Abstract
12(S)-Lipoxygenase (LOX) and its product 12(S)-hydroxyeicosatetraenic (HETE) acid have been implicated in angiogenesis and tumour invasion in several tumour types while their role in colorectal cancer progression has not yet been studied. We have analysed 12(S)-LOX expression in colorectal tumours and found gene expression up-regulated in colorectal cancer specimens for which the pathology report described involvement of inflammation.
Using cell line models exposed to 12(S)-HETE or over-expressing 12(S)-LOX malignant cell growth as well as tumour cell migration was found to be stimulated. Specifically, Caco2 and SW480 cells over-expressing 12(S)-LOX formed fewer colonies from sparse cultures, but migrated better in filter-migration assays. SW480 LOX cells also had higher anchorage-independent growth capacity and a higher tendency to metastasise in vivo. Knock-down or inhibition of 12(S)-LOX inhibited cell migration and anchorage-independent growth in both 12(S)-LOX transfectants and SW620 cells that express high endogenous levels of 12(S)-LOX. On the cell surface E-cadherin and integrin-β1 expression were down-regulated in a 12(S)-LOX-dependent manner disturbing cell–cell interactions. The results demonstrate that 12(S)-LOX expression in inflammatory areas of colorectal tumours has the capacity to induce an invasive phenotype in colorectal cancer cells and could be targeted for therapy.
Abbreviations: AA, arachidonic acid; HETE, hydroxyeicosatetraenic acid; LOX, lipoxygenase.
Keywords: Lipoxygenase, 12(S)-HETE, Cell migration, Malignant phenotype, E-cadherin, Integrin
Highlights
► 12(S)-LOX is up-regulated in inflammatory areas of colorectal tumours. ► 12(S)-HETE and 12(S)-LOX over-expression stimulate malignant growth and migration. ► 12(S)-LOX over-expressing SW480 cells have a higher tendency to metastasise. ► Blocking 12(S)-LOX activity or expression inhibits malignant growth and migration.
Introduction
Lipoxygenases (LOX) and their eicosanoid products are involved in many physiological and pathophysiological processes like growth, differentiation, vascularisation, inflammation and arteriosclerosis [1,2]. Specifically, 12- and 15-LOX enzymes have been described as regulators of inflammation and immune-response [3]. Leukocyte, reticulocyte and epidermal forms of the enzyme are well characterised as to their product pattern and physiological function [3–5]. By contrast, the platelet-derived 12(S)-LOX (ALOX12) is still insufficiently understood. It produces almost exclusively 12(S)-hydroxyeicosatetraenic acid (HETE) [6,7] that has been shown to act as a growth and/or survival factor in gastric [8], pancreatic [9], melanoma and prostate tumour cells [10,11] and to induce a metastatic phenotype in prostate cancer [12]. In addition 12(S)-HETE may also affect other tissue components to enhance neovascularisation and tumour progression [10,12–15]. For this reason 12(S)-LOX is regarded as a protumorigenic gene [16]. Protumorigenic roles of LOX-enzymes are also suggested by the observation that inhibitors of LOX isoenzymes like quercetin, nordihydroguaretic acid or baicalein can protect from carcinogenesis in both the skin and the colon [17–19]. Such inhibitors are not specific for individual LOX enzymes, however, so that a more specific approach is needed to demonstrate the actual role of 12(S)-LOX in tumour development.
In colorectal cancer protumorigenic impact has been unequivocally demonstrated for cyclooxygenase 2 (COX-2) and its prostaglandin products [20] as well as for 5-LOX and its product leukotrien B4 [21,22]. Concerning 12(S)-LOX a genetic polymorphism that produces a more active enzyme is related to a higher cancer risk [23] suggesting that there is a protumorigenic impact of 12(S)-LOX on CRC. The pathophysiological impact of the enzyme on CRC has not yet been analysed in any detail, however. This study therefore, undertakes to determine the expression of 12(S)-LOX in colorectal carcinomas as compared to normal mucosa as well as the cell biological consequences of 12(S)-HETE and 12(S)-LOX expression in colorectal tumour cell lines.
Materials and methods
Tissue specimen
Tissue specimens were obtained from colorectal carcinomas and normal tumour-free mucosa at the resection margin from patients suffering from colorectal cancer. The study has been approved by the local ethics committee and the patients had given their informed consent. The tissue specimens were collected immediately after surgery and frozen in liquid N2 until extraction.
Cell lines and transfection
SW480, SW620, and Caco2 colon carcinoma cells were obtained from the American Type Culture Collection. The cell lines were kept under standard tissue culture conditions using Minimal Essential Medium (MEM) containing 10% fetal calf serum (FCS).
12(S)-LOX over-expressing SW480 and Caco2 cells were produced by electroporation as described before [24]. Populations stably over-expressing 12(S)-LOX were cultivated in the presence of 1.6 and 0.2 mg/ml geneticin (G418) respectively.
Eicosanoid mediators, inhibitors and cell treatment
12(S)-HETE (0.1 mg/ml in ethanol) was purchased from Cayman chemicals (Ann Arbor, MI). Arachidonic acid (AA) and baicalein were obtained from Sigma (St. Louis, MO). Cells were plated at a density of 5 × 104 in 24-well plates and left to attach for 24 h before exposure to the compounds diluted into HEPES-buffered MEM containing 1 mg/ml bovine serum albumin (BSA). Experiments on 12(S)-LOX transfectants and their respective controls were done in the presence of 10 μM AA to provide sufficient substrate for eicosanoid synthesis.
Growth parameters
Viable cell number was determined by neutral red uptake as reported previously [24].
For clonogenicity assays cells were plated at a density of 100 cells/well (SW480) or 200 cells/well (Caco2, SW620) into six-well plates in growth medium. Unattached cells were removed 24 h later and the cultures then left to grow for 7 days. The number of colonies was assessed after staining with crystal violet.
Anchorage-independent growth was determined from 5000 cells/well in 0.25% agar prepared in RPMI medium containing 20% FCS and incubated for 2–3 weeks before counting the number of colonies microscopically.
