Abstract
Using standard morphological methods, we describe one new Leptopharynx species and a new subspecies of L. costatus, both from soil of the neotropic region. Further, we studied two populations of L. costatus costatus. Leptopharynx brasiliensis nov. spec., which was discovered in the Mato Grosso, Brazil, is a large member (60 μm) of the genus with an enormous oral basket. It differs from similar congeners in having six monokinetids in kinety 6, widely spaced kinetids in kinety 1, and an average of 294 kinetids. Leptopharynx costatus gonohymen nov. subspec., which was discovered in southern Florida, makes a small (35 μm) and a large morph (55 μm) both with narrow oral basket. The small morph is inseparable from the small morph of L. costatus costatus, while the large morph has right-angled adoral membranelles and widely (vs. narrowly) spaced kinetids in kinety 1. The small morphs of a Brazilian and an Austrian L. costatus match Mexican and other European populations, all having on average 181–187 kinetids. As yet, we know four morphs of L. costatus that differ by body size (small vs. large), the oral basket (narrow vs. wide), membranelle 1 (present vs. absent), and the arrangement of the membranelles (flat vs. angled).
Keywords: Brazil, Florida, Leptopharynx costatus-complex, Leptopharynx costatus costatus nov. stat., Neotropic region, Soil ciliates
Introduction
This paper continues a series of studies on the genus Leptopharynx Mermod, 1914, which is globally distributed in limnetic and, especially, terrestrial habitats (Alekperov 1993; Foissner 1989, 1998; Foissner et al. 1994, 2011; Kahl 1926, 1931; Njiné 1979; Omar and Foissner, 2011; Prelle 1961; Thompson 1972). As yet, few members of the genus have been investigated with modern methods and described thoroughly (for a brief review, see Foissner et al. 2011). The two new taxa described in the present paper support the species features used by Foissner et al. (2011) and Omar and Foissner (2011). Leptopharynx brasiliensis, which was discovered in the Mato Grosso, a large, seasonally flooded area in Brazil, is rather similar to L. australiensis Omar and Foissner, 2011 from jungle soil of Australia. These species are possibly examples for post-Gondwanan speciation from a common ancestor. The second taxon, Leptopharynx costatus gonohymen, which was discovered in soil from Florida, USA, was classified as a subspecies of L. costatus, mainly because of the unique arrangement of adoral membranelles 2 and 3.
Leptopharynx brasiliensis was associated with a small morph of L. costatus. Thus we performed a detailed morphometric analysis on the Brazilian and an Austrian population of L. costatus. This and literature data (Omar and Foissner, 2011) showed an extraordinary result: five populations from Austria, Germany, Mexico, and Brazil have a nearly identical average total number of basal bodies: 181–187.
The subspecies concept has been hotly discussed (for a review, see Mayr 1963), but a biogeographic component was usually included. Unfortunately, biogeography of most protists is in its infancy (Foissner 2006). Thus, we followed Foissner et al. (2002), who characterized protist subspecies by distinct morphometrical differences and/or qualitative characters whose taxonomic value is still doubtful or not known.
Material and Methods, Terminology
For details on samples and locations, see the individual species descriptions. Leptopharynx brasiliensis and L. costatus gonohymen were reactivated from the resting cysts of air-dried soil samples from Brazil and Florida, USA, respectively, using the non-flooded Petri dish method (NFPM). Briefly, the NFPM involves placing 50–500 g litter and soil in a Petri dish (13–18 cm wide, 2–3 cm high) and saturating, but not flooding it, with distilled water. Such a culture is analysed for ciliates by inspecting about 2 ml of the run-off on days 2, 7, 14, 21, and 28; for a detailed description of the NFPM, see Foissner et al. (2002).
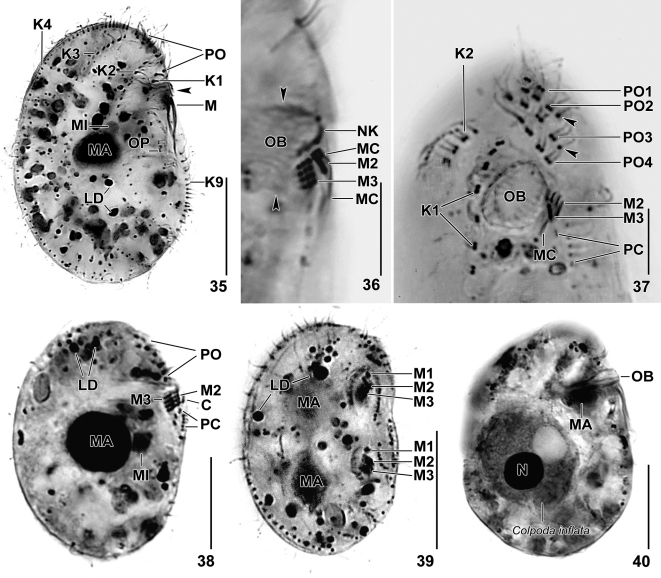
Both species were observed in vivo and in protargol preparations (Foissner 1991); Leptopharynx brasiliensis was investigated also with the Chatton–Lwoff silver nitrate method. Counts and measurements on silvered specimens were conducted at a magnification of 1000×. The “total number of basal bodies” excludes those of the adoral membranelles, which are difficult to count. In vivo measurements were performed at magnifications of 40–1000×. Drawings of live specimens were based on free-hand sketches and micrographs, while those of impregnated cells were made with a drawing device.
Basal terminology is according to Corliss (1979) and Lynn (2008). We propose the terms “group A, B and C basal bodies”, to designate small rows of basal bodies or granules in the surroundings of the adoral membranelles (Fig. 6). The terms “microstome” and “macrostome” refer to small- and large-mouthed shapes in a polymorphic life cycle. Such a life cycle has been shown in Leptopharynx costatus (Foissner et al. 2011) but not (yet) in other species of the genus. In L. costatus, macrostomy is associated with large body size, but not always. Thus, it is convenient to use “small morph” and “large morph” in species descriptions at the present state of knowledge.
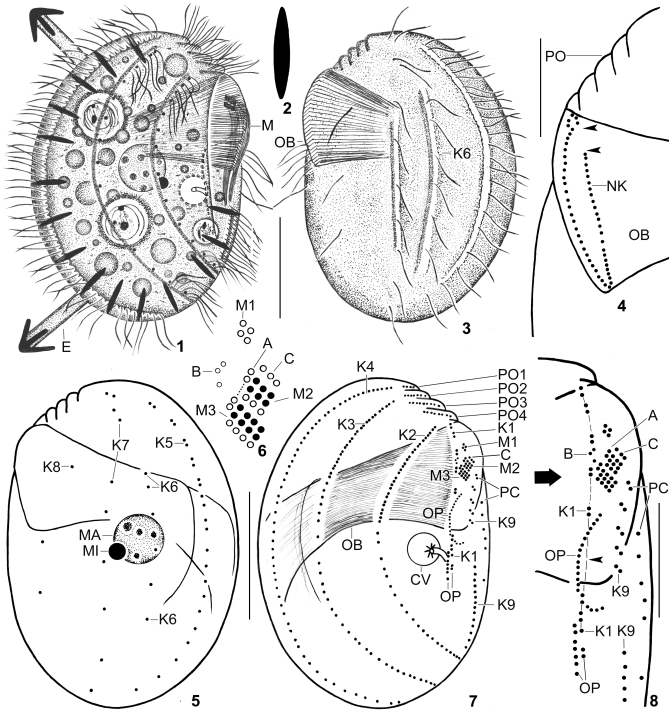
Figs 1–8.
Leptopharynx brasiliensis from life (1–3) and after protargol impregnation (4–8). 1, 3. Right and left side view of representative specimens. Note the margin of a hyaline plate on the ventral side (hatched line), the left side ciliation, the two furrows extending on the left side and containing kinety 6 as well as the middle portion of kinety 7, the distinctly oblique preoral region, and the large oral basket. 2. A resting extrusome, 6–7 μm long. 4. Left side view of the oral basket opening, showing the break of the nasse kinetosomes in the left anterior portion (arrowheads) and the curl-like pattern at the right end. 5–8. Left and right side view (5, 7), adoral membranelles (6), and the arrangement of the basal bodies on the ventral side (8) of the holotype specimen, length 52 μm. The hatched line in (8) connects the basal bodies of kinety 1. Note the wide break between the fifth and sixth dikinetid (arrowhead). Open circles in (6) indicate non-ciliated basal bodies. A, group A basal bodies; B, group B granules; C, group C basal bodies; CV, contractile vacuole; E, exploding extrusome; K1–9, somatic kineties; M(1–3), adoral membranelles; MA, macronucleus; MI, micronucleus; NK, nasse kinetosomes; OB, oral basket; OP, oral primordium; PC, postoral complex; PO (1–4), preoral kineties. Scale bars 20 μm (1, 3, 5, 7), and 10 μm (4, 8).
