Abstract
The understanding of the influence of toxic elements on root anatomy and element distribution is still limited. This study describes anatomical responses, metal accumulation and element distribution of rooted cuttings of Salix caprea after exposure to Cd and/or Zn. Differences in the development of apoplastic barriers and tissue organization in roots between two distinct S. caprea isolates with divergent Cd uptake and accumulation capacities in leaves might reflect an adaptive predisposition based on different natural origins. Energy-dispersive X-ray spectroscopy (EDX) revealed that Cd and Zn interfered with the distribution of elements in a tissue- and isolate-specific manner. Zinc, Ca, Mg, Na and Si were enriched in the peripheral bark, K and S in the phloem and Cd in both vascular tissues. Si levels were lower in the superior Cd translocator. Since the cuttings originated from stocks isolated from polluted and unpolluted sites we probably uncovered different strategies against toxic elements.
Keywords: Apoplastic barriers, Salix caprea (willow), EDX, Natural variation, Root anatomy
Highlights
► We describe responses in roots of S. caprea exposed to Cd and Zn. ► Apoplastic barrier development varied among isolates from differently polluted sites. ► EDX analyses revealed variations of element distributions in root tissues. ► Si weight% was lower in the isolate with a higher Cd translocation capacity. ► S. caprea isolates possessed different strategies to respond to Cd and Zn.
S. caprea altered element distribution and translocation, apoplastic barrier development and root anatomy upon Cd and/or Zn exposure.
1. Introduction
Various pollutants, including toxic metals, are present in soils throughout the world. This phenomenon is common on soils which have developed on naturally enriched rock substrates. Another source of soil pollution can be attributed to human activities, including mining, metal and ore processing, and industrial and agricultural activities (Padmavathiamma and Li, 2007). During recent years efforts have been made to rehabilitate contaminated sites. Phytoremediation activities have been carried out on some polluted sites. Several plant species have been identified that extract, tolerate and/or hyperaccumulate pollutants from soils in their above-ground tissues (Shah and Nongkynrih, 2007; Verbruggen et al., 2009; Rascio and Navari-Izzo, 2011). The accumulation of metals and the final concentration in tissues often exceed one hundred-times the concentration of common plant species (Maestri et al., 2010). Major disadvantages of these species (e.g. Thlaspi caerulescens, Arabidopsis halleri) are their slow growth, low biomass production and short life cycle. Therefore, researchers over the world are interested in how to make the process of phytoextraction more effective (Zhao and McGrath, 2009). One of the possible solutions is the utilization of fast growing woody plant species with high biomass production and high genetic variability (Pulford and Watson, 2003). Several studies about different woody species have been published in recent years dealing with their uptake, translocation and accumulation capacity, as well as tolerance to various metals. Trees from the Salicaceae family, especially the genera Salix and Populus, might be suitable candidates for these purposes (e.g. Greger and Landberg, 1999; Sebastiani et al., 2004; Vysloužilová et al., 2006; Dos Santos Utmazian and Wenzel, 2007; Unterbrunner et al., 2007; Wieshammer et al., 2007; Jensen et al., 2009; Vollenweider et al., 2011). Besides the metal extraction, Salix caprea represents a suitable candidate for remediation because of fast colonization of mineral substrates, high biomass production and landscape restoration.
Variability in the uptake capacity of several elements has been reported between populations or clones of the same species, sometimes originating from different environmental conditions. Metallicolous and non-metallicolous populations of the heavy metal tolerant plant T. caerulescens have shown different responses to soil Cd and Zn contamination (Escarré et al., 2000; Lombi et al., 2000; Jiménez-Ambriz et al., 2007; Dechamps et al., 2007). Similarly, Zacchini et al. (2009) compared characteristics important for phytoextraction such as metal tolerance, translocation and accumulation of Cd in poplar hybrids (Populus x canadensis, P. x deltoides, P. x generosa, P. x nigra, P. x alba, P. x trichocarpa) and willow (S. alba) clones. Their results show that willows have a higher Cd tolerance than poplars. A similar study was published by Dos Santos Utmazian and Wenzel (2007) on six different willows (S. babylonica, S. caprea, S. dasyclados, S. matsudana x alba, S. purpurea, S. smithiana) and two poplar species (Populus tremula, P. nigra) and discovered that S. smithiana leaves accumulated the highest Cd concentration. Differences in the tolerance and accumulation capacity of Cd, Cu and Zn between clones of five Salix species (S. viminalis, S. dasyclados, S. daphnoides, S. purpurea and S. triandra) grown in metal polluted and unpolluted areas were compared by Landberg and Greger (1996). Clones from polluted areas accumulated more metals in roots while the transport to shoots was decreased when compared with clones from unpolluted areas. However, these authors found no difference in the concentration of metals in stems between these two groups of Salix species.
Puschenreiter et al. (2010) analyzed 170 S. caprea isolates of four metal polluted and three uncontaminated middle European sites to reveal potential selective effects of long-term heavy metal contaminations on the genetic structure and Zn/Cd accumulation capacity. They found differentiation of metallicolous and non-metallicolous S. caprea populations based on phenotypic characteristics and nuclear microsatellite (SSR) markers (Puschenreiter et al., 2010).
The root is the first plant part to encounter soil heavy metal pollution; therefore the molecular uptake mechanisms at the root–soil interface are the subject of intensive study in hyperaccumulating species (Lombi et al., 2000; Hanikenne et al., 2008; Verbruggen et al., 2009). Concerning the uptake and translocation of heavy metals, there is very limited knowledge about their relation to root tissue organization and development in the woody plants used for phytoremediation. Lux et al. (2004) described differences in the anatomy and apoplastic barrier development of adventitious roots of willows (S. viminalis, S. viminalis x schwerinii, S. daphnoides) with contrasting characteristics of Cd accumulation and Cd sensitivity. They show that both the Casparian strips and suberin lamellae developed more distantly from root tips in high Cd than in low accumulating and translocating cuttings. However, the influence of Cd and/or Zn on the development of root tissues of genetically distinct S. caprea plants with different Zn and Cd translocation capabilities has not been evaluated (Puschenreiter et al., 2010). Therefore the present study focuses on anatomical differences and metal accumulation of root tissues of cuttings from S. caprea plants originating from polluted and uncontaminated areas. The development of apoplastic barriers affecting radial transport of elements across the root (Casparian strips and suberin lamellae), changes in tissue proportions, and metal localization within root tissues were compared. The aim of this work was to get a better understanding of the involvement of root tissues in the uptake, translocation and accumulation of Cd and Zn in a woody species promising for phytoextraction, S. caprea.