Cell migration assay
Cell migration was analysed by filter migration assay as described before [25]. Specifically, 0.5 × 105 cells/cm² were seeded into 8-μm-pore-size filters (Becton Dickinson-Falcon, Franklin Lakes, NJ) and migration periods of 24 h (SW480, SW620) or 48 h (Caco2) were permitted.
Tumour growth in vivo
SW480-LOX and SW480-co cells were harvested, washed with PBS, and suspended in Ringer's solution. 1 × 106 cells in 50 μl Ringer's solution were subcutaneously injected into the rear flanks of immunodeficient SCID/Balb/c recipient mice (female, aged 4 weeks, Harlan Winkelmann, Borchen, Germany). Tumour formation was monitored periodically by palpation, and the tumour size was determined using a Vernier caliper. Tumour volume was calculated using the formula (smaller diameter2 × larger diameter)/2. All experiments were performed in triplicate and carried out according to the Austrian and FELASA guidelines for animal care and protection. Tissue sections of experimental tumours were analysed by immunohistochemistry using antibodies directed against cytokeratin 20.
Mouse lungs were prepared for immunohistochemistry and stained with a monoclonal antibody recognising Ki67 (Novacastra, Leica Microsystems, Wetzlar, Germany). Serial sections were scored for Ki67-positive cells/field of vision and the following scores were assigned: < 1 cell/field = 0, 1–5 cells/field = 1; 5–10 cells/field = 2; 10–20 cells/field = 3; > 20 cells/field = 4.
Knock-down of 12(S)-LOX expression
To knock down 12(S)-LOX expression 400 pmol (Caco2 transfectants), 10 nmol (SW480 transfectants) or 30 nmol (SW620 cells) of an antisense phosphothioate oligonucleotide directed against 12(S)-LOX with the sequence 5′-CTCAGGAGGGTGTAAACA-3′ [26] was introduced by lipofection. Lipofectamin (Invitrogen Life Technologies, Carlsbad, CA) was used for Caco2 cells and siLentFect (BioRad, Hercules, CA) for SW480 and SW620 cells. Controls were transfected with a scrambled oligonucleotide, the sequence of which was 5′-AAGATT GCGCGACGATGA-3′ [26]. To determine the efficiency of 12(S)-LOX down-regulation the production of 12(S)-HETE was determined 48 and 96 h after transfection.
Reverse transcription-PCR analysis of gene expression
Total RNA was isolated from subconfluent cultures or frozen colon tissue specimens using Trifast (PeqLab, Erlangen, G) and chloroform extraction. 5 μg aliquots were used to prepare cDNA using RevertAid MMuLV reverse transcriptase (Fermentas, Burlington, Ontario) and random hexamer primers (GE Healthcare, Piscataway, NJ, USA).
The expression of 12(S)-LOX mRNA, E-cadherin, integrin β1 and GAPDH was then determined by real time PCR on an ABI PRISM 7000 system (Applied Biosystems, Foster City, CA, USA) using Taqman Gene expression assays listed in Table 2 of supplemental materials. Quantification of gene expression was calculated by the ΔΔCt method using GAPDH as the internal control gene. Selected experiments were also done by standard RT-PCR using the primers and conditions listed in Table 1 of supplemental materials.
E-cadherin and integrin β1 on the cell surface
Cells were harvested by limited trypsinisation and washed thoroughly. 106 cells were then re-suspended in 100 μl PBS. For detection of E-cadherin cells were incubated with 10 μg/ml mouse anti-human E-Cadherin (HECD-1; Alexis, San Diego) for 2 h on ice followed by a 1-h incubation with 20 μl of a phycoerythrin-labelled second antibody (Rat anti mouse—PE, Becton Dickinson). Detection of integrin β1 was done using a PE-labelled antibody recognising total integrin β1 (clone MAR4, Becton Dickinson). Control samples were incubated with control IgG. Analysis was performed using a FACScalibur (Becton Dickinson).
Western blot
Whole cell lysates were obtained by homogenising cultures in RIPA buffer (50 mM Tris/HCl pH 7.4, 500 mM NaCl, 1% NP-40, 0.5% Na-DOC, 0.1% SDS, 0.05% NaN3) supplemented with complete protease inhibitor mix (1:50; Roche Diagnostics, Mannheim, Germany). Integrin β1 protein levels were determined by western blotting from 50 μg of protein per lane using primary antibodies directed against integrin β1 (1:1000; 4706, Cell Signalling, Boston, MA) and β-actin (1:5000; AC-15, Sigma, St. Louis, MO, USA). Secondary antibodies were conjugated to horseradish peroxidase and detection achieved by chemiluminescence.
12(S)-HETE ELISA
5 × 104 cells/ well were plated into 24 well plates and 10 μM AA (Sigma) was added 24 h later. 12(S)-HETE production was determined from culture supernatants using an indirect ELISA assay purchased from R&D (Minneapolis, MN) according to the manufacturer's instructions. 100 μl of the supernatant were added to the plates together with an alkaline phosphatase-labelled HETE as a tracer and polyclonal anti-12(S)-HETE antibody. After 2 h of incubation at room temperature wells were washed extensively and bound tracer was detected photometrically.
Results
Expression of 12(S)-LOX in colorectal tumours
Expression of 12(S)-LOX was determined by quantitative RT-PCR from 50 colorectal tumour specimens. For 16 of these tumours the pathology report described ulcerated areas and/or inflammatory cells recruited into the tumour tissue. Based on the role of 12-LOX enzymes in inflammation regulation we chose to analyse these tumours separately from the remaining 34 tissue specimens for which no signs of inflammation was reported. Quantification was obtained relative to the house keeping gene glycerinaldehyd-3-phosphat-dehydrogenase (GAPDH) and normalised to the respective normal, tumour-free tissue at the resection margin. The mean relative expression for the 16 tumour specimens was 2.29 ± 0.52 (range 0.004–7.24; higher than normal mucosa at p = 0.0121) with 12(S)-LOX mRNA up-regulated in 10/16 specimens and down-regulated in only 2/16. No up-regulation and even down-regulation was observed in tumour-specimens for which little or no inflammatory component was described in the pathology report (mean relative LOX-expression 0.381 ± 0.380; range 0.00001–1.14). Calculation relative to ß-actin and microglobulin as alternative house keeping genes did not alter the results (data not shown).