Results
Leptopharynx brasiliensis Foissner and Omar nov. spec. (Figs 1–16; Table 1)
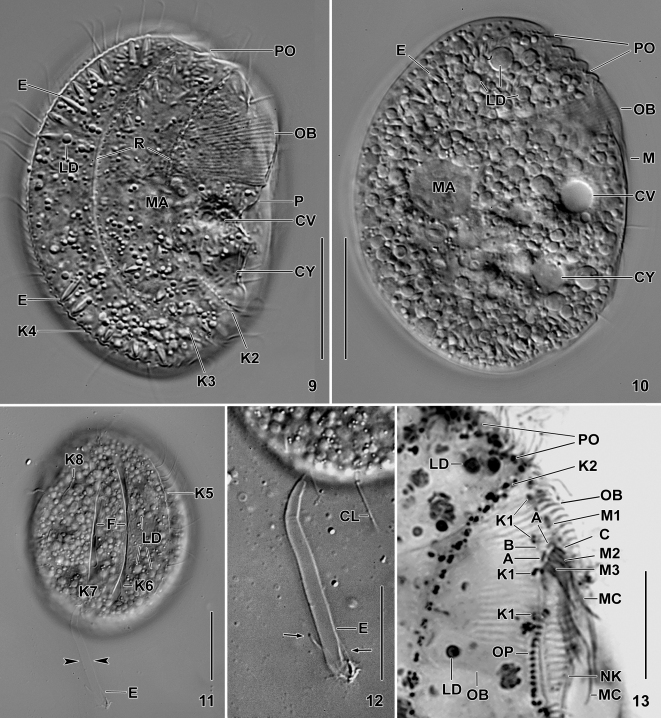
Figs 9–13.
Leptopharynx brasiliensis from life (9–12) and after protargol impregnation (13). 9, 10. Right side view at two focal planes. Note, inter alia, the enormous oral basket, the distinctly oblique and serrated preoral region, the cortical ridges right of kineties 2 and 3, the margin of a hyaline plate on the ventral side (9), and the countless lipid droplets in the cytoplasm (10). 11, 12. Left side view showing the furrows containing kinety 6 and the middle portion of kinety 7. Note the thick, exploded extrusome (arrowheads), shown at higher magnification in (12). Arrows denote the rod-shaped arms. 13. Right side view of oral region. Note the enormous oral basket, the widely spaced kinetids of kinety 1, the group A–C basal bodies, and the upper part of the oral primordium. A, group A basal bodies; B, group B granules; C, group C basal bodies; CL, cilium; CV, contractile vacuole; CY, cytopyge; E, extrusomes; F, furrows; K1–8, somatic kineties; LD, lipid droplets; M(1–3), adoral membranelles; MA, macronucleus; MC, membranellar cilia; NK, nasse kinetosomes; OB, oral basket; OP, oral primordium; P, margin of a hyaline plate; PC, postoral complex; PO, preoral kineties; R, cortical ridges. Scale bars 20 μm (9, 10, 12), 25 μm (11), and 10 μm (13).
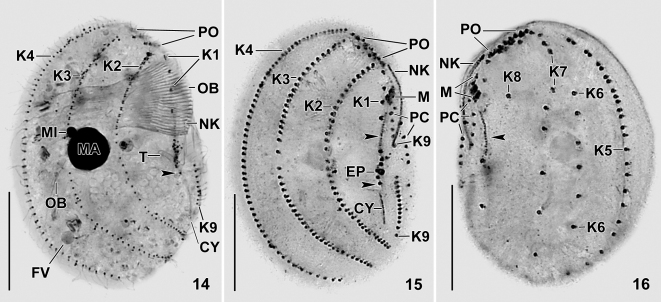
Figs 14–16.
Leptopharynx brasiliensis after protargol impregnation (14) and after Chatton–Lwoff silver nitrate impregnation (15, 16). 14. Right side view of a paratype specimen showing the ciliary pattern, the distinctly oblique preoral region, the widely spaced kinetids in kinety 1, and the conspicuous oral basket. The arrowhead marks the posterior portion of the oral primordium. 15, 16. Right and left side view of paratype specimens, showing the ciliary pattern. Note the widely spaced kinetids of kinety 1, the pair-like arranged kinetids in the anterior portion of kinety 5, and the six kinetids comprising kinety 6. Arrowheads denote the oral primordium. CY, cytopyge; EP, excretory pore; FV, food vacuole; K1–9, somatic kineties; M, adoral membranelles; MA, macronucleus; MI, micronucleus; NK, nasse kinetosomes; OB, oral basket; PC, postoral complex; PO, preoral kineties; T, excretory tube. Scale bars 20 μm.
Table 1.
Morphometric data on Leptopharynx brasiliensis (upper line) and a small morph of L. costatus costatus contained in the same slides (lower line).
| Characteristicsa | M | SD | SE | CV | Min | Max | n | |
|---|---|---|---|---|---|---|---|---|
| Body, length in protargol preparations | 54.0 | 54.0 | 5.1 | 1.1 | 9.5 | 42.0 | 61.0 | 21 |
| 34.1 | 33.0 | 3.3 | 0.7 | 9.7 | 30.0 | 41.0 | 21 | |
| Body, width in protargol preparations | 38.0 | 39.0 | 4.0 | 0.9 | 10.5 | 29.0 | 45.0 | 21 |
| 23.8 | 24.0 | 2.3 | 0.5 | 9.5 | 20.0 | 28.0 | 21 | |
| Body, length in Chatton–Lwoff silver nitrate preparations | 56.8 | 57.0 | 5.0 | 1.1 | 8.8 | 48.0 | 63.0 | 21 |
| 37.7 | 38.0 | 2.0 | 0.4 | 5.3 | 33.0 | 41.0 | 21 | |
| Body, width in Chatton–Lwoff silver nitrate preparations | 39.3 | 39.0 | 4.9 | 1.1 | 12.4 | 31.0 | 47.0 | 21 |
| 26.1 | 26.0 | 1.8 | 0.4 | 6.7 | 22.0 | 29.0 | 21 | |
| Body length: width, ratio in protargol preparations | 1.4 | 1.4 | 0.1 | 0.1 | 4.6 | 1.3 | 1.7 | 21 |
| 1.4 | 1.4 | 0.1 | 0.1 | 3.5 | 1.3 | 1.5 | 21 | |
| Body length: width, ratio in Chatton–Lwoff silver nitrate preparations | 1.5 | 1.5 | 0.1 | 0.1 | 8.1 | 1.3 | 1.8 | 21 |
| 1.5 | 1.4 | 0.1 | 0.1 | 4.4 | 1.3 | 1.6 | 21 | |
| Anterior body end to anteriormost adoral membranelle, distanceb | 10.6 | 10.0 | 1.4 | 0.3 | 13.6 | 8.0 | 13.0 | 21 |
| 11.0 | 10.0 | 1.7 | 0.4 | 16.0 | 9.0 | 14.0 | 21 | |
| Body length: anterior body end to anteriormost adoral membranelle, ratiob | 5.2 | 5.0 | 6.0 | 0.1 | 12.0 | 3.8 | 6.5 | 21 |
| 3.3 | 3.3 | 0.3 | 0.1 | 9.3 | 2.6 | 3.8 | 21 | |
| Anterior body end to macronucleus, distance | 21.5 | 21.0 | 2.5 | 0.5 | 11.4 | 16.0 | 25.0 | 21 |
| 12.1 | 12.0 | 1.2 | 0.3 | 9.8 | 10.0 | 14.0 | 21 | |
| Anterior body end to excretory pore of contractile vacuole, distance | 29.5 | 29.0 | 2.8 | 0.6 | 9.5 | 24.0 | 33.0 | 21 |
| 17.5 | 17.0 | 1.8 | 0.4 | 10.3 | 15.0 | 21.0 | 21 | |
| Macronucleus, length | 9.2 | 9.0 | 0.9 | 0.2 | 9.5 | 7.0 | 11.0 | 21 |
| 7.6 | 8.0 | 0.7 | 0.2 | 8.8 | 7.0 | 9.0 | 21 | |
| Macronucleus, width | 8.9 | 9.0 | 1.0 | 0.2 | 11.7 | 6.0 | 11.0 | 21 |
| 7.3 | 7.0 | 0.7 | 0.2 | 9.8 | 6.0 | 9.0 | 21 | |
| Micronucleus, diameter | 2.0 | 2.0 | – | – | – | 2.0 | 3.0 | 21 |
| 2.1 | 2.0 | – | – | – | 2.0 | 3.0 | 21 | |
| Oral basket, width | 17.4 | 18.0 | 1.6 | 0.4 | 9.2 | 14.0 | 19.0 | 21 |
| 4.1 | 4.0 | – | – | – | 4.0 | 5.0 | 21 | |
| Somatic kineties, number | 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 21 |
| 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 21 | |
| Somatic kinety 1, number of dikinetids | 7.0 | 7.0 | 0.0 | 0.0 | 0.0 | 7.0 | 7.0 | 21 |
| 7.0 | 7.0 | 0.0 | 0.0 | 0.0 | 7.0 | 7.0 | 21 | |
| Somatic kinety 1, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 | |
| Somatic kinety 2, number of dikinetids | 13.5 | 14.0 | 0.9 | 0.2 | 6.5 | 12.0 | 15.0 | 21 |
| 6.3 | 6.0 | 0.6 | 0.1 | 8.9 | 5.0 | 7.0 | 21 | |
| Somatic kinety 2, number of monokinetids | 22.2 | 22.0 | 2.1 | 0.5 | 9.4 | 17.0 | 25.0 | 21 |
| 11.6 | 11.0 | 1.1 | 0.2 | 9.6 | 9.0 | 13.0 | 21 | |
| Somatic kinety 3, number of dikinetids | 12.2 | 12.0 | 1.3 | 0.3 | 10.3 | 10.0 | 15.0 | 21 |
| 3.7 | 3.0 | 1.1 | 0.2 | 28.4 | 2.0 | 5.0 | 21 | |
| Somatic kinety 3, number of monokinetids | 34.4 | 34.0 | 2.4 | 0.5 | 6.9 | 30.0 | 39.0 | 21 |
| 26.7 | 27.0 | 2.5 | 0.6 | 9.4 | 21.0 | 32.0 | 21 | |
| Somatic kinety 4, number of monokinetids (does not have dikinetids) | 62.7 | 63.0 | 3.8 | 0.8 | 6.0 | 57.0 | 72.0 | 21 |
| 38.3 | 38.0 | 3.3 | 0.7 | 8.6 | 34.0 | 44.0 | 21 | |
| Somatic kinety 5, number of monokinetids (does not have dikinetids) | 24.7 | 25.0 | 1.3 | 0.3 | 5.2 | 21.0 | 26.0 | 21 |
| 13.3 | 13.0 | 1.4 | 0.3 | 10.4 | 11.0 | 17.0 | 21 | |
| Somatic kinety 6, number of monokinetids (does not have dikinetids) | 6.1 | 6.0 | 0.4 | 0.1 | 7.2 | 6.0 | 8.0 | 21 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | |
| Somatic kinety 7, number of monokinetids (does not have dikinetids) | 9.7 | 10.0 | – | – | – | 9.0 | 10.0 | 21 |
| 9.4 | 9.0 | – | – | – | 9.0 | 10.0 | 21 | |
| Somatic kinety 8, number of monokinetids (does not have dikinetids) | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 | |
| Somatic kinety 9, number of monokinetids in posterior segment | 13.2 | 14.0 | 1.2 | 0.3 | 8.9 | 11.0 | 15.0 | 21 |
| 6.7 | 7.0 | 0.7 | 0.1 | 9.9 | 6.0 | 8.0 | 21 | |
| Preoral ciliary rows, number | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | |
| Preoral kinety 1, number of dikinetids (does not have monokinetids) | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | |
