Abstract
Lipolysis is defined as the catabolism of triacylglycerols stored in cellular lipid droplets. Recent discoveries of essential lipolytic enzymes and characterization of numerous regulatory proteins and mechanisms have fundamentally changed our perception of lipolysis and its impact on cellular metabolism. New findings that lipolytic products and intermediates participate in cellular signaling processes and that “lipolytic signaling” is particularly important in many nonadipose tissues unveil a previously underappreciated aspect of lipolysis, which may be relevant for human disease.
Main Text
From Fat Ferments to Lipases: A Short Historical Overview
Lipolysis describes the hydrolysis of triacylglycerols (TGs), commonly referred to as fat. In the mid-19th century the great French physiologist Claude Bernard (Bernard, 1856) noted that the pancreatic juice of mammals was able to efficiently degrade fat in the form of butter and oil. His observation led to the partial characterization of a pancreatic fat-splitting ferment by Balser (Balser, 1882), Langerhans (Langerhans, 1890), and Flexner (Flexner, 1897). The importance of lipolysis to general metabolism became apparent when Whitehead (Whitehead, 1909) discovered that fat (TGs) could not enter cells in its unhydrolyzed form. The absolute requirement for TG hydrolysis for the cellular uptake or release of fatty acids (FAs) and glycerol defines three processes in vertebrate physiology where lipolysis is essential: gastrointestinal lipolysis mediates the catabolism of dietary fat; vascular lipolysis is responsible for the hydrolysis of lipoprotein-associated TGs in the blood; and intracellular lipolysis catalyzes the breakdown of TGs stored in intracellular lipid droplets (LDs) for subsequent export of FAs (from adipose tissue) or their metabolism (in nonadipose tissues).
Although fundamental aspects of lipolysis were understood early on, it took more than a century to identify, isolate, and characterize the main fat-splitting ferments, which have been called lipases since 1900. The chief lipolytic enzymes of the gastrointestinal tract are lingual lipase, gastric lipase, pancreatic lipase, and pancreatic lipase-related proteins 1, 2, and 3. Vascular TG hydrolysis depends on lipoprotein lipase (LPL) and hepatic TG lipase. Pancreatic and vascular lipases are structurally related and prototypic for the pancreatic lipase family. Intracellular lipolysis of TGs involves neutral (pH-optimum around pH 7) and acid lipases present in lysosomes (pH optimum between pH 4–5). Well-characterized neutral TG hydrolases include adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), whereas lysosomal acid lipase (LAL) is the most important lipase in lysosomes. Besides an active serine site, these enzymes share no obvious structural homologies and are unrelated to the pancreatic lipase family.
Lipolysis is the catabolic branch of the FA cycle that provides FAs in times of metabolic need and removes them when they are present in excess. FAs are essential as substrates for energy production and the synthesis of most lipids, including membrane lipids and lipids involved in cellular signaling. Accordingly, all uni- and multicellular organisms are able to synthesize FAs de novo (endogenous FAs) from carbohydrate and/or protein metabolites. Despite their fundamental physiological importance, an oversupply of FAs is highly detrimental. Increased concentrations of nonesterified FAs disrupt the integrity of biological membranes, alter cellular acid-base homeostasis, and elicit the generation of harmful bioactive lipids. These effects, in turn, impair membrane function and induce endoplasmic reticulum (ER) stress, mitochondrial dysfunction, inflammation, and cell death. Collectively, these deleterious effects are subsumed under the term lipotoxicity (Unger et al., 2010). As a countermeasure, essentially all cells are able to detoxify nonesterified FAs by esterification with glycerol to yield inert TGs. Additionally, higher organisms store FAs in a specialized organ (i.e., adipose tissue), which supplies FAs to other high-demand tissues, such as liver and muscle (exogenous FAs). The carefully regulated balance of FA esterification and TG hydrolysis creates an efficient buffer system, allowing sufficient FA flux without nonphysiological increases in cellular nonesterified FA concentrations. Moreover, FA cycling creates metabolic intermediates that can be utilized in anabolic processes or as extra- or intracellular signaling molecules.
This review focuses on the catabolic branch of the FA cycle. We summarize recent advances in understanding the enzymatic mechanisms of the lipolytic process and the (patho)physiological impact of lipolysis on energy homeostasis and cellular signaling.
Neutral Lipolysis: Three Steps, Three Enzymes
Neutral hydrolysis of TGs to FAs and glycerol requires three consecutive steps that involve at least three different enzymes: ATGL catalyzes the initial step of lipolysis, converting TGs to diacylglycerols (DGs); HSL is mainly responsible for the hydrolysis of DGs to monoacylglycerols (MGs) and MG lipase (MGL) hydrolyzes MGs. In adipose tissue, ATGL and HSL are responsible for more than 90% of TG hydrolysis (Schweiger et al., 2006). Although most nonadipose tissues also express ATGL and HSL, expression levels are low in some tissues, raising the question of whether other lipases are additionally required for efficient lipolysis.
ATGL: the Initial Step of Lipolysis
ATGL is the newest member of the lipolytic enzyme trio. The enzyme, first described in 2004 (Jenkins et al., 2004; Villena et al., 2004; Zimmermann et al., 2004), belongs to the family patatin domain-containing proteins including nine human and eight murine members. The patatin domain was originally discovered in lipid hydrolases of the potato and other plants and named after the most abundant protein of the potato tuber, patatin. Because some members of the family act as phospholipases, the proteins were officially named patatin-like phospholipase domain-containing protein A1 to A9 (PNPLA1–9) (Wilson et al., 2006). Unfortunately, this name is misleading because it implies that all members of this family are phospholipases. However, several PNPLA proteins have only minor or no phospholipase activity. A more appropriate designation for the family should be considered.
ATGL (officially annotated as PNPLA2) preferentially hydrolyzes TGs. Orthologous enzymes are found in essentially all eukaryotic species, including vertebrates, invertebrates, plants, and fungi. The human protein comprises 504 amino acids (AA). In analogy with patatin, the active site of the enzyme contains an unusual catalytic dyad (S47 and D166) within the patatin domain (Rydel et al., 2003). This domain comprises 180 AA and is embedded within a 250 AA α−β−α sandwich structure at the protein's NH2-terminal half. The COOH-terminal half has a predominantly regulatory function and contains a predicted hydrophobic region for LD binding (Duncan et al., 2010; Schweiger et al., 2008). Loss of this region increases the specific in vitro activity of ATGL against artificial TG substrates but blunts the intracellular activity due to the inability of the truncated enzyme to bind to cellular LDs.
Regulation of ATGL
Regulation of ATGL expression and enzyme activity is complex (Lass et al., 2011). ATGL mRNA expression is elevated by peroxisome proliferator-activated receptor (PPAR) agonists, glucocorticoids, and fasting, whereas insulin and food intake decrease expression. More recently, it was shown that mTOR complex 1-dependent signaling reduces ATGL mRNA levels (Chakrabarti et al., 2010). Conversely, activation of FoxO1 by SIRT1-mediated deacetylation activates lipolysis by increasing ATGL mRNA levels. SIRT1 silencing has the opposite effect (Chakrabarti et al., 2011). The abundance of ATGL (and HSL) mRNA does not always correlate with cellular lipase activity. For example, isoproterenol and tumor necrosis factor-α reduce ATGL (and HSL) mRNA levels in adipocytes (Kralisch et al., 2005) but, conversely, stimulate lipase activities and FA and glycerol release. The discrepancy between enzyme mRNA levels and activities is explained by the extensive posttranslational regulation of ATGL and HSL (discussed below). Thus, cellular lipase mRNA levels are inadequate as indicators of enzyme activities.
At least two serine residues in ATGL can be phosphorylated (S406 and S430 in the murine enzyme) (Bartz et al., 2007). In contrast to HSL (see below), ATGL modification is not protein kinase A (PKA)-dependent (Zimmermann et al., 2004). Instead, the laboratory of H.-S. Sul recently showed that AMP-activated kinase (AMPK) phosphorylates S406, leading to increased hydrolytic activity of murine ATGL (Ahmadian et al., 2011). The role of AMPK in the regulation of lipolysis has been controversial, with data showing that AMPK induces (Gaidhu et al., 2009; Yin et al., 2003), inhibits (Daval et al., 2005; Gauthier et al., 2008), or has no effect (Chakrabarti et al., 2011) on lipolysis. Interestingly, in the nematode Caenorhabiditis (C.) elegans, AMPK-mediated phosphorylation of ATGL-1 (the worm ortholog of mammalian ATGL) at different phosphorylation sites inhibits TG hydrolysis (Narbonne and Roy, 2009), delays the consumption of TG stores, and prolongs life span during the stress-induced dauer larval stage. The functional role of the second phosphorylation site (S430) is not known.