Biological effects of 12(S)-HETE on colorectal cancer cells
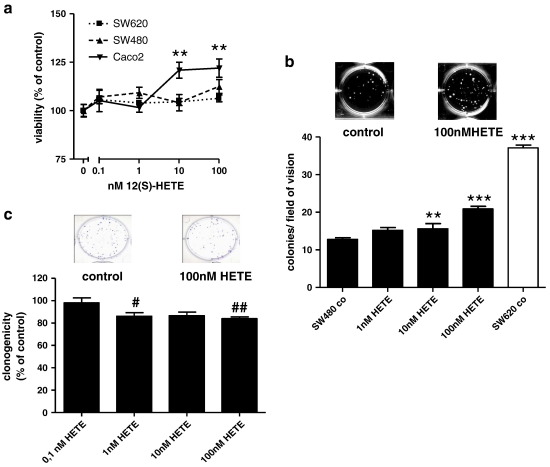
To investigate the biological impact of 12(S)-LOX expression and the 12(S)-HETE product on colorectal cancer cells cell line models were chosen due to their endogenous 12(S)-LOX expression levels (see supplemental materials). To assess the biological effects of the 12(S)-LOX product 12(S)-HETE in colorectal tumour cells the mediator was diluted into the medium of SW620, SW480 and Caco2 cultures and cell number was determined 24 h later. Viability was increased by 12(S)-HETE in the highly differentiated, slowly growing Caco2 cells about 25% above the control. The eicosanoid did not enhance viability in the rapidly growing SW480 and SW620 cells (Fig. 1a). However, growth of SW480 cells in soft agar was stimulated by 12(S)-HETE in a concentration dependent manner (p < 0.001 by one-way ANOVA) reaching 1.6-fold at a concentration of 100 nM. SW620 cells that were obtained from a lymph node metastasis served as positive control for agar growth (Fig. 1b). Caco2 cells did not produce sufficient agar colonies to be quantified (data not shown). By contrast, clonogenicity of SW480 cells was inhibited by 12(S)-HETE (Fig. 1c; p < 0.05 by one-way ANOVA). Effects of 12(S)-HETE on the migration of SW480 or Caco2 cells were not detected (data not shown).
Fig. 1.
Effects of 12(S)-HETE on cell number a: Cells were plated in 24-well plates at 5 × 104 cells/well and left to attach for 24 h before exposure to 12(S)-HETE. The number of viable cells was determined by neutral red uptake and calculated relative to controls. ** represents an increase as compared to the respective controls at p < 0.01. b: 5000 SW480 or SW620 cells were suspended in agar medium containing appropriate 12(S)-HETE concentrations to determine anchorage-independent growth. SW620 cells were used as a positive control for agar growth. After 3 weeks colonies were scored at 4-fold magnification in 2 independent experiments using triplicate cultures. All data points represent the mean ± SD, the overall induction was significant at p < 0.001 by one-way ANOVA, for single column comparison ** and *** indicate an increase above control at p < 0.01 and p < 0.001 respectively. c: 100 SW480 cells/well were seeded into 6-well plates using medium supplemented with 12(S)-HETE at the concentrations indicated and left to attach overnight. The medium together with any cells that had not attached was removed. After the medium change cultures were maintained in standard growth medium for 1 week. Colonies were fixed, stained with crystal violet and counted. All data points represent the mean ± SD of 2 independent experiments, the overall decrease was significant at p < 0.05 by one-way ANOVA and for single column comparisons # and ## indicate a decrease as compared with control at p < 0.05 or 0.01 respectively.
Over-expression of 12(S)-LOX in colorectal tumour cells
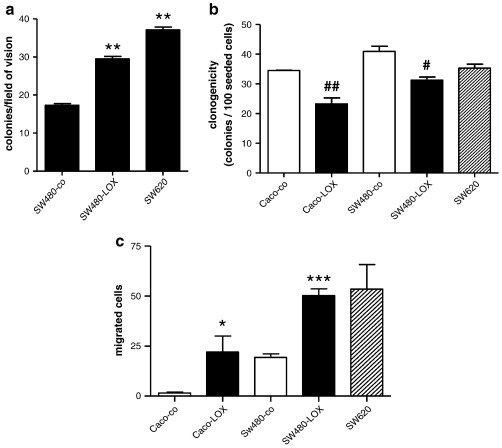
To achieve sustained 12(S)-HETE production 12(S)-LOX over-expressing cells were constructed from both Caco2 and SW480 cells. The SW480-LOX cultures have been characterised previously [24]. Stable Caco-LOX transfectants expressed 14-fold higher levels of 12(S)-LOX compared to vector-transfected cells and secreted about 5 nM 12(S)-HETE into their medium upon AA addition (supplemental materials Figure 1s). That amount of mediator was sufficient to induce increased viability: in the presence of AA to support 12(S)-HETE production both over-expressing cell lines achieved a growth and/or survival advantage over the respective controls (supplemental materials Figure 2s). The growth promoting effect was even more obvious in soft agar media. Colony number obtained from SW480-LOX was increased 1.7-fold above the controls and almost reached the number of colonies derived from untransfected SW620, which is a more malignant cell clone producing higher amounts of 12(S)-HETE (Fig. 2a). The growth of Caco-LOX cells was not sufficient to be analysed in soft agar assays. By contrast, colony formation was inhibited by 33% in Caco2 transfectants and by 24% in SW480-LOX cells which was equal to colony formation in SW620 cultures (Fig. 2b). Cell migration was stimulated in 12(S)-LOX transfectants as compared to controls so that Caco-LOX cells migrated like SW480 controls and SW480-LOX cells displayed activity similar to SW620 cells (Fig. 2c).
Fig. 2.
