white matter. Band heterotopia was described as a symmetrical thick band of gray matter with smooth inner and outer margins that lies between layers of white matter[10-13]. The purpose of this study is to evaluate MRI features of different types of gray matter heterotopia.
MATERIALS AND METHODS
A consecutive series of patients with gray matter heterotopia during the period from June 2005 and December 2009 were studied. Medical records and MRI studies of all patients were reviewed. MRI morphologic findings of heterotopia were recorded along with the presence and type of associated cranial malformations. Available clinical and electrophysiological data were also recorded. All patients presented with a history of seizures, their clinical histories were reviewed with specific attention to the following; type of seizures, age at seizure onset, response to antiepileptic medications, associated cognitive or motor deficits; antenatal and perinatal history, developmental milestones, school performance and family history. Full systemic and neurologic examinations were performed during the visits to the neurology clinic. Electroencephalography (EEG) was carried out for each patient using the international 10-20 system. Head computed tomography (CT) scans were available for 12 patients. Cranial MRI studies were performed using a 1.5-T scanner (Symphony, Siemens Medical Systems, Erlangen, Germany). The following series of images were acquired: a- Sagittal, and axial T1WI: TR: 500-600 ms, TE: 14-20 ms. b- Axial and coronal T2WI: TR: 2500-4000 ms, TE: 80-120 ms. c- Axial FLAIR (Fluid-attenuated inversion recovery): TR: 7000-9000 ms, TE: 90-140 ms, TI: 2500 ms. d- Axial and coronal inversion-recovery (IR): TR/TI/TE (7000/400/70 ms). Advanced gradient echo sequence providing high contrast between white and gray matter is used as Fast Low Angle Shot and was used in 10 cases and turbo IR images were performed for 8 patients. Intravenous Gadolinium-DTPA (0.1 mmol/kg) T1WI in three planes was obtained for 9 patients as a space occupying mass was suspected on plain MRI. In all series, the thickness/gap was 5/1 mm, the matrix was 256 × 256 and the field of view was 23 cm. According to Barkovich[1], we classified heterotopia into three groups; subependymal, subcortical and band heterotopia.
RESULTS
During the study period, we included 20 patients with an MRI diagnosis of gray matter heterotopia. The mean age (± SD) of these patients was 14.6 (± 2.17) years, with a range from 9 mo to 39 years. Fourteen patients were females and 6 were males. All patients had a history of epileptic seizures. Patients were classified into three groups (Tables 1, 2 and 3).
Table 1.
Data of 12 patients with subependymal heterotopia
| Patient/sex/age (yr) | Seizure type | Developmental milestones | Neurological examination | EEG findings | MRI characteristics of heterotopia |
| 1/F/8 | Simple partial motor | Normal | Normal | Focal spikes | Unilateral nodule affecting frontal horn of right lateral ventricle |
| 2/F/15 | Complex partial | Delayed speech | Mild spasticity | Focal spikes | Bilateral nodules affecting both trigones |
| 3/F/22 | Tonic-clonic | Delayed walking | Spasticity | General spike-wave | Bilateral nodules affecting body of both lateral ventricles |
| 4/F/14 | Mixed | Learning disability | Hyperactivity, spasticity | General slow wave | Bilateral nodules affecting body of right lateral ventricle |
| 5/M/12 | Complex partial | Delayed speech | Spasticity | Focal spikes | Unilateral coalescent nodules affecting frontal horn of left lateral ventricle |
| 6/F/10 | Simple partial motor | Normal | Normal | Focal spikes | Bilateral nodules affecting trigone and occipital horns |
| 7/M/39 | Complex partial | Delayed walking | Hyperactive stretch reflexes | Focal spikes | Bilateral nodules affecting trigone and occipital horns |
| 8/F/26 | Simple partial motor | Normal | Ataxia | Focal spikes | Unilateral nodules affecting trigone of right lateral ventricle, Dandy Walker cyst |
| 9/F/18 | Simple partial motor | Normal | Spasticity | Focal spikes | Unilateral nodules affecting body of left lateral ventricle |
| 10/M/13 | Tonic-clonic | Delayed speech | Normal | General slow wave | Bilateral nodules affecting body of both lateral ventricles |
| 11/F/28 | Clonic | Learning disability | Hyperactivity | Slow wave | Bilateral nodules affecting trigone and occipital horns and ventricular dilatation |
| 12/M/10 | Tonic-clonic | Normal | Normal | General spike-wave | Bilateral nodules affecting trigone and occipital horns |
M: Male; F: Female; EEG: Electroencephalography; MRI: Magnetic resonance imaging.