2. Materials and methods
2.1. Origin of the plant material
All experiments were performed with two genetically distinct S. caprea isolates which differed in the ability to tolerate and accumulate Zn and Cd in above-ground tissues in perlite cultures. The term “isolate” refers to an individual plant collected in a specific area as part of a group of 20–25 individuals representing the population at this site (Puschenreiter et al., 2010). Isolate KH21 originated from an old mining area (Kutná Hora, Czech Republic); it was formerly characterized as accumulating high amounts of Zn and Cd. Isolate F20 originated from a non-polluted control area (Forchtenstein, Austria); it accumulated less Zn and Cd than KH21 but had a higher biomass production in perlite-based hydroponics (Puschenreiter et al., 2010). All cuttings of both S. caprea isolates used in our experiments were obtained from a stock cutting cultivated in non-polluted control soil at the AGES (Vienna, Austria).
2.2. Cultivation of plant material
Green cuttings of approx. 10 cm length were rooted in a mixture of perlite and coarse sand (20:80) in a greenhouse (AGES and BOKU, Vienna, Austria). Rooting was initiated in tap water for 60 days at 24/18 °C day/night, a 12 h photoperiod, 60% relative humidity and 200 μM m−2 s−1 PAR.
For the effect of Zn and Cd exposure on root anatomy and the distribution of minerals in root tissues, each pre-rooted cutting with new shoot of 15–20 cm length and 2 – 5 adventitious roots of an average total length of 250 mm was transferred to 1 L pots filled with pure perlite. The plants were watered twice a week with a nutrient solution containing (in μM) 1000 Ca(NO3)2, 500 Mg(SO4)2, 50 KH2PO4, 100 KCl, 5 H3BO3, 0.2 H24Mo7N6O24, 10 MnSO4, 2.5 CuSO4, 0.25 NiSO4, 2.5 ZnSO4 and 50 Fe (III)-EDDHA (ethylenediamine-di(o-hydroxyphenylacetic acid)). Solution pH was adjusted to 6.0 with 1 mM MES as potassium salt (Shen et al., 1997). Four different treatments were used: 1) Standard nutrient solution, 2) Nutrient solution with 0.5 mg Cd/l in the form of CdNO3•4 H2O (Cd), 3) Nutrient solution with 5 mg/l Zn in the form of ZnSO4•7 H2O (Zn), and 4) Nutrient solution with 0.5 mg Cd/l and 5 mg/l Zn (Cd + Zn). Six pots with one plant in each were prepared per treatment. Plants were cultivated for 14 weeks.
For the analysis of the development of Casparian strips and suberin lamellae, rooted cuttings were transferred to 0.5 L pots and watered twice a week for three months with the solutions described above. Six pots with one plant in each were prepared per treatment. Plants for apoplastic barrier analysis were cultivated for 6 weeks.
2.3. Quantification of Zn and Cd
For the analysis of Zn and Cd concentration in below- and above-ground tissues, plant material was divided into roots, shoots and leaves and washed with distilled water before drying at 80 °C. After recording the DW, samples were ground in a metal-free mill (IKA® – Werke, MF 10) and digested in a mixture of HNO3 (puriss. p. a., Sigma–Aldrich, Austria) and HClO4 (puriss. p. a., Sigma–Aldrich, Austria) (4:1, v/v) using an automated heating block (Digester DK 42/26, Velp Scientifica, Milano, Italy). Zinc and Cd concentrations were measured with a flame atomic absorption spectrometer (AAS, Perkin Elmer 2100). For quality assurance, six biological replicate samples, blanks and standardized reference materials were included in all analyses.
2.4. Quantification of root tissues
To determine if anatomical changes of the main root tissues correlate with the different origin and metal translocation behavior of the two S. caprea isolates, the areas of different tissues were quantified on transverse roots sections at a distance of 1.2–1.5 cm from the apex. Root cells at this distance are already developed and well differentiated and this part of the root mostly contributes to the uptake of elements. The root segments were fixed in 2% glutaraldehyde and 0.2% osmium tetroxide. After dehydration with ethanol the samples were embedded in Spurr resin (Serva). Approximately 2 μm thick semi-thin sections were prepared on microtome Microm HM36 and stained with toluidine blue and basic fuchsine according to Lux (1981).
Sections from eight different roots were analyzed with a Zeiss Axioskop 2+ microscope (Zeiss, Germany), equipped with Olympus DP72 camera. The tissue areas (total root area, area of the central cylinder, area of xylem elements) were analyzed with the image analysis software Lucia G 4.80 (LIM, Czech Republic).
2.5. Analysis of the development of apoplastic barriers
To determine the developmental point where Casparian strips and suberin lamellae start to differentiate in roots, and if the developmental program of Casparian strips and suberin lamellae differentiation varies between isolates from an unpolluted (F20) and a heavy metal polluted site (KH21), six different roots from each isolate and each treatment were stained with fluorescent dyes for Casparian strips and suberin lamellae visualization.
Free hand sections were made at regular distances from the apex to the base of the root (in 1 mm distance for the first 10 mm from the root apex and later in 1 cm distance for the rest of the root). Casparian strips were visualized by staining with 0.2% Berberine hemisulphate and post-staining with 0.1% toluidine blue. Suberin lamellae were stained with 0.2% Fluorol Yellow 088 according to Brundrett et al. (1991) and Lux et al. (2005). All sections were observed with a Zeiss Axioskop 2+ epifluorescence microscope (Zeiss, Germany).
2.6. SEM coupled with X-ray analysis
Approximately 2 mm long root segments were cut from the basal part of roots and immediately frozen in liquid nitrogen according to Markhart and Läuchli (1982) and McCully et al. (2010). The frozen segments were freeze-dried in a vacuum freeze dryer. Dried segments were coated with carbon and fixed on aluminum stubs covered with a carbon sticker. Surface conductivity was increased by coating with carbon; sputter coating was stopped as soon as the carbon film showed a golden to bluish color, resulting in a uniform thickness of the coating (approx. 60 nm). Cross sections of root tissues and the distribution of eight elements (Zn, Cd, Ca, K, S, Si, Mg, Na) were observed and analyzed with the Phillips XL 20 scanning electron microscope equipped with an EDX analyzer. All samples were observed at 1.200× magnification at 30 kV, working distance 12.0 mm. Data were collected for 100 live seconds and areas of 50 × 50 μm were analyzed. As uneven surfaces may heavily distort EDX measurements (Goldstein, 2003), only flat parts of the samples were selected for analysis.
The element distribution was analyzed from three different roots of each treatment. In total, 24 different roots were investigated by measuring thirty different spots on each root. Raw data were processed with the Genesis Spectrum 5.11 (EDAX, USA) and all the values are expressed as weight% of total analyzed elements. Zinc and Cd were selected because of the main interest of this paper and the other investigated elements share chemical and physical similarities with Zn and Cd or are important macro- and micronutrients and beneficial elements.