Biological impact of 12(S)-LOX expression. a: 5000 cells each of SW480-co and SW480-LOX were suspended in agar medium to determine anchorage-independent growth and colonies were scored after 3 weeks. SW620 cells were used as a positive control. The results represent means ± SD of at least 3 independent experiments using triplicate cultures. ** indicates an increase above SW480-co at p < 0.01 by unpaired t-test. b: Cells were plated at 100 (SW480-co, SW480-LOX) or 200 (Caco-co, Caco-LOX, SW620) cells/well in 6-well plates for assessment of clonogenicity. Medium was changed and un-attached cells removed after 24 h. Colonies were counted after 7 days in culture. Results were calculated per 100 cells and presented as mean ± SD of at least 3 independent experiments with # and ## representing a decrease as compared to the respective control at p < 0.05 and 0.01 respectively by unpaired t-test. c: Cell migration was determined by filter migration assay. After a migration period of 24 h (SW480 transfectants, SW620) or 48 h (Caco2 transfectants) cells on the bottom of the membrane (Caco2 transfectants) or in the lower chamber (SW480 transfectants and SW620) were fixed, stained with crystal violet and counted. The results represent the mean ± SD of 3 independent experiments using duplicate cultures. * and *** indicate an increase above control at p < 0.05 and p < 0.001 respectively by unpaired t-test.
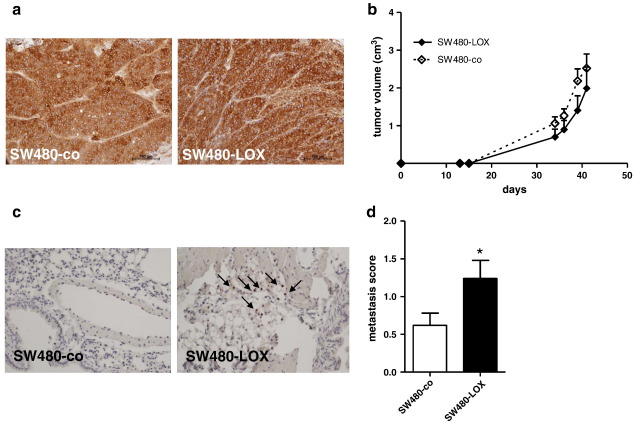
To assess the cells' malignant properties in vivo they were injected s.c. into the rear flank of SCID mice and tumour growth monitored for 7 weeks. Both LOX- and control (co)-transfectants formed tumours of epithelioid, cytokeratin 20 expressing cells (Fig. 3a) and local tumour growth did not differ between both groups (Fig. 3b). Metastatic potential was determined from the presence of Ki67-positive tumour cells in the lungs of tumour bearing mice. Single metastatic cells were identified in the vicinity of major vessels (Fig. 3c) and scored from serial sections as described in Materials and methods. The resulting mean score in SW480-LOX groups was about double the score in SW480-co mice (Fig. 3d; p = 0.0281).
Fig. 3.
12(S)-LOX induced tumour growth and metastasis. 106 cells each of SW480-co and SW480-LOX cells were injected s.c. into SCID-mice. Tumour growth was monitored regularly and the mice sacrificed when tumour size reached 5 cm3 or after 9 weeks whichever came first. The tumours were fixed in 4% formalin and analysed by immunohistochemistry using antibodies directed against cytokeratin 20 (a). Tumour growth did not differ between the SW480-LOX and SW480-co group (b). Lungs were isolated from the tumour-bearing mice bearing SW480 tumours and fixed with formalin. Serial sections were stained using antibodies recognising Ki67 to detect tumour cells (arrows in c). Ki67-positive tumour cells in the lungs were scored according to the criteria given in materials and methods (d). * indicates a significant increase as compared to control at p = 0.0281 by Mann–Withney test.
Inhibition of 12(S)-HETE production
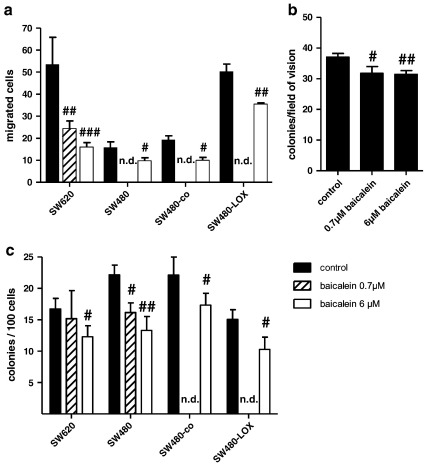
For inhibition of 12(S)-LOX-activity the flavonoid baicalein was used that reduced 12(S)-HETE production with an IC50 of 0.7 μM and decreased cell viability with an IC50 in the range of 6–8 μM [24]. In addition the inhibitor blocked cell migration in untransfected SW480 and SW620 cells as well as SW480-co and SW480-LOX transfectants (Fig. 4a). Baicalein effects on anchorage-independent growth were determined in SW620 cells and were also inhibited (Fig. 4b). Unexpectedly, clonogenicity was also inhibited by 0.7 μM as well as 6 μM baicalein (Fig. 4c) which may be due to effects of baicalein on other cellular targets that also cause loss of viability (e.g. growth signalling).
Fig. 4.
Biological impact of 12(S)-LOX inhibition by baicalein. a: Cell migration was determined as described in Fig. 2 but in the presence of baicalein at 0.7 μM (hatched bars) or 6 μM (open bars). SW620 and SW480 groups were obtained from untransfected cultures and SW480-LOX and SW480-co groups represent LOX transfectants and vector controls. The results represent means ± SD of 3 independent experiments using duplicate cultures. #, ## and ### indicate a decrease as compared to control at p < 0.05, p < 0.01 and p < 0.001 respectively. n.d = not determined. b: 5000 SW620 cells were suspended in soft agar medium containing baicalein at the indicated concentrations. Number of colonies was scored microscopically after 2 weeks of growth. The results shown represent the pooled data from 3 experiments using triplicate cultures and #, ## indicate a decrease as compared to control at p < 0.05 and p < 0.01 respectively. c: Clonogenicity was determined as described in Fig. 2. Baicalein was present during the attachment period, but not during the growth period. The results represent means ± SD of 3 independent experiments using triplicate cultures. n.d = not determined; # and ## indicate a decrease as compared to control at p < 0.05 and p < 0.001 respectively.