Table 2.
Data of 5 patients with subcortical heterotopia
| Patient/sex/age (yr) | Seizure type | Developmental milestones | Neurological examination | EEG findings | MRI characteristics of heterotopia |
| 1/M/27 | Complex partial | Delayed motor | Left hemiparesis | Focal spikes, slow wave | Large, nodular mass at right fronto-parietal lobes, extends across midline and ACC |
| 2/F/15 | Mixed | Delayed walking | spasticity | Focal spikes, slow waves | Small, multiple nodular, right frontal and parietal lobes |
| 3/F/9 | General tonic-clonic | Delayed speech and motor | Right hemipariesis | Spike-wave | Small, multiple, nodular, left parietal and ACC |
| 4/F/7 | General tonic-clonic | Delayed speech and motor | Attention deficit, hyperactivity, bilateral spasticity | General spike-wave | Bilateral curvilinear |
| 5/M/1 | Mixed | Delayed walking | Hyperactive reflexes | General slow wave | Mixed nodular and curvilinear and distorted ventricles |
M: Male; F: Female; EEG: Electroencephalography; MRI: Magnetic resonance imaging; ACC: Anterior corpus callosum.
Table 3.
Data of 3 patients with band heterotopia
| Patient/ sex/age | Seizure type | Developmental milestones | Neurological examination | EEG findings | MRI characteristics of heterotopia |
| 1/F/13 yr | Mixed | Delayed speech, motor | Nystagmus, dysartheria, spasticity | Generalized spike-wave | Bilateral diffuse thick bands at fronto-parietal lobes and pachygyria |
| 2/F/4 yr | Tonic-clonic, myoclonic | Delayed motor | Microcephaly, bilateral spasticity | Generalized slow waves, polyphasic waves | Bilateral diffuse thick bands at both occipital lobes and pachygyria |
| 3/F/9 mo | Infantile spasms | Delayed speech, motor | Spasticity, hyperactive reflexes | hypsarrhythmia | Bilateral diffuse thick bands at both fronto-parietal lobes and lissencephaly |
M: Male; F: Female; EEG: Electroencephalography; MRI: Magnetic resonance imaging.
Subependymal heterotopia
This group included 12 patients (8 females and 4 males), aged 8 to 39 years with a mean age (± SD) of 17.9 (± 2.66) years. Details of the clinical, EEG and MRI findings of these patients are shown in Table 1. Subependymal heterotopia (SEH) had the MRI appearance of round to ovoid subependymal nodules, located just beneath and abutted the ependymal lining of the lateral ventricles and protruding slightly into its lumen resulting in an irregular ventricular outline. The number and size of heterotopia varied widely, from small nodules to a thick layer of coalescent nodules of gray matter lining the lateral ventricles. The nodules were isointense to the cortical gray matter on all MRI sequences (Figure 1). Contrast-enhanced T1WI studies were obtained in 4 patients, and the nodules showed no enhancement. The nodules were bilateral in 8 patients and unilateral in 4 patients. The trigone and occipital horns of lateral ventricles were the commonest location of subependymal nodules followed by the body and frontal region of the lateral ventricle. Associated brain anomalies were detected in only two patients, one had ventricular dilation and the other had Dandy Walker cyst.
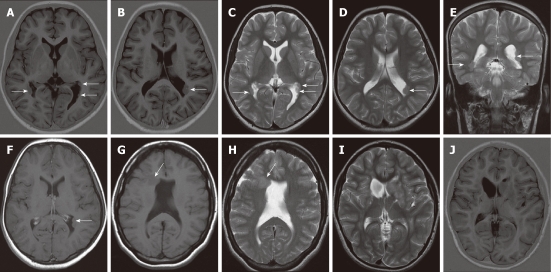
Figure 1.