| Preoral kinety 2, number of dikinetids | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 | |
| Preoral kinety 2, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 | |
| Preoral kinety 3, number of dikinetids | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | |
| Preoral kinety 3, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 | |
| Preoral kinety 4, number of dikinetids (for monokinetids, see postoral complex) | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | |
| Oral primordium, number of dikinetids in posterior part | 3.4 | 3.0 | – | – | – | 3.0 | 4.0 | 21 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | |
| Oral primordium, number of monokinetids in posterior part | 0.9 | 1.0 | – | – | – | 0.0 | 1.0 | 21 |
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21 | |
| Oral primordium, number of granules (basal bodies?) in anterior part | 20.3 | 21.0 | 1.8 | 0.4 | 8.8 | 16.0 | 23.0 | 21 |
| Present but too faintly impregnated | ||||||||
| Adoral membranelle 1, number of basal bodies | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 |
| Not present | ||||||||
| Adoral membranelle 2, number of basal body rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| 2.2 | 2.0 | – | – | – | 2.0 | 3.0 | 21 | |
| Adoral membranelle 2, number of basal bodies | 11.4 | 11.0 | 1.1 | 0.2 | 9.4 | 11.0 | 14.0 | 21 |
| 9.4 | 8.0 | 2.1 | 0.5 | 22.0 | 8.0 | 15.0 | 21 | |
| Adoral membranelle 3, number of basal body rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 | |
| Adoral membranelle 3, number of basal bodies | 13.7 | 14.0 | 0.9 | 0.2 | 6.6 | 11.0 | 14.0 | 21 |
| 12.7 | 12.0 | 1.3 | 0.3 | 10.3 | 12.0 | 15.0 | 21 | |
| Left row of postoral complex, number of monokinetidsc | 6.1 | 6.0 | – | – | – | 6.0 | 7.0 | 21 |
| 6.0 | 6.0 | 0.0 | 0.0 | 0.0 | 6.0 | 6.0 | 21 | |
| Right row of postoral complex, number of dikinetidsd | 4.6 | 5.0 | 0.6 | 0.1 | 12.8 | 4.0 | 6.0 | 21 |
| 3.0 | 3.0 | 0.3 | 0.1 | 10.5 | 2.0 | 4.0 | 21 | |
| Right row of postoral complex, number of monokinetidsd | 0.5 | 0.0 | – | – | – | 0.0 | 1.0 | 21 |
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21 | |
| Basal bodies, total numbere | 293.8 | 294.0 | 9.2 | 2.0 | 3.1 | 275.0 | 314.0 | 21 |
| 186.1 | 183.0 | 8.8 | 1.9 | 4.7 | 174.0 | 201.0 | 21 | |
Data based, if not mentioned otherwise, on mounted, protargol-impregnated, and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. CV, coefficient of variation in %; M, median; Max, maximum; Min, minimum; n, number of specimens investigated; SD, standard deviation; SE, standard error of mean; , arithmetic mean.
Membranelle 1 is the anteriormost membranelle in Leptopharynx brasiliensis, while membranelle 2 is the anteriormost in L. costatus costatus.
Without basal bodies of group C.
This is the anterior segment of somatic kinety 9.
Except of basal bodies of adoral membranelles.
Diagnosis
Size about 60 × 40 μm in vivo; body semidiscoidal with distinctly oblique, serrate preoral region confluent with distal end of oral basket. Somatic ciliature of costatus type, i.e., with postoral complex and nine ciliary rows, of which kineties 1, 2 and 3 have dikinetids anteriorly. Kinety 1 consisting of widely spaced, ciliated dikinetids; kinety 6 composed of six monokinetids; a total of 294 basal bodies on average. Adoral membranelle 1 consisting of four basal bodies, membranelles 2 and 3 each composed of three rows of basal bodies. Possibly produces only large-mouthed cells with oral basket about 17 μm wide.
Type locality
Dusty, light brown soil with some litter and fine roots in the surroundings of kilometer 42 of the Transpantaneira Road between the cities of Poconé and Porto Jofre, near the Pousada Rio Claro, Pantanal wetland, Mato Grosso, Brazil, S16°39′ W56°45′.
Type material
A holotype slide with protargol-impregnated specimens and six paratype slides with protargol-impregnated and Chatton–Lwoff silver nitrate-impregnated specimens have been deposited in the Biology Centre of the Museum of Upper Austria, Linz (LI). The holotype and important paratype specimens have been marked by black ink circles on the coverslip.
Etymology
Named after the country in which discovered.
Description
Size 45–70 × 30–50 μm, usually about 60 × 40 μm, as calculated from some measurements of live specimens and values shown in Table 1; rather similar in protargol and silver nitrate preparations, where the length: width ratio is higher (1.5) than in protargol-prepared cells (1.4), corresponding to the better fixation (osmium acid). Body semidiscoidal with conspicuous, serrate preoral truncation extending to body midline in an angle of about 45°. Dorsal side distinctly convex, ventral flat to slightly convex. Body thin, leaf-like flattened laterally (Figs 1, 3, 5, 9, 10, 14–16). Nuclear apparatus usually in or near body centre, right or left of body's midline, anterior third frequently covered by the oral basket (Table 1; Figs 1, 5, 10, 14). Macronucleus comparatively small, i.e., occupies only about 17% of body length, usually spherical, with pale nucleoli about 2 μm across. Micronucleus attached to macronucleus at various positions, spherical. Contractile vacuole in or near mid-body, right of anterior half of oral primordium, with distinct tube recognizable in protargol preparations; contains fibre bundles forming star-like pattern around tube base (Figs 7, 14). Cytopyge posterior of contractile vacuole; in silver nitrate preparations represented by a thick, short silverline extending between posterior portion of kineties 2 and 9 (Table 1; Figs 1, 7, 9, 10, 14, 15). Extrusomes as in Leptopharynx bromelicola, i.e., left of kineties, bluntly fusiform and compact, 6–7 μm long when resting, while up to 40 × 3–4 μm and with four rod-shaped arms when exploded (Figs 1, 2, 11, 12). Cortex as in L. costatus, i.e., rigid and glossy. Right and left body side with a conspicuous ridge and furrow pattern recognizable in vivo and in some protargol-impregnated specimens; right side with two narrow ridges right of kineties 2 and 3 (Figs 1, 9); ridges accompanying kineties 4 and 5 on dorsal margin and thus appearing less distinct when specimens are viewed laterally; kinety 6 and middle part of kinety 7 each extend in a furrow accompanied by a sharp ridge right of kineties (Figs 3, 11). Details of ventral side difficult to observe, possibly organized as follows (Figs 1, 9): (i) conspicuous ridges or furrows along preoral kineties; (ii) a sharp line produced by the edge of the right side, extending left of kinety 1 and between posterior portion of kineties 2 and 9; (iii) the margin of a hyaline plate commencing left of the oral basket and then merging with the distinct postoral furrow containing the oral primordium; (iv) a flat ridge left of posterior portion of kinety 9. Cytoplasm colourless, contains about 10 μm-sized food vacuoles, in well-fed specimens studded with lipid droplets up to 5 μm across (Figs 1, 10, 11). Feeds on small flagellates, possibly also on bacteria.