ATGL requires a coactivator protein, comparative gene identification-58 (CGI-58), for full hydrolase activity (Lass et al., 2006). CGI-58 was originally discovered in a screen comparing the proteomes of humans and C. elegans. The gene is now officially named α/β hydrolase domain-containing protein-5 (ABHD5), owing to the presence of an α/β hydrolase domain commonly found in esterases, thioesterases, and lipases. However, CGI-58 is unlikely to exhibit hydrolase activity due to the fact that asparagine-153 (N153) in CGI-58 replaces a nucleophilic serine residue that is required in the active site of enzymatically functional members of the esterase/thioesterase/lipase family. Interestingly, substitution of N153 by serine does not convert CGI-58 into a lipid hydrolase for TGs, DGs, MGs, or short FA-glycerol esters (A. Lass et al., unpublished). In the epidermis of the skin, CGI-58 may also stimulate another, currently unknown, TG hydrolase distinct from ATGL (Radner et al., 2010). Importantly, two laboratories showed that CGI-58, at least in vitro, exhibits enzymatic activity as an acylCoA-dependent acylglycerol-3-phosphate acyltransferase (AGPAT) (Ghosh et al., 2008; Montero-Moran et al., 2010). The physiological role of this activity requires clarification. It may affect phosphatidic acid or lysophosphatidic acid signaling (Brown et al., 2010).
Recently, Yang et al. (Yang et al., 2010b) discovered a specific peptide inhibitor for ATGL. The protein was originally identified in blood mononuclear cells to act at the G0 to G1 transition of the cell cycle. Consistent with this function, it was named G0G1 switch protein 2 (G0S2). Human and murine G0S2 have a predicted primary structure of 103 AA. The protein is found in many tissues, with highest concentrations in adipose tissue and liver. Consistent with lipase activities, G0S2 expression is very low in adipose tissue during fasting but increases after feeding (Yang et al., 2010b). Conversely, fasting or PPARα-agonists increase hepatic G0S2 expression (Zandbergen et al., 2005). The protein localizes to multiple cellular compartments, including LDs, cytoplasm, ER, and mitochondria. The different localizations may reflect multiple functions for G0S2 in regulating lipolysis, the cell cycle, and, possibly, apoptosis via its ability to interact with the mitochondrial antiapoptotic factor Bcl2 (Welch et al., 2009). In vitro, LD binding and inhibition of ATGL depend on a physical interaction between the NH2-terminal region of G0S2 (involving AA 27–42) and the patatin domain of ATGL (Lu et al., 2010). Elucidation of whether G0S2 also regulates tissue-specific lipolysis in vivo will require the characterization of G0S2 transgenic and knockout mouse models.
LD-associated proteins participate in the CGI-58-mediated regulation of ATGL (Figure 1, left). In hormonally nonstimulated white and brown adipocytes, perilipin-1 interacts with CGI-58, preventing its binding to and, thus, induction of ATGL. Upon β-adrenergic stimulation, protein kinase A (PKA) phosphorylates perilipin-1 at multiple sites, including the critical serine-517 residue, causing the release of CGI-58. The effector then binds and stimulates ATGL (Granneman et al., 2009; Miyoshi et al., 2007). Thus, β-adrenergic stimulation of PKA induces ATGL activity by phosphorylation of perilipin-1 and not by direct enzyme phosphorylation. Consistent with this model, frameshift mutants of perilipin-1 in humans (L404fs and V398fs) fail to bind CGI-58, leading to unrestrained lipolysis, partial lipodystrophy, hypertriglyceridemia, and insulin resistance (Gandotra et al., 2011). ATGL-mediated TG hydrolysis in nonadipose tissues with high FA oxidation rates, such as muscle and liver, follows another mechanism. In these tissues, perilipin-1 is replaced by perilipin-5 (Figure 1, right). During fasting, perilipin-5 recruits both ATGL and CGI-58 to LDs by direct binding of the enzyme and its coactivator. Formation of the ternary complex involves the COOH-terminal region of perilipin-5 (AA 200–463) (Granneman et al., 2011). The role of perilipin-5 within this complex is still a matter of discussion. Recent data suggest that it is involved in the interaction of LDs with mitochondria and inhibits ATGL-mediated TG hydrolysis (Wang et al., 2011). Whether perilipins-2, -3, and -4 also interact with either ATGL or CGI-58 is disputed. Overexpression of perilipin-2 in hepatocyte cell lines inhibits ATGL activity by restricting its physical access to LDs. Direct protein-protein interaction is probably not required (Bell et al., 2008; Listenberger et al., 2007).
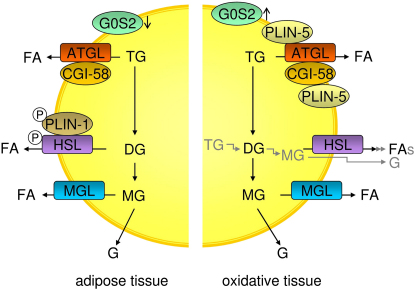
Figure 1.
Lipolysis in Adipose and Oxidative Tissues during Fasting
In adipose tissues, beta-adrenergic stimulation of lipolysis leads to the consecutive hydrolysis of TG and the formation of FAs and glycerol. The process requires three enzymes: ATGL cleaves the first esterbond in TGs, HSL hydrolyzes DGs, and MGL MGs. For full hydrolytic activity, ATGL interacts with its coactivator protein CGI-58, whereas HSL is phosphorylated, translocates to the LD, and interacts with phosphorylated PLIN-1. Expression of the ATGL inhibitor G0S2 during fasting is low in adipose and high in oxidative tissues (e.g., liver). In oxidative tissues PLIN-1 is not present on LDs. Instead, PLIN-5 is expressed and interacts with both ATGL and CGI-58, facilitating LD localization of these proteins. ATGL, adipose triglyceride lipase; CGI-58, comparative gene identification-58; DG, diacylglycerol; FA, fatty acid; G, glycerol; G0S2, G0/G1 switch gene 2; HSL, hormone-sensitive lipase; MG, monoacylglycerol; MGL, monoglyceride lipase; PLIN-1, perilipin-1; PLIN-5, perilipin-5; TG, triacylglycerol.
Recently, several groups reported that pigment epithelium-derived factor (PEDF) induces TG hydrolysis in adipose tissue, muscle, and liver, via ATGL (Borg et al., 2011; Chung et al., 2008). PEDF is a widely expressed 50 kD protein of the noninhibitory serpin family of serine protease inhibitors (Filleur et al., 2009). It exhibits a large spectrum of bioactivities, including antiangiogenic, antitumorigenic, neuroprotective, antioxidative, and antiinflammatory effects. PEDF binds to ATGL and activates its enzymatic activity (Borg et al., 2011; Notari et al., 2006). Although the mechanism remains to be clarified, ATGL activation by PEDF may be involved in the pathogenesis of insulin resistance and the development of hepatosteatosis.
Another important aspect of ATGL regulation was identified by genetic analyses of LD formation in Drosophila (D.) melanogaster L2 cells (Beller et al., 2008; Guo et al., 2008). Compelling results showed that ATGL delivery to LDs requires functional vesicular transport. In the absence of essential protein components of the transport machinery, such as ADP-ribosylation factor 1 (ARF1), small GTP-binding protein 1 (SAR1), the guanine-nucleotide exchange factor Golgi-Brefeldin A resistance factor (GBF1), or deficiency of the coatamer protein coat-complex I and II, ATGL translocation to LDs is blocked and the enzyme remains associated with the ER (Soni et al., 2009). The process requires physical binding of ATGL to GBF1 (Ellong et al., 2011).
HSL: the Main DG Lipase
In the early 1960s, it was noted that a lipolytic activity present in adipose tissue was induced by hormonal stimulation. A landmark paper published by D. Steinberg's group (Vaughan et al., 1964) described the isolation and characterization of both HSL and MGL. Although this classic work originally noted that HSL is a much better DG hydrolase than TG hydrolase, standard textbook knowledge perpetuated the conclusion that HSL was rate-limiting for the catabolism of fat stores in adipose and many nonadipose tissues. This view required revision when HSL-deficient mice efficiently hydrolyzed TGs (Osuga et al., 2000). HSL-deficient mice showed no signs of TG accumulation in either adipose or nonadipose tissues; instead, they accumualted large amounts of DGs in many tissues, suggesting that in vivo the enzyme was more important as a DG- than a TG-hydrolase (Haemmerle et al., 2002). Although originally somewhat controversial (Rydén et al., 2007), it is now accepted that ATGL is responsible for the initial step of lipolysis in human adipocytes, and that HSL is rate-limiting for the catabolism of DGs (Bezaire et al., 2009). In addition to DGs, HSL also hydrolyzes ester bonds of many other lipids (e.g., TGs, MGs, cholesteryl esters, and retinyl esters) and short-chain carbonic acid esters (Fredrikson et al., 1986).