2.7. Statistical analysis
Significances according to Student's T-tests and Pearson correlations were calculated using the Statgraphics (Statgraphics Centurion XV v. 15.2.05, StatPoint, Inc.) and Excel (Microsoft Office 2003) programs. A p-value <0.05 was defined as significant. The principal component analysis was performed in Simca-P 11 (Umetrics, Umeå, Sweden) on log-transformed weight% data of all analyzed elements (Zn, Cd, Ca, K, S, Si, Mg, Na). Loadings plots are based on a correlation matrix, scores scatter plots on a covariance matrix. Ellipses in scores scatter plots represent the 95% confidence interval according to Hotelling's T2 distribution. Data points outside the ellipses represent outliers.
All experiments have been performed on six biological replicates/clones with an adequate number of repetitions (see above) for statistical analysis.
3. Results
3.1. Zn and Cd accumulation varied between roots and shoots
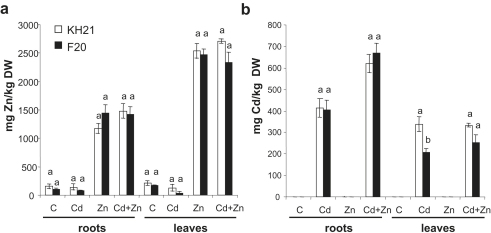
Leaves accumulated considerably more Zn than roots in both isolates. This was observed in Zn and also in the combined Cd + Zn treatment (Fig. 1a). However, the differences between the isolates were not significant. Similarly, the Zn concentration was not different in leaves and roots of both isolates when grown in control conditions or excess Cd (Fig. 1a). The Cd concentration was higher in roots than in leaves. No significant differences were found between the Cd concentrations in roots of the isolates. However, significantly more Cd was detected in leaves of isolate KH21 from the polluted site compared to isolate F20 from the control site. The same tendency was observed when plants were treated with both metals (Cd + Zn) together, but the difference was not significant (Fig. 1b).
Fig. 1.
Zinc (a) and Cadmium (b) concentration (mg kg−1) in roots and leaves of the two S. caprea isolates (KH21 and F20) cultivated for 14 weeks in standard nutrient solution (C) or with either supplemented 0.5 mg l−1 Cd (Cd) or 5 mg l−1 Zn (Zn) or both metals (Cd + Zn). Data are means ± SE. Different letters indicate significant differences between isolates. Twelve roots of six biological replicates of each isolate were evaluated.
3.2. The development of Casparian strips and suberin lamellae varied between S. caprea isolates
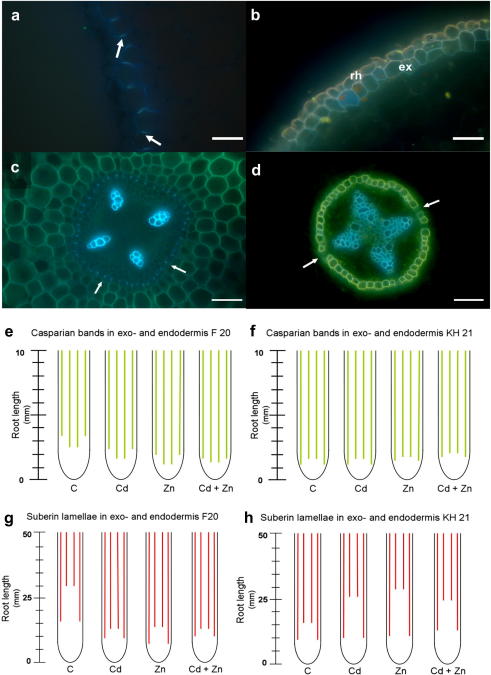
In order to determine the developmental point where the Casparian strips (Fig. 2a,c) and suberin lamellae (Fig. 2b,d) start to differentiate, the exo- and endodermis of roots were stained with fluorescent dyes.
Fig. 2.
Development of apoplastic barriers in roots. Fully developed Casparian strips (blue dots marked with white arrows) in the exodermis (a) and endodermis (c) stained with Berberine hemisulphate, and suberin lamellae (white or yellow-framed cells) in the exodermis (b) and endodermis (d) of S. caprea roots stained with Fluorol yellow 088. Some passage cells in the endodermis (non stained cells marked with white arrows) that are oriented opposite to the xylem poles (blue) can be still observed (d). Abbreviations: rh – rhizodermis, ex – exodermis. Bars, 50 μm. Schemes of the development of Casparian strips (e,f, green lines) and suberin lamellae (g,h, red lines) in exo- and endodermis of the root of S. caprea isolates F20 (e,g) and KH21 (f,h). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the roots of both isolates Casparian strips developed in the exodermis as well as in the endodermis. A striking difference between isolates F20 and KH21 could be detected in the Casparian strips development already in control conditions (Fig. 2e,f; Table S1); in F20, the Casparian strips differentiated first in the endodermis and later in the exodermis. In contrast, the Casparian strip development in KH21 followed an opposite pattern in these two tissues and differentiated first in the exodermis and later in the endodermis. Another difference was in the starting point of the Casparian strip development from the root apex; in F20 the zone of exo- and endodermal Casparian strip development was between 2.5 and 3.5 mm from the root apex while in KH21 this distance was shorter, between 1.0 and 2.0 mm from the root apex (Fig. 2e,f).
The formation of Casparian strips in the exodermis and endodermis of roots after exposure to Zn or/and Cd also varied between the isolates; while the Casparian strips differentiated in the exo- and endodermis closer to the apex in F20 roots exposed to Zn or Cd, this was not the case in KH21. Exposure to Zn or Cd, as well as the combined treatment, had the same effect indicating that the development of apoplastic barriers relates to the presence of these metals. The unresponsiveness of KH21 could indicate a limit in the root apex beyond which the differentiation cannot take place.
Similar to the Casparian strips, the suberin lamellae developed differently in exo- and endodermis of the two isolates. For both isolates, the suberin lamellae form earlier in the exodermis than in the endodermis. However, the distance of suberin lamellae development from the root apex was different between the two isolates. While the exodermal suberin lamellae of F20 started approximately 15 mm from the apex, the distance was only 10 mm in KH21. The endodermal suberin lamellae were detectable 30 mm from the root apex in F20 under control conditions, while in KH21 the distance was only 15 mm (Fig. 2g,h).
Upon heavy metal exposure the exodermal suberin lamellae started at around 10 mm from the root apex in both isolates. Metal treatment affected the development of the endodermal suberin lamellae in F20 and KH21 in an opposite manner. While for F20 the onset of endodermal suberin lamellae differentiation shifted towards the root apex in roots exposed to heavy metals, in KH21 the onset of endodermal suberin lamellae development was at about 25 mm and shifted further from the apex in roots exposed to heavy metals. Interestingly, the same effects were observed in Zn, Cd, and the combined treatment (Fig. 2g,h).