Somatic cilia about 8 μm long in protargol preparations. Invariably nine somatic and four preoral ciliary rows with a total of 294 basal bodies on average (Table 1; Figs 1, 3, 5, 7, 9, 14–16). Kineties 2–5 and 7 bipolar, rows 1, 6, 8 and 9 shortened anteriorly and/or posteriorly. Kinety 1 extends at right margin of ventral side and ends underneath mid-body, composed of conspicuously widely spaced dikinetids and one monokinetid at posterior end; a wide break between fifth and sixth dikinetid; cilia of third dikinetid often lacking, anterior cilium of a few other dikinetids shortened or lacking in some specimens. Kineties 2 and 3 on right body side, fully ciliated, consist of narrowly spaced dikinetids in anterior portion, of widely spaced monokinetids in middle portion, and of narrowly spaced monokinetids in posterior region; kinety 3 commences with a single monokinetid. Kineties 4 and 5 limit dorsal margin of right and left body side, respectively; kinety 4 composed of narrowly spaced, ciliated monokinetids throughout; kinety 5 composed of widely spaced, ciliated monokinetids, forming pair-like pattern in anterior half. Kinety 6 on left body side, usually consisting of six widely spaced, ciliated monokinetids in second and third quarter of body; number very stable, i.e., of more than 100 specimens observed, only one showed eight monokinetids. Kinety 7 composed of widely spaced, ciliated monokinetids, forming pair-like pattern in anterior half; first and second pair obliquely arranged, usually dislocated to the left and then easily confused with kinety 6. Kinety 8 begins in second quarter of body, consists of three very widely spaced, ciliated monokinetids. Kinety 9 on ventral side of body, commences underneath adoral membranelles with 4–6 likely barren dikinetids, sometimes followed by one monokinetid, interrupted in mid-body (see postoral complex) and then extending to posterior body margin with an average of 13 ciliated monokinetids (Table 1; Figs 7, 8, 14, 15).
Four slightly oblique preoral kineties on ventral side, composed of ciliated dikinetids and some ciliated monokinetids at left end. Postoral complex as in L. costatus, i.e., composed of the monokinetidal posterior portion of preoral kinety 4 and the dikinetidal anterior portion of somatic kinety 9, as we know from the ontogenesis (paper in preparation); dikinetids widely spaced and obliquely arranged, first dikinetid usually slightly dislocated to the left (Table 1; Figs 1, 7–10, 14–16).
Oral apparatus conspicuous due to the very large oral basket, occupies anterior half of body within a deepened, fusiform oral field. Three narrowly spaced adoral membranelles obliquely arranged to main body axis between anterior half of oral basket and left body margin (Table 1; Figs 1, 8–10, 13–16). Membranelle 1 (M1) anterior of M2 and M3, composed of four barren basal bodies; membranelles 2 and 3 close together, distinctly larger than M1. Membranelle 3 larger than M2, each consisting of three rows of basal bodies with about 20 μm long cilia in vivo; individual rows composed of an average of four and five basal bodies in M2 and M3, respectively; right row of M2 and M3 barren. Right of M2 and M3 the barren group A basal bodies slightly dislocated anteriorly, forming an interrupted row; right of group A the minute, faintly impregnated B granules (basal bodies?), possibly belonging to the oral primordium or remnants of a paroral; anterior of M2 the group C basal bodies that belong to the postoral complex (Figs 6–8, 13).
Oral basket very conspicuous because long axis 14–19 μm wide in protargol preparations, occupying almost one third of body length; laterally flattened; extends to body midline, where it abruptly curves to dorsal posterior body end and nematodesmata become rather disordered (Table 1; Figs 1, 3, 7, 9, 13, 14). Nasse kinetosomes faintly impregnated with protargol, not at distal end of basket rods but subapically at base of rod angles, absent from left anterior region of basket opening, making a curl-like pattern at right end (Figs 4, 13–16). Oral primordium in postoral furrow, consists of two parts (Table 1; Figs 7, 8, 13–15): anterior part extends left and underneath of oral basket, forming a convex row composed of deeply impregnated granules (basal bodies?); posterior part right of somatic kinety 1 in laterally oriented specimens, composed of 3–4 partially ciliated dikinetids and one monokinetid at posterior end; fourth dikinetid, if present at all, left of row formed by other dikinetids.
Occurrence and ecology
As yet found only at type locality as described in “Diagnosis” section. We did not try to produce resting cysts. However, the species must be able to do this because the vegetative specimens were reactivated from air-dried soil.
Leptopharynx costatusMermod, 1914
Improved diagnosis (includes two subspecies and four morphs described in Table 4)
Table 4.
Comparison of main characteristics in four Leptopharynx costatus populations. Those features marked with a dot define distinct morphs of L. costatus.
| Characteristics | L. costatus costatus (Germany) | L. costatus costatus (Mexico) | L. costatus costatus (Austrian Alps) | L. costatus costatus (Brazil) | L. costatus gonohymen (Florida) |
|---|---|---|---|---|---|
| • Small morph with narrow oral basket | Present | Present | Present | Present | Present |
| • Large morph with wide oral basket | Presenta | Present | Absent | Not observed | Absent |
| Large morph with narrow oral basket | Absent | Absent | Does not apply | Not observed | Present |
| Adoral membranelle 1 in small morph with narrow oral basket | Absent | Absent | Absent | Absent | Absent |
| • Adoral membranelle 1 in large morph with wide oral basket | Present | Absent | Does not apply | Does not apply | Does not apply |
| Adoral membranelle 1 in large morph with narrow oral basket | Does not apply | Does not apply | Absent | Does not apply | Absent |
| Adoral membranelle 1 in dividers | Present | Present | Present | Not observed | Present |
| • Orientation of membranelles 2 and 3 | Flat | Flat | Flat | Flat | Right-angled |
| Average total number of basal bodies in small morph | 185b | 185b | 187 c | 186 | 162 |
| Average total number of basal bodies in large morph | 265b | 248b | Does not apply | Does not apply | 256 |
See Figs 33, 34.
From Table 3 in Omar and Foissner (2011).
Small and large specimens together. When they are separated, 155 and 202 basal bodies are obtained, respectively.
Size of small morphs (SMs) on average 31 × 22 μm (22–41 × 15–30 μm; six populations), that of large morphs (LMs) 44 × 29 μm (37–48 × 25–31 μm; three populations) in protargol preparations. Body outline elliptical to semidiscoidal with slightly to moderately oblique preoral region. Small morphs with nine somatic kineties, LMs with 9 or 10. Kineties 1, 2 and 3 with dikinetids anteriorly; kinety 1 consisting of narrowly spaced, ciliated dikinetids in SMs and of narrowly or widely spaced dikinetids in LMs; kinety 6 composed of two monokinetids; kinety 9 far underneath of adoral membranelles and without dikinetids; a total average of 162–187 and 248–265 basal bodies in SMs and LMs, respectively. Preoral kineties on ventral side, kinety 4 discontinuous. Postoral complex present. Two adoral membranelles in SMs, two or three in LMs, membranelles 2 and 3 flat or right-angled in LMs. Oral basket narrow in SMs and narrow or wide in LMs. Oral primordium left of kinety 1.
Subspecies assigned
Leptopharynx costatus costatus Mermod, 1914 (nominotypical subspecies); Leptopharynx costatus gonohymen nov. subspec.
Leptopharynx costatus costatusMermod, 1914 nov. stat.
Diagnosis
Nine somatic and four preoral kineties with an average of 181–187 basal bodies in SMs, while 9 or 10 kineties and 248–265 basal bodies in LMs. Kinety 1 consisting of narrowly spaced dikinetids in both morphs. Adoral membranelle 1 absent in SMs, while present or absent in LMs; membranelles 2 and 3 form a flat ciliary field. Oral basket on average 3–4 μm and 10 μm wide in SMs and LMs, respectively.
Type locality
In a moor in the surroundings of the village of Sainte-Croix (Jura Vaudois), Switzerland, E6°30′ N46°49′.
Type material
Not available. In a forthcoming study, we shall suggest neotypification with the German population mentioned by Foissner et al. (2011).
A Brazilian population of Leptopharynx costatus costatus (Table 1)
We found a small morph of L. costatus costatus in the slides containing L. brasiliensis. This population is highly similar to three other populations of L. costatus costatus from Europe (Foissner 1989 and below) and Mexico (Foissner et al. 2011), all having an average total number of 181–187 basal bodies.
Three voucher slides with protargol-impregnated specimens have been deposited at the same repository as L. brasiliensis. These slides are in the series typifying L. brasiliensis.
An Austrian population of Leptopharynx costatus costatus (Table 2)
Table 2.
Morphometric data on large specimens (upper line) and small specimens (lower line) of an alpine population of Leptopharynx costatus costatus.