The HSL expression profile essentially mirrors that of ATGL. Highest mRNA and protein concentrations are found in white adipose tissue (WAT) and brown adipose tissue (BAT); low expression is detected in many other tissues, including muscle, testis, steroidogenic tissues, and pancreatic islets (Holm et al., 2000). Alternative exon usage leads to tissue-specific differences in mRNA and protein size (Holst et al., 1996). In adipose tissue, the HSL protein comprises 768 AA. Unlike ATGL, with orthologous enzymes found across all eukarya, HSL is less ubiquitous phylogenetically. For example, no HSL ortholog is known in birds, C. elegans, D. melanogaster, and Saccharomyces (S.) cerevisiae. Interestingly, the closest structural relatives to HSL are found in prokaryotes (e.g., lipase 2 in Moraxella TA144) (Langin et al., 1993). Functional studies have delineated in HSL an NH2-terminal lipid-binding region, the α/β hydrolase fold domain including the catalytic triad, and the regulatory module containing all known phosphorylation sites important for regulation of enzyme activity (Holm et al., 2000).
Regulation of HSL
Since ATGL and HSL hydrolyze TGs in a coordinated manner, it is not unexpected that they share many regulatory similarities. In adipose tissue, HSL enzyme activity is strongly induced by β-adrenergic stimulation, whereas insulin has a strong inhibitory effect. The mechanisms of enzyme regulation, however, differ markedly between the two lipases. While β-adrenergic stimulation regulates ATGL primarily via recruitment of the coactivator CGI-58 (see above), HSL is a major target for PKA-catalyzed phosphorylation (Strålfors and Belfrage, 1983). Other kinases, including AMPK, extracellular signal-regulated kinase, glycogen synthase kinase-4, and Ca2+/calmodulin-dependent kinase, also phosphorylate HSL to modulate its enzyme activity (Lass et al., 2011). The enzyme has at least five potential phosphorylation sites, of which S660 and S663 appear to be particularly important for hydrolytic activity (Anthonsen et al., 1998). Enzyme phosphorylation affects enzyme activity moderately (an approximate 2-fold induction). For full activation, HSL must gain access to LDs, which, in adipose tissue, is mediated by perilipin-1. Simultaneously with HSL, PKA also phosphorylates perilipin-1 on six consensus serine residues. As a result, HSL binds to the NH2-terminal region of perilipin-1, thereby gaining access to LDs (Miyoshi et al., 2007; Shen et al., 2009; Wang et al., 2009). Together, HSL-phosphorylation and enzyme translocation to LDs coupled with ATGL activation by CGI-58 result in a more than 100-fold increase in TG hydrolysis in adipocytes (Figure 1). This activation process is modulated by other factors. For example, receptor-interacting protein 140 (RIP-140) was shown to induce lipolysis by binding to perilipin-1, increasing HSL translocation to LDs, and activating ATGL via CGI-58 dissociation from perilipin-1 (Ho et al., 2011). In nonadipose tissues, such as skeletal muscle, HSL is activated by phosphorylation in response to adrenaline and muscle contraction (Watt et al., 2006). These tissues lack perilipin-1, and it remains to be determined which alternative mechanisms regulate HSL access to LDs.
Insulin-mediated deactivation of lipolysis is associated with transcriptional downregulation of ATGL and HSL expression (Kershaw et al., 2006; Kralisch et al., 2005). Additionally, insulin signaling results in phosphorylation and activation of various phosphodiesterase (PDE) isoforms by PKB/AKT (Enoksson et al., 1998), PDE-catalyzed hydrolysis of cAMP, and inhibition of PKA. These actions halt lipolysis by preventing phosphorylation of both HSL and perilipin-1, activation and translocation of HSL, and activation of ATGL by CGI-58. In addition to its peripheral action, insulin also acts centrally via the sympathetic nervous system to inhibit lipolysis in WAT. Elegant studies by Scherer and coworkers (Scherer et al., 2011) showed that increased insulin levels in the brain inhibit HSL and perilipin phosphorylation, leading to reduced HSL and ATGL activities.
MGL: the Final Step in Lipolysis
MGL is considered to be the rate-limiting enzyme for the breakdown of MGs derived from extracellular TG hydrolysis (by LPL), intracellular TG hydrolysis (by ATGL and HSL), and intracellular phospholipid hydrolysis (by phospholipase C and membrane-associated DG lipase α and β). The enzyme localizes to cell membranes, cytoplasma (Sakurada and Noma, 1981), and LDs (unpublished data). MGL received significant attention following the realization that glycerophospholipid-derived MG 2-arachidonylglycerol (2-AG) is a major agonist for endocannabinoid signaling and is inactivated by the hydrolytic activity of MGL (see MG-signaling, below). The enzyme is ubiquitously expressed with highest expression levels in adipose tissue. The importance of MGL for efficient degradation of MGs was recently confirmed in mutant mouse models (Chanda et al., 2010; Schlosburg et al., 2010; Taschler et al., 2011). Lack of MGL impairs lipolysis and is associated with increased MG levels in adipose and nonadipose tissues alike.
MGL shares homology with esterases, lysophospholipases, and haloperoxidases, and contains a consensus GXSXG motif within a catalytic triad (S122, A239, and H269 for mouse MGL) that is typical of lipases and esterases. Very recently, the crystal structure of MGL was solved (Bertrand et al., 2010; Labar et al., 2010). The enzyme exhibits the classic fold of the α/β hydrolases, crystallizes as a dimer, and exhibits a wide, hydrophobic access to the catalytic site. An apolar helix-domain lid covers the active site and mediates the interaction of MGL with membrane structures and the recruitment of substrate.
Other Lipases Implicated in TG Catabolism
Experiments with ATGL-deficient mice and small-molecule HSL inhibitors revealed that ATGL and HSL are responsible for more than 90% of the lipolytic activity in WAT and cultured adipocytes (Schweiger et al., 2006). In nonadipose tissues, the contribution of other neutral lipases to the catabolism of stored TGs may be more prominent. For example, in the liver of fasted mice, ATGL accounts for less than 50% of neutral TG hydrolase activity (Reid et al., 2008). This activity is physiologically important because ATGL-deficient mice develop hepatosteatosis (Haemmerle et al., 2006; Wu et al., 2011). However, the pronounced remaining activity of hepatic TG hydrolase(s) in ATGL-deficient mice indicates that other lipases contribute to the highly dynamic turnover of TGs. This view is supported by the observation that mice lacking ATGL in the liver have no apparent defect in VLDL biogenesis (Wu et al., 2011), although assembly and secretion of hepatic VLDL particles require substantial mobilization of hepatic TG stores. Because HSL is also poorly expressed in hepatocytes, the existence of alternative hepatic DG hydrolases seems likely.
Several members of the carboxylesterase/lipase family and the PNPLA family have been suggested as potential TG hydrolases. One of them, carboxyl esterase-3/triglyceride hydrolase-1 (Ces-3/Tgh-1, ortholog of human Ces-1), has gained major interest because the recent characterization of Ces-3/Tgh-1-deficient mice provided compelling evidence that the enzyme participates in the assembly and secretion of hepatic VLDL (Wei et al., 2010). How this biological function conforms to the strict luminal localization of Tgh-1 in the ER remains to be elucidated.