3.3. Root anatomy differed between S. caprea isolates and upon heavy metal exposure
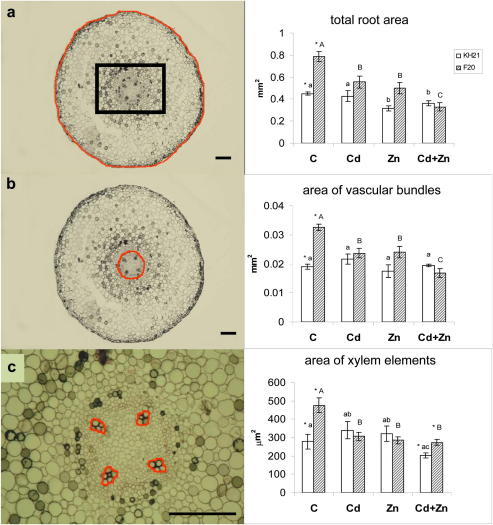
To determine if anatomical changes of the main root tissues correlate with the different origin and metal translocation behavior of the two S. caprea isolates, the areas of different tissues were quantified on transverse roots sections. The total areas of the root (Fig. 3a), vascular bundles (Fig. 3b) and xylem elements (Fig. 3c) were significantly larger in F20 than KH21 under control conditions, indicating that all tissues contribute to the thicker roots of F20. While in F20 a significant decrease was evident for the total root area, the area of vascular bundles and the area of xylem elements after metal exposure, only the total area of KH21 roots decreased in the Zn and Cd + Zn treatments (Fig. 3a). The area of the vascular bundles remained constant while the area of xylem elements slightly increased with Zn or Cd treatment, but decreased if Zn and Cd were offered together (Fig. 3b,c).
Fig. 3.
Representative semi-thin cross section of a S. caprea root approx. 1.5 cm from the apex from which the quantitative anatomical measurements were made; the total root area (a), the area of vascular bundles (b), and area of xylem elements in vascular bundles (c). Red lines bound the measured areas. Black frame on (a) indicates the area of the root which is shown in (c). Bars, 100 μm. S. caprea isolates KH21 and F20 were cultivated for 14 weeks in standard nutrient solution (C), and in solution containing Cd, Zn or both metals (Cd + Zn). Data are means ± SE. Eight biological replicates for each treatment were evaluated. Small and capital letters indicate significant differences among the treatments in KH21 and F20, respectively, and asterisks indicate significant differences between the isolates in the same treatment. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Elemental distribution in different root tissues
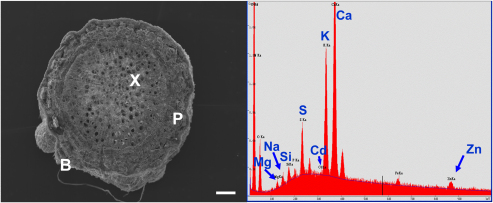
Roots analyzed by EDX had already undergone secondary thickening. Three different zones could be clearly distinguished: the central part of the root consists of secondary xylem (Fig. 4a, X) and is surrounded by secondary phloem (Fig. 4a, P). The outer part of the root, here called outer bark or rhytidome (Fig. 4a, B), is highly heterogeneous and includes secondary (periderm) and primary vascular bundles, the endodermis, the primary cortex and the exodermis. Cells of this zone were collapsed and apparently dead. The distribution of Zn, Cd, Ca, K, S, Si, Mg and Na in this zone was analyzed by EDX (Fig. 4b, Table S2).
Fig. 4.
Cross section of a secondary thickened root of S. caprea in SEM. Representative EDX spectrum from the peripheral zone of the root showing the characteristic peaks of Zn, Cd, Ca, K, S, Si, Mg and Na. Abbreviations: X, secondary xylem; P, secondary phloem; B, peripheral zone or bark of the root. Bar, 100 μm.
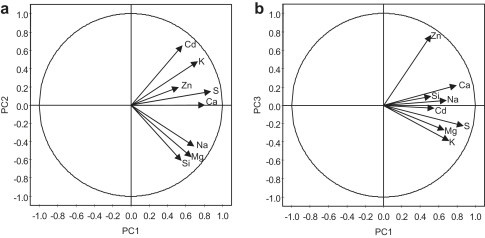
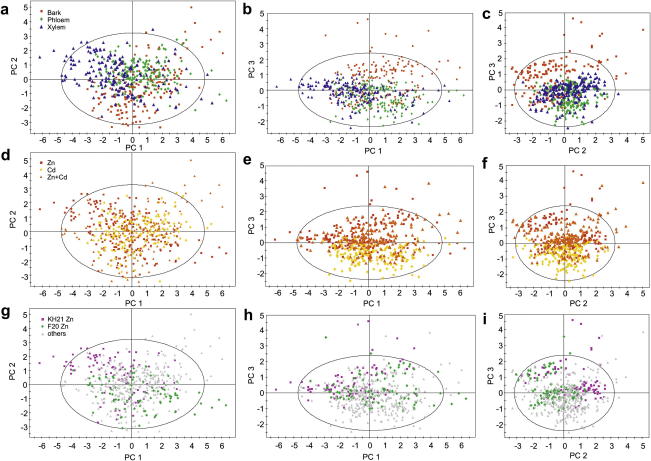
To determine which parameter (tissues, treatment and/or isolate) affected the variation in the EDX data most, the weight% values were subjected to a principal component analysis (PCA) with all elements as variables. The analysis revealed three significant principal components (PCs) (Fig. 5). The first principal component (PC1) (45.5% of the total variance) was interpreted as “responsiveness to metal treatment”. All investigated elements correlated with this axis (Fig. 5a), indicating that the element distribution was severely altered by metal treatments. The second PC explains 20.8% of the total variance. The main descriptors of PC2 are Cd and Si, correlating oppositely with this axis (Fig. 5a). This corresponds to the lower Cd translocation capacity of F20 (Fig. 1b) along with its generally higher Si weight% compared to KH21 (Fig. 7) Thus this axis represents “isolate specificity”. The third PC, contributing 11.5% of the total variance, was most influenced by Zn (Fig. 5b). It represents the “tissue-specific element accumulation”. The scores scatter plots show that tissue-specific clusters form along all three PCs (Fig. 6a–c). PC3 is able to separate the effects of the Cd from the Zn and Zn/Cd treatments (Fig. 6e,f). The PC1/PC3 and PC2/PC3 plot also shows that the Cd treatment largely overlaps with the xylem while the Zn treatment correlates with the bark values and the Cd/Zn treatment with the phloem (Fig. 6c,f). To visualize isolate-specific clusters, KH21 and F20 were differently labeled (Fig. 6g–i; Fig. S1a–f). In this way a weak separation can be visualized in the Zn and Cd + Zn treatment of plot PC1/PC2. This separation is best noticeable in PC2 (Fig. 6d,f,g; Fig. S1d,f).
Fig. 5.
Correlation-scaled loadings plots showing the influence of the measured variables (element weight%) on the principal components (PCs) obtained by PCA. Single elements are represented by arrows in planes with the first three significant PCs as coordinates. The arrow length in the direction of the axis represents the strength of the correlation with the PC, thus its influence on the PC. The circle represents the maximum, a correlation coefficient of 1. a, Plane PC1/PC2. b, Plane PC1/PC3.