| Characteristicsa | M | SD | SE | CV | Min | Max | n | % increaseb | |
|---|---|---|---|---|---|---|---|---|---|
| Body, length | 40.8 | 41.0 | 2.2 | 0.6 | 5.4 | 36.0 | 45.0 | 12 | 79.7 |
| 22.7 | 21.0 | 3.3 | 1.3 | 14.4 | 19.0 | 28.0 | 6 | ||
| Body, width | 30.2 | 30.0 | 1.5 | 0.4 | 4.9 | 27.0 | 32.0 | 12 | 101.3 |
| 15.0 | 14.0 | 2.8 | 1.1 | 18.4 | 13.0 | 20.0 | 6 | ||
| Body length: width, ratio | 1.4 | 1.4 | 0.1 | 0.1 | 3.0 | 1.3 | 1.4 | 12 | −6.6 |
| 1.5 | 1.5 | 0.1 | 0.1 | 5.9 | 1.4 | 1.6 | 6 | ||
| Anterior body end to adoral membranelles, distance | 12.8 | 13.0 | 1.1 | 0.3 | 8.7 | 11.0 | 15.0 | 12 | 120.7 |
| 5.8 | 6.0 | 1.3 | 0.5 | 22.8 | 4.0 | 8.0 | 6 | ||
| Body length: anterior body end to adoral membranelles, ratio | 3.2 | 3.1 | 0.3 | 0.1 | 7.9 | 2.9 | 3.7 | 12 | −20 |
| 4.0 | 3.9 | 0.5 | 0.2 | 12.5 | 3.5 | 4.8 | 6 | ||
| Anterior body end to macronucleus, distance | 13.6 | 13.0 | 1.3 | 0.4 | 9.7 | 11.0 | 16.0 | 12 | 63.8 |
| 8.3 | 8.0 | 1.0 | 0.4 | 12.4 | 7.0 | 1.0 | 6 | ||
| Anterior body end to excretory pore of contractile vacuole, distance | 20.4 | 20.0 | 1.9 | 0.6 | 9.2 | 18.0 | 23.0 | 9 | |
| Not recognizable | |||||||||
| Macronucleus, length | 9.2 | 9.0 | 0.8 | 0.2 | 9.1 | 8.0 | 10.0 | 12 | 58.6 |
| 5.8 | 6.0 | 0.8 | 0.3 | 12.9 | 5.0 | 7.0 | 6 | ||
| Macronucleus, width | 8.4 | 8.0 | – | – | – | 8.0 | 9.0 | 12 | 68 |
| 5.0 | 5.0 | 0.0 | 0.0 | 0.0 | 5.0 | 5.0 | 6 | ||
| Micronucleus, diameter | 2.1 | 2.0 | – | – | – | 2.0 | 3.0 | 12 | 16.6 |
| 1.8 | 1.8 | – | – | – | 1.5 | 2.0 | 6 | ||
| Oral basket, width | 3.4 | 3.0 | – | – | – | 3.0 | 4.0 | 12 | 21.4 |
| 2.8 | 3.0 | – | – | – | 2.0 | 3.0 | 6 | ||
| Somatic kineties, number | 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 12 | 0.0 |
| 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 6 | ||
| Somatic kinety 1, number of dikinetids | 7.0 | 7.0 | 0.0 | 0.0 | 0.0 | 7.0 | 7.0 | 12 | 0.0 |
| 7.0 | 7.0 | 0.0 | 0.0 | 0.0 | 7.0 | 7.0 | 6 | ||
| Somatic kinety 1, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 12 | 0.0 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 6 | ||
| Somatic kinety 2, number of dikinetids | 6.6 | 6.0 | 0.7 | 0.2 | 10.2 | 6.0 | 8.0 | 12 | 57.1 |
| 4.2 | 4.0 | – | – | – | 4.0 | 5.0 | 6 | ||
| Somatic kinety 2, number of monokinetids | 12.7 | 12.0 | 1.7 | 0.5 | 13.2 | 9.0 | 15.0 | 12 | 76.4 |
| 7.2 | 7.0 | 1.7 | 0.7 | 24.0 | 5.0 | 10.0 | 6 | ||
| Somatic kinety 3, number of dikinetids | 4.3 | 4.0 | 0.9 | 0.3 | 20.5 | 3.0 | 6.0 | 12 | 53.6 |
| 2.8 | 3.0 | – | – | – | 2.0 | 3.0 | 6 | ||
| Somatic kinety 3, number of monokinetids | 25.2 | 26.0 | 3.1 | 0.9 | 12.2 | 19.0 | 29.0 | 12 | 93.8 |
| 13.0 | 11.0 | – | – | – | 7.0 | 24.0 | 6 | ||
| Somatic kinety 4, number of monokinetids (does not have dikinetids) | 42.1 | 42.0 | 3.6 | 1.0 | 8.4 | 37.0 | 48.0 | 12 | 45.2 |
| 29.0 | 27.0 | 5.7 | 2.3 | 19.5 | 23.0 | 36.0 | 6 | ||
| Somatic kinety 5, number of monokinetids (does not have dikinetids) | 13.0 | 13.0 | 1.2 | 0.4 | 9.3 | 12.0 | 16.0 | 12 | 30.0 |
| 10.0 | 10.0 | 1.3 | 0.5 | 12.7 | 8.0 | 12.0 | 6 | ||
| Somatic kinety 6, number of monokinetids (does not have dikinetids) | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 12 | 0.0 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 6 | ||
| Somatic kinety 7, number of monokinetids (does not have dikinetids) | 10.2 | 10.0 | 0.8 | 0.2 | 8.2 | 9.0 | 12.0 | 12 | 9.7 |
| 9.3 | 9.0 | 0.5 | 0.2 | 5.5 | 9.0 | 10.0 | 6 | ||
| Somatic kinety 8, number of monokinetids (does not have dikinetids) | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 12 | 0.0 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 6 | ||
| Somatic kinety 9, number of monokinetids in posterior segment | 7.3 | 7.0 | 1.2 | 0.4 | 16.8 | 6.0 | 9.0 | 12 | 32.7 |
| 5.5 | 5.0 | 1.2 | 0.5 | 22.3 | 5.0 | 8.0 | 6 | ||
| Preoral ciliary rows, number | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 12 | 0.0 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 6 | ||
| Preoral kinety 1, number of dikinetids (does not have monokinetids) | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 12 | 0.0 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 6 | ||
| Preoral kinety 2, number of dikinetids | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 12 | 0.0 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 6 | ||
| Preoral kinety 2, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 12 | 0.0 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 6 | ||
| Preoral kinety 3, number of dikinetids | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 12 | 0.0 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 6 | ||
| Preoral kinety 3, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 12 | 0.0 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 6 | ||
| Preoral kinety 4, number of dikinetids (for monokinetids, see postoral complex) | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 12 | 0.0 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 6 | ||
| Oral primordium, number of dikinetids in posterior part | 4.3 | 4.0 | 0.5 | 0.1 | 11.4 | 4.0 | 5.0 | 12 | 22.9 |
| 3.5 | 3.0 | 0.6 | 0.2 | 15.7 | 3.0 | 4.0 | 6 | ||
| Oral primordium, number of monokinetids in posterior part | 0.9 | 1.0 | – | – | – | 0.0 | 0.1 | 12 | 200 |
| 0.3 | 0.0 | – | – | – | 0.0 | 1.0 | 6 | ||
| Adoral membranelle 2, number of basal body rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 12 | 0.0 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 6 | ||
| Adoral membranelle 2, number of basal bodies | 12.0 | 12.0 | 0.0 | 0.0 | 0.0 | 12.0 | 12.0 | 12 | 0.0 |
| 12.0 | 12.0 | 0.0 | 0.0 | 0.0 | 12.0 | 12.0 | 6 | ||
| Adoral membranelle 3, number of basal body rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 12 | 0.0 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 6 | ||
| Adoral membranelle 3, number of basal bodies | 12.0 | 12.0 | 0.0 | 0.0 | 0.0 | 12.0 | 12.0 | 12 | 0.0 |
| 12.0 | 12.0 | 0.0 | 0.0 | 0.0 | 12.0 | 12.0 | 6 | ||
| Left row of postoral complex, number of monokinetids c | 6.1 | 6.0 | – | – | – | 6.0 | 7.0 | 12 | 1.6 |
| 6.0 | 6.0 | 0.0 | 0.0 | 0.0 | 6.0 | 6.0 | 6 | ||
| Right row of postoral complex, number of dikinetids d | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 12 | 0.0 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 6 | ||
| Basal bodies, total number e | 201.9 | 202.0 | 10.5 | 3.0 | 5.2 | 183.0 | 218.0 | 12 | 30.0 |
| 155.3 | 150.0 | 15.1 | 6.2 | 9.7 | 144.0 | 185.0 | 6 | ||
Data based on mounted, protargol-impregnated, and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. CV, coefficient of variation in %; M, median; Max, maximum; Min, minimum; n, number of specimens investigated; SD, standard deviation; SE, standard error of mean; , arithmetic mean.
The increase in the mean value for the large specimens relative to the small specimens.
Without basal bodies of group C.
This is the anterior segment of somatic kinety 9.
Except of basal bodies of adoral membranelles.
This population is from the same area as that studied by Foissner (1989), i.e., from an about 2000 m high mountain (Stubnerkogel) in the outskirts of the village of Gastein. At first glance, the alpine specimens appear as a polymorphic population of L. costatus costatus because of the high size variability: 19–45 μm × 13–32 μm including many lengths in between, e.g., 28 μm, 36 μm, and 40 μm. However, all specimens are narrow-mouthed with the oral basket 2–4 μm wide. Furthermore, we calculated the total number of basal bodies for small (<30 μm) and large (>30 μm) specimens separately, obtaining 155 and 202. When the average of these counts is calculated, 187 is obtained, matching perfectly the number of other populations of L. costatus costatus with narrow oral basket.
One voucher slide with protargol-impregnated specimens has been deposited at the same repository as L. brasiliensis. This slide also contains vouchers for Dimacrocaryon amphileptoides amphileptoides and Microdileptus breviproboscis, both described in a forthcoming study (Vďačný and Foissner, submitted).