Structural relatives of ATGL within the PNPLA family were also considered as potential TG hydrolases. For example, PNPLA4 and -5 exhibit TG-hydrolase, DG transacylase, and retinylester hydrolase activity in vitro (Kienesberger et al., 2009b). Whether these activities are also relevant in vivo remains to be determined. The member with highest homology to ATGL is adiponutrin (PNPLA3), with over 50% AA identity within the patatin domain. Adiponutrin was originally discovered as a nutritionally regulated adipose-specific transcript of unknown function (Baulande et al., 2001). Interest in adiponutrin increased tremendously when H. Hobbs and colleagues found a strong genetic association between a nonsynonymous AA change (I148M) in adiponutrin and susceptibility to develop nonalcoholic fatty liver disease (NAFLD) (Romeo et al., 2008). Several other groups confirmed and extended this important finding by showing robust associations of I148M with alcoholic- and nonalcoholic liver disease, hepatic fibrosis, and liver cirrhosis (Krawczyk et al., 2011; Tian et al., 2010; Yuan et al., 2008). The close similarity to ATGL as well as the presence of conserved structural motifs typical for lipases/esterases (α−β−α sandwich structure and the GXSXG motif within a catalytic dyad) suggest a lipase function for adiponutrin. In accordance with this assumption, several groups reported that adiponutrin acts as a TG hydrolase and additionally exhibits DG transacylase activity (He et al., 2010; Huang et al., 2011; Jenkins et al., 2004; Lake et al., 2005). However, the expression profile generated in response to fasting/feeding and the induction of adiponutrin gene expression by SREBP1a/c and CHREBP argued against an in vivo role of adiponutrin in lipolysis (Dubuquoy et al., 2011; He et al., 2010; Kershaw et al., 2006; Lake et al., 2005). Pinpointing the functional role of adiponutrin was also confounded when PNPLA3-deficient mice exhibited no detectable phenotype in lipid, lipoprotein, or energy metabolism (Basantani et al., 2011; Chen et al., 2010). Overexpression of the I148M variant of adiponutrin caused TG accumulation (He et al., 2010), whereas overexpression of wild-type adiponutrin in the liver created no obvious phenotype (He et al., 2010). Overall, the biochemical and (patho)physiological function of adiponutrin remains unclear.
Autophagy and Acid Lipolysis
In addition to classical lipolysis by extralysosomal neutral lipases, TGs and cholesteryl esters can also be hydrolyzed by LAL. LAL is thought to catabolize primarily lipoprotein-associated lipids subsequent to their receptor-mediated endocytosis and fusion with lysosomes. Accordingly, the contribution of LAL activity to lipolysis of intracellular LDs was not considered relevant. Given the lysosomal localization of LAL, it was not intuitively obvious how lipids from LDs would enter lysosomes. Addressing this, Singh and colleagues reported compelling evidence linking lipolysis to macroautophagy (Singh et al., 2009a). Macroautophagy is a lysosomal pathway that degrades superfluous or damaged organelles as well as cytoplasmic inclusions, such as misfolded protein aggregates (Levine and Kroemer, 2008). These cytoplasmic cargos are trapped inside double-membrane vesicles (autophagosomes) that ultimately fuse with lysosomes, where their contents are degraded. Subsequently, lipids and AA are released into the cytosol and contribute to energy and AA supply in times of starvation. Thus, along with lipolysis, macroautophagy is one of two conserved responses to organismal and cellular fasting.
Singh and coworkers (Singh et al., 2009a) found that, in addition to conventional neutral lipases, autophagy of LDs is required for fasting-induced lipolysis in murine liver and cultured hepatocytes. They showed that recruitment of microtubule-associated protein 1 light chain 3 (LC-3) and formation of a regional membrane through conjugation of autophagy-related protein 7 (Atg7) gives rise to double-membrane vesicles that engulf portions of cytoplasmic LDs (autolipophagosomes). The autolipophagosomes ultimately fuse with lysosomes, where their lipid content is degraded by LAL. Consistent with a role for lipoautophagy during starvation, Singh et al. found that deletion of Atg7 causes lipid accumulation in the liver. Chronic fat feeding impairs the autophagic removal of lipid stores in the liver, prompting excessive hepatic lipid deposition. Hotamisligil and colleagues (Yang et al., 2010a) strengthened these findings by showing that hepatic Atg7 expression is severely impaired in ob/ob mice, contributing to the hepatosteatosis in these animals. Liver fat accumulation was reduced by liver-specific restoration of Atg7 expression.
While the effects of autophagy in genetically obese mice and in mice fed high-fat diets seem consistent, a role for autophagy in lipid metabolism of normal mice remains controversial. First, autophagy appears to be predominantly relevant in the liver after abnormally long fasting periods. Normally, during shorter fasting periods the hepatic fat content increases due to the induction of adipose tissue lipolysis and increased FA supply to the liver. It is unlikely that autophagy would be induced under this condition. Consistent with this prediction, Hotamisligil and colleagues did not observe hepatic steatosis or changes in serum TG or FA levels in lean mice following siRNA-mediated suppression of Atg7, arguing that lipoautophagy is not involved (Yang et al., 2010a). Second, hepatosteatosis as a result of Atg7 deficiency was observed in some but not all studies. Although Atg7 deficiency leads to severe liver enlargement, it is controversial whether TGs accumulate. In fact, Uchiyama and colleagues reported that hepatic Atg7 deletion upon starvation inhibits LD formation both in vivo and in vitro, which leads to a lower hepatic TG content (Shibata et al., 2009, 2010). Consistent with a role for LC3 in LD formation, RNAi-mediated suppression of LC3-expression prevented LD formation in a panel of different (hepatic and nonhepatic) cell lines. Interestingly, LC3 localized to the surface of LDs, and the authors argued that lipidation of LC3 by phosphatidylethanolamine (formation of LC3-II), which is the initial step in autophagosome formation during autophagy, is also required for LD formation. A potential role of autophagy in lipogenesis but not in lipolysis is consistent with the finding that external administration of FAs induces, rather than inhibits, autophagy when LDs are formed in the fasting liver (Tang et al., 2011).
A role for autophagy in lipogenesis also became evident from analysis of adipose tissue in Atg7-deficient mice. Assuming a lipolytic defect, we speculated that ablation of autophagy in adipocytes would result in an obese phenotype (Zechner and Madeo, 2009). In contrast, however, adipose-specific knockout of Atg7 resulted in lean mice with reduced adipose mass, enhanced insulin sensitivity, and an elevated rate of β-oxidation (Singh et al., 2009b). The adipocytes contained smaller, multilocular LDs and exhibited normal basal lipolysis. In line with this, inhibition of autophagy in cultured adipocytes using Atg7 siRNA blocked TG accumulation (Singh et al., 2009b). Taken together, these data argue for a currently poorly understood role of autophagy in the biogenesis of LDs. Interestingly, while adipose-specific Atg7-deficient mice display reduced WAT mass, BAT mass increases (Baerga et al., 2009; Singh et al., 2009b). Singh et al. argued that inhibition of white adipocyte differentiation may lead to a defect in lipogenesis or that blocked autophagy may promote WAT to BAT transdifferentiation.
In summary, autophagy may have pleiotropic roles in lipid metabolism depending on the cell- or tissue-type. In the liver, autophagy may contribute to lipolysis under a high-fat diet or prolonged fasting. Alternatively, autophagy may promote lipid accumulation under normal fasting conditions. In WAT, autophagy appears to be involved in adipocyte differentiation and lipogenesis but not in lipolysis. The role of autophagy in the breakdown of fat in other lipolytically active tissues, such as muscle, macrophages, and steroidogenic cells remains to be determined.
Lipolysis: A Role in Lipid-Mediated Signaling?
Textbook knowledge tells us that TGs are the most efficient way to store large amounts of FAs as an energy reserve. Consistent with this view, cellular LDs were long seen as a relatively inert storage depot for fat in adipose tissue that is mobilized at times of increased energy demand. This view changed when it was realized that 1) LDs are present in essentially all cell types, including those for which mere energy storage does not seem to be the main purpose; 2) LDs, particularly in nonadipose tissues, undergo very dynamic changes of formation and degradation; and 3) LDs represent a reservoir of bioactive lipids and lipid-derived hormones in adipose and nonadipose tissues.
A role for neutral lipid metabolism in signaling gained substantial interest when it was noted that increased cellular TG concentrations are strongly associated with insulin resistance in skeletal muscle and liver (Cohen et al., 2011; Kelley et al., 2002). The relatively inert nature of TGs makes it unlikely that they interfere directly with insulin signaling. The concept that TGs themselves are not the culprit was also supported by the so-called athletes' paradox. Endurance athletes accumulate more TGs in LDs of skeletal myocytes than do untrained individuals, yet their muscle is highly insulin-sensitive. Similarly, mice that lack ATGL accumulate large amounts of fat in numerous tissues (including skeletal muscle, cardiac muscle, liver, kidneys, and macrophages) but exhibit increased insulin sensitivity (Haemmerle et al., 2006; Kienesberger et al., 2009a). Increased insulin sensitivity is observed despite the fact that ATGL-deficiency leads to an insulin-secretion defect in pancreatic islets (Peyot et al., 2009). In humans, ATGL-deficiency leads to neutral lipid storage disease with myopathy (NLSDM), with a similar lipid phenotype as observed in ATGL-deficient mice (Fischer et al., 2007). Patients lacking the ATGL coactivator CGI-58 not only develop neutral lipid storage disease but also exhibit a severe skin defect (neutral lipid storage disease with ichthyosis, NLSDI) (Lefèvre et al., 2001). To date, defective pancreatic insulin production and alterations in insulin sensitivity have not been reported in patients with NLSDM or NLSDI. In conclusion, cellular TG content can be a marker of insulin resistance under certain physiological conditions but is not a regulator of insulin signaling.