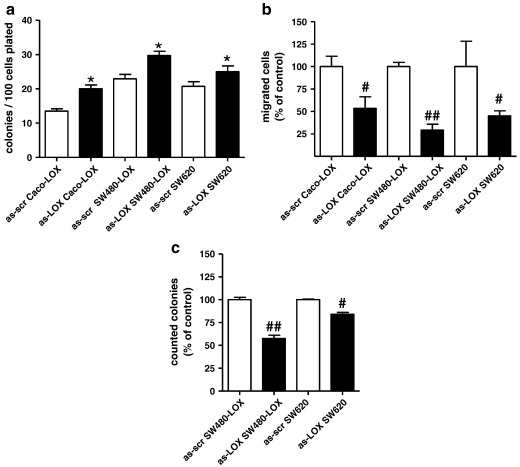
To obtain a more specific inhibition 12(S)-LOX expression was knocked down by anti-sense nucleotides as described by Tang et al. [26]. In SW620 cells this caused an 80% reduction of 12(S)-LOX mRNA and 12(S)-HETE production that was maintained for 4 days (supplemental materials Figure 3s a, b). Knock down in 12(S)-LOX over-expressing SW480 and Caco2 cells was less efficient but did reach a ≥ 50% reduction after 2 transfections both on the RNA- and the HETE-production level (supplemental materials Figure 3s c, d, e). This procedure caused an increase in clonogenicity (Fig. 5a) as well as a reduction in migration (Fig. 5b) and in anchorage-independent growth (Fig. 5c).
Fig. 5.
Knock-down of 12(S)-LOX expression. Anti-sense oligonucleotides were introduced into the cells by lipofection according to the transfection scheme described in supplemental materials. This achieved a ≥ 50% reduction of 12(S)-LOX expression and 12(S)-HETE production (supplemental materials Figure 4s). At the time point when expression was at the minimum cells were (a) plated for clonogenicity assays, (b) placed into filter inserts for migration assays and (c) suspended in soft agar medium to determine anchorage-independent growth. All data points represent the mean ± SEM of at least 2 independent experiments using triplicate cultures. * indicates increased above control at p < 0.05; #, and ## a decrease as compared to control at p < 0.05 and 0.01 respectively.
Expression of genes related to cell interactions
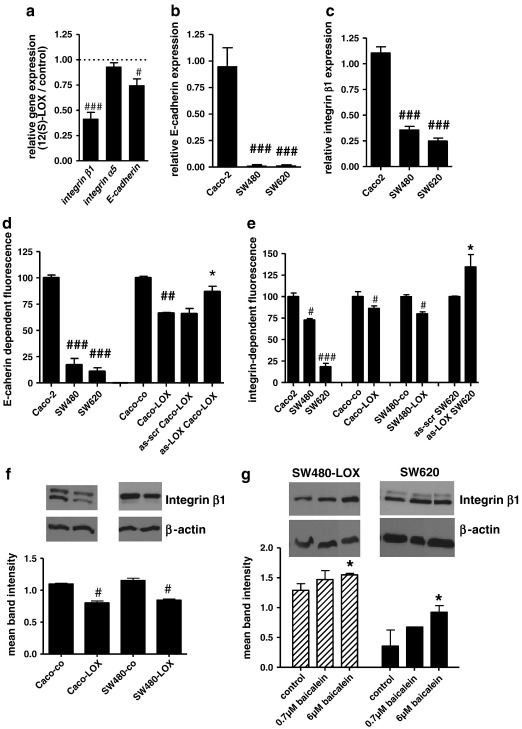
For an initial analysis of gene expression Caco2 cells transiently over-expressing 12(S)-LOX were used because of their high HETE production upon AA addition (about 20 nM; see supplemental materials Figure 2s b). RNA was isolated 24 h after supplementation of the culture medium with 20 μM AA and 96 metastasis-related genes were assessed using a focused micro-array. 12 of these genes produced signals robust enough for analysis and were significantly down-regulated compared to control (supplemental materials Table 3). Prominent among the down-regulated genes were the cell surface molecules integrin β1 (ITGB1), integrin α5 (ITGA5), and E-cadherin (CDH1). The effect on expression of these three genes was verified by standard RT-PCR. While integrin α5 did not appear down regulated in the PCR reaction, the inhibitory effect could be verified for E-cadherin and integrin β1 (Fig. 6a).
Fig. 6.
Differential expression of cell surface molecules. a: RNA was isolated from Caco2-LOX and Caco2-co cultures and expression of E-cadherin, integrin α5 and integrin β1 determined by qRT-PCR. # and ## represent a decrease as compared to control at p < 0.05 and 0.01 by Mann–Withney test. b, c: RNA was isolated for the determination of E-cadherin (b) and integrin β1 (c) expression by qRT-PCR. Results are shown relative to GAPDH expression. ### indicates a decrease as compared to Caco2 at p < 0.001 by unpaired t-test. d, e: Untransfected cultures (Caco2, SW480, SW620) and Caco2 transfectants (Caco-co, Caco-LOX) were harvested at semi-confluence. In Caco-LOX cells 12(S)-LOX expression was knocked down by lipofection with an anti-sense oligonucleotide (as-scr Caco-LOX and as-LOX Caco-LOX) and cultures were used at the time-point of minimal 12(S)-LOX expression. E-cadherin (d) and integrin β1 (e) on the cell surface was determined by FACS analysis. The mean fluorescence intensity was used as a measure of protein expression. The data were normalised to Caco2 or the respective control for better comparison. All data represent pooled results from 3 independent experiments using duplicate measurements. #, ##, ### indicate a decrease as compared to the respective controls at p < 0.05; 0.01 or 0.001. * represents an increase at p < 0.05 by Mann–Withney test. f: Cultures were grown to semi-confluence and then homogenised in lysis buffer for determination of integrin β1 by western blotting. The figure depicts representative blots above the column of semi-quantitative assessment of band intensity. Results are pooled from at least 2 experiments using duplicate lanes and # indicates a decrease at p < 0.05. g: SW620 and SW480-LOX cells were grown to semi-confluence and then exposed to 0.7 μM or 6 μM baicalein in serum-free medium. Protein was harvested and integrin β1 level determined as described above. All data points represent the mean ± SEM of 2 independent experiments using duplicate cultures. * increased above control at p < 0.05.
E-cadherin expression was easily detectable on both the RNA and protein level in Caco2 cells but not in SW480 and SW620 cultures (Figs. 6b, d). E-cadherin protein on the cell surface of SW480 and SW620 was about 10% the amount seen on Caco2 cells (Fig. 6d). Over-expression of 12(S)-LOX in Caco2 cells induced a 40% reduction of E-cadherin at the cell surface that was reversible upon knock down of 12(S)-LOX expression (Fig. 6d).