Fig. 7.
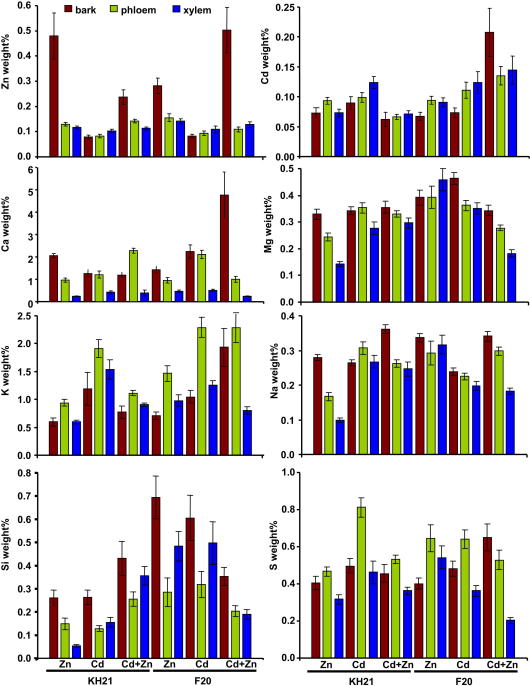
Distribution of Zn, Cd, Ca, Mg, K, Na, Si and S investigated by EDX analysis in secondary thickened roots of S. caprea isolates KH21 and F20, cultivated for 14 weeks in solution containing Zn, Cd or both metals (Cd + Zn). Data are means ± SE. Twelve roots of six biological replicates of each isolate were evaluated.
Fig. 6.
Principal component analysis (PCA) of the element distribution data. Color-coded are the tissues, the treatments and the isolates in the Zn treatment. The scatter plots illustrate the effects of the three significant PCs being the coordinates of PC1 and PC2 in a,d,g, of PC1 and PC3 in b,e,h and of PC2 and PC3 in (c,f,i).
PCA of all measured elements revealed a grouping in treatment-, isolate- and tissue-specific clusters. But since the PCA cannot visualize the effect of the individual elements, we analyzed their weight% distribution relative to the tissues and the isolates separately (Fig. 7). As already noticeable in the PCA the weight% of Zn was highest in the peripheral bark zone in both isolates. However, an isolate-specific response was revealed since KH21 had less weight% of Zn in the combined treatment while F20 had an increased Zn weight%.
Cd had a slightly higher weight% in the xylem tissues except in F20 upon the combined Zn + Cd treatment where the Cd weight% was increased in peripheral bark zone. This different pattern of the Cd distribution is consistent with the finding that the isolate from the unpolluted site (F20) accumulated less Cd in the above-ground tissues (Fig. 1).
For calcium (Ca), a descending trend was observed from the peripheral bark zone towards the xylem in both isolates. However, the isolates differed with a higher weight% distribution in F20 upon Cd exposure and a dramatic increase in the peripheral bark zone for F20 upon the combined treatment with Zn + Cd. Pearson correlation analyses showed that the distribution of Ca and Zn is positively correlated and stronger for Zn uptake in F20 than in KH21 isolate (Fig. S2a–d). Addition of Cd even increased this correlation in root tissues of F20 (Fig. S2d). In KH21 the Zn/Ca correlation is lost in the presence of Cd (Fig. S2b).
The magnesium (Mg) weight% distributions had a tendency of descending from the peripheral bark zone to the xylem in KH21 while in F20 this tendency was not detectable in Zn treatment.
A similar general gradient was detected for sodium (Na) but the weight% levels were different between the isolates for the Zn and the Cd treatment (Fig. 7). The weight% of potassium (K) was generally highest in the secondary phloem and was increased upon Cd treatment in both isolates and in F20 upon Cd + Zn treatment (Fig. 7). Furthermore, a positive correlation existed between K and Cd but not Zn (Fig. S2e,f).
In both isolates the silicon (Si) weight% was highest in the peripheral bark zone (Fig. 7), but with a clear difference between the isolates. Upon Cd or Zn treatment the values of Si were higher in F20 compared to KH21. However, this effect was lost upon exposure of Cd + Zn together.
Sulfur (S) distribution was elevated in the phloem and most prominent in KH21 upon Zn treatment. While for most treatments and in both isolates S was higher in the peripheral bark zone than in the xylem, this tendency was exaggerated in F20 upon Zn + Cd and shifted upon Zn treatment (Fig. 7).
In summary, the analyses of the individual elements in different tissues and isolates showed that their distributions are altered upon Cd, Zn and Cd + Zn treatment in most cases in an isolate-specific manner.
4. Discussion
To better understand uptake and accumulation of elements into shoots, knowledge of root anatomy and physiology is essential. Elements are transported radially from the rhizodermis through the apoplast or symplast across the cortex to the xylem and the shoot. Uptake is controlled by apoplastic barriers in the endo- and exodermis (White, 2001; Ma and Peterson, 2003; Baxter et al., 2009; Schreiber, 2010). First, a mixture of lignin and suberin is deposited into the radial and transversal cell walls (Casparian strips), which is usually followed by lamellar suberin deposition on the inner surface of exo- and endodermal cell walls (suberin lamellae) and, in some species, also by secondary thickening of the walls (Von Guttenberg, 1968). The development of these apoplastic barriers is variable and often differs between plant species and environmental conditions (Zimmerman and Steudle, 1998; Seago et al., 1999; Enstone and Peterson, 2005; Redjala et al., 2011).
We observed that the initiation of the Casparian strip and suberin lamellae formation varied between two S. caprea isolates originating from different environments. The KH21 isolate, collected from metal polluted soil and previously characterized by high Cd and Zn accumulation capacities (Puschenreiter et al., 2010), develops Casparian strips and suberin lamellae closer to the root apex compared to the genetically distinct F20 isolate which originated from unpolluted soil and showed phenotypic differences to KH21. Furthermore, F20 first developed Casparian strips in endodermis and later in exodermis, oppositely to KH21. Suberin lamellae developed first in exodermis and later in endodermis in both isolates. Similarly, differences in the development of apoplastic barriers between isolates of S. viminalis differing in metal tolerance and accumulation were also observed by Lux et al. (2004). Therefore we propose that apoplastic barrier development is an adaptive trait and can vary between different isolates of the same species.
Similarly, we have observed that anatomical reactions to heavy metal exposure differed between isolates. In F20, exposure to Cd, Zn or Cd + Zn resulted in earlier development of both barriers while the development in KH21 was not influenced by metal exposure. The behavior of F20 is consistent with several studies showing that roots exposed to Cd develop earlier apoplastic barriers (Schreiber et al., 1999; Martinka and Lux, 2004; Zelko and Lux, 2004; Vaculík et al., 2009). Accelerated apoplastic barrier development was also induced by higher salinity (Reinhardt and Rost, 1995; Karahara et al., 2004). Thus the typical reaction is to minimize the uptake of pollutants by developing the exo- and endodermis closer to the root apex. This is consistent with the finding that the concentration of Cd in leaves was lower in F20 than in KH21. On the other hand, delayed development of the endodermal suberin lamellae of KH21 upon exposure to heavy metals could be the reason of the higher accumulation capacity of Cd in shoots. However, the understanding of the delay in apoplastic barriers development after metal stress in isolate KH21 originating from metal polluted soil remains still poorly understood.
