Abstract
The rods of anti-parallel myosin molecules overlap at the centre of bipolar myosin filaments to produce an M-region (bare zone) that is free of myosin heads. Beyond the M-region edges, myosin molecules aggregate in a parallel fashion to yield the bridge regions of the myosin filaments. Adjacent myosin filaments in striated muscle A-bands are cross-linked by the M-band. Vertebrate striated muscle myosin filaments have a 3-fold rotational symmetry around their long axes. In addition, at the centre of the M-region, there are three 2-fold axes perpendicular to the filament long axis, giving the whole filament dihedral 32-point group symmetry. Here we describe the three-dimensional structure obtained by a single-particle analysis of the M-region of myosin filaments from goldfish skeletal muscle under relaxing conditions and as viewed in negative stain. This is the first single-particle reconstruction of isolated M-regions. The resulting three-dimensional reconstruction reveals details to about 55 Å resolution of the density distribution in the five main nonmyosin densities in the M-band (M6′, M4′, M1, M4 and M6) and in the myosin head crowns (P1, P2 and P3) at the M-region edges. The outermost crowns in the reconstruction were identified specifically by their close similarity to the corresponding crown levels in our previously published bridge region reconstructions. The packing of myosin molecules into the M-region structure is discussed, and some unidentified densities are highlighted.
Keywords: 3D reconstruction, fish skeletal muscle, bare zone region, M-region, Myosin filament
Abbreviations: D32, dihedral 32-point group; MM-CK, muscle-specific form of creatine kinase; 3D, three-dimensional; 1D, one-dimensional; 2D, two-dimensional
Introduction
Bipolar myosin filaments in the striated muscle A-band1 are built from myosin molecules, each of which has a 1600 -Å-long rod region (a two-chain coiled-coil α-helical structure), on one end of which are two elongated globular myosin heads (cross-bridges). The heads are ATPases and interact with actin filaments to produce force and movement.2 In vertebrate striated muscle myosin filaments, the myosin molecules pack together into bipolar filaments, with myosin rods forming the filament backbone and with myosin heads in quasi-helical arrays on the filament surface.1,3 The backbone also has on its surface additional protein components such as titin4,5 and C-protein.6 The central region of myosin filaments where rod packing is anti-parallel is called the bare zone or M-region (Fig. 1a).1,7,8 It was originally called the bare zone, since there are no myosin heads in this region (especially in synthetic filaments). However, in muscle, this part of the filament is not at all bare; there are other proteins on the filament surface, such as the M-band proteins and titin, that make the term M-region more appropriate. A possible molecular packing scheme for the myosin molecules in the M-region was proposed by Squire,9 as was a general structure with dihedral 32-point group (D32) symmetry proposed by Luther et al.10
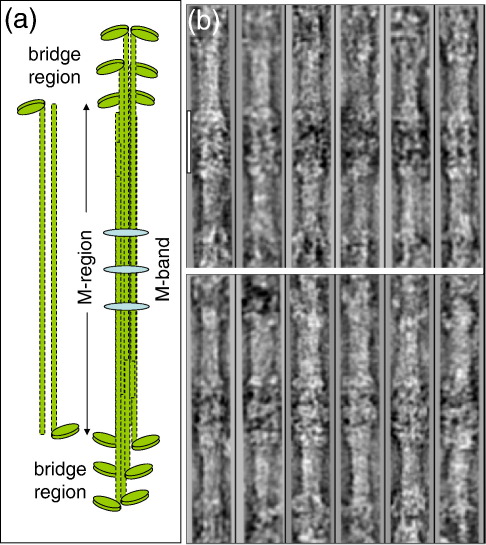
Fig. 1.
(a) Schematic diagram showing how myosin molecules, each with a tail and two heads, pack to form bipolar myosin filaments with a central bare zone (after Huxley1). (b) Montage of 12 of the M-region particles used in this study. Each image has a length of about 2200 Å. Scale bar represents 440 Å.
The M-band in the middle of the M-region in vertebrate striated muscles contains myomesin, M-protein, and the C-terminal region of titin. Each of these three proteins is an assembly of IgI-like and fibronectin-3-like domains.4,5,11–23 The M-band also contains the muscle-specific form of creatine kinase (MM-CK17) and the proteins obscurin and obscurin-like-1.24,25 Since the M-region part of titin also contains a kinase domain perhaps near M6,19,26 it is clear that the M-band possesses not only a structural role but also important metabolic and regulatory properties. In addition, there are diseases associated with mutations in the M-band proteins (e.g., Lange et al.,18 Fukuzawa et al.,24 Ehler and Gautel,27 and Ottenheijm et al.28).
Myosin filaments have two main structural regions: the cross-bridge region and the bare zone or M-region. We have previously investigated the three-dimensional (3D) structure of the cross-bridge region of myosin filaments from fish skeletal muscle in the relaxed state by both X-ray modelling29 and electron microscopy, combined with single-particle analysis.30 Electron microscopy and single-particle analysis have also been used to study the structure of vertebrate cardiac myosin filaments.31,32
In the present study, the M-regions of myosin filaments isolated from goldfish skeletal muscle under relaxing conditions and as viewed in negative stain by electron microscopy were selected, treated as segments, and subjected to 3D single-particle analysis. The final 3D reconstruction reveals details of density distributions in the five main nonmyosin densities at the M-band (M6′, M4′, M1, M4 and M6). The analysis maintained the well-documented axial spacing of the M-band density peaks and also revealed additional densities (e.g., M3, M3′, M8, M8′ and projecting density at M9). Two crown levels at the M-region edge (probably P2 and P3) were identified clearly by comparison with the corresponding crown levels in our previously published bridge region reconstructions.30,31 Another crown level was tentatively identified as P1. The centres of mass of opposite P1 (P1,P1′) levels are about 1540 Å apart, with the 1600-Å rods probably fully overlapped and with the projecting heads tilting back slightly towards the M-band. In addition, there are densities (e.g., M3, M8 and M9) whose protein composition has yet to be identified.
Results
Selection, alignment and classification of M-region segments
From a data set of 29 electron micrographs containing 80 full-length myosin filaments (see Fig. 1a and b in AL-Khayat et al.30), 72 M-regions that contained significant details and were uncontaminated by other filaments or particles were selected for further analysis. Each selected particle/segment was approximately 2300 Å in length and contained a small number of crown levels at each end of the M-region (i.e., close to P1, P2 and P37,8). Particles were floated in 300 × 300 pixel boxes. From the original 72 M-regions and by further careful assessment of quality, symmetry, and detail, including straightness in such long particles and the absence of other contaminating filaments, the final number of images used was 19. This is effectively multiplied by 6 (i.e., giving 114 nonequivalent particles) due to the D32 symmetry of the system. From trials, it became evident that single-particle analysis with a few really good particles was much more effective, self-consistent, and informative than adding in extra particles at the expense of quality. A large number of particles is not essential per se for a useful reconstruction; it is the quality of the particles that is paramount. Figure 1b shows examples of the M-region particles used in the final reconstruction. Within these particles, M-band protein density is visible in the middle of the M-region, and myosin head density in the proximal parts of the bridge regions appears at the M-region ends.