Similarly, integrin β1 which is part of the fibronectin and collagen I receptors was expressed in the cell lines in different amounts (Caco2 > SW480 > SW620) at both the RNA and the protein levels (Figs. 6c, e). Over-expression of 12(S)-LOX in both Caco2 and SW480 cells reduced integrin β1 at the cell surface by 20% (Fig. 6e) and total integrin β1 by 30% (Fig. 6f). By contrast, inhibition of 12(S)-LOX by either knock down (SW620, Fig. 6e) or exposure to baicalein (both SW480-LOX and SW620) increased integrin protein (Fig. 6g).
Discussion
12(S)-LOX (ALOX12) introduces a hydroxyl-group at position 12 of arachidonic acid to produce 12(S)-HETE [2,27], a mediator involved in tumour progression and metastasis in melanoma [28,29], prostate cancer [30], pancreatic cancer [31] as well as in angiogenesis in several tumour types reviewed in [32]. In prostate cancer 12(S)-LOX-over expression was correlated with advanced disease [13,33]. Our study now shows up-regulation of 12(S)-LOX in colorectal tumours for which an inflammatory component was described in the pathology report but not in tumours with little signs of inflammation. The study did not use specimens from IBD or IBD-related cancer. A correlation with staging or grading parameters could not be observed. This is in agreement with a recent survey of eicosanoid levels in colorectal tumours that did not report a general increase in 12(S)-HETE [34] and may indicate that both enzyme and product may be derived from or produced as a reaction to the inflammatory tumour microenvironment.
In colorectal cancer cell lines 12(S)-LOX expression was generally low except for SW620 cells that were obtained from a lymph node metastasis. This cell line produced about twice the amount of 12(S)-HETE when compared with SW480 cells that originated from the respective primary tumour and the well differentiated Caco2 cells. Other cell lines derived from metastases (e.g. T84) did not produce more LOX or HETE. For this two explanations are possible: (1) 12(S)-LOX expression is not tumour cell autonomous, but arises from the tumour microenvironment or (2) 12(S)-LOX expression is transiently up-regulated in response to an inflammatory micro-environment.
Independent of their source the results obtained with our in vitro models suggests a role of the enzyme and its product in tumour progression and metastasis. 12(S)-HETE increased cell viability in standard cultures of the well differentiated, slowly growing cell line Caco2. In cultures of SW480 and SW620 cells that already have a much higher growth potential the mediator had no additional impact on growth under standard conditions, but it strongly supported anchorage-independent growth of SW480 cells. Effective concentrations in both cases were 10 and 100 nM. Similarly, over-expression of 12(S)-LOX in Caco2 or SW480 cells conferred a growth/survival advantage in standard cultures and increased growth of SW480-LOX over-expressing cells in soft agar media. Over-expressing cell lines secreted about 5–10 nM 12(S)-HETE into their media, which on the low end of the concentration range for which biological effects of exogenous 12(S)-HETE have been observed in our cells. As endogenous HETE has a limited half-life when exposed to oxygen, it may well be less effective than HETE continuously produced in an autocrine manner.
Both our results from HETE-exposed cells and from 12(S)-LOX over-expressers confirm reports assigning survival activity to 12(S)-HETE in other tumour types [26,35,36]. In addition, 12(S)-LOX expression reduced clonogenicity which under our conditions—removal of non-attached cells after 24 h—largely reflects decreased cell attachment. Concomitantly cell migration in Caco2 and SW480 cells was stimulated effectively increasing their capability to invade. The combined results from the functional assays (clonogenicity, anchorage-independent growth, cell migration) define a malignant phenotype increasing in the sequence Caco2 < SW480 < SW620. Over-expression of 12(S)-LOX reduced clonogenicity but stimulated anchorage-independent growth and cell migration. In summary these biological effects shift the tumour cell characteristics towards higher degrees of malignancy. An impact on the expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) that also indicate tumour progression was not observed in our analysis (data not shown).
In vivo SW480-LOX cells did not form larger tumours than the respective controls, which seems to indicate that the growth and survival impact of 12(S)-HETE is not sustained in vivo. With regard to this observation it has to be taken into account that SW480 cells already have a very high potential for local tumour growth so that it may not be possible to increase it further—similar to the absence of a 12(S)-HETE growth effect in standard cultures. LOX-over-expressing SW480 cells did, however, have a stronger tendency to metastasise in line with their increased growth capacity in soft agar and enhanced migratory potential. Again this indicates a more aggressive phenotype. A reverse shift was achieved by inhibition of LOX-activity by baicalein or by knock-down of 12(S)-LOX expression in SW620 cells as well as Caco2-LOX and SW480-LOX transfectants demonstrating LOX-dependency of the effects.
Pro-metastatic effects of 12(S) HETE have also been described for breast cancer cells where the mediator comes from 15-LOX-1 (ALOX15) and enhanced intravasation into lymph vessels [15]. The HETE induces migration of the lymph endothelial cells in an NFκB-dependent manner in these cells [37]. In prostate cancer 12(S)-LOX-derived 12(S)-HETE increased anchorage-independent growth [33], similar to our observations. Attachment and spreading on the matrix proteins fibronectin and collagen I was increased in the 12-LOX over-expressing prostate cells [33]. By contrast, 12(S)-LOX over-expressing colon cancer cells displayed decreased colony formation using an assay format reflecting both cell attachment and growth potential. As viability assays indicate a positive effect of 12(S)-HETE on growth and survival, we do conclude that in contrast to prostate cancer cells colon carcinoma cells decrease their interaction with the culture substrate in response to the mediator. This is also supported by the down-regulation of integrin β1 protein which is part of the receptors for both fibronectin and collagen I [38] by 12(S)-LOX over-expression in both Caco2 and SW480 cells. Integrin β1 has been shown to inhibit cell migration [39] and is lost in advanced colorectal tumours [40]. Its down-regulation was reversed by both baicalein and 12(S)-LOX knock-down indicating that 12(S)-HETE is instrumental in this process.