Changes in root tissue areas might also be the consequence of continuous exposure to metal polluted soils. Lux et al. (2004) showed that willow clones characterized by high tolerance to Cd had a higher proportion of epi-, exo- and endodermal tissues when compared with the sensitive clones, which had a higher proportion of mid-cortex. We found that KH21 produced thinner roots with a smaller area of vascular bundles and xylem elements when compared with F20. However, when the same isolates were exposed to elevated metal concentrations, the reaction of roots was different. In roots of F20 the total area and areas of central cylinder and xylem elements decreased in comparison to control conditions. This was not, or to a lesser extent, detectable in KH21. We suppose that the differences in the root anatomy of the Salix isolates treated with metals reflect an adaptive predisposition and depend on the different natural origins.
Cd-induced differences in the proportions of specific root tissues (rhizodermis–cortex–vascular bundles) or in the size and shape of individual cells have been reviewed recently by Lux et al. (2011). Described syndromes of Cd toxicity on roots are a decrease of root number, length and dry mass as well as enlargement of the root diameter. Lunáčková et al. (2003) reported that roots of S. alba and Populus x euramericana became shorter and thicker upon Cd treatment. Similarly, Maksimović et al. (2007) observed increased root diameter of maize seedlings after Cd application. However, the reactions of root tissues to Cd mostly differ among plant species and used Cd concentrations (e.g. Lux et al., 2011). Conversely, Cd, Zn and Cd + Zn treatment in our experiments decreased the total area of the S. caprea roots in F20. The total root area of KH21 was unaffected when Cd was applied, although after Zn and Zn + Cd application the total area of root decreased. Similar to our findings Vázquez et al. (1992) reported that the root diameter of bean (Phaseolus vulgaris) remained unaffected, although the number of cortical parenchyma cells decreased when Cd was applied. Also Florijn et al. (1993) did not find any changes in the root diameter, length or surface area in two different maize inbred lines exposed to Cd. A decrease of the root diameter was noticed by Gratão et al. (2009) after Cd application to the tomato cultivar Micro-Tom. We expected that unresponsiveness of KH21 tissues to elevated Cd concentration probably related with higher Cd accumulation capacity. This pattern of root tissue development probably disappeared when plants were exposed to Zn or Cd + Zn.
There is little knowledge on the influence of Zn on root tissue proportions. Rosolem et al. (2005) found that the total area of the root, the diameter of the vascular bundles, the number of xylem strands and the area they occupied in stele were higher in coffee plants grown without Zn in the medium than in plants exposed to Zn. A similar decrease of tissue areas after Zn application was observed in our experiments in F20 while KH21 differed only in the total root area.
Balanced Zn concentration and distribution is essential for optimal growth and function of metabolic processes (Broadley et al., 2007). Similar to findings of Di Baccio et al. (2009) for poplar, MacFarlane and Burchett (2000) for gray mangrove and Brunner et al. (2008) for spruce exposed to elevated Zn, in our experiments most of the Zn accumulated in the peripheral tissues of the secondary thickened root. Therefore we assume that the peripheral bark zone of secondary thickened roots serves as an effective barrier for radial Zn transport to vascular bundles.
Freeze drying of the samples prevented leakage of elements during fixation, allowing us to detect tissue-specific distribution of elements with low abundance such as Cd (Fig. 4b). In contrast to Zn, most of the Cd was detected in the xylem in both isolates treated with Cd. However, when both metals Cd + Zn were applied to KH21, no significant differences in the Cd distribution between the peripheral zone and vascular bundles were observed. In contrast, Coccoza et al. (2008) found that in poplar roots exposed to higher doses of Cd this element accumulated predominantly in cells surrounding the central cylinder. Nevertheless, in the combined Cd + Zn treatment, double values of Cd in each investigated tissue of F20 were detected when compared with KH21. Together with the observations that the Cd weight% in KH21 tissues is lower as in F20 (Table S2) we assume that KH21 is more efficiently translocating Cd to the shoot, especially when excess of Zn is present. These differences in Cd localization within root tissues probably relate with the uptake and translocation of Cd and Zn, and might reflect the situation on the site of KH21's origin in natural conditions, which is adapted to a soil containing up to 84 mg total Cd kg−1 and 8.6 g total Zn kg−1 (Puschenreiter et al., 2010).
The levels of Zn and Ca are positively correlated in both isolates when treated with excess Zn. In the combined Cd + Zn treatment, this correlation is lost only in KH21. Cadmium is among others transported into the root via Ca uptake and translocation pathways (Lux et al., 2011). KH21 accumulates more Cd in the shoot than F20, therefore it is possible that higher amounts of Cd are taken up via Ca transport mechanisms. Cadmium competes with Ca uptake, which might explain the lower Ca concentration in the combined Zn + Cd treatment. Furthermore, a positive correlation exists between K and Cd, but not Zn, distribution in root tissues. It was recently found that K supplement alleviated Cd toxicity in soybean (Shamsi et al., 2010), which might be also one possible tolerance mechanism in S. caprea.
Silicon repressed root-to-shoot translocation of Cd in Brassica chinensis and increased Cd tolerance (Song et al., 2009). Consistently, F20, which translocates less Cd into shoots, accumulates significantly more Si than KH21 in all root tissues. Proposed mechanisms which reduce translocation to the shoot are Si co-precipitation with Cd and Zn in roots or physical blockage of metal flow across cell walls (Neumann and zur Nieden, 2001; Shi et al., 2005). We suggest that low Si weight% in KH21 roots allows higher shoot Cd uptake.
5. Conclusion
EDX analyses show that Cd and Zn interfere differently with the element uptake systems in S. caprea, and that for some elements this effect is isolate-specific. Variation in the organization of root tissues, development of apoplastic barriers and the distribution pattern of Zn and Cd and other elements in S. caprea isolates originating from polluted and unpolluted sites support the hypothesis that these features are results of environmental adaptation. Understanding the molecular basis of these adaptive traits might help to increase the tolerance and heavy metal accumulation capacities of S. caprea for phytoremediation technologies.
Acknowledgment
This work was supported by the FWF project L433_B17 to MTH, and by the Slovak Research and Development Agency APVV under the contract Nr. APVV (0140-10), and VEGA (1/0472/10 and 1/0817/12) to MV and AL, and by COST Action FA0905. The authors thank Dr. Marie-Luise Weidinger for assistance with preparation of samples for EDX analysis, Dr. Irene Lichtscheidl for providing her expertise on heavy metal analyses in plants and valuable comments on the manuscript and Franz Hadacek for sharing the SIMCA-P software for PC-analyses. The authors are grateful to Dr. Phillip White for valuable and stimulating comments and suggestions.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.envpol.2011.12.031.