Lipolysis-Derived FAs and PPAR Signaling
Besides their powerful role as energy substrates and precursors of other lipids, FAs are directly involved in cellular signaling pathways and regulation of gene transcription. FAs or FA derivatives can bind to and activate members of the nuclear receptor family of transcription factors that control the expression of genes involved in lipid and energy homeostasis and inflammation. The best-studied FA-activated nuclear receptors are the PPARs. The PPAR family consists of four members: PPARα, PPARγ-1 and -2, and PPARδ (also designated PPARβ). PPARα and PPARδ are highly expressed in oxidative tissues and regulate genes involved in substrate delivery, substrate oxidation, and oxidative phosphorylation (OXPHOS). In contrast, PPARγ is more important in lipogenesis and lipid synthesis, with highest expression levels in WAT. The full transcriptional activity of PPARs requires the binding of cognate lipid ligands, heterodimerization with another nuclear receptor (retinoid-X receptor, RXR), and interaction with a number of transcriptional coactivators, including PPARγ coactivator-1 (PGC-1). In addition to FAs, other lipid ligands also have been described to activate PPARs, such as acyl-CoAs, glycerol-phospholipids, and eicosanoids.
FAs involved in signaling originate from import of exogenous FAs (from circulating FA-albumin complexes or from LPL-mediated hydrolysis of plasma VLDL and chylomicrons) or from endogenous de novo synthesis. Recently it was shown that neither source of FA can generate PPAR ligands directly; rather, a cycle of FA esterification and rehydrolysis is required (Haemmerle et al., 2011) (Figure 2). As a consequence, lipolysis-impaired ATGL-deficient mice exhibit a severe defect in PPARα signaling in oxidative tissues such as liver (Ong et al., 2011), macrophages (Chandak et al., 2010), and BAT (Ahmadian et al., 2011). The most dramatic phenotype is observed in cardiac muscle (Haemmerle et al., 2011). The reduced expression of PPARα target genes in ATGL knockout animals causes severe mitochondrial dysfunction, decreased rates of substrate oxidation and OXPHOS, massive cardiac lipid accumulation, and lethal cardiomyopathy within a few months after birth. HSL deficiency is also associated with moderately decreased PPARα target-gene expression but does not generate a comparable cardiac phenotype, indicating the specific importance of ATGL activity in the generation of PPARα ligands or ligand precursors.
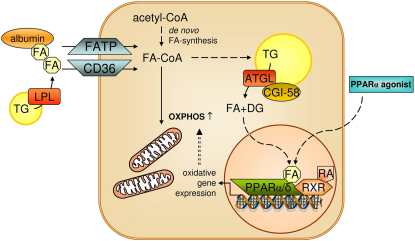
Figure 2.
ATGL-Mediated Lipolysis Is Required for PPAR Signaling and OXPHOS
Fatty acids from exogenous or endogenous sources are activated to acyl-CoAs, which are subject to mitochondrial oxidation or TG formation. ATGL-mediated lipolysis of TG generates lipolytic products (FA and DG), which may act directly (e.g., FA) or after conversion (e.g., DG to phospholipids) as ligands for nuclear receptors (for details see text). Activation of nuclear receptor PPARα via lipolytic cleavage of TGs is required for normal mitochondrial function and OXPHOS. In ATGL-deficient mice, defective PPARα activation and OXPHOS can be restored by treatment with PPARα agonists. ATGL, adipose triglyceride lipase; CD36, cluster of differentiation 36; DG, diacylglycerol; FA, fatty acid; FATP, fatty acid transport protein; LPL, lipoprotein lipase; OXPHOS, oxidative phosphorylation; PPARα/δ, peroxisome proliferator-activated receptor alpha/delta; RA, retinoic acid; RXR, retinoid X receptor; TG, triacylglycerol.
NLSDM or NLSDI in humans due to the deficiency of ATGL or CGI-58, respectively, may also lead to reduced PPARα signaling and defective OXPHOS. Although not proven experimentally, this assumption stems from the finding that NLSDM patients also develop systemic TG accumulation and cardiomyopathy. In many patients, this condition is lethal if they do not undergo heart transplantation (Hirano et al., 2008). Importantly, at least in mice, the mitochondrial defect can be prevented by application of PPARα activators, such as Wy16453 or fenofibrate (Haemmerle et al., 2011). Treatment of ATGL-deficient mice leads to increased PPARα signaling, disappearance of cardiac steatosis, and improved mitochondrial function and OXPHOS, as well as prolonged survival. Whether pharmacological PPARα activation will also be beneficial for patients with NLSDM is currently not known but remains a promising possibility.
In addition to the instrumental role of ATGL in PPARα signaling, the enzyme may also affect PPARγ function. Festuccia et al. (Festuccia et al., 2006) showed that rosiglitazone-mediated PPARγ activation and lipid accumulation are associated with increased lipolysis in WAT of rats and that increased lipolysis was due to induction of ATGL and MGL. Although it seems counterintuitive that lipolysis is induced during increased TG synthesis, it is conceivable that this step is required to promote PPARγ-activated expression of lipogenic genes. Moreover, the fact that HSL-deficiency also leads to downregulation of PPARγ target-gene expression in WAT (Shen et al., 2011; Zimmermann et al., 2003) suggests that lipolysis is involved in PPARγ signaling.
Lipolysis-Derived FAs and Insulin Signaling
It is well established that both plasma and cellular FA concentrations correlate positively with increased insulin resistance (Boden and Shulman, 2002). Several mechanisms are discussed. Increased cellular concentrations of nonesterified FAs, particularly palmitate, can drive the synthesis of lipotoxic lipids such as ceramides, which interfere with functional insulin signaling (Summers, 2010). Additionally, FAs can directly or indirectly—via increased production of reactive oxygen species—activate redox-sensitive serine kinases, which, in turn, inactivate the insulin response (Vallerie and Hotamisligil, 2010). Yet, not all FA species seem to have the same inhibitory effect on insulin signaling. Whereas palmitate consistently decreases the insulin response, palmitoleate actually enhances the insulin signal in liver and muscle (Cao et al., 2008). Palmitoleate is mostly generated by de novo synthesis in adipose tissue. Its effects on insulin signaling in liver and muscle suggest that this FA is a lipokine with endocrine function. Additionally, it is conceivable that hepatic palmitoleate also contributes to insulin sensitization in a paracrine fashion. Whether lipolysis contributes to the generation of palmitoleate or other FAs with a downstream effect on insulin signaling requires additional studies. ATGL-deficient mice have low plasma FA concentrations and are highly insulin-sensitive, arguing for a protective role of reduced lipolysis and low FA levels. Whether low plasma FAs in HSL-deficient mice also affect insulin sensitivity is controversial (Mulder et al., 2003; Park et al., 2005; Voshol et al., 2003).
Lipolysis-Derived DGs Are Unlikely to Affect PKC Signaling
The potential of DGs to act as second messengers was discovered approximately 50 years ago when researchers realized that DGs affect many metabolic and mitogenic activities via activation of protein kinase-C (PKC). Metabolic regulation involves suppression of insulin signaling via phosphorylation of insulin receptor substrate-1, leading to insulin resistance in muscle and liver (Samuel et al., 2010). Only one DG stereoisomer, 1,2-diacyl-sn-glycerols (1,2-DGs), is able to activate PKCs, whereas the others, 1,3-diacyl-sn-glycerols (1,3-DGs) and 2,3-diacyl-sn-glycerols (2,3-DGs), lack this bioactivity (Boni and Rando, 1985). 1,2-DGs activate conventional and novel PKC isoforms after recruitment of the enzymes to the plasma membrane by receptor of activated C kinase (RACK) proteins (Turban and Hajduch, 2011). Accordingly, both stereo-specific and location-specific preconditions are required for DGs to activate PKCs. Three potential sources exist for the generation of 1,2-DGs (Figure 3). Classical signaling 1,2-DGs derive from phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol-4,5-phosphate in the plasma membrane. Vertebrates have 13 isoforms of PLC grouped within 6 isotypes. Both products of the enzymatic reaction, 1,2-DGs and inositol-1,4,5-phosphate (IP3), are potent second messengers. Whereas 1,2-DGs remain plasma membrane-associated, IP3 dissociates and induces Ca2+ release from the ER, which is required in addition to 1,2-DGs for activation of conventional PKCs.