A circular mask was applied to the data set of 19 images, which were then rotationally and translationally aligned to an initial reference corresponding to the average of all the segments. Since the selected filament images were already quite accurately rotationally and translationally aligned, the angular range in the alignment was restricted to ± 15° to preserve the polarity of the filaments. The alignment was achieved by multireference alignment,33 but the axial shift along the filament long axis was restricted to between − 50 Å and + 50 Å so as not to allow the protein levels to get out of step. The alignment was further checked thoroughly by calculating the one-dimensional (1D) density profiles for the individual particles which were then compared, and the positions of the peaks were calculated. The Cross-Corr program (Knupp, C., unpublished), which was especially developed for this purpose, was used to align the filaments by cross-correlation and to sum the 1D profiles to give optimised averages. The shift values needed to superimpose similar peaks in each profile were found for each bare zone image. This shift was then applied to the corresponding particles. The sum of the shifted particles, together with the corresponding 1D profile, was then recalculated in order to check that they were all aligned.
Angular assignment and refinement
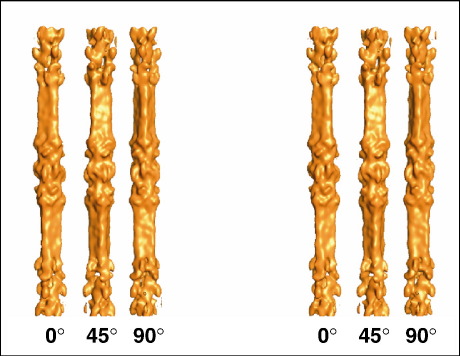
As explained by AL-Khayat et al.,30,31 because the aligned M-region segments are likely to be related by rotation about a single axis (the filament long axis), with little out-of-plane rotation normal to this axis, ab initio angular assignment by standard angular reconstitution is not possible.34 The approach adopted here (as suggested by Paul et al.34) was based on the use of a starting 3D model generated here by reconstructing one class with Euler (viewing) angles α, β and γ of 0°, 90° and 0°, respectively, and by imposing a 3-fold rotational symmetry around the long axis. An anchor set of two-dimensional (2D) projections from this model was calculated as reference from which to determine projection angles of the images of the individual segments. By convention, the three Euler angles are α, β and γ, where α is the rotation angle in the plane of the image (i.e., the plane of the electron microscopy grid), β is the out-of-plane tilt angle (to allow for the fact that the filaments may not lie perfectly flat on the grid or the grid itself may not be flat) and γ is the rotation angle around the filament long axis. During the procedure, a new 3D map was calculated from the particle images using the Euler angles assigned to them. Two-dimensional reprojections of this new 3D map were then used to make a new set of reference images with which to realign the original raw image data using multireference alignment and to carry out further angular reconstitution/assignment. This procedure was repeated about 20 times, with the reprojection images gradually improving. Refinement was stopped when no further significant changes in the reprojection images were observed and when the assigned angles had become stable. When it became evident that the filaments did have perpendicular 2-fold rotation axes, the full D32 symmetry was imposed. The final 3D reconstruction was obtained using the weighted backprojection method of Paul et al.34 and is shown in Fig. 2 in stereo view as surface representations that are vertically oriented and rotated in steps of 45° around the filament axis and in Fig. 3 as cross-sectional slices. The threshold used to calculate the surface representation corresponds to the theoretical volume of the rod part of myosin molecules based on there being nine whole molecules per 429-Å repeat in the bridge region, plus an additional 15% to account for nonmyosin proteins (titin, M-protein, obscurin, myomesin, etc.) present in the M-region of the myosin filament. The reconstruction shows a filament length of about 2200 Å.
Fig. 2.
Surface views of the final 3D reconstruction of the M-region of fish skeletal muscle myosin filaments from the single-particle electron microscopy analysis with D32 symmetry imposed. Three stereo views with rotational steps of 45° are shown. The maps were filtered from 150 Å to 55 Å resolution and are displayed using PyMOL.35
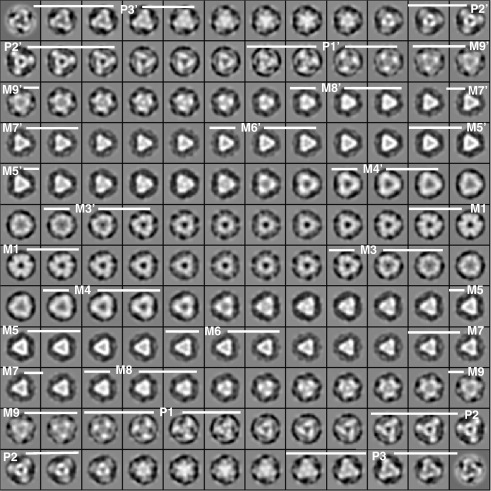
Fig. 3.
Cross-sectional slices through the 3D reconstruction shown in Fig. 2 (protein in white). The images are shown in successive slices 7.54 Å thick and spaced 2 × 7.54 Å apart from one end of the M-region to the other. The top left corner corresponds to the bottom edge of the M-region map in Fig. 2. Successive slices are read from left to right and from top to bottom through the final bottom right-hand slice, which corresponds to the top of Fig. 2. Various M-region positions are indicated.