Leptopharynx costatus gonohymen Foissner and Omar nov. subspec. (Figs 17–32, 35–40; Table 3)
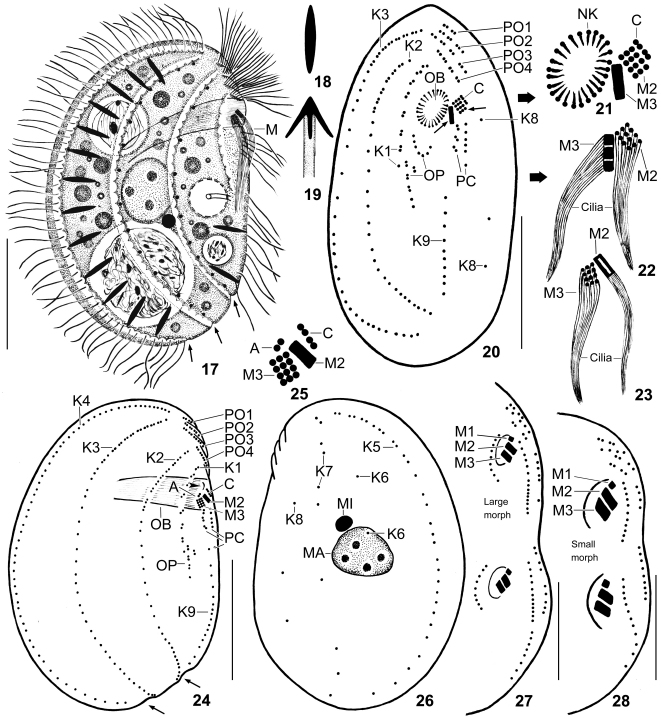
Figs 17–28.
Leptopharynx costatus gonohymen from life (17–19) and after protargol impregnation (20–28). 17. Right side view of a representative specimen of the large morph, length 55 μm. Note the deep furrows right of kineties 2–4 and the narrow oral basket. Arrows mark notches formed by the furrows of the right side. 18, 19. A resting (5–6 μm) and an exploded extrusome. 20–22. Ventral view of a paratype specimen of the large morph. Note the right-angled M2 and M3 (small arrows and (21, 22)) and the broadly elliptical oral basket opening. 23–26. Right and left side view (24, 26) and adoral membranelles (23, 25) of the hapantotype specimen for the large morph, length 53 μm. Arrows mark notches formed by the right side furrows. Arrowhead denotes a line produced by the nasse kinetosomes. Note the narrow oral basket and the right-angled M2 and M3, forming a triangular space in between. Compared to the ventral view (20, 21), the lateral view shows M2 from the narrow side and M3 from the wide side. 27, 28. When dividing, both morphs show three adoral membranelles in proter and opisthe. A, group A basal bodies; C, group C basal bodies; K1–9, somatic kineties; M(1–3), adoral membranelles; MA, macronucleus; MI, micronucleus; NK, nasse kinetosomes; OB, oral basket; OP, oral primordium; PC, postoral complex; PO1–4, preoral kineties. Scale bars 20 μm (17, 24, 26), 15 μm (20, 27), and 10 μm (28).
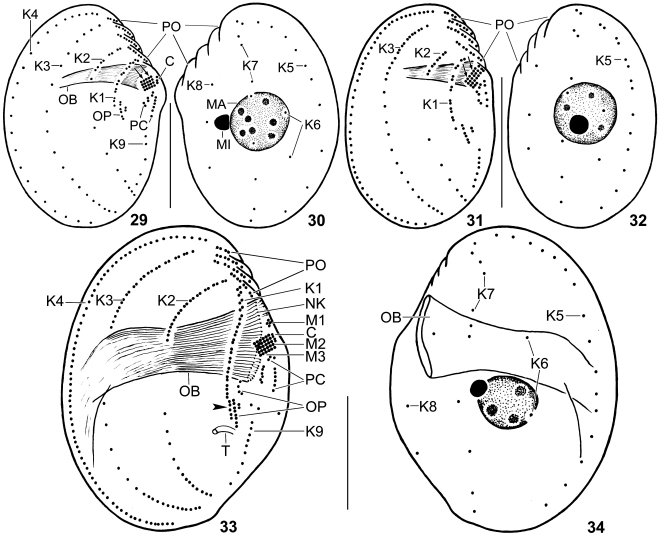
Figs 29–34.
Leptopharynx costatus gonohymen(29–32) and Leptopharynx costatus costatus(33, 34) after protargol impregnation. 29, 30. Right and left side view of the hapantotype specimen for the small morph (length 32 μm), which looks like the small morph of L. costatus costatus. Note the moderately oblique preoral region; the absence of dikinetids in kinety 3; and the flat orientation of M2 and M3 (arrows). 31, 32. Right and left side view of a small (26 μm) specimen. Although being small, this could be a transition stage to the large morph because it has dikinetids in kinety 3, pair-like monokinetids in kinety 5, and a less oblique preoral area. 33, 34. Right and left side view of large morph of the German population of L. costatus costatus, showing the wide oral basket, the flat M2 and M3, the presence of M1, and the slightly dislocated posterior kinetids of K1 (arrowhead). C, group C basal bodies; K1–9, somatic kineties; M1–3, adoral membranelles; MA, macronucleus; MI, micronucleus; NK, nasse kinetosomes; OB, oral basket; OP, oral primordium; PC, postoral complex; PO, preoral kineties; T, excretory tube. Scale bars 15 μm.
Figs 35–40.
Leptopharynx costatus gonohymen after protargol impregnation. 35, 36. Right side view of the hapantotype specimen for the large morph. Note the right-angled M2 and M3, shown at higher magnification in (36). Arrowheads denote the narrow oral basket. 37. Ventral view of a paratype specimen, showing the right-angled M2 and M3, the broadly elliptical oral basket opening, the widely spaced kinetids of kinety 1, and the preoral kineties. Arrowheads mark the monokinetids at end of preoral kineties 2 and 3. 38. Right side view of the hapantotype specimen for the small morph, having the same body shape and oral apparatus (flat adoral membranelles) as the small morph of L. costatus costatus. 39. Right side view of a divider of the small morph, showing the three adoral membranelles in both proter and opisthe. 40. Right side view of a large morph specimen. Although having a narrow oral basket, it can ingest large prey, viz., Colpoda inflata. Note the anteriorly dislocated macronucleus. C, group C basal bodies; K1–4, 9, somatic kineties; LD, lipid droplets; M(1–3), adoral membranelles; MA, macronucleus; MC, membranellar cilia; MI, micronucleus; N, macronucleus of prey ciliate, Colpoda inflata; NK, nasse kinetosomes; OB, oral basket; OP, oral primordium; PC, postoral complex; PO(1–4), preoral kineties. Scale bars 20 μm (35, 40), 15 μm (38, 39), and 5 μm (36, 37).
Table 3.
Morphometric data on large morph (upper line) and small morph (lower line) of Leptopharynx costatus gonohymen.
| Characteristicsa | M | SD | SE | CV | Min | Max | n | % increaseb | |
|---|---|---|---|---|---|---|---|---|---|
| Body, length | 46.2 | 46.0 | 5.8 | 1.3 | 12.5 | 37.0 | 57.0 | 21 | 68.0 |
| 27.5 | 27.0 | 3.3 | 0.8 | 12.1 | 23.0 | 33.0 | 19 | ||
| Body, width | 31.5 | 31.0 | 4.8 | 1.1 | 15.2 | 24.0 | 43.0 | 21 | 59.9 |
| 19.7 | 20.0 | 3.3 | 0.8 | 16.7 | 13.0 | 25.0 | 19 | ||
| Body length: width, ratio | 1.5 | 1.5 | 0.1 | 0.1 | 5.9 | 1.3 | 1.6 | 21 | 7.1 |
| 1.4 | 1.4 | 0.1 | 0.1 | 9.9 | 1.2 | 1.8 | 19 | ||
| Anterior body end to adoral membranelles, distance | 10.4 | 10.0 | 1.5 | 0.3 | 14.8 | 8.0 | 13.0 | 21 | 44.4 |
| 7.2 | 7.0 | 1.7 | 0.5 | 23.2 | 5.0 | 10.0 | 11 | ||
| Body length: anterior body end to adoral membranelles, ratio | 4.5 | 4.4 | 0.5 | 0.1 | 10.2 | 3.8 | 5.3 | 21 | 12.5 |
| 4.0 | 3.7 | 1.0 | 0.3 | 25.7 | 2.8 | 6.6 | 11 | ||
| Anterior body end to macronucleus, distance | 17.9 | 17.0 | 2.8 | 0.6 | 15.9 | 13.0 | 23.0 | 21 | 75.5 |
| 10.2 | 10.0 | 1.5 | 0.4 | 14.5 | 8.0 | 13.0 | 11 | ||
| Anterior body end to excretory pore of contractile vacuole, distance | 23.7 | 24.0 | 2.6 | 0.6 | 11.0 | 18.0 | 28.0 | 21 | – |
| Not recognizable | |||||||||
| Macronucleus, length | 9.5 | 10.0 | 1.3 | 0.3 | 13.9 | 8.0 | 13.0 | 21 | 33.8 |
| 7.1 | 7.0 | 1.1 | 0.3 | 16.0 | 5.0 | 9.0 | 11 | ||
| Macronucleus, width | 7.9 | 8.0 | 1.3 | 0.3 | 16.0 | 5.0 | 10.0 | 21 | 19.7 |
| 6.6 | 6.0 | 0.9 | 0.3 | 14.3 | 5.0 | 8.0 | 11 | ||
| Micronucleus, diameter | 2.2 | 2.0 | – | – | – | 1.0 | 3.0 | 21 | 10.0 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 11 | ||
| Oral basket, width | 5.2 | 5.0 | 0.6 | 0.1 | 11.6 | 4.0 | 6.0 | 21 | 73.3 |
| 3.0 | 3.0 | 0.5 | 0.1 | 14.9 | 2.0 | 4.0 | 11 | ||
| Somatic kineties, number | 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 21 | 0.0 |
| 9.0 | 9.0 | 0.0 | 0.0 | 0.0 | 9.0 | 9.0 | 11 | ||
| Somatic kinety 1, number of dikinetids | 7.1 | 7.0 | 0.2 | 0.1 | 3.1 | 7.0 | 8.0 | 21 | 1.4 |
| 7.0 | 7.0 | 0.0 | 0.0 | 0.0 | 7.0 | 7.0 | 11 | ||
| Somatic kinety 1, number of monokinetids | 1.0 | 1.0 | – | – | – | 0.0 | 2.0 | 21 | 0.0 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 11 | ||
| Somatic kinety 2, number of dikinetids | 7.9 | 8.0 | 1.2 | 0.3 | 15.2 | 6.0 | 10.0 | 21 | 102.6 |
| 3.9 | 4.0 | 0.9 | 0.3 | 24.2 | 2.0 | 6.0 | 11 | ||
| Somatic kinety 2, number of monokinetids | 18.7 | 18.0 | 2.9 | 0.6 | 15.4 | 13.0 | 24.0 | 21 | 128.0 |
| 8.2 | 8.0 | 1.5 | 0.5 | 18.8 | 6.0 | 12.0 | 11 | ||
| Somatic kinety 3, number of dikinetids | 7.8 | 8.0 | 1.2 | 0.3 | 15.5 | 6.0 | 11.0 | 21 | 457.1 |
| 1.4 | 1.0 | – | – | – | 0.0 | 5.0 | 11 | ||
| Somatic kinety 3, number of monokinetids | 31.0 | 31.0 | 4.2 | 0.9 | 13.7 | 24.0 | 38.0 | 21 | 66.7 |
| 18.6 | 19.0 | 4.6 | 1.4 | 24.8 | 11.0 | 28.0 | 11 | ||
| Somatic kinety 4, number of monokinetids (does not have dikinetids) | 57.0 | 56.0 | 6.3 | 1.4 | 11.1 | 48.0 | 71.0 | 21 | 93.9 |