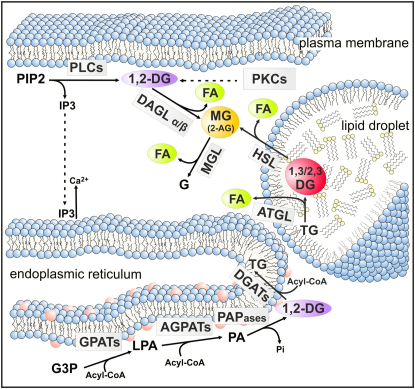
Figure 3.
Lipolysis and Lipid Signaling
Lipid intermediates involved in cellular signaling are generated by anabolic and catabolic reactions in distinct cellular compartments. 1,2-DGs, the ligands of conventional and novel PKCs, are formed at the plasma membrane by PLC-mediated degradation of PIP2. This reaction also generates IP3, a signaling molecule, which leads to Ca2+ efflux from the ER. De novo synthesis of 1,2-DGs at the ER may also contribute to PKC activation. FAs are ligands for nuclear receptors. They are generated by de novo synthesis or hydrolysis of neutral lipids or phospholipids. 2-AG is an important MG involved in endocannabinoid signaling. It originates from membrane-associated phospholipid hydolysis by PLCs and the subsequent hydrolysis of DGs by DAGLs. The contribution of TG hydrolysis by ATGL and HSL to cellular 2-AG concentrations is not known. The 2-AG signal is inactivated by MGL. AGPAT, acyl-CoA acylglycerol-3-phosphate acyltransferase; 2-AG, 2-arachidonoyl-glycerol; ATGL, adipose triglyceride lipase; DAGL, diacylglycerol lipase; DG, diacylglycerol; 11, 2-DG, diacyl-sn1,2-glycerol, DGAT, acyl-CoA: diacylglycerol acyltransferase; FA, fatty acid; G, glycerol; G3P, glycerol-3-phosphate; GPAT, glycerol-3-phosphate acyltransferase; HSL, hormone-sensitive lipase; IP3, inositol-1,4,5-trisphosphate; LPA, lysophosphatidic acid; MG, monoacylglycerol; MGL, monoglyceride lipase; PA, phosphatidic acid; PAPase, PA phosphohydrolase; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; TG, triacylglycerol.
Although PLCs generate the “correct” stereoisomer (1,2-DGs) in the “correct” cellular location for PKC activation, it remains controversial whether other cellular sources also contribute to the production of signaling 1,2-DGs. De novo synthesis via dual acylation of sn-glycerol-3-phosphate by acyl-CoA glycerol-3-phosphate acyltransferases (GPATs) and acyl-CoA acylglycerol-3-phosphate acyltransferases (AGPATs), and subsequent dephosphorylation of phosphatidic acid by phosphatidic acid phosphohydrolases (PAPases) also leads to the formation of 1,2-DGs (Figure 3). However, this pathway of DG synthesis is restricted to ER membranes. Accordingly, a model proposing activation of PKC by ER-associated 1,2-DGs needs to include PKC localization to the ER or contact sites between the plasma membrane and the ER membrane.
The third potential source of DGs derives from the lipolysis of LD-associated TGs by ATGL (Figure 3). The stereospecificity of ATGL has not been reported. However, our unpublished observations showed that the enzyme preferentially hydrolyzes sn-1 and sn-2 ester bonds but not sn-3 esters. This indicates that ATGL generates 1,3-DGs and 2,3-DGs but not 1,2-DGs. In accordance with the subsequent hydrolysis of 1,3-DGs and 2,3-DGs by HSL, this enzyme has a stereo-preference for the hydrolysis of FAs in the sn-3 position of DGs (Rodriguez et al., 2010). In TGs, HSL preferably hydrolyses sn-1(3) ester bonds (Fredrikson and Belfrage, 1983). Therefore, neither the ATGL nor the HSL reaction generate 1,2-DGs on LDs. Additionally, it is questionable whether LD-associated DGs would dissociate from LDs to participate in the recruitment and activation of PKC at the plasma membrane. From all this, it seems unlikely that lipolytically generated DGs act as signaling mediators.
In a recent review, Shulman and colleagues (Samuel et al., 2010) summarized numerous animal and human studies providing evidence that cellular DG concentrations account for the development of lipid-induced insulin resistance in type 2 diabetes, lipodystrophy, and other conditions. Consistent with this hypothesis, mice lacking DG-kinase-δ, the major enzyme that inactivates the DG signal, have increased DG levels and increased insulin resistance (Chibalin et al., 2008). However, considering the structural complexity of DG species and their localization in different cellular compartments, a general correlation between total cellular DG concentrations and insulin resistance seems unlikely. This is supported by studies where increased total cellular DG concentrations in mutant mouse models were not associated with insulin resistance. For example, HSL-deficient mice accumulate large amounts of DGs in adipose and many nonadipose tissues due to defective DG catabolism. Yet, most studies agree that this does not lead to PKC hyperactivation or a severely defective insulin response (Mulder et al., 2003; Park et al., 2005; Voshol et al., 2003). Similarly, CGI-58 silencing in the liver leads to increased DG levels, but normal (chow diet) or increased (high-fat diet) glucose tolerance and insulin sensitivity (Brown et al., 2010).
Taken together, determination of the specific 1,2-DG concentrations in the plasma membrane may provide a more reliable predictor for lipid-induced, PKC-mediated insulin resistance than total cellular DG concentrations.
Monoacylglycerol Signaling and Lipolysis
The signaling potential of MGs was recognized when it was found that the phospholipid-derived MG 2-AG activates cannabinoid receptors (CBR), thereby regulating food intake, lipid metabolism, and energy homeostasis. The endocannabinoid system (ECS) refers to a group of neuromodulatory lipids (endocannabinoids, ECs), two G protein-coupled receptors (CBR1 and CBR2), and enzymes involved in the synthesis and degradation of ECs (Di Marzo, 2009). The best-characterized ECs are N-arachidonoyl ethanolamine (AEA, anandamide) and 2-AG. Their biological effect is mimicked by Δ9-tetrahydrocannabinol (THC), the major psychoactive component of marijuana. The ECS regulates a diverse spectrum of physiological processes, including motor function, pain, appetite, cognition, emotional behavior, and immunity. In the nervous system, 2-AG acts as a retrograde messenger, inhibiting presynaptic neurotransmitter release (Alger and Kim, 2011). It is produced postsynaptically and traverses the synaptic cleft to stimulate presynaptic CBR1. Subsequently, 2-AG is internalized into the presynaptic terminal and inactivated by the MGL reaction, forming glycerol and arachidonic acid. The ECS is active in neurons and nonneuronal cells such as immune cells, hepatocytes, and adipocytes. Treatment of obese patients with the CBR1-antagonist rimonabant (Christopoulou and Kiortsis, 2011) and studies with animal models lacking CBR1 revealed that blockade of the ECS reduces food intake, decreases lipogenesis, and increases energy consumption. Conversely, an overactive ECS has a central orexigenic effect and reduces energy expenditure, promoting lipid deposition in peripheral tissues like the liver and WAT (Cota, 2008). Because of these biological effects, the ECS has been linked to the pathogenesis of metabolic diseases. Obese patients may have an overactive ECS, which stimulates appetite and promotes lipid deposition (Perkins and Davis, 2008).
It is generally assumed that signaling 2-AG originates from the degradation of glycerophospholipids containing arachidonic acid in the sn-2 position (Figure 3). Various isoforms of PLC generate 1,2-DGs (see above), which are subsequently hydrolyzed by DG lipase (DAGL) to 2-AG. Whether HSL also participates in the hydrolysis of plasma membrane-associated 1,2-DGs to generate 2-AG is not known. Current evidence suggests that at least two isoforms of DAGL (DAGLα and DAGLβ) exist in the brain and liver (Bisogno et al., 2003). Mice lacking DAGLα exhibit a substantial decrease in brain and spinal cord 2-AG levels, whereas DAGLβ appears to be more important in peripheral tissues, such as the liver (Gao et al., 2010). Whether the catabolism of arachidonic acid-containing TGs in LDs by ATGL and HSL also contributes to the cellular 2-AG and arachidonic acid pool is not known.
Recent studies using an MGL-specific small-molecule inhibitor (JZL184) and MGL knockout mice provided compelling evidence that MGL is the major enzyme in the degradation of 2-AG and other MGs esterified with long-chain FAs (Chanda et al., 2010; Schlosburg et al., 2010; Taschler et al., 2011). Animals lacking MGL or mice treated with JZL184 show abnormally high amounts of various MG species in the brain and peripheral tissues. In the brain, lack of MGL activity leads to a more than 20-fold increase in 2-AG, suggesting that MGL-deficiency could lead to hyperactivation of the ECS. Indeed, JZL184 treatment of mice provoked cannabimimetic effects in mice, including analgesia, hypothermia, and hypomotility (Long et al., 2009). However, genetic ablation of MGL in mice did not result in a hyperactive ECS or any obvious phenotype. This surprising observation was explained by desensitization of brain CBR1 leading to functional antagonism, and highlights the important role of 2-AG as a retrograde neurotransmitter (Chanda et al., 2010; Schlosburg et al., 2010). Obviously, increased brain 2-AG concentrations provoke counter-regulatory mechanisms similar to those observed when animals are chronically fed CBR agonists (Lichtman and Martin, 2005).