Identification of density levels
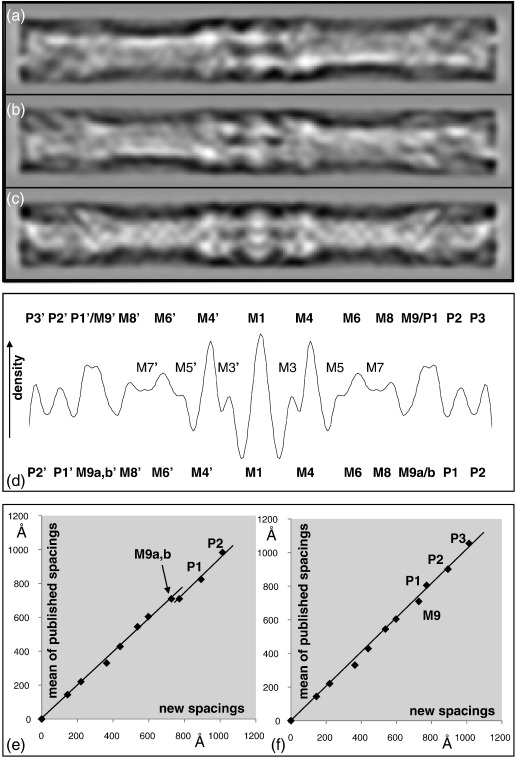
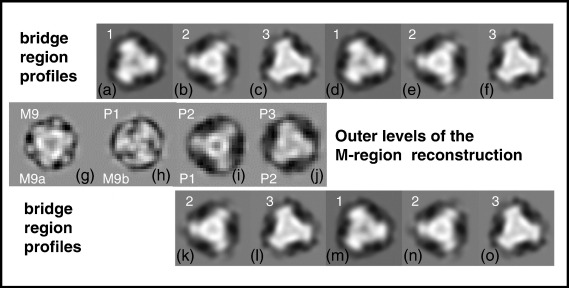
The axial positions of the various density peaks, as seen in the 2D and 1D projections in Fig. 4, are listed in Table 1, where they are compared with measurements from other published studies. It is evident that there is very good correlation, for example, between the measurements of Sjostrom and Squire7 and those presented here, especially for the M-band densities. However, at the M-region edges, there is some ambiguity about the identification of crown levels from the axial positions alone. There are four strong density levels close to the M-region edges. These will be either myosin head crowns or the location of M9, a nonmyosin protein that has yet to be identified but was seen in the analysis reported by Luther et al.10 and is the first of the 11, 429-Å-spaced stripes seen through the A-band, of which the last seven (sometimes more) are C-protein locations.7,40–43 Some of this ambiguity can be removed if the cross-sectional profiles at the various peripheral density levels in the new reconstruction (Fig. 5g–j) are compared to corresponding levels in our previously published bridge region reconstruction (Fig. 5a–c in AL-Khayat et al.30). Here the outermost density level in our reconstruction (Fig. 5j) appears very similar indeed to what we called the level 3 crown in the C-zone (Fig. 5c). In the same way, the next level from the end (Fig. 5i) appears very much like what we called level 2 in the C-zone (Fig. 5b). That the match is so good and that the previous study used many more particles than used here illustrate the quality of the present reconstruction using only a small number of particles but with the benefit of high imposed symmetry.
Fig. 4.
(a–c) Three 2D projections of the final reconstruction in Fig. 2. (a and b) Shown down one of the 2-fold axes but from opposite ends (the two views are mirror images) and (c) perpendicular to a 2-fold axis, giving mirror symmetry. (d) One-dimensional density profile through the M-region showing the main peaks and labelling in accordance with Sjostrom and Squire.7 (e) The measured positions of the M-region features (Table 1, column C) compared with the average of previously published estimates (Table 1, column O) in two interpretations: (e) where the outer two features in the reconstruction are taken to be crowns P1 and P2 (interpretation 2) and (f) where the reconstruction is taken to show the three crown levels P1, P2 and P3 (interpretation 1). There is an unattractive dog leg labelled M9a,b in the plot in (e) that makes (f) the preferable option.
Table 1.
Axial positions of the various density peaks, as seen in the 2D and 1D projections in Fig. 4 and compared to other studies
| A | B | C | D | E | F | G | H | I | J | K | L | N | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interpretation 1 with three crowns at the periphery of the reconstruction | |||||||||||||
| M1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| M2 | 89 | 88 | 93 | 97 | 91 | 98 | 93 | ||||||
| M3 | 144 | 145 | 151 | 139 | 140 | 149 | 137 | 147 | 144 | ||||
| M4a | 219 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | |
| M5 | 362 | 364 | 340 | 337 | 322 | 322 | 331 | 329 | 330 | ||||
| M6 | 437 | 439 | 437 | 423 | 408 | 434 | 418 | 440 | 429 | 434 | 428 | ||
| M7 | 535 | 537 | 544 | 539 | 526 | 560 | 556 | 542 | 545 | ||||
| M8 | 595 | 598 | 608 | 605 | 600 | 604 | |||||||
| M9 | 724 | 727 | 692 | 724 | 699 | 682 | 729 | 691 | 708 | 752 | 707 | 709 | |
| P1 | 768 | 772 | 810 | 800 | 805 | ||||||||
| P2 | 889 | 893 | 940 | 862 | 901 | ||||||||
| P3 | 1009 | 1014 | 1083 | 1025 | 1054 | ||||||||
| Interpretation 2 with two crowns at the periphery of the reconstruction | |||||||||||||
| M1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M2 | 89 | 88 | 93 | 97 | 91 | 98 | 93 | ||||||
| M3 | 144 | 145 | 151 | 139 | 140 | 149 | 137 | 147 | 144 | ||||
| M4a | 219 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | 220 | |
| M5 | 362 | 364 | 340 | 337 | 322 | 322 | 331 | 329 | 330 | ||||
| M6 | 437 | 439 | 437 | 423 | 408 | 434 | 418 | 440 | 429 | 434 | 428 | ||
| M7 | 535 | 537 | 544 | 539 | 526 | 560 | 556 | 524 | 545 | ||||
| M8 | 595 | 598 | 608 | 605 | 600 | 604 | |||||||
| M9a | 724 | 727 | 692 | 724 | 699 | 682 | 729 | 691 | 708 | 752 | 707 | 709 | |
| M9b | 768 | 772 | 692 | 724 | 699 | 682 | 729 | 691 | 708 | 752 | 707 | 709 | |
| P1 | 889 | 893 | 810 | 862 | 800 | 824 | |||||||
| P2 | 1009 | 1014 | 940 | 1025 | 983 | ||||||||
Column A: Line numbering.7
Column B: Raw spacings from the new M-region reconstruction (this study; goldfish).
Column C: New M-region reconstruction corrected to M4 = 220 Å.
Column D: Human m. tibialis anterior. Data adapted from Sjostrom and Squire.7
Column E: Plaice fin assuming, in interpretation 1, that P1 was hidden in the peak that they labelled M9 and normalised to M4 = 220 Å. Data adapted from Cantino et al.36
Column F: Rabbit psoas. Estimate to the proximal edge of the bare zone (770 Å); 30 Å has been added. Data adapted from Craig and Offer.37
Columns G–L: Rabbit cardiac (G), guinea pig cardiac (H), beef cardiac (I), rat cardiac (J), carp cardiac (K), plaice cardiac (L). Data adapted from Pask et al.38
Column N: Chicken pectoralis type 2. Data adapted from Edman et al.39
Column O: Mean spacings from column D to column N.
Used for calibration.
Fig. 5.
Top (a–f): Crown profiles from the 3D reconstruction of the fish muscle bridge region by AL-Khayat et al.30 Centre (g–j): Profiles at the outer end of the new M-region 3D reconstruction shown in Fig. 2. Bottom (k–o): Interpretation where the first crown (P1; i) corresponds to crown 2 in the bridge region reconstruction (k), and where the second crown (P2; j) corresponds to crown 3 in the bridge region (l); hence, only the first two crown levels P1 and P2 are seen in the M-region reconstruction [cf. the bottom labelling in (g)–(j), with the less favored interpretation shown in Fig. 4e]. There is a very good correlation between bridge region profile 2 (b or k) and bridge region profile 3 (c or l) and the outer two profiles in the M-region reconstruction [(i) and (j)]. With the preferred interpretation [top labelling in (g)–(j)], the three outer projecting densities in the 3D reconstruction (P1, P2 and P3) correspond to crown levels 1, 2 and 3 of the bridge region (cf. Fig. 4f). The difference between the level 1 crown in (a) and the P1 level in the new reconstruction (h) is attributed to the different nonmyosin proteins located at these positions, to C-protein (MyBP-C) in the C-zone, and to an unknown protein here at M9/P1.