| 29.4 | 27.0 | 6.6 | 1.5 | 22.4 | 20.0 | 46.0 | 19 | ||
| Somatic kinety 5, number of monokinetids (does not have dikinetids) | 28.1 | 28.0 | 4.5 | 1.0 | 16.0 | 23.0 | 40.0 | 21 | 140.2 |
| 11.7 | 12.0 | 1.9 | 0.6 | 16.2 | 8.0 | 14.0 | 11 | ||
| Somatic kinety 6, number of monokinetids (does not have dikinetids) | 2.2 | 2.0 | – | – | – | 2.0 | 4.0 | 21 | 4.8 |
| 2.1 | 2.0 | 0.3 | 0.1 | 14.4 | 2.0 | 3.0 | 11 | ||
| Somatic kinety 7, number of monokinetids (does not have dikinetids) | 10.2 | 10.0 | 0.5 | 0.1 | 5.0 | 9.0 | 11.0 | 21 | 4.0 |
| 9.8 | 10.0 | 0.4 | 0.1 | 4.1 | 9.0 | 10.0 | 11 | ||
| Somatic kinety 8, number of monokinetids (does not have dikinetids) | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 | −3.2 |
| 3.1 | 3.0 | 0.3 | 0.1 | 9.8 | 3.0 | 4.0 | 11 | ||
| Somatic kinety 9, number of monokinetids in posterior segment | 10.1 | 10.0 | 1.8 | 0.4 | 17.7 | 8.0 | 16.0 | 21 | 74.1 |
| 5.8 | 6.0 | 0.8 | 0.2 | 12.9 | 5.0 | 7.0 | 11 | ||
| Preoral ciliary rows, number | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | 0.0 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 11 | ||
| Preoral kinety 1, number of dikinetids (does not have monokinetids) | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | 0.0 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 11 | ||
| Preoral kinety 2, number of dikinetids | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 | 0.0 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 11 | ||
| Preoral kinety 2, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 | 0.0 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 11 | ||
| Preoral kinety 3, number of dikinetids | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | 0.0 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 11 | ||
| Preoral kinety 3, number of monokinetids | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 | 0.0 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 11 | ||
| Preoral kinety 4, number of dikinetids (for monokinetids, see postoral complex) | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 | 0.0 |
| 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 11 | ||
| Oral primordium, number of dikinetids in posterior part | 4.1 | 4.0 | – | – | – | 4.0 | 5.0 | 21 | 7.9 |
| 3.8 | 4.0 | – | – | – | 3.0 | 4.0 | 11 | ||
| Oral primordium, number of monokinetids in posterior part | 1.1 | 1.0 | – | – | – | 1.0 | 2.0 | 21 | – |
| Not present | |||||||||
| Oral primordium, number of granules (basal bodies?) in anterior part | 4.8 | 5.0 | 0.9 | 0.3 | 18.2 | 4.0 | 6.0 | 11 | – |
| Present but too faintly impregnated | |||||||||
| Adoral membranelle 2, number of basal body rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 | 25.0 |
| 2.4 | 2.0 | – | – | – | 2.0 | 3.0 | 11 | ||
| Adoral membranelle 3, number of basal body rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 | 0.0 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 11 | ||
| Left row of postoral complex, number of monokinetids c | 6.1 | 6.0 | – | – | – | 6.0 | 7.0 | 21 | 3.4 |
| 5.9 | 6.0 | – | – | – | 5.0 | 6.0 | 11 | ||
| Right row of postoral complex, number of dikinetids d | 3.1 | 3.0 | – | – | – | 3.0 | 4.0 | 21 | 3.3 |
| 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 11 | ||
| Basal bodies, total number e | 256.2 | 255.0 | 20.3 | 4.4 | 7.9 | 231.0 | 302.0 | 21 | 58.5 |
| 161.6 | 160.0 | 10.1 | 3.1 | 6.3 | 148.0 | 180.0 | 11 | ||
Data based on mounted, protargol-impregnated, and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. CV, coefficient of variation in %; M, median; Max, maximum; Min, minimum; n, number of specimens investigated; SD, standard deviation; SE, standard error of mean; , arithmetic mean.
The increase in the mean value for the large morph relative to the small morph.
Without basal bodies of group C.
This is the anterior segment of somatic kinety 9.
Except of basal bodies of adoral membranelles.
Diagnosis
Nine somatic and four preoral ciliary rows with an average of 162 and 256 basal bodies in SMs and LMs, respectively. Kinety 1 consisting of widely spaced dikinetids in LMs. Adoral membranelle 1 absent in SMs and LMs; membranelles 2 and 3 right-angled to each other in LMs. Oral basket on average 3 and 5 μm wide in SMs and LMs, respectively.
Type locality
Leaves, soil and lichens from middle and southern Florida, USA (for details, see “Occurrence and ecology” section).
Type material
A hapantotype and six paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Museum of Upper Austria, Linz (LI). The hapantotypes (one each for the small and large morph on the same slide) and important paratype specimens have been marked by black ink circles on the coverslip.
Etymology
Composite of the Greek substantive he gonía (angle), the thematic vowel ·o-, and the Greek noun hymen (membrane), referring to the angled adoral membranelles.
Description
Small and large morphs of this subspecies are distinguished mainly by the arrangement of the adoral membranelles: flat vs. right-angled. In most other features, it is highly similar to L. costatus costatus, as described by Kahl (1931), Prelle (1961), and Foissner (1989). Thus, we do not provide a full description but emphasize some additional observations, the data in Table 3, and the figures.
-
(i)
Size of SMs in vivo about 35 × 25 μm, while about 55 × 35 μm in LMs, with an extreme of up to 70 μm as calculated from some in vivo measurements and the data shown in Table 3.
-
(ii)
Right body surface of LMs with three deep furrows accompanying kineties 2, 3 and 4 (Fig. 17).
-
(iii)
In spite of the narrow oral basket (up to 5 μm), the LMs contain up to 40 μm-sized food vacuoles with almost undigested ciliates, e.g., Colpoda inflata (Fig. 40).
-
(iv)
Invariably nine somatic and four preoral ciliary rows with a total of 162 and 256 basal bodies on average in SMs and LMs, respectively (Table 3). Dikinetids of kinety 1 widely spaced. Kinety 3 commences with a single monokinetid and lacks dikinetids in some SM specimens. Monokinetids of kinety 5 more or less pair-like arranged in anterior half of LMs and in some transition specimens. Kinety 6 composed of two, very rarely of three or four widely spaced, ciliated monokinetids in mid-body, sometimes in anterior or posterior half of cell (Table 3; Figs 20, 24, 26, 29–32, 35, 37).
-
(v)
Adoral membranelle 1 absent from both SMs and LMs, but present in dividers (Figs 27, 28, 39); M2 and M3 form a flat ciliary field in the SMs, while right-angled and forming a triangular space in between in the LMs. Membranelle 2 composed of 2 or 3 and 3 rows of basal bodies in SMs and LMs, respectively; M3 consists of three rows with about 15 μm long cilia. Right of M2 and M3 the barren group A basal bodies slightly dislocated anteriorly, forming a short, sometimes interrupted row in some LM specimens; anterior of M2 the group C basal bodies, consisting of two dikinetids or one monokinetid and one dikinetid, belonging to the postoral complex (Fig. 25). Oral basket 3 μm and 5 μm wide on average in SMs and LMs, respectively; extends to dorsal side of cell, does not curve posteriorly; 8 μm wide in one out of 100 specimens (Table 3; Figs 17, 20–25, 27–29, 31, 35–40).