Although MGL-deficiency in mice does not produce cannabimimetic effects, the lack of MGL activity substantially affects lipolysis and metabolism in adipose tissue and nonadipose tissues (Taschler et al., 2011). MGL deficiency results in the accumulation of MGs and a reduction of circulating TG and glycerol levels in fasted animals. Unexpectedly, and in contrast to the proposed role of the ECS in obesity-related metabolic diseases, MGL knockout mice exhibit improved insulin sensitivity and glucose tolerance when fed a high-fat diet. The cause for this finding may be complex, considering that MGL-deficiency is associated with desensitized CBRs. Investigation of mice lacking MGL, specifically in the brain or peripheral tissues, should help to unravel the question of whether central or peripheral effects cause attenuation of insulin resistance.
Lipolysis in the Cell Cycle, Cancer, and Cachexia: A Question of Lipid Signaling?
The first observation to indicate that lipolysis is linked to efficient cell-cycle progression was reported in the yeast S. cerevisiae. Yeast expresses three TG lipases of the patatin domain-containing family termed Tgl3 to 5 (Czabany et al., 2007). Tgl4 is a functional ortholog of mammalian ATGL (Kurat et al., 2006). Deletion of Tgl3 and Tgl4 abolishes virtually all cellular TG lipase activity and causes a marked delay of entry into the cell division cycle of starved cells upon refeeding (Kurat et al., 2009). Tgl4 is activated via phosphorylation by the cyclin-dependent kinase Cdk1/Cdc28 (ortholog of mammalian Cdc2). Concomitantly, Cdk1/Cdc28 inhibits lipogenesis by phosphorylation of phosphatidic acid phosphohydrolase (Pah1). This suggests that TG levels oscillate during the cell-division cycle to either deposit de novo synthesized FAs in TGs or, conversely, to provide FAs during phases of increased demand. Tgl4 phosphorylation and activation occur at the G1/S transition of the cell-division cycle, which coincides with bud emergence and requires increased amounts of membrane lipids. Pah1 phosphorylation and inactivation occur at the G2/M transition of the cell cycle, indicating that a window exists during the cell cycle in which both the initial step of lipogenesis and lipolysis may operate in parallel. This is feasible because both activities are confined to different organelles, namely the ER and LDs, respectively. The specific checkpoint proteins that regulate cell-cycle progression in response to lipolysis are currently unknown. Recent evidence shows that the synthesis of phosphatidylinositol (PI), a precursor for signaling molecules in cell-cycle regulation in yeast (e.g., IP3 and inositol-containing ceramides), strongly depends on intact lipolysis (Gaspar et al., 2011). Thus, cell cycle-regulated TG lipolysis may provide critical precursors for signaling molecules for cell division.
A highly interesting study recently demonstrated that MGL promotes the oncogenic properties of mammalian cancer cells (Nomura et al., 2010). The authors demonstrated that overexpression or disruption of MGL activity increased or decreased, respectively, the proliferation of cancer cells, and that MGL is highly expressed in aggressive tumor cell lines or primary tumors. The study also provided evidence that MGL influences tumor proliferation by metabolic effects rather than by CBR-dependent mechanisms. The lack of MGL reduced cellular nonesterified FA concentrations. Growth of tumor cells in response to MGL-knockdown was not impaired after addition of exogenous nonesterified FAs or in mice fed a high-fat diet. This led to the conclusion that MGL affects the concentration of FA-derived tumorigenic lipid metabolites, such as LPA and PGE2. Although the involved lipid signal(s) requires identification, these studies clearly designate MGL as an interesting target for cancer therapy.
Lipolytic signaling may also be causally involved in the pathogenesis of cancer-associated cachexia (CAC). In a recent study, Das et al. (Das et al., 2011) demonstrated that ATGL-deficient mice are protected against tumor-induced loss of adipose tissue and skeletal muscle. HSL-deficient mice were also protected, although to a lesser degree. ATGL-deficient mice maintained body weight and composition despite increased circulating factors that induce lipolysis, muscle proteolysis, and apoptosis (e.g., TNFα, interleukin-6, and zinc-α-glycoprotein 1). This suggests that lipolysis is integrated in a signal transduction network that eventually leads to the loss of adipose tissue and muscle. This view is also consistent with the observation that the activity of lipolytic enzymes and release of FAs and glycerol are increased in adipose tissue of cancer patients with cachexia (Agustsson et al., 2007; Das et al., 2011; Rydén et al., 2008). The nature of the lipolytic signal involved is currently unknown. It is also unclear whether the signal originates from lipolysis in one tissue (such as adipose tissue) and promotes wasting in an endocrine manner or whether wasting requires autonomous lipolysis in all tissues that are affected by wasting. Although the underlying mechanism is not yet defined, this study suggests that inhibition of lipolysis may help to prevent cachexia in patients with cancer or other chronic diseases.
Conclusion
Recent discoveries of enzymes and regulatory factors have led to a revision of our perception of lipolysis. The complexity of the process and its regulation are still only partially understood. Additionally, we have just begun to address the role of lipolytic products and intermediates in cellular signaling. Important topics for future investigations include: 1) better understanding of the biochemical factors and processes that coordinately regulate the lipolytic machinery in response to hormonal activators and inhibitors, 2) the physiological function of lipolysis in numerous nonadipose tissues and the tissue-specific differences in lipolytic mechanisms, and 3) the characterization of lipolytic signals and the molecular mechanisms of their effects on gene transcription, the cell cycle, and cell growth. The recent examples of lipases affecting tumor proliferation or cancer-associated cachexia emphasize the potential importance of lipolysis in human disease.
Acknowledgments
This work was supported by the grants P20602, P18434, P21296, F30 SFB LIPOTOX, the Doktoratskollegs W901 and W1226, and the Wittgenstein Award Z136, which are funded by the Austrian Science Foundation. Funding was also provided by the grant “GOLD: Genomics Of Lipid-associated Disorders,” which is part of the Austrian Genome Project “GEN-AU: Genome Research in Austria” funded by the Austrian Ministry of Science and Research and the FFG. Additional support was obtained from the European commission grant agreements no. 202272 (LipidomicNet), the City of Graz and the Province of Styria. We thank Dr. Ellen Zechner, Mag. Caroline Schober-Trummler, and Mag. Dr. Tarek Mustafa for careful and critical reading of the manuscript.