We note ambiguity at the third level from the end of the reconstruction (Fig. 5h). Is this level only part of M9, or is there another myosin head crown very close to M9? It is hard to tell from a comparison of the profile here with level 1 of our previous bridge region reconstruction (Fig. 5a), but this is perhaps not surprising. C-protein is located at level 1 in the C-zone of the bridge region, whereas the level close to M9 in the M-region would be unique not only by having another myosin head crown on only one side of it but also by being very close to another nonmyosin protein (M9), which is certainly different from C-protein.
This ambiguity is reduced when the measured spacings of the M-region features in the axial density profile in Fig. 4d from the middle of the M-band are compared with the results from previous studies, as in Table 1. Here, to be consistent with previous studies, many of which calibrated their measurements using 220 Å as the position of the strong M4 M-bridge lines, we have rescaled our own results very slightly to 220 Å from the observed spacing of 219 Å. The two alternative models are illustrated in Fig. 4e and f, as well as in Fig. 5. The plots in Fig. 4e and f compare the peak spacings from the middle of M1 in the present study with the average of similar measurements from a number of previous studies. In Fig. 4e, which assumes that we are seeing only two crown levels (which must be P1 and P2) at the M-region edge, we can see that there is a dog leg in the plot at around M9, and this appears unsatisfactory. However, if part of the density near M9 is taken to be the first crown level (P1) in the bridge region, then the results in Fig. 4f are obtained, and here the dog leg has disappeared. We conclude that it is very likely that the mass near M9 is indeed partly due to the first crown of myosin heads and that our reconstruction includes the three crown levels P1, P2 and P3, as in the top row of Fig. 5. This would put the innermost myosin head crown at around 770 Å from the M-band centre and very close indeed (about 45 Å) to the extra protein at M9. We will assume the presence of the three crown levels P1, P2 and P3 in the discussion of the new reconstruction that follows.
Discussion
Details of the M-band
The M-band is the structure that defines the relative rotations of adjacent myosin filaments in the A-band hexagonal lattice (Pask et al.,38 based on Luther and Squire44). It consists of sets of cross-links that join the myosin filaments in the middle of the A-band. The major interactions are observed at M1 at the filament centre in some muscles, at M4 220 Å from M1 in all muscles, and also in some muscles at M6. M-bands can therefore have all five cross-link levels, only the central three cross-link levels, or four cross-link levels if M1 is absent but M6 is present. Since M4 is the level that is always present, the M4 interaction primarily determines whether the A-band has a superlattice or a simple lattice structure, as shown by Luther et al.10 and Pask et al.38 Our current map and its cross-sectional slices through the fish bare zone region (Fig. 3) show that the M1 profile has a rather circular but hollow central core and has its main outer density projecting in six directions, confirming previous observations. The M4 and M4′ profiles are triangular, with the main projecting densities located on the points of the triangles rather than on the sides. This agrees with the observations of Luther et al.10 and with those of Luther and Crowther,45 who reconstructed cross-sectional slices through the fish skeletal simple lattice M-band from oblique (slightly tilted) sections.
Although myomesin forms much of the M4 lines, it also extends across the M-band from M4′ to M4,14,15 possibly forming, together with the M-band part of titin, the axially aligned M-filaments seen by Luther and Squire.46 Other M-band proteins apart from myosin, titin and myomesin are M-protein, obscurin, obscurin-like-1 and MM-CK. M-protein is thought to be located at the M1 position and is only found in fast fibres and cardiac fibres, thus explaining why M1 density is weak or absent in micrographs of slow fibres (four-line M-bands38). The appearance of a strong M1 line in our map (Fig. 4a–d) confirms that there is likely to be M-protein, or its equivalent, in fish fibres. MM-CK appears to be located around the M1–M4 region of the M-band, where it interacts with both myomesin and M-protein.17 The appearance of strong M4 and M4′ lines in our map suggests that all of these three proteins (myomesin, MM-CK and titin) are present. Moreover, M1 is present—but the M6 and M6′ lines are usually absent—in very fast muscles, giving rise to a three-line M-band. Here, in addition to the M4 lines, we see a strong M1 line and also weak density at M6 and M6′ (Fig. 4d), consistent with the fact that fish skeletal muscle is of intermediate speed between slow and fast fibres and has a 3 + 2-line (not quite a five-line) M-band. It is important to note that various nonmyosin M-region protein components already identified by antibody labelling come from studies on mammalian striated muscles, but we expect equivalent proteins to exist in the M-region of skeletal fish muscle based on the structural similarity observed between the fish skeletal muscle M-region and images from mammalian vertebrate muscle.
The three crown levels
As discussed above, we have identified levels P2 and P3 at the edge of the M-region (Fig. 5g–i, top) as being almost exactly equivalent to levels 2 and 3 in our published reconstruction of the bridge region in the fish muscle A-band.30 The fact that the profiles in Fig. 5 are so similar not only says a great deal about the quality and reliability of the new M-region reconstruction but also shows that the bridge region crown levels where the extra proteins do not reside (i.e., crown levels other than level 1) are likely to be similar all along the bridge region. The various extra proteins at level 1 appear to be the cause of the major perturbation in the otherwise helical array.30 It is not clear which protein the extra density at M9 is due to, but it must be perturbing the P1 crown structure (Fig. 5h, top) to make it different from the level 1 crowns in the rest of the bridge region (Fig. 5a), particularly where C-protein/X-protein are located.
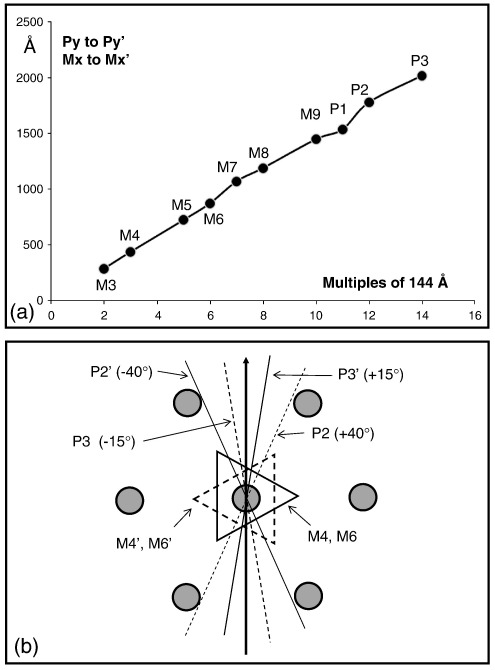
In determining how myosin molecules pack into the filament backbone, it is of interest to ask about the angles of rotation between projecting heads at equivalent positions on each side of the M-region. We have attempted to define this for the P2 and P3 levels at the edge of the new reconstruction, and the results are shown in Fig. 6b. For completeness, the equivalent angular changes at M4,M4′ and M6,M6′ are also given, although these appear to be close to 60°, as required for fitting into the hexagonal M-band lattice.