Occurrence and ecology
Leptopharynx costatus gonohymen was found in soil samples from Florida, USA. One sample contained a mixture of leaves, soil and lichens from beneath a tree in the surroundings of the town of St. Petersburg; the other sample was a mixture of leaves, bark and soil from beneath a tree in the surroundings of the town of St. Augustine. Both samples were small and thus united. Accordingly, the type locality cannot be fixed exactly.
Discussion
Comparison of Leptopharynx brasiliensis with similar species
We discovered L. brasiliensis in a soil sample containing a small morph of L. costatus Mermod, 1914 (Table 1), suggesting that the larger and wide-mouthed cells could be a large morph of L. costatus. However, this can be excluded for the following reasons (Foissner et al. 2011; Omar and Foissner, 2011): (i) the absence of transitions in all main features of more than 200 silver-impregnated specimens from both species; (ii) the number of monokinetids in kinety 6 (6 in L. brasiliensis vs. 2 in the small and large morph of L. costatus); (iii) the widely (vs. narrowly) spaced kinetids in kinety 1 (cp. Figs 7, 33; Table 5); (iv) the distinctly (vs. slightly to moderately) oblique preoral region; and (v) the location of the oral primordium (right vs. left of kinety 1).
Table 5.
Comparison of the number and spacing of kinetids in kinety 1 in the large morphs of L. costatus gonohymen and the German population of L. costatus costatus.
| Characteristics | L. costatus gonohymena | L. costatus costatusb |
|---|---|---|
| Body, length | 46.2 (37–57) | 38.7 (30–45) |
| Kinety 1, number of dikinetids | 7.1 (7–8) | 13.3 (11–15) |
| Kinety 1, adapted number of dikinetids c | 8.5 | 13.3 |
| Kinety 1, number of monokinetids | 1.0 | 1.6 (0–2) |
| Kinety 1, length | 13.8 (12–16) | 16.5 (12–20) |
| Body, adapted length d | 8.5 | 16.0 |
Data from Table 2.
Data based on 15 mounted, protargol-impregnated, and randomly selected large morph specimens from a pure culture. Numbers in parentheses are extreme values.
Number of dikinetids when kinety length is adapted to that of L. costatus costatus.
Number of dikinetids when body length of L. costatus costatus is adapted to that of L. costatus gonohymen.
Leptopharynx brasiliensis differs from L. australiensis Omar and Foissner, 2011 mainly in body size (60 × 40 μm vs. 40 × 25 μm), the number of monokinetids in kinety 6 (6 vs. 2), and the total number of basal bodies (294 vs. 184). Another curious feature, viz., the group B granules right of the adoral membranelles 2 and 3, is present in both species, while absent in L. bromelicola, L. bromeliophilus, and L. costatus, as verified by the investigation of the type slides.
Both, L. australiensis and L. brasiliensis have a conspicuous feature in common, viz., a strongly receding and thus long and prominent preoral region. This feature separates both from L. eurystoma (Kahl, 1931) Foissner et al., 2011 and L. euglenivorus Kahl, 1926, both having an only slightly receding preoral portion. Possibly, there are further, as yet unknown differences because both have not yet been redescribed with modern methods. See Omar and Foissner (2011) for further considerations. Leptopharynx brasiliensis is also similar to the African L. macrostoma Njiné, 1979. They differ mainly in the number of monokinetids in kinety 6 (6 vs. 2), the dikinetids in kinety 4 (absent vs. present), the shape of the preoral region (distinctly vs. slightly oblique), and the total number of basal bodies (294 vs. 406, as counted from the figures provided by Njiné 1979).
In one of more than 100 L. brasiliensis specimens, the oral basket was as small as in the small morph of L. costatus, indicating that L. brasiliensis can produce small-mouthed cells; this specimen was also distinctly smaller, i.e., 33 × 23 μm, and thus matched well the Hungarian L. stenostomatus, which Gellért (1942) discovered in the green algal cover of bark. However, L. stenostomatus has kinety 1 densely ciliated, while that of L. brasiliensis is very loosely ciliated. Although it cannot be excluded that the wide spacing is a stretching effect in the large L. brasiliensis, it is unlikely because the large morph of L. costatus has the kinetids of kinety 1 much more narrowly spaced than the small morph (Figs 24, 29, 31, 33). Thus and because both occur in different habitats and biogeographic regions, we find it unlikely that L. stenostomatus is the small morph of L. brasiliensis.
Leptopharynx costatus
The present and former investigations (Foissner et al. 2011; Omar and Foissner, 2011) showed that L. costatus makes four morphs described in Table 4. We studied four populations of L. costatus costatus, all being similar in having a small morph with narrow oral basket and a total of 181–187 basal bodies on average. Likewise, the large morphs, if present, are similar in the total number of basal bodies, i.e., 248–265 on average. Thus, the average total number of basal bodies is an important feature for recognizing Leptopharynx species, as already suggested by Omar and Foissner (2011).
The alpine population looks like a fifth morph of L. costatus costatus because it contains up to 45 μm long specimens with narrow oral basket. However, on average the population has the same total number (187) of basal bodies as other small morphs of L. costatus costatus, suggesting that the high size variability (19–45 × 13–32 μm) is a phenotypic character activated under certain environmental conditions.
The alpine population is highly similar to L. costatus gonohymen in having large specimens with narrow oral basket (Table 4). However, the large specimens of the alpine population differ from the large morph of L. costatus gonohymen by the orientation of adoral membranelles 2 and 3 (forming a flat field vs. right-angled) and the total number of basal bodies (187 vs. 256).
The subspecies Leptopharynx costatus gonohymen
The Floridian population is unique within the L. costatus-complex and within the genus (as far as detailed data are available) in having right-angled adoral membranelles 2 and 3. Further, the morphometric data show that the spacing and number of the dikinetids of kinety 1 is quite different in the large morphs of L. costatus costatus and L. costatus gonohymen (Table 5). Thus, we consider the Floridian population as a distinct subspecies, even if the small morph lacks these features and is thus indistinguishable from the small morph of L. costatus costatus, although there is some indication that the average total number of basal bodies is different: 162 vs. 181–187.
To overcome identification problems, we suggest applying the “complex terminology”, i.e., to use “Leptopharynx costatus-complex” for all populations that were not tested for the occurrence of a large morph with angled adoral membranelles.
Acknowledgements
Financial support was provided by the Austrian Science Fund (FWF, project P20360-B17). The technical assistance of Robert Schörghofer, Andreas Zankl, and Mag. Barbara Harl is gratefully acknowledged. Special thanks to Miss Gerlinde Fischer for collecting samples from Florida, USA, and to Mag. Birgit Weissenbacher and Mag. Maria Pichler for collecting the Brazilian sample. Thanks also to Mag. Remigius Geiser for help with nomenclature.
References
- Alekperov I.K. Free-living ciliates in the soils of St. Petersburg parks (Protozoa) Zoosyst. Rossica. 1993;2:13–28. [Google Scholar]
- Corliss J.O. 2nd ed. Pergamon Press; Oxford/New York/Toronto/Sydney/Paris/Frankfurt: 1979. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. [Google Scholar]
- Foissner W. Morphologie und Infraciliatur einiger neuer und wenig bekannter terrestrischer und limnischer Ciliaten (Protozoa, Ciliophora) Sber. Akad. Wiss. Wien. 1989;196:173–247. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Eur. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. Eur. J. Protistol. 1998;34:195–235. [Google Scholar]
- Foissner W. Biogeography and dispersal of micro-organisms: a review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- Foissner W., Berger H., Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems – Band III: Hymenostomata, Prostomatida, Nassulida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1994;1/94:1–548. [Google Scholar]
- Foissner W., Agatha S., Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W., Wolf K., Yashchenko V., Stoeck T. Description of Leptopharynx bromelicola n. sp. and characterization of the genus Leptopharynx Mermod, 1914 (Protista, Ciliophora) J. Eukaryot. Microbiol. 2011;58:134–151. doi: 10.1111/j.1550-7408.2011.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellért J. Életegyüttes a fakéreg zöldporos bevonatában. Acta Sci. math. -nat. Univ. Kolozsvár. 1942;8:1–36. [Google Scholar]
- Kahl A. Neue und wenig bekannte Formen der holotrichen und heterotrichen Ciliaten. Arch. Protistenk. 1926;55:197–438. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt Dtl. 1931;21:181–398. [Google Scholar]
- Lynn D.H. 3rd ed. Springer; Dordrecht: 2008. The Ciliated Protozoa, Characterization, Classification, and Guide to the Literature. [Google Scholar]
- Mayr E. Harvard University Press; Cambridge: 1963. Animal Species and Evolution. [Google Scholar]
- Mermod G. Recherches sur la faune infusoriennes des tourbiéres et des eaux voisines de Sainte-Croix (Jura vaudois) Revue suisse Zool. 1914;22:31–114. [Google Scholar]
- Njiné T. Structure et morphogenése buccales du cilié Leptopharynx (Mermod, 1914) Protistologica. 1979;15:459–465. [Google Scholar]
- Omar A., Foissner W. Description of Leptopharynx bromeliophilus nov. spec. and Leptopharynx australiensis nov. spec. (Ciliophora, Nassulida) Acta Protozool. 2011;50:89–103. [Google Scholar]
- Prelle A. Contribution a l’étude de Leptopharynx costatus (Mermod) (infusoire cilié) Bull. Biol. Fr. Belg. 1961;95:731–752. [Google Scholar]
- Thompson J.C. Ciliated protozoa of the Antarctic Peninsula. Antarct. Res. Ser. 1972;20:261–288. [Google Scholar]