In Caco2 cells not only integrin β1 was subject to down-regulation by 12(S)-LOX, but E-cadherin was similarly reduced diminishing cell-cell contacts in a 12(S)-LOX-dependent manner. Like many other malignant cells the more undifferentiated cell lines SW480 and SW620 did no longer express detectable amounts of E-cadherin, as it is lost with tumour progression and epithelial mesenchymal transition [41].
In summary, our results show the induction of a migratory, metastatic phenotype in 12(S)-LOX transfected colorectal tumour cells. This is a pre-requisite for metastasis while the cells move towards the metastatic site.
Conflict of interest statement
None of the authors have any conflict of interest to declare.
Acknowledgments
This work was supported by a grant from the Austrian Science Fund (P16328).
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.yexcr.2011.12.017.
Appendix A. Supplementary data
Supplementary materials.
References
- 1.Silverman E.S., Drazen J.M. The biology of 5-lipoxygenase: function, structure, and regulatory mechanisms. Proc. Assoc. Am. Physicians. 1999;111:525–536. doi: 10.1046/j.1525-1381.1999.t01-1-99231.x. [DOI] [PubMed] [Google Scholar]
- 2.Funk C.D., Chen X.S., Johnson E.N., Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68–69:303–312. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 3.Kühn H., O'Donnell V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn H. Biologic relevance of lipoxygenase isoforms in atherogenesis. Expert Rev. Cardiovasc. Ther. 2005;3:1099–1110. doi: 10.1586/14779072.3.6.1099. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn H., Walther M., Kuban R.J. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 6.Aleem A.M., Jankun J., Dignam J.D., Walther M., Kühn H., Svergun D.I., Skrzypczak-Jankun E. Human platelet 12-lipoxygenase, new findings about its activity, membrane binding and low-resolution structure. J. Mol. Biol. 2008;376:193–209. doi: 10.1016/j.jmb.2007.11.086. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimoto T., Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68–69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 8.Wong B.C., Wang W.P., Cho C.H., Fan X.M., Lin M.C., Kung H.F., Lam S.K. 12-Lipoxygenase inhibition induced apoptosis in human gastric cancer cells. Carcinogenesis. 2001;22:1349–1354. doi: 10.1093/carcin/22.9.1349. [DOI] [PubMed] [Google Scholar]
- 9.Ding X.Z., Iversen P., Cluck M.W., Knezetic J.A., Adrian T.E. Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 1999;261:218–223. doi: 10.1006/bbrc.1999.1012. [DOI] [PubMed] [Google Scholar]
- 10.Timar J., Raso E., Dome B., Li L., Grignon D., Nie D., Honn K.V., Hagmann W. Expression, subcellular localization and putative function of platelet-type 12-lipoxygenase in human prostate cancer cell lines of different metastatic potential. Int. J. Cancer. 2000;87:37–43. doi: 10.1002/1097-0215(20000701)87:1<37::aid-ijc6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Timar J., Raso E., Honn K.V., Hagmann W. 12-Lipoxygenase expression in human melanoma cell lines. Adv. Exp. Med. Biol. 1999:469617–469622. doi: 10.1007/978-1-4615-4793-8_89. [DOI] [PubMed] [Google Scholar]
- 12.Timar J., Raso E., Fazakas Z.S., Silletti S., Raz A., Honn K.V. Multiple use of a signal transduction pathway in tumor cell invasion. Anticancer Res. 1996;16:3299–3306. [PubMed] [Google Scholar]
- 13.Gao X., Grignon D.J., Chbihi T., Zacharek A., Chen Y.Q., Sakr W., Porter A.T., Crissman J.D., Pontes J.E., Powell I.J., Honn K.V. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–237. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 14.Honn K.V., Tang D.G., Grossi I.M., Renaud C., Duniec Z.M., Johnson C.R., Diglio C.A. Enhanced endothelial cell retraction mediated by 12(S)-HETE: a proposed mechanism for the role of platelets in tumor cell metastasis. Exp. Cell Res. 1994;210:1–9. doi: 10.1006/excr.1994.1001. [DOI] [PubMed] [Google Scholar]
- 15.Kerjaschki D., Bago-Horvath Z., Rudas M., Sexl V., Schneckenleithner C., Wolbank S., Bartel G., Krieger S., Kalt R., Hantusch B., Keller T., Nagy-Bojarszky K., Huttary N., Raab I., Lackner K., Krautgasser K., Schachner H., Kaserer K., Rezar S., Madlener S., Vonach C., Davidovits A., Nosaka H., Hammerle M., Viola K., Dolznig H., Schreiber M., Nader A., Mikulits W., Gnant M., Hirakawa S., Detmar M., Alitalo K., Nijman S., Offner F., Maier T.J., Steinhilber D., Krupitza G. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Invest. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furstenberger G., Krieg P., Muller-Decker K., Habenicht A.J. What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int. J. Cancer. 2006;119:2247–2254. doi: 10.1002/ijc.22153. [DOI] [PubMed] [Google Scholar]
- 17.Deschner E.E., Ruperto J.F., Wong G.Y., Newmark H.L. The effect of dietary quercetin and rutin on AOM-induced acute colonic epithelial abnormalities in mice fed a high-fat diet. Nutr. Cancer. 1993;20:199–204. doi: 10.1080/01635589309514287. [DOI] [PubMed] [Google Scholar]
- 18.Deschner E.E., Ruperto J., Wong G., Newmark H.L. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991;12:1193–1196. doi: 10.1093/carcin/12.7.1193. [DOI] [PubMed] [Google Scholar]
- 19.Furstenberger G., Muller-Decker K., Scholz K., Loschke M., Lehmann W.D., Marks F. Different expression of prostaglandin-H synthase isozymes and lipoxygenases during multistage carcinogenesis in mouse skin. Adv. Exp. Med. Biol. 1997:419–424. doi: 10.1007/978-1-4615-5325-0_56. [DOI] [PubMed] [Google Scholar]