Fig. 6.
(a) Various M-region distances between quasi-equivalent points Mx–Mx′ and Py–Py′ plotted against multiples of 144 Å. There is a very clear 144-Å repeat through the M-region, as previously found by Sjostrom and Squire.7 (b) Illustration of rotations between equivalent cross-sectional levels in Fig. 3. The triangular profiles at M4 and M6 are 60° (180°) rotated from those at M4′ and M6′. P2 and P3 projections have azimuths that are about 55° apart from each other, as in the bridge region reconstructions of AL-Khayat et al.,30,31 and are + 40° and − 15°, respectively, from the (vertical) 2-fold axis, with P2′ and P3′ being symmetrically placed at − 40° and + 15°. Unfortunately, the head mass azimuth in P1 is not clear, since much of the observed mass must come from part of the M9 protein.
Anti-parallel molecular packing in the backbone
Anti-parallel myosin molecules interact at the M-region. The myosin rod is about 1096 amino acids long; assuming that these are all folded into a conventional coiled-coil structure, this would make the rod length just over 1600 Å long. Since we have concluded that the first crown level P1 at the edge of the M-region is about 770 Å from M1, giving a total length of 1540 Å from P1 to P1′, the P1 heads must be very close to the C-terminal tails of the anti-parallel myosin molecules. The current view on how myosin heads pack into the crowns in resting muscle is that they fold back a little against the rod, as in the vertebrate smooth muscle heavy meromyosin structure observed by Wendt et al.47 and as also seen in tarantula thick filament reconstructions by Woodhead et al.48 This view, at least on some crowns, is supported by reconstructions of vertebrate cardiac muscle thick filaments.31,32 This could explain why the P1–P1′ separation is slightly shorter than the myosin rod length; the anti-parallel rods would be almost completely overlapped to give a dimer 1600 Å long, and then the heads at each end would each tilt back by 30 Å to put the crown centres of mass at positions 1540 Å apart.
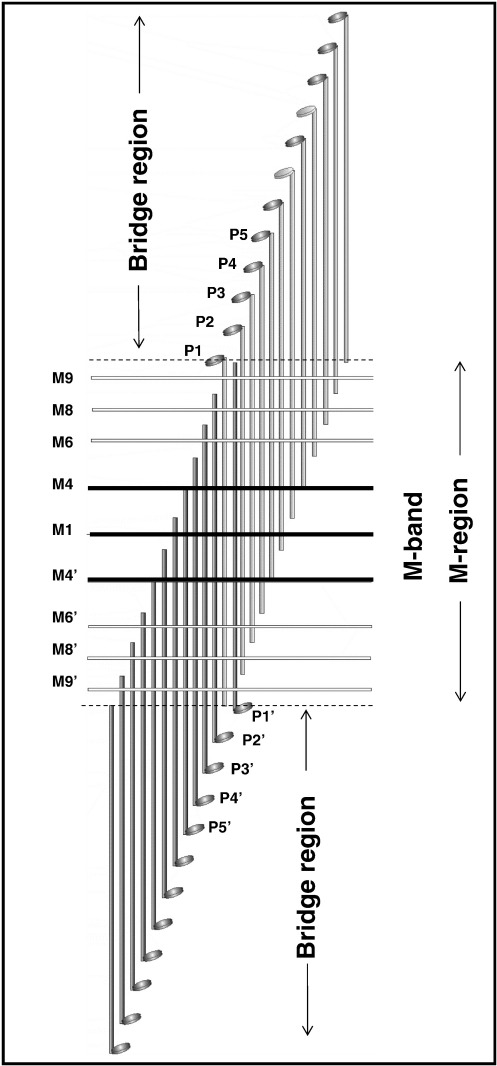
We know that successive crowns are approximately spaced at 144-Å intervals, so we would expect the C-terminal rod ends to be similarly spaced through the M-region. Since we see density features throughout the M-region, it is of interest to ask (as Sjostrom and Squire7 did) whether these also show an obvious 144-Å repeat through the M-region and, if so, whether these features are actually positioned near the molecular ends. There is a conserved sequence at the C-terminus of myosin that is known to be essential for filament assembly.49 Locating the molecular ends in our reconstruction is therefore of considerable interest. Figure 6a shows the observed separation of equivalent features across the M-region (e.g., P1–P1′, M6–M6′, etc.) plotted against multiples of 144 Å. There is quite a good correlation, especially if the back tilt and the axial perturbation of the crowns are allowed for. The rod length of 1600 Å is just over 11 Å × 144 Å. Working back from crown P1 (as in Fig. 7) shows that the prominent M4 bridging densities are coincident with the molecular ends of myosin molecules whose crowns are observed at P5,P5′, whereas M1, M6, M8 and M9 are all positioned halfway between successive rod C-termini. So there is a very clear 144 -Å repeat through the M-region, but only M4 appears to be located at a molecular end close to the C-terminal assembly sequence.
Fig. 7.
Schematic diagram of the molecular overlaps of one-third of the myosin molecules in the M-region illustrating the positions of the various M-region features relative to myosin rod molecular ends. The rods are taken to be about 1600 Å long, and the heads in relaxed muscle are tilted back slightly to give an apparent M-region length (centres of mass from P1 to P1′) of 1540 Å. The other two-thirds of the M-region myosins would be related to this scheme (which only shows the rod axial positions, not the lateral packing) by the 3-fold rotation of D32.
The bare region backbone structure
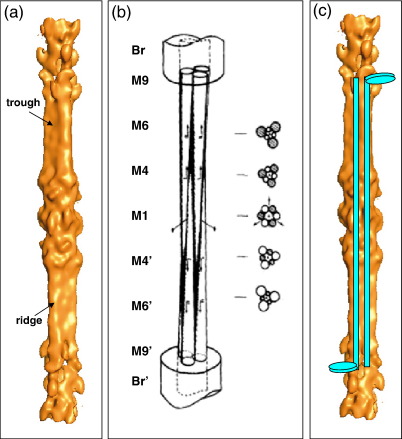
Since the M-region contains myosin rods that are anti-parallel and is of D32 symmetry, and based on their cross-sectional images at various positions through the M-region, Luther et al. proposed a molecular packing scheme for the M-region involving three molecular groups of parallel rods interdigitating with three similar groups with opposite polarities.10 Their model is reproduced here as Fig. 8b. An attractive feature of this model is that each molecular group tapers down from being a cluster of about 11 rods at M9 to being a cluster of about 6 rods at M1 and down to 1 rod at M9′ (see our Fig. 7). Therefore, in each bare region, defined as the part between M4 and M9,44 there would be three large assemblies related by 3-fold rotational symmetry interdigitating with three much smaller assemblies of anti-parallel rods, giving the whole profile a triangular shape. On opposite sides of the M-band, as observed, the triangular bare region profiles and the M-bridges from them would point in opposite directions [i.e., they would be rotated by 60° (180°)]. Additionally at M1, there would be six assemblies of equal size—three assemblies of one polarity and three assemblies of opposite polarity, consistent with the D32 symmetry—giving the backbone profile at this point a ring-like (pseudo-6-fold) shape. The new reconstruction appears to be entirely consistent with this model and even shows how a backbone ridge on one side of the M-region (Fig. 8a) becomes a trough on the other side. Cross sections of the reconstruction (Fig. 3) effectively reproduce the transition from the ring-like (pseudo-6-fold) shape characteristic of the six equal-size assemblies of the model at M1 to the triangular profile observed at M4 and beyond, where the model is dominated by the three large assemblies (Fig. 8b). Observations of thick filaments fraying into three strands on either side of M150 would appear to be consistent with this type of organisation. Figure 8c shows schematically how two myosin molecules would fit into this structure. The cross-sectional backbone shapes at various positions through the reconstruction (Fig. 3) are entirely as expected from the model of Luther et al.10
Fig. 8.