Subependymal heterotopia. A, B: Axial inversion-recovery magnetic resonance (MR) images; C-E: Axial (C, D) and coronal T2 W (E) images showing multiple bilateral subependymal gray matter heterotopic nodules protruding into and indenting the trigone of the lateral ventricles and the left occipital horn (arrows). The nodules are isointense to cortical gray matter; F: Axial contrast-enhanced T1WI shows no enhancement of the nodules; G, H: Axial T1W and T2W MR images revealing unilateral focal subependymal gray matter nodule (arrow) protruding into and indenting the frontal horn of the right lateral ventricle. The nodule is isointense to the cortical gray matter. Mild ventricular dilatation is noted with absent septum pelucidum; I, J: Axial T2 WI and inversion-recovery MR image showing a unilateral, large subependymal heterotopic gray matter mass projecting into the frontal horn of the left lateral ventricle. The mass is isointense to the cortical gray matter.
Subcortical heterotopia
This group included 5 patients (3 females and 2 males), aged from 1 year to 27 years with a mean age (± SD) of 11.8 (± 4.4) years. Details of the clinical, EEG and MRI findings of these patients with subcortical heterotopia (SCH) are shown in Table 2. SCH appeared on MRI as regions of gray matter signal intensity within the cerebral hemispheric white matter. Their sizes varied from a few centimeters to large focal lesions that appeared as a mass with distortion of the adjacent ventricle but no enhancement after contrast. Three patients were diagnosed as having purely nodular SCH as there was no definite contiguity of the heterotopia with the cerebral cortex (Figure 2). The affected area of the cerebral hemisphere was reduced in size compared with the normal contralateral hemisphere. One patient had curvilinear SCH (Figure 3) as the heterotopic tissue had the appearance of enfolded cortex and showed contiguity with the cortex. One patient had mixed regions of both nodular and curvilinear SCH (Figure 4) as the gray matter nodules were seen in deep portions within the cerebral hemispheric white matter and the curvilinear portions were seen in the superficial portion and showed contiguity with the cortex. Three patients had associated brain abnormalities; two patients had agenesis of the corpus callosum and the third had ventricular distortion.
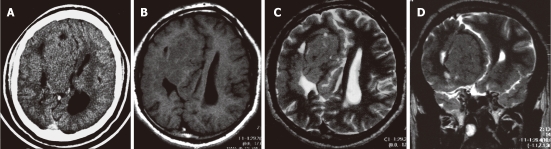
Figure 2.
Subcortical nodular heterotopia. A: Contrast-enhanced axial computed tomography shows a large right fronto-parietal, non-enhancing mass exerting mass effect on the right lateral ventricle; B-D:Non-contrast enhanced magnetic resonance imaging (B), axial T1W, (C) axial T2W and (D) coronal T2W images showing a large subcortical nodular mass, isointense to the cortical gray matter. The overlying cortex is thin and the corpus callosum is agenetic.
Figure 3.
Subcortical curvilinear heterotopia. A, B: Axial T2W and coronal T2W images showing bilateral curvilinear heterotopia within the white matter. The heterotopic tissue is convoluted and contiguous with the overlying cortex. Linear and punctuate cerebrospinal fluid signal are seen within the heterotopic tissue. The cerebral cortex shows pachygyria.
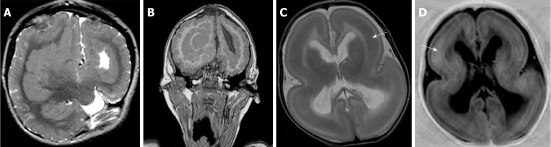
Figure 4.