Appendix. Supplementary data
References
- Baxter I., Hosmani P.S., Rus A., Lahner B., Borevitz J.O., Muthukumar B., Mickelbart M.V., Schreiber L., Franke R.B., Salt D.E. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genetics. 2009;5:e1000492. doi: 10.1371/journal.pgen.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley M.R., White P.J., Hammond J.P., Zelko I., Lux A. Zinc in plants. New Phytologist. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Brundrett M.C., Kendrick B., Peterson C.A. Efficient lipid staining in plant material with Sudan red 7B or Fluorol yellow 088 in polyethylene glycol–glycerol. Biotechnic and Histochemistry. 1991;66:111–116. doi: 10.3109/10520299109110562. [DOI] [PubMed] [Google Scholar]
- Brunner I., Luster J., Günthardt-Goerg M.S., Frey B. Heavy metal accumulation and phytostabilisation potential of tree fine roots in a contaminated soil. Environmental Pollution. 2008;152:559–568. doi: 10.1016/j.envpol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Coccoza C., Minnoci A., Tognetti R., Iori V., Zacchini M., Scarascia Mungozza G. Distribution and concentration of cadmium in root tissue of Populus alba determinated by scanning electron microscopy and energy-dispersive x-ray microanalysis. iForest. 2008;1:96–103. [Google Scholar]
- Dechamps C., Lefèbvre C., Noret N., Meerts P. Reaction norms of life history traits in response to zinc in Thlaspi caerulescens from metalliferous and nonmetalliferous sites. New Phytologist. 2007;173:191–198. doi: 10.1111/j.1469-8137.2006.01884.x. [DOI] [PubMed] [Google Scholar]
- Di Baccio D., Tognetti R., Minnocci A., Sebastiani L. Responses of the Populus x euramericana clone I-214 to excess zinc: carbon assimilation, structural modifications, metal distribution and cellular localization. Environmental and Experimental Botany. 2009;67:153–163. [Google Scholar]
- Dos Santos Utmazian M.N., Wenzel W.W. Cadmium and zinc accumulation in willow and poplar species grown on polluted soils. Journal of Plant Nutrition and Soil Science. 2007;70:265–272. [Google Scholar]
- Enstone D.E., Peterson C.A. Suberin lamella development in maize seedlings roots grown in aerated and stagnant conditions. Plant, Cell and Environment. 2005;25:444–455. [Google Scholar]
- Escarré J., Lefèbvre C., Gruber W., Leblanc M., Lepart J., Rivière Y., Delay B. Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: implications for phytoremediation. New Phytologist. 2000;145:429–437. doi: 10.1046/j.1469-8137.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Florijn P.J., Nelemans J.A., Van Beusichem M.L. Evaluation of structural and physiological plant characteristics in relation to the distribution of cadmium in maize inbred lines. Plant and Soil. 1993;154:103–109. [Google Scholar]
- Goldstein J. Kluwer; New York: 2003. Scanning Electron Microscopy and X-ray Microanalysis. 689 pp. [Google Scholar]
- Gratão P.L., Monteiro C.C., Rossi M.L., Martinelli A.P., Peres L.E.P., Medici L.O., Lea P.J., Azevedo R.A. Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environmental and Experimental Botany. 2009;67:387–394. [Google Scholar]
- Greger M., Landberg T. Use of willow in phytoextraction. International Journal of Phytoremediation. 1999;1:115–123. doi: 10.1080/15226514.2014.1003785. [DOI] [PubMed] [Google Scholar]
- Hanikenne M., Talke I.N., Haydon M.J., Lanz C., Nolte A., Motte P., Kroymann J., Weigel D., Krämer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;458:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- Jensen J.K., Holm P.E., Nejrup J., Larsen M.B., Borggaard O.K. The potential of willow for remediation of heavy metal polluted calcareous urban soils. Environmental Pollution. 2009;157:931–937. doi: 10.1016/j.envpol.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ambriz G., Petit C., Bourrié I., Dubois S., Olivieri I., Ronce O. Life history variation in the heavy metal tolerant plant Thlaspi caerulescens growing in a network of contaminated and noncontaminated sites in southern France: role of gene flow, selection and phenotypic plasticity. New Phytologist. 2007;173:199–215. doi: 10.1111/j.1469-8137.2006.01923.x. [DOI] [PubMed] [Google Scholar]
- Karahara I., Ikeda A., Kondo T., Uetake Y. Development of the Casparian strip in primary root of maize under salt stress. Planta. 2004;219:41–47. doi: 10.1007/s00425-004-1208-7. [DOI] [PubMed] [Google Scholar]
- Landberg T., Greger M. Differences in uptake and tolerance to heavy metals in Salix caprea from unpolluted and polluted areas. Applied Geochemistry. 1996;11:175–180. [Google Scholar]
- Lombi E., Zhao F.J., Dunham S.J., McGrath S.P. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi geosingense. New Phytologist. 2000;145:11–20. [Google Scholar]
- Lunáčková L., Šottníková A., Masarovičová E., Lux A., Streško V. Comparison of cadmium effect on willow and poplar in response to different cultivation conditions. Biologia Plantarum. 2003;47:403–411. [Google Scholar]
- Lux A. A rapid method for staining semithin sections of plant material. Biologia. 1981;36:753–757. (In Slovak) [Google Scholar]
- Lux A., Martinka M., Vaculík M., White P.J. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany. 2011;62:21–37. doi: 10.1093/jxb/erq281. [DOI] [PubMed] [Google Scholar]
- Lux A., Morita S., Abe J., Ito K. An improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany. 2005;96:989–996. doi: 10.1093/aob/mci266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A., Šottníková A., Opatrná J., Greger M. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiologia Plantarum. 2004;120:537–545. doi: 10.1111/j.0031-9317.2004.0275.x. [DOI] [PubMed] [Google Scholar]
- Ma F., Peterson C.A. Recent insights into the development, structure and chemistry of the endodermis and exodermis. Canadian Journal of Botany. 2003;81:405–421. [Google Scholar]
- MacFarlane G.R., Burchett M.D. Cellular distribution of copper, lead and zinc in the grey mangrove, Avicena marina (Forsk.) Vierh. Aquatic Botany. 2000;68:45–59. [Google Scholar]
- Maestri E., Marmiroli M., Visioli G., Marmiroli N. Metal tolerance and hyperaccumulation: cost and trade-offs between traits and environment. Environmental and Experimental Botany. 2010;68:1–13. [Google Scholar]
- Maksimović I., Kastori R., Krstic L., Lukovic J. Steady presence of cadmium and nickel affects root anatomy, accumulation and distribution of essential ions in maize seedlings. Biologia Plantarum. 2007;51:589–592. [Google Scholar]