Enlarged view of the new M-region reconstruction showing (a) the ridges and troughs at corresponding positions in the upper and lower halves of the M-region; (b) the previously published model of Luther et al.,10 including various cross-sectional views as seen in sections, with molecular groups of opposite polarity shown as open and filled circles; and (c) the reconstruction in (a) with fully overlapped anti-parallel myosin molecules superimposed.
A second consideration in determining the M-region is how to fit all the myosin rods into the backbone volume observed. Squire9 considered this, thought it hard based on what was known at the time to fit in so many rods, and postulated that there might be missing crown levels in the early part of the proximal zone of the bridge region (what would now be called P2, P3 and P5).7,8 By antibody labelling, Craig and Offer then showed that there only appears to be one missing crown and that this is at the other end of the bridge region near the A-band edges (D19).37 So, if there are no missing crowns in the proximal zone, can all the myosin rods be fitted into the observed M-region reconstruction? From Fig. 7, which represents only one third of the molecules in the M-region and beyond (with the other two thirds being related to what is illustrated by the 3-fold rotational symmetry of the D32), it can be seen that there are 36 rods in any cross section throughout the M-region. Myosin rods, like paramyosin (which is a similar two-chain coiled-coil α-helical molecule), pack with an intermolecular packing distance of around 20 Å.51–53 The packing could be anything between hexagonal (the closest packing) to tetragonal (the loosest packing). The cross-sectional area occupied per molecule therefore lies between 346.4 Å2 and 400 Å2. The total backbone cross-sectional area occupied by myosin rods is 36 times this, giving 12,470–14,400 Å2. In the cross sections of Fig. 3 and also in the observations of Luther et al., the backbone profile is like a hollow circle near M1 (between M1 and M3) and a slightly hollow triangle at around M6–M8 where the backbone is at its smallest.10 Using the profiles in Fig. 3 as guide and avoiding M1 itself where the M1 M-bridging proteins are known to be located, we determined that the estimated backbone areas are about 24,000 Å2 at M2–M3 (a circle of 174 Å diameter and 58 Å core diameter) and about 15,500 Å2 at M6–M8 (a triangle of 190 Å side and 44 Å core diameter). At M6–M8, where the backbone may actually be relatively bare, the estimated area of 15,500 Å2 is almost exactly sufficient to house 36 myosin rods spaced 20 Å apart, giving an area of 12,470–14,400 Å2 with only a small excess to spare. On the other hand, at M2–M3, the observed area is about 50% more than needed to house the myosin rods. If, for example, there were six titin molecules running axially along the filament backbone surface, each of roughly 40 Å diameter, then this would provide an extra area of 7540 Å2, far too much to be accommodated at M6–M8 but compatible with the extra area at M2–M3. On the other hand, additional molecules of myomesin, M-protein or both, which have domain structures similar to those of titin (see summary in Squire et al.42), would also provide the necessary extra area. This area is approximately the observed area of 23,900 Å2 minus the theoretical rod area of 12,470–14,400 Å2, giving 9500–11,430 Å2 for nonmyosin proteins. Extra axially aligned myomesin or M-protein-like molecules would give additional areas of about 3770 Å2 for 3 molecules, 7540 Å2 for 6 molecules, 11,310 Å2 for 9 molecules, and 15,080 Å2 for 12 molecules.
In summary, the observed backbone profiles seem to be able to accommodate all of the expected myosin rods if there are no gaps in the crowns; however, at M6–M8, they fill the space almost completely, leaving little for titin or any other extra protein at this position. There is a possibility15 that titin leaves the thick filament surface at the M-region edges at say, M9, and moves to the position of the M-filaments, halfway between the myosin filaments seen by Luther and Squire.46 On the other hand, each half of the M-band region between M1 and M4 (i.e., not at the bridge levels themselves) has sufficient cross-sectional area to accommodate about 6 or 9 (possibly 12) extra proteins like myomesin or M-protein running along the backbone surface.
Of additional interest is that in Fig. 4c, for example, there appears to be regular backbone structural detail within the M4–M8 region with sets of axial spacings close to 70 Å (which are axially displaced by 35 Å), but it is not yet clear what these features signify. In summary, by applying a single-particle analysis of isolated M-regions for the first time, we believe that the new M-region reconstruction will provide considerable constraints on building more detailed molecular models of the myosin filament M-region and on further defining the 3D structure of the M-band. The current study has therefore provided further evidence illustrating the power of single-particle analysis in solving a structure like the myosin filament M-region, which does not have helical or viral-like symmetry and does not have a single repeating axial structure.
Materials and Methods
Myosin filaments isolated from goldfish (Carassius auratus) in relaxing solution, as described by Kensler and Stewart, were applied to a thin carbon film support on holey carbon grids and negatively stained.54 Only filaments positioned over the holes were used for analysis. Electron micrographs were collected on a JEOL 1200 electron microscope at an accelerating voltage of 80 kV under minimal dose conditions at a magnification of 20,000×.