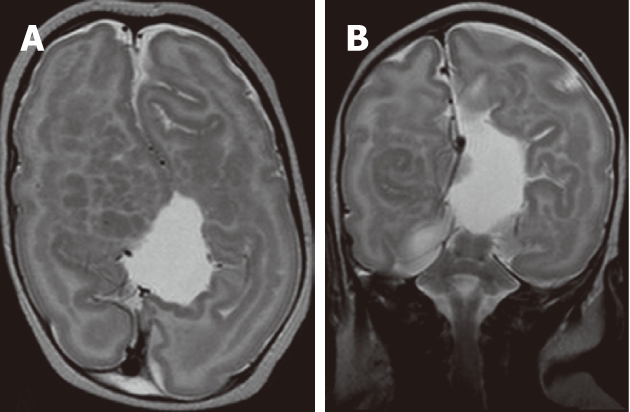
Mixed nodular and curvilinear subcortical heterotopia. A, B: Axial T2W and coronal T2W images showing multiple nodular and curvilinear heterotopia within the white matter bilaterally. The overlying cortex shows pachygyria. The right cerebral hemisphere is smaller compared to the left cerebral hemisphere. The corpus callosum is agenetic with distorted lateral ventricles.
Band heterotopia
This group included 3 patients, all were females; aged from 9 mo to 13 years with a mean age (± SD) of 5.9 (± 3.6) years. Details of the clinical, EEG and MRI findings of these patients with band heterotopia (BH) are shown in Table 3. Band heterotopia appeared as smooth, bilateral and symmetric ribbons of gray matter found in the central white matter between the cerebral cortex and the ventricular surface and separated from the cortex by normally myelinated white matter. BH was present in both cerebral hemispheres and had the same MRI signal intensity as the cortical gray matter on all MRI sequences (Figure 5). Two patients had associated pachygyria and one patient had lissencephaly. All patients in this group were developmentally delayed.
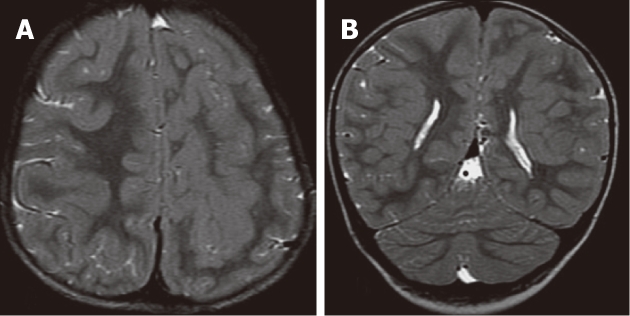
Figure 5.
Band heterotopia. A, B: Axial T2W and coronal inversion-recovery images showing bilateral thick bands of heterotopia, isointense to cortical gray matter within the white matter. Note the pachygyria and small left cerebral hemisphere; C, D: Axial T2W and inversion-recovery images showing bilateral, symmetric, continuous, smooth, thick bands of gray matter (arrow) outlined by thin layers of white matter and seems like a “double cortex”. Note the very thin, smooth cerebral cortex with absent cortical sulci (lissencephaly).
DISCUSSION
Malformations of cortical development are more common than was recognized in the era before MRI, as heterotopia on CT may be difficult to visualize[1,14]. Heterotopia is the most frequently occurring anomaly affecting cortical development. It is considered to be one of the most common congenital disorders in familial and early onset epilepsy[14-18]. MRI classification of gray matter heterotopia into subependymal, subcortical, and band types has been considered useful because patients in these three groups have different clinicoradiologic presentations and different underlying genetic disorders[3,5,19]. This classification of heterotopia may be useful in predicting patient outcome[2]. Several authors have reported that patients in all three heterotopia groups are very likely to develop epilepsy which is more commonly found in females than males[1,3,11,14]. In our study, all patients had a history of seizures and the female to male ratio was 14:6. In the current study, the three types of heterotopia were detected by MRI; SEH was the commonest type, followed by SCH, while BH was the least common type. On MRI; the heterotopic tissue was isointense with gray matter on all MR pulse sequences. The inversion recovery sequence was considered useful for the demonstration of heterotopic gray matter and the assessment of cortical thickness as it provides a strong contrast between gray and white matter. A similar frequency and MRI appearance has been reported in the literature[1-5].