References
- Agustsson T., Rydén M., Hoffstedt J., van Harmelen V., Dicker A., Laurencikiene J., Isaksson B., Permert J., Arner P. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67:5531–5537. doi: 10.1158/0008-5472.CAN-06-4585. [DOI] [PubMed] [Google Scholar]
- Ahmadian M., Abbott M.J., Tang T., Hudak C.S., Kim Y., Bruss M., Hellerstein M.K., Lee H.Y., Samuel V.T., Shulman G.I. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B.E., Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonsen M.W., Rönnstrand L., Wernstedt C., Degerman E., Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Baerga R., Zhang Y., Chen P.H., Goldman S., Jin S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5:1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser W.A. Ueber Fettnekrose, eine zuweilen tödtliche Krankheit des Menschen. Virchows Arch. 1882;90:520–535. [Google Scholar]
- Bartz R., Zehmer J.K., Zhu M., Chen Y., Serrero G., Zhao Y., Liu P. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J. Proteome Res. 2007;6:3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- Basantani M.K., Sitnick M.T., Cai L., Brenner D.S., Gardner N.P., Li J.Z., Schoiswohl G., Yang K., Kumari M., Gross R.W. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res. 2011;52:318–329. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulande S., Lasnier F., Lucas M., Pairault J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J. Biol. Chem. 2001;276:33336–33344. doi: 10.1074/jbc.M105193200. [DOI] [PubMed] [Google Scholar]
- Bell M., Wang H., Chen H., McLenithan J.C., Gong D.W., Yang R.Z., Yu D., Fried S.K., Quon M.J., Londos C., Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller M., Sztalryd C., Southall N., Bell M., Jäckle H., Auld D.S., Oliver B. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C. Academie des Sciences; Paris: 1856. Mémoire sur le pancréas et sur la role du suc pancréatique dans les phénomèmes digestifs particulièrement dans la digestion des matières grasses neutres. [Google Scholar]
- Bertrand T., Augé F., Houtmann J., Rak A., Vallée F., Mikol V., Berne P.F., Michot N., Cheuret D., Hoornaert C., Mathieu M. Structural basis for human monoglyceride lipase inhibition. J. Mol. Biol. 2010;396:663–673. doi: 10.1016/j.jmb.2009.11.060. [DOI] [PubMed] [Google Scholar]
- Bezaire V., Mairal A., Ribet C., Lefort C., Girousse A., Jocken J., Laurencikiene J., Anesia R., Rodriguez A.M., Ryden M. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J. Biol. Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T., Howell F., Williams G., Minassi A., Cascio M.G., Ligresti A., Matias I., Schiano-Moriello A., Paul P., Williams E.J. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G., Shulman G.I. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- Boni L.T., Rando R.R. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J. Biol. Chem. 1985;260:10819–10825. [PubMed] [Google Scholar]
- Borg M.L., Andrews Z.B., Duh E.J., Zechner R., Meikle P.J., Watt M.J. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–1466. doi: 10.2337/db10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.M., Betters J.L., Lord C., Ma Y., Han X., Yang K., Alger H.M., Melchior J., Sawyer J., Shah R. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Gerhold K., Mayers J.R., Wiest M.M., Watkins S.M., Hotamisligil G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P., English T., Shi J., Smas C.M., Kandror K.V. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59:775–781. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P., English T., Karki S., Qiang L., Tao R., Kim J., Luo Z., Farmer S.R., Kandror K.V. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 2011;52:1693–1701. doi: 10.1194/jlr.M014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P.K., Gao Y., Mark L., Btesh J., Strassle B.W., Lu P., Piesla M.J., Zhang M.Y., Bingham B., Uveges A. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol. Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- Chandak P.G., Radovic B., Aflaki E., Kolb D., Buchebner M., Fröhlich E., Magnes C., Sinner F., Haemmerle G., Zechner R. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 2010;285:20192–20201. doi: 10.1074/jbc.M110.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Chang B., Li L., Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–1142. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalin A.V., Leng Y., Vieira E., Krook A., Björnholm M., Long Y.C., Kotova O., Zhong Z., Sakane F., Steiler T. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Christopoulou F.D., Kiortsis D.N. An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J. Clin. Pharm. Ther. 2011;36:10–18. doi: 10.1111/j.1365-2710.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Chung C., Doll J.A., Gattu A.K., Shugrue C., Cornwell M., Fitchev P., Crawford S.E. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J. Hepatol. 2008;48:471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front. Horm. Res. 2008;36:135–145. doi: 10.1159/000115362. [DOI] [PubMed] [Google Scholar]
- Czabany T., Athenstaedt K., Daum G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta. 2007;1771:299–309. doi: 10.1016/j.bbalip.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Das S.K., Eder S., Schauer S., Diwoky C., Temmel H., Guertl B., Gorkiewicz G., Tamilarasan K.P., Kumari P., Trauner M. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- Daval M., Diot-Dupuy F., Bazin R., Hainault I., Viollet B., Vaulont S., Hajduch E., Ferré P., Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Dubuquoy C., Robichon C., Lasnier F., Langlois C., Dugail I., Foufelle F., Girard J., Burnol A.F., Postic C., Moldes M. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J. Hepatol. 2011;55:145–153. doi: 10.1016/j.jhep.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Duncan R.E., Wang Y., Ahmadian M., Lu J., Sarkadi-Nagy E., Sul H.S. Characterization of desnutrin functional domains: critical residues for triacylglycerol hydrolysis in cultured cells. J. Lipid Res. 2010;51:309–317. doi: 10.1194/jlr.M000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellong E.N., Soni K.G., Bui Q.T., Sougrat R., Golinelli-Cohen M.P., Jackson C.L. Interaction between the triglyceride lipase ATGL and the Arf1 activator GBF1. PLoS ONE. 2011;6:e21889. doi: 10.1371/journal.pone.0021889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoksson S., Degerman E., Hagström-Toft E., Large V., Arner P. Various phosphodiesterase subtypes mediate the in vivo antilipolytic effect of insulin on adipose tissue and skeletal muscle in man. Diabetologia. 1998;41:560–568. doi: 10.1007/s001250050947. [DOI] [PubMed] [Google Scholar]
- Festuccia W.T., Laplante M., Berthiaume M., Gélinas Y., Deshaies Y. PPARgamma agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia. 2006;49:2427–2436. doi: 10.1007/s00125-006-0336-y. [DOI] [PubMed] [Google Scholar]
- Filleur S., Nelius T., de Riese W., Kennedy R.C. Characterization of PEDF: a multi-functional serpin family protein. J. Cell. Biochem. 2009;106:769–775. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- Fischer J., Lefèvre C., Morava E., Mussini J.M., Laforêt P., Negre-Salvayre A., Lathrop M., Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- Flexner S. On the Occurrence of the Fat-Splitting Ferment in Peritoneal Fat Necroses and the Histology of These Lesions. J. Exp. Med. 1897;2:413–425. doi: 10.1084/jem.2.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson G., Belfrage P. Positional specificity of hormone-sensitive lipase from rat adipose tissue. J. Biol. Chem. 1983;258:14253–14256. [PubMed] [Google Scholar]
- Fredrikson G., Tornqvist H., Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 1986;876:288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- Gaidhu M.P., Fediuc S., Anthony N.M., So M., Mirpourian M., Perry R.L., Ceddia R.B. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J. Lipid Res. 2009;50:704–715. doi: 10.1194/jlr.M800480-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandotra S., Lim K., Girousse A., Saudek V., O'Rahilly S., Savage D.B. Human frameshift mutations affecting the carboxyl terminus of perilipin increase lipolysis by failing to sequester the adipose triglyceride lipase (ATGL) coactivator, AB-hydrolase containing 5 (ABHD5) J Biol Chem. 2011;286:34998–35006. doi: 10.1074/jbc.M111.278853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Vasilyev D.V., Goncalves M.B., Howell F.V., Hobbs C., Reisenberg M., Shen R., Zhang M.Y., Strassle B.W., Lu P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar M.L., Hofbauer H.F., Kohlwein S.D., Henry S.A. Coordination of storage lipid synthesis and membrane biogenesis: evidence for cross-talk between triacylglycerol metabolism and phosphatidylinositol synthesis. J. Biol. Chem. 2011;286:1696–1708. doi: 10.1074/jbc.M110.172296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M.S., Miyoshi H., Souza S.C., Cacicedo J.M., Saha A.K., Greenberg A.S., Ruderman N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.K., Ramakrishnan G., Rajasekharan R. YLR099C (ICT1) encodes a soluble Acyl-CoA-dependent lysophosphatidic acid acyltransferase responsible for enhanced phospholipid synthesis on organic solvent stress in Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:9768–9775. doi: 10.1074/jbc.M708418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman J.G., Moore H.P., Krishnamoorthy R., Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J. Biol. Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman J.G., Moore H.P., Mottillo E.P., Zhu Z., Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J. Biol. Chem. 2011;286:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Walther T.C., Rao M., Stuurman N., Goshima G., Terayama K., Wong J.S., Vale R.D., Walter P., Farese R.V. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T.M., Wagner E.F., Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P.C., Zierler K. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., McPhaul C., Li J.Z., Garuti R., Kinch L., Grishin N.V., Cohen J.C., Hobbs H.H. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Ikeda Y., Zaima N., Sakata Y., Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N. Engl. J. Med. 2008;359:2396–2398. doi: 10.1056/NEJMc0805305. [DOI] [PubMed] [Google Scholar]
- Ho P.C., Chuang Y.S., Hung C.H., Wei L.N. Cytoplasmic receptor-interacting protein 140 (RIP140) interacts with perilipin to regulate lipolysis. Cell. Signal. 2011;23:1396–1403. doi: 10.1016/j.cellsig.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Osterlund T., Laurell H., Contreras J.A. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu. Rev. Nutr. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- Holst L.S., Langin D., Mulder H., Laurell H., Grober J., Bergh A., Mohrenweiser H.W., Edgren G., Holm C. Molecular cloning, genomic organization, and expression of a testicular isoform of hormone-sensitive lipase. Genomics. 1996;35:441–447. doi: 10.1006/geno.1996.0383. [DOI] [PubMed] [Google Scholar]
- Huang Y., Cohen J.C., Hobbs H.H. Expression and characterization of a PNPLA3 isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol Chem. 2011;286:37085–37093. doi: 10.1074/jbc.M111.290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C.M., Mancuso D.J., Yan W., Sims H.F., Gibson B., Gross R.W. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- Kelley D.E., Goodpaster B.H