Micrographs analysed in our earlier work30 were also used here. However, in order to better digitise the raw data, we rescanned the micrographs using a Nikon Super Coolscan-8000 scanner in 16-bit grey-scale mode. Twenty-nine electron micrographs were digitised at a step size of 6.35 μm/pixel. Digitised images (for a typical whole micrograph, see Fig. 1a in AL-Khayat et al.30) were saved in TIFF format and converted into MRC format for preprocessing with the MRC suite of programs55 and with locally developed software. The filament images were scaled to achieve the same magnification and sampling (Å/pixel) using the MRC programs for selecting individual full-length filaments (80 in total) (see Fig. 1b in AL-Khayat et al.30), which were then cut into two half filaments, with the whole M-region included in each. Half filaments (i.e., from the M-band to the pointed end of the myosin filament) were then rotated to make each filament image vertical with the M-region at the bottom (see Fig. 2a in AL-Khayat et al.30). Selected half filaments were floated in 2048 square arrays, and their Fourier transforms were computed. The spacing of the 71.5-Å meridional reflection (the sixth order of the 430-Å repeat) was used to calibrate the magnification and to adjust the sampling of each original full filament to exactly 7.54 Å/pixel. The resulting filament images were read into IMAGIC,33 where all further single-particle analyses were performed. The M-regions (80 in total) were cut out from each individual full filament, rotated to be vertical, and positioned with their M-bands at the centre (Fig. 1b). The modified exact filter method for backprojection described by Paul et al. was used for calculating the 3D reconstruction.34 This allowed the thickness of the central section to be adjusted, taking into account the fact that the diameter of the filament is much less than the size of the cube. Three-dimensional structures were visualised with IMAGIC and PyMOL.35
Acknowledgements
This work was supported by a British Heart Foundation Fellowship to H.A.A. (FS/07/017/22951). E.P.M. was supported by a Wellcome Trust University Award (066418) and a British Heart Foundation project grant (PG/07/076/23480). R.W.K. was supported by Minorities Basic Research Support Grant S06 GM08224 from the National Institutes of Health and, in part, by funding from “Research Centres in Minority Institutions” Award G12RR-03051 from the National Centre for Research Resources, National Institutes of Health. J.M.S. was supported by the European MYORES Network.
Edited by W. Baumeister
References
- 1.Huxley H.E. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J. Mol. Biol. 1963;16:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- 2.Huxley H.E. The mechanism of muscular contraction. Science. 1969;164:1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- 3.Squire J.M. General model of myosin filament structure: II. Myosin filaments and crossbridge interactions in vertebrate striated and insect flight muscles. J. Mol. Biol. 1972;72:125–138. doi: 10.1016/0022-2836(72)90074-5. [DOI] [PubMed] [Google Scholar]
- 4.Labeit S., Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 5.Trinick J. Cytoskeleton—titin as a scaffold and a spring. Curr. Biol. 1996;6:258–260. doi: 10.1016/s0960-9822(02)00472-4. [DOI] [PubMed] [Google Scholar]
- 6.Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils: extraction, purification and characterisation. J. Mol. Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- 7.Sjostrom M., Squire J.M. Fine structure of the A-band in cryo-sections: I. The structure of the A-Band of human skeletal muscle from ultrathin cryo-sections negatively stained. J. Mol. Biol. 1977;109:49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- 8.Sjostrom M., Squire J.M. Cryo-ultramicrotomy and myofibrillar fine structure. J. Microsc. 1977;111:239–278. doi: 10.1111/j.1365-2818.1977.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 9.Squire J.M. General model of myosin filament structure: III. Molecular packing arrangements in myosin filaments. J. Mol. Biol. 1973;77:291–323. doi: 10.1016/0022-2836(73)90337-9. [DOI] [PubMed] [Google Scholar]
- 10.Luther P.K., Munro P.M.G., Squire J.M. Three-dimensional structure of the vertebrate muscle A-band: III. M-region structure and myosin filament symmetry. J. Mol. Biol. 1981;151:703–730. doi: 10.1016/0022-2836(81)90430-7. [DOI] [PubMed] [Google Scholar]
- 11.Grove B.K., Kurer V., Lehner C., Doetschman T.C., Perriard J.C., Eppenberger H.M. A new 185,000-dalton skeletal muscle protein detected by monoclonal antibodies. J. Cell Biol. 1984;98:518–524. doi: 10.1083/jcb.98.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grove B.K., Cerny L., Perriard J.C., Eppenberger H.M. Myomesin and M-protein: expression of two M-band proteins in pectoral muscle and heart during development. J. Cell Biol. 1985;101:1413–1421. doi: 10.1083/jcb.101.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Ven P.F.M., Obermann W.M.J., Weber K., Furst D.O. Myomesin, M-protein and the structure of the sarcomeric M-band. Adv. Biophys. 1996;33:91–99. doi: 10.1016/0065-227x(96)81666-2. [DOI] [PubMed] [Google Scholar]
- 14.Obermann W.M., Gautel M., Steiner F., van der Ven P.F., Weber K., Furst D.O. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250 kD carboxy-terminal region of titin by immunoelectron microscopy. J. Cell Biol. 1996;134:1441–1453. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obermann W.M.J., Gautel M., Weber K., Furst D.O. Molecular structure of the sarcomeric M-band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 1997;16:211–220. doi: 10.1093/emboj/16.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auerbach D., Bantle S., Keller S., Hinderling V., Leu M., Ehler E., Perriard J.C. Different domains of the M-band protein myomesin are involved in myosin binding and M-band targeting. Mol. Biol. Cell. 1999;10:1297–1308. doi: 10.1091/mbc.10.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornemann T., Kempa S., Himmel M., Hayeb K., Furst D.O., Wallimann T. Muscle-type creatine kinase interacts with central domains of the M-band proteins myomesin and M-protein. J. Mol. Biol. 2003;332:877–887. doi: 10.1016/s0022-2836(03)00921-5. [DOI] [PubMed] [Google Scholar]
- 18.Lange S., Agarkova I., Perriard J.C., Ehler E. The sarcomeric M-band during development and in disease. J. Muscle Res. Cell Motil. 2005;26:375–379. doi: 10.1007/s10974-005-9019-4. [DOI] [PubMed] [Google Scholar]
- 19.Lange S., Xiang F., Yakovenko A., Vibola A., Heckman P., Rostkova E. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1633. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- 20.Agarkova I., Ehler E., Lange S., Schoenauer R., Perriard J.C. M-band: a safeguard for sarcomere stability. J. Muscle Res. Cell Motil. 2003;24:191–203. doi: 10.1023/a:1026094924677. [DOI] [PubMed] [Google Scholar]