SEH is characterized by periventricular nodules adjacent to the lateral ventricular walls just beneath and abutting the ependyma giving the nodular ventricular wall, and is not enhanced after intravenous contrast administration. This allows these nodules to be distinguished from the subependymal nodules of tuberous sclerosis that do not follow gray matter signal, and enhance after contrast administration[1,2,10-13]. SEH may be isolated or may develop in conjunction with other central nervous system malformations. It can be subdivided genetically into X-linked and non-X-linked inheritance patterns. They can be subdivided anatomically into unilateral focal, bilateral focal and bilateral diffuse groups. Some patients with SEH are neurologically and developmentally normal[1,12,20].
In this series, patients with SEH had a relatively late onset of seizures of different types. Simple partial seizure was the commonest type reported. Five patients had normal development and the remaining patients had mild developmental delay. The most common EEG abnormality was focal spikes. These findings are in agreement with other studies[1,2,8,10,11,15].
The present study, like many others, has demonstrated that MRI is excellent in the detection and characterization of SEH nodules[1,2,11,21-23]. The nodules are most likely to be bilateral and commonly at the trigone and occipital horns of lateral ventricles. It was reported that SEH caused little or no distortion of the remaining brain, whereas focal SCH caused marked distortion of the ventricles and diminished hemisphere size. In addition, surrounding white matter is normal in SEH, whereas most of the hemispheres containing SCH had qualitatively diminished white matter[1,3]. In our study, only 18% of patients with SEH had associated brain anomalies. Mithchell et al[12] reported that SEH was occasionally accompanied by mild ventricular dilation.
In the present study, MRI showed SCH in five patients. Three patients had purely nodular SCH, one patient had curvilinear SCH and another patient had mixed nodular and curvilinear SCH. Barckovich et al[3] reported that SCH usually consists of swirling, heterogeneous, nodular or curvilinear masses of gray matter containing blood vessels and cerebrospinal fluid (CSF). It is reported that SCH extends through the white matter, from the ventricular surface to the cerebral cortex. In the current study, the affected area of the hemisphere was reduced in size compared with the normal contralateral hemisphere in three patients. Three of the five patients with SCH had associated brain abnormalities. In the present study, as in other studies, patients with SCH had a higher prevalence of developmental delay and motor dysfunction compared to the SEH group and had variable motor and intellectual disturbances, depending on the size of the lesion and the effect on the underlying cortex[3,5]. There were no differences in clinical manifestations among the different types of SCH.
Band heterotopia or double cortex syndrome usually occurs in females and very rarely in males. MRI shows the characteristic appearance of BH as a smoothly marginated layer of gray matter coursing parallel to the lateral ventricle, separated from the overlying cortex and underlying ventricle by layers of white matter. Bands are neither convoluted nor contiguous with the overlying cortex. They do not contain blood vessels or CSF. The thicker the band of heterotopic neurons; the worse the disability and increased prevalence of developmental delay[1,4,9]. In the present study, MRI showed band heterotopia in three females. Two patients had associated pachygyria and one had lissencephaly. All patients in this group had severe developmental delay.
The assessment of neuronal heterotopias and their pathophysiological significance in epilepsy are still the subject of debate. Many reports either classify all neuronal migration disorders together, or lump them with other cortical dysgeneses. Also, the mechanisms by which heterotopic gray matter results in seizures are unclear and future respective strategies will have to take into account the multiplicity of heterotopic lesions, their localization, and their effects on overlying cortical functional organization. In our study, the clinical, EEG, psychometric, and neuroimaging features of 20 patients with gray matter heterotopia are described and compared with other studies with the aim of reducing debate and emphasizing the role of conventional and advanced MRI sequences in differentiating between various types of gray matter heterotopia. The severity of the clinical manifestations of heterotopia is related to the location and pattern of heterotopia. Determination of the heterotopia type and its extent is useful for management planning and predicting prognosis.
COMMENTS
Background
Gray matter heterotopia is a relatively common form of neuronal migration disorders. The pathogenic mechanisms are not fully understood, but they lead to distinct clinicoradiological syndromes. Neuroradiologists, basing their ideas on magnetic resonance imaging (MRI) appearances, have classified heterotopia into subependymal, subcortical and band heterotopia.