- 20.Dubois R.N. Review article: cyclooxygenase–a target for colon cancer prevention. Aliment. Pharmacol. Ther. 2000;14(Suppl. 1):64–67. doi: 10.1046/j.1365-2036.2000.014s1064.x. [DOI] [PubMed] [Google Scholar]
- 21.Bortuzzo C., Hanif R., Kashfi K., Staiano-Coico L., Shiff S.J., Rigas B. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim. Biophys. Acta. 1996;1300:240–246. doi: 10.1016/0005-2760(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 22.Cianchi F., Cortesini C., Magnelli L., Fanti E., Papucci L., Schiavone N., Messerini L., Vannacci A., Capaccioli S., Perna F., Lulli M., Fabbroni V., Perigli G., Bechi P., Masini E. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol. Cancer Ther. 2006;5:2716–2726. doi: 10.1158/1535-7163.MCT-06-0318. [DOI] [PubMed] [Google Scholar]
- 23.Tan W., Wu J., Zhang X., Guo Y., Liu J., Sun T., Zhang B., Zhao D., Yang M., Yu D., Lin D. Associations of functional polymorphisms in cyclooxygenase-2 and platelet 12-lipoxygenase with risk of occurrence and advanced disease status of colorectal cancer. Carcinogenesis. 2007;28:1197–1201. doi: 10.1093/carcin/bgl242. [DOI] [PubMed] [Google Scholar]
- 24.Bednar W., Holzmann K., Marian B. Assessing 12(S)-lipoxygenase inhibitory activity using colorectal cancer cells overexpressing the enzyme. Food Chem. Toxicol. 2007;45:508–514. doi: 10.1016/j.fct.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Sonvilla G., Allerstorfer S., Heinzle C., Stattner S., Karner J., Klimpfinger M., Wrba F., Fischer H., Gauglhofer C., Spiegl-Kreinecker S., Grasl-Kraupp B., Holzmann K., Grusch M., Berger W., Marian B. Fibroblast growth factor receptor 3-IIIc mediates colorectal cancer growth and migration. Br. J. Cancer. 2010;102:1145–1156. doi: 10.1038/sj.bjc.6605596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang D.G., Chen Y.Q., Honn K.V. Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5241–5246. doi: 10.1073/pnas.93.11.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brash A.R. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 28.Honn K.V., Timar J., Rozhin J., Bazaz R., Sameni M., Ziegler G., Sloane B.F. A lipoxygenase metabolite, 12-(S)-HETE, stimulates protein kinase C-mediated release of cathepsin B from malignant cells. Exp. Cell Res. 1994;214:120–130. doi: 10.1006/excr.1994.1240. [DOI] [PubMed] [Google Scholar]
- 29.Honn K.V., Tang D.G., Grossi I., Duniec Z.M., Timar J., Renaud C., Leithauser M., Blair I., Johnson C.R., Diglio C.A., Kimler V.A., Taylor J.D., Marnett L.J. Tumor cell-derived 12(S)-hydroxyeicosatetraenoic acid induces microvascular endothelial cell retraction. Cancer Res. 1994;54:565–574. [PubMed] [Google Scholar]
- 30.Nie D., Tang K., Szekeres K., Li L., Honn K.V. Eicosanoid regulation of angiogenesis in human prostate carcinoma and its therapeutic implications. Ann. N. Y. Acad. Sci. 2000;905:165–176. doi: 10.1111/j.1749-6632.2000.tb06548.x. [DOI] [PubMed] [Google Scholar]
- 31.Ding X.Z., Tong W.G., Adrian T.E. 12-lipoxygenase metabolite 12(S)-HETE stimulates human pancreatic cancer cell proliferation via protein tyrosine phosphorylation and ERK activation. Int. J. Cancer. 2001;94:630–636. doi: 10.1002/ijc.1527. [DOI] [PubMed] [Google Scholar]
- 32.Nie D., Honn K.V. Eicosanoid regulation of angiogenesis in tumors. Semin. Thromb. Hemost. 2004;30:119–125. doi: 10.1055/s-2004-822976. [DOI] [PubMed] [Google Scholar]
- 33.Nie D., Nemeth J., Qiao Y., Zacharek A., Li L., Hanna K., Tang K., Hillman G.G., Cher M.L., Grignon D.J., Honn K.V. Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clin. Exp. Metastasis. 2003;20:657–663. doi: 10.1023/a:1027302408187. [DOI] [PubMed] [Google Scholar]
- 34.Shureiqi I., Chen D., Day R.S., Zuo X., Hochman F.L., Ross W.A., Cole R.A., Moy O., Morris J.S., Xiao L., Newman R.A., Yang P., Lippman S.M. Profiling Lipoxygenase Metabolism in Specific Steps of Colorectal Tumorigenesis. Cancer Prev. Res. 2010;3:829–838. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pidgeon G.P., Tang K., Cai Y.L., Piasentin E., Honn K.V. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing alpha(v)beta(3) and alpha(v)beta(5) integrin expression. Cancer Res. 2003;63:4258–4267. [PubMed] [Google Scholar]
- 36.Szekeres C.K., Trikha M., Honn K.V. 12(S)-HETE, pleiotropic functions, multiple signaling pathways. Adv. Exp. Med. Biol. 2002;507:509–515. doi: 10.1007/978-1-4615-0193-0_78. [DOI] [PubMed] [Google Scholar]
- 37.Vonach C., Viola K., Giessrigl B., Huttary N., Raab I., Kalt R., Krieger S., Vo T.P., Madlener S., Bauer S., Marian B., Hammerle M., Kretschy N., Teichmann M., Hantusch B., Stary S., Unger C., Seelinger M., Eger A., Mader R., Jager W., Schmidt W., Grusch M., Dolznig H., Mikulits W., Krupitza G. NF-kappaB mediates the 12(S)-HETE-induced endothelial to mesenchymal transition of lymphendothelial cells during the intravasation of breast carcinoma cells. Br. J. Cancer. 2011;105:263–271. doi: 10.1038/bjc.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takada Y., Ye X., Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kren A., Baeriswyl V., Lehembre F., Wunderlin C., Strittmatter K., Antoniadis H., Fassler R., Cavallaro U., Christofori G. Increased tumor cell dissemination and cellular senescence in the absence of beta1-integrin function. EMBO J. 2007;26:2832–2842. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pignatelli M., Smith M.E., Bodmer W.F. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br. J. Cancer. 1990;61:636–638. doi: 10.1038/bjc.1990.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavallaro U., Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.