- Markhart A.H., Läuchli A. The comparison of three freezing methods for electron probe X-ray microanalysis of hydrated barley root tissue. Plant Science Letters. 1982;25:29–36. [Google Scholar]
- Martinka M., Lux A. Response of roots of three populations of Silene dioica to cadmium treatment. Biologia. 2004;59:185–189. [Google Scholar]
- McCully M., Canny M.J., Huang C.X., Miller C., Brink F. Cryo-scanning electron microscopy (CSEM) in the advancement of functional plant biology: energy dispersive X-ray microanalysis (CDEX) applications. Functional Plant Biology. 2010;37:1011–1040. [Google Scholar]
- Neumann D., zur Nieden U. Silicon and heavy metal tolerance of higher plants. Phytochemistry. 2001;56:685–692. doi: 10.1016/s0031-9422(00)00472-6. [DOI] [PubMed] [Google Scholar]
- Padmavathiamma P.K., Li L.Y. Phytoremediation technology: hyper-accumulation metals in plants. Water Air and Soil Pollution. 2007;184:105–126. [Google Scholar]
- Pulford I.D., Watson C. Phytoremediation of heavy metal-contaminated land by trees – a review. Environment International. 2003;29:529–540. doi: 10.1016/S0160-4120(02)00152-6. [DOI] [PubMed] [Google Scholar]
- Puschenreiter M., Türktaş M., Sommer P., Wieshammer G., Laaha G., Wenzel W.W., Hauser M.-T. Differentiation of metallicolous and non-metallicolous Salix caprea populations based on phenotypic characteristics and nuclear microsatellite (SSR) markers. Plant, Cell and Environment. 2010;33:1641–1655. doi: 10.1111/j.1365-3040.2010.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascio N., Navari-Izzo F. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Science. 2011;180:169–181. doi: 10.1016/j.plantsci.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Redjala T., Zelko I., Sterckeman T., Legué V., Lux A. Relationship between root structure and root cadmium uptake in maize. Environmental and Experimental Botany. 2011;71:241–248. [Google Scholar]
- Reinhardt D.H., Rost T.L. Salinity accelerates endodermal development and induces an exodermis in cotton seedling roots. Environmental and Experimental Botany. 1995;35:563–574. [Google Scholar]
- Rosolem C.A., Sacramento L.V.S., Oliveira D.M.T. Kinetics of zinc uptake and anatomy of roots and leaves of coffee trees as affected by zinc nutrition. Journal of Plant Nutrition. 2005;28:2101–2112. [Google Scholar]
- Schreiber L. Transport barriers made of cutin, suberin and associated waxes. Trends in Plant Science. 2010;15:546–553. doi: 10.1016/j.tplants.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Schreiber L., Hartmann K., Skrabs M., Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany. 1999;50:1267–1280. [Google Scholar]
- Seago J.L., Peterson C.A., Enstone D.E., Scholey C.A. Development of the endodermis and hypodermis of Typha glauca Godr. and Typha angustifolia. Canadian Journal of Botany. 1999;77:122–134. [Google Scholar]
- Sebastiani L., Scebba F., Tognetti R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoides x maximowiczii) and I-214 (P. x euramericana) exposed to industrial waste. Environmental and Experimental Botany. 2004;52:79–88. [Google Scholar]
- Shamsi I.H., Jiang L.X., Wei K., Jilani G., Hua S.J., Zhang G.P. Alleviation of cadmium toxicity in soybean by potassium supplementation. Journal of Plant Nutrition. 2010;33:1926–1938. [Google Scholar]
- Shah K., Nongkynrih J.M. Metal hyperaccumulation and bioremediation. Biologia Plantarum. 2007;51:618–634. [Google Scholar]
- Shen Z.G., Zhao F.J., McGrath S.P. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant, Cell and Environment. 1997;20:898–906. [Google Scholar]
- Shi X., Zhang C., Wang H., Zhang F. Effect of Si on the distribution of Cd in rice seedlings. Plant and Soil. 2005;272:53–60. [Google Scholar]
- Song A., Li Z., Zhang J., Xue G., Fan F., Liang Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. in attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. Journal of Hazardous Materials. 2009;172:74–83. doi: 10.1016/j.jhazmat.2009.06.143. [DOI] [PubMed] [Google Scholar]
- Unterbrunner R., Puschenreiter M., Sommer P., Wieshammer G., Tlustoš P., Zupan M., Wenzel W.W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environmental Pollution. 2007;148:107–114. doi: 10.1016/j.envpol.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Vaculík M., Lux A., Luxová M., Tanimoto E., Lichtscheidl I. Silicon mitigates cadmium inhibitory effects in young maize plants. Environmental and Experimental Botany. 2009;67:52–58. [Google Scholar]
- Vázquez M.D., Poschenrieder C., Barceló J. Ultrastructural effects and localization of low cadmium concentrations in bean roots. New Phytologist. 1992;120:215–226. [Google Scholar]
- Verbruggen N., Hermans C., Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist. 2009;181:759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- Vollenweider P., Menard T., Günthardt-Goerg M. Compartmentation of metals in foliage of Populus tremula grown on soils with mixed contamination. I. From the tree crown to leaf cell level. Environmental Pollution. 2011;159:324–336. doi: 10.1016/j.envpol.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Von Guttenberg H. Gebrüder Borntraegern; Berlin – Stuttgart: 1968. Der primäre Bau der Angiospermenwurzel. (Handbuch der Pflanzenanatomie). 472 pp. [Google Scholar]
- Vysloužilová M., Puschenreiter M., Wieshammer G., Wenzel W.W. Rhizosphere characteristics, heavy metal accumulation and growth performance of two willow (Salix x rubens) isolates. Plant, Soil and Environment. 2006;52:353–361. [Google Scholar]
- White P.J. The pathways of calcium movement to the xylem. Journal of Experimental Botany. 2001;52:891–899. doi: 10.1093/jexbot/52.358.891. [DOI] [PubMed] [Google Scholar]
- Wieshammer G., Unterbrunner R., Banares Garcia T., Zivkovic M.F., Puschenreiter M., Wenzel W.W. Phytoextraction of Cd and Zn from agricultural soils by Salix ssp. and intercropping of Salix caprea and Arabidopsis halleri. Plant and Soil. 2007;298:255–264. [Google Scholar]
- Zacchini M., Pietrini F., Mugnozza G.S., Iori V., Pietrosanti L., Massacci A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollution. 2009;197:23–34. [Google Scholar]
- Zelko I., Lux A. Effect of cadmium on Karwinskia humboldtiana roots. Biologia. 2004;59:205–209. [Google Scholar]
- Zhao F.-J., McGrath S.P. Biofortification and phytoremediation. Current Opinion in Plant Biology. 2009;12:373–380. doi: 10.1016/j.pbi.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Zimmerman H.M., Steudle E. Apoplastic transport across young maize roots: effect of the exodermis. Planta. 1998;206:7–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.