Research frontiers
The assessment of neuronal heterotopias and their pathophysiological significance in epilepsy are still the subject of debate. The mechanisms by which heterotopic gray matter results in seizures are unclear. In this study, the clinical, electroencephalography, psychometric and neuroimaging features of 20 patients with gray matter heterotopia are described and compared with other studies with the aim of reducing debate and emphasizing the role of conventional and advanced MRI sequences in differentiating between various types of gray matter heterotopia.
Innovations and breakthroughs
This recent study highlighted the usefulness of MRI in differentiating between shape, location and extent of various types of gray matter heterotopia. The authors emphasized that the severity of the clinical manifestations of heterotopia is related to the location and pattern of heterotopia.
Applications
Determination of the type, extent and location of gray matter heterotopia are useful for management planning and predicting prognosis.
Terminology
Gray matter heterotopia is a neuronal migration disorder in which collections of cortical neurons are found in an abnormal location. Subependymal heterotopia consists of small foci of gray matter that are located in a subependymal location near the ventricular wall. Subcortical heterotopia occurs as masses of gray matter within the deep and subcortical white matter. Band heterotopia is a symmetrical thick band of gray matter that lies between layers of the white matter.
Peer review
This is a well-written account of MRI of brain heterotopia.
Footnotes
Peer reviewer: Tarik F Massoud, MB, BCh, BAO, LRCPI, LRCSI, MA, MD, PhD, FRCR, Department of Radiology, University of Cambridge, Addenbrooke’s Hospital, Box 219, Hills Road, Cambridge CB2 2QQ, United Kingdom
S- Editor Cheng JX L- Editor Webster JR E- Editor Xiong L
References
- 1.Barkovich AJ, Kuzniecky RI. Gray matter heterotopia. Neurology. 2000;55:1603–1608. doi: 10.1212/wnl.55.11.1603. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Kjos BO. Gray matter heterotopias: MR characteristics and correlation with developmental and neurologic manifestations. Radiology. 1992;182:493–499. doi: 10.1148/radiology.182.2.1732969. [DOI] [PubMed] [Google Scholar]
- 3.Barkovich AJ. Morphologic characteristics of subcortical heterotopia: MR imaging study. AJNR Am J Neuroradiol. 2000;21:290–295. [PMC free article] [PubMed] [Google Scholar]
- 4.D'Agostino MD, Bernasconi A, Das S, Bastos A, Valerio RM, Palmini A, Costa da Costa J, Scheffer IE, Berkovic S, Guerrini R, et al. Subcortical band heterotopia (SBH) in males: clinical, imaging and genetic findings in comparison with females. Brain. 2002;125:2507–2522. doi: 10.1093/brain/awf248. [DOI] [PubMed] [Google Scholar]
- 5.Barkovich AJ. Subcortical heterotopia: a distinct clinicoradiologic entity. AJNR Am J Neuroradiol. 1996;17:1315–1322. [PMC free article] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Kuzniecky RI, Dobyns WB, Jackson GD, Becker LE, Evrard P. A classification scheme for malformations of cortical development. Neuropediatrics. 1996;27:59–63. doi: 10.1055/s-2007-973750. [DOI] [PubMed] [Google Scholar]
- 7.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. Classification system for malformations of cortical development: update 2001. Neurology. 2001;57:2168–2178. doi: 10.1212/wnl.57.12.2168. [DOI] [PubMed] [Google Scholar]
- 8.Huttenlocher PR, Taravath S, Mojtahedi S. Periventricular heterotopia and epilepsy. Neurology. 1994;44:51–55. doi: 10.1212/wnl.44.1.51. [DOI] [PubMed] [Google Scholar]