- 21.Agarkova I., Perriard J.C. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Schoenauer R., Bertoncini P., Machaidze G., Aebi U., Perriard J.C., Hegner M., Agarkova I. Myomesin is a molecular spring with adaptable elasticity. J. Mol. Biol. 2005;349:367–379. doi: 10.1016/j.jmb.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Schoenauer R., Lange S., Hirschy A., Ehler E., Perriard J.C., Agarkova I. Myomesin 3, a novel structural component of the M-band in striated muscle. J. Mol. Biol. 2008;376:338–351. doi: 10.1016/j.jmb.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 24.Fukuzawa A., Lange S., Holt M., Vihola A., Carmignac V., Ferreiro A. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band—implications for hereditary myopathies. J. Cell Sci. 2008;121:1841–1851. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- 25.Pernigo S., Fuzukawa A., Bertz M., Holt M., Rief M., Steiner R.A., Gautel M. Structural insights into M-band assembly and mechanics from the titin-obscurin-like-1 complex. Proc. Natl Acad. Sci. 2010;107:2908–2913. doi: 10.1073/pnas.0913736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayans O., van der Ven P.F., Wilm M., Mues A., Young P., Furst D.O. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–869. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- 27.Ehler E., Gautel M. The sarcomere and sarcomerogenesis. Adv. Exp. Med. Biol. 2008;642:1–14. doi: 10.1007/978-0-387-84847-1_1. [DOI] [PubMed] [Google Scholar]
- 28.Ottenheijm C.A., Hidalgo C., Rost K., Gotthardt M., Granzier H. Altered contractility of skeletal muscle in mice deficient in titin's M-band region. J. Mol. Biol. 2009;393:10–26. doi: 10.1016/j.jmb.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AL-Khayat H.A., Squire J.M. Refined structure of bony fish muscle myosin filaments from low-angle X-ray diffraction data. J. Struct. Biol. 2006;155:218–229. doi: 10.1016/j.jsb.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.AL-Khayat H.A., Morris E.P., Kensler R.W., Squire J.M. 3D structure of relaxed fish muscle myosin filaments by single particle analysis. J. Struct. Biol. 2006;155:202–217. doi: 10.1016/j.jsb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 31.AL-Khayat H.A., Morris E.P., Kensler R.W., Squire J.M. Myosin filament 3D structure in mammalian cardiac muscle. J. Struct. Biol. 2008;163:117–126. doi: 10.1016/j.jsb.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoghbi M.E., Woodhead J.L., Moss R.L., Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl Acad. Sci. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Heel M., Gowen B., Matadeen R., Orlova E.V., Finn R., Pape T. Single-particle electron cryo-microscopy: towards atomic resolution. Q. Rev. Biophys. 2000;33:307–369. doi: 10.1017/s0033583500003644. [DOI] [PubMed] [Google Scholar]
- 34.Paul D., Patwardhan A., Squire J.M., Morris E.P. Single particle analysis of filamentous and highly elongated macromolecular assemblies. J. Struct. Biol. 2004;148:236–250. doi: 10.1016/j.jsb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 35.DeLano W.L. DeLano Scientific; San Carlos, CA: 2002. The PyMOL User's Manual. [Google Scholar]
- 36.Cantino M.E., Chew M.W., Luther P.K., Morris E., Squire J.M. Structure and nucleotide-dependent changes of thick filaments in relaxed and rigor plaice fin muscles. J. Struct. Biol. 2002;137:164–175. doi: 10.1006/jsbi.2002.4474. [DOI] [PubMed] [Google Scholar]
- 37.Craig R., Offer G. Axial arrangement of crossbridges in thick filaments of vertebrate skeletal muscle. J. Mol. Biol. 1976;102:325–332. doi: 10.1016/s0022-2836(76)80057-5. [DOI] [PubMed] [Google Scholar]
- 38.Pask H.T., Jones K.L., Luther P.K., Squire J.M. M-band structure, M-bridge interactions and contraction speed in vertebrate cardiac muscles. J. Muscle Res. Cell Motil. 1994;15:633–645. doi: 10.1007/BF00121071. [DOI] [PubMed] [Google Scholar]
- 39.Edman A.C., Squire J.M., Sjostrom M. Fine structure of the A-band in cryo-sections: diversity of M-band structure in chicken breast muscle. J. Ultrastruct. Mol. Struct. Res. 1988;100:1–12. doi: 10.1016/0889-1605(88)90054-7. [DOI] [PubMed] [Google Scholar]
- 40.Hanson J., O'Brien E.J., Bennett P.M. Structure of the myosin-containing filament assembly (A-segment) separated from frog skeletal muscle. J. Mol. Biol. 1971;58:865–871. doi: 10.1016/0022-2836(71)90045-3. [DOI] [PubMed] [Google Scholar]
- 41.Bennett P., Craig R., Starr R., Offer G. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J. Muscle Res. Cell Motil. 1986;7:550–567. doi: 10.1007/BF01753571. [DOI] [PubMed] [Google Scholar]
- 42.Squire J.M., AL-Khayat H.A., Knupp C., Luther P.K. Molecular architecture in muscle contractile assemblies. Adv. Protein Chem. 2005;71:17–87. doi: 10.1016/S0065-3233(04)71002-5. [DOI] [PubMed] [Google Scholar]
- 43.Luther P.K., Bennett P.M., Knupp C., Craig R., Padron R., Harris S.P. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J. Mol. Biol. 2008;384:60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luther P.K., Squire J.M. Three-dimensional structure of the vertebrate muscle A-band: II. The myosin filament superlattice. J. Mol. Biol. 1980;141:409–439. doi: 10.1016/0022-2836(80)90254-5. [DOI] [PubMed] [Google Scholar]
- 45.Luther P.K., Crowther R.A. Three-dimensional reconstruction from tilted sections of fish muscle M-band. Nature. 1984;307:566–568. doi: 10.1038/307566a0. [DOI] [PubMed] [Google Scholar]
- 46.Luther P.K., Squire J.M. Three-dimensional structure of the vertebrate muscle M-region. J. Mol. Biol. 1978;125:313–324. doi: 10.1016/0022-2836(78)90405-9. [DOI] [PubMed] [Google Scholar]
- 47.Wendt T., Taylor D., Trybus K.M., Taylor K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc. Natl Acad. Sci. 2001;98:4361–4366. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodhead J.L., Zhao F.Q., Craig R., Egelman E.H., Alamo L., Padrón R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 49.Cohen C., Parry D.A.D. A conserved C-terminal assembly region in paramyosin and myosin rods. J. Struct. Biol. 1998;122:180–187. doi: 10.1006/jsbi.1998.3983. [DOI] [PubMed] [Google Scholar]
- 50.Maw M.C., Rowe A.J. Fraying of A-filaments into three subfilaments. Nature. 1980;286:412–414. doi: 10.1038/286412a0. [DOI] [PubMed] [Google Scholar]
- 51.Elliott A., Lowy J., Parry D.A., Vibert P.J. Puzzle of the coiled coils in the alpha-protein paramyosin. Nature. 1968;218:656–659. doi: 10.1038/218656a0. [DOI] [PubMed] [Google Scholar]
- 52.Squire J.M. Muscle myosin filaments: internal structure and crossbridge organisation. Comments Mol. Cell. Biophys. 1986;3:155–177. [Google Scholar]
- 53.Chew M.W.K., Squire J.M. Packing of α-helical coiled-coil molecules invertebrate muscle thick filaments. J. Struct. Biol. 1995;115:233–249. doi: 10.1006/jsbi.1995.1048. [DOI] [PubMed] [Google Scholar]
- 54.Kensler R.W., Stewart M. An ultrastructural study of the cross-bridge arrangement in the fish skeletal muscle thick filament. J. Cell Sci. 1989;94:391–401. doi: 10.1242/jcs.94.3.391. [DOI] [PubMed] [Google Scholar]
- 55.Crowther R.A., Henderson R., Smith J.M. MRC image processing programs. J. Struct. Biol. 1996;116:9–16. doi: 10.1006/jsbi.1996.0003. [DOI] [PubMed] [Google Scholar]