- 9.Ono J, Mano T, Andermann E, Harada K, Sakurai K, Ikeda T, Yoshihara N, Shimizu K, Okada S, Andermann F. Band heterotopia or double cortex in a male: bridging structures suggest abnormality of the radial glial guide system. Neurology. 1997;48:1701–1703. doi: 10.1212/wnl.48.6.1701. [DOI] [PubMed] [Google Scholar]
- 10.Dubeau F, Tampieri D, Lee N, Andermann E, Carpenter S, Leblanc R, Olivier A, Radtke R, Villemure JG, Andermann F. Periventricular and subcortical nodular heterotopia. A study of 33 patients. Brain. 1995;118(Pt 5):1273–1287. doi: 10.1093/brain/118.5.1273. [DOI] [PubMed] [Google Scholar]
- 11.Aghakhani Y, Kinay D, Gotman J, Soualmi L, Andermann F, Olivier A, Dubeau F. The role of periventricular nodular heterotopia in epileptogenesis. Brain. 2005;128:641–651. doi: 10.1093/brain/awh388. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell LA, Simon EM, Filly RA, Barkovich AJ. Antenatal diagnosis of subependymal heterotopia. AJNR Am J Neuroradiol. 2000;21:296–300. [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrini R, Dobyns WB. Bilateral periventricular nodular heterotopia with mental retardation and frontonasal malformation. Neurology. 1998;51:499–503. doi: 10.1212/wnl.51.2.499. [DOI] [PubMed] [Google Scholar]
- 14.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–1887. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Sheen V. Periventricular heterotopia. Epilepsy Behav. 2005;7:143–149. doi: 10.1016/j.yebeh.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Fauser S, Schulze-Bonhage A, Honegger J, Carmona H, Huppertz HJ, Pantazis G, Rona S, Bast T, Strobl K, Steinhoff BJ, et al. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain. 2004;127:2406–2418. doi: 10.1093/brain/awh277. [DOI] [PubMed] [Google Scholar]
- 17.Hannan AJ, Servotte S, Katsnelson A, Sisodiya S, Blakemore C, Squier M, Molnár Z. Characterization of nodular neuronal heterotopia in children. Brain. 1999;122(Pt 2):219–238. doi: 10.1093/brain/122.2.219. [DOI] [PubMed] [Google Scholar]
- 18.Smith AS, Weinstein MA, Quencer RM, Muroff LR, Stonesifer KJ, Li FC, Wener L, Soloman MA, Cruse RP, Rosenberg LH. Association of heterotopic gray matter with seizures: MR imaging. Work in progress. Radiology. 1988;168:195–198. doi: 10.1148/radiology.168.1.3132731. [DOI] [PubMed] [Google Scholar]
- 19.Mai R, Tassi L, Cossu M, Francione S, Lo Russo G, Garbelli R, Ferrario A, Galli C, Taroni F, Citterio A, et al. A neuropathological, stereo-EEG, and MRI study of subcortical band heterotopia. Neurology. 2003;60:1834–1838. doi: 10.1212/01.wnl.0000065884.61237.24. [DOI] [PubMed] [Google Scholar]
- 20.Sisodiya SM, Free SL, Thom M, Everitt AE, Fish DR, Shorvon SD. Evidence for nodular epileptogenicity and gender differences in periventricular nodular heterotopia. Neurology. 1999;52:336–341. doi: 10.1212/wnl.52.2.336. [DOI] [PubMed] [Google Scholar]
- 21.El-Serougy LG, Azab AH, Saad M. Gray matter heterotopia: MRI diagnostic criteria correlated with clinical assessment. Egypt J Radiol Nuc Med. 2002;33:65–77. [Google Scholar]
- 22.Canal N, Frattola L, Smirne S. The metabolism of cyclic-3’-5’-adenosine monophosphate (cAMP) in diseased muscle. J Neurol. 1975;208:259–265. doi: 10.1007/BF00312801. [DOI] [PubMed] [Google Scholar]
- 23.Chang BS, Ly J, Appignani B, Bodell A, Apse KA, Ravenscroft RS, Sheen VL, Doherty MJ, Hackney DB, O’Connor M, et al. Reading impairment in the neuronal migration disorder of periventricular nodular heterotopia. Neurology. 2005;64:799–803. doi: 10.1212/01.WNL.0000152874.57180.AF. [DOI] [PubMed] [Google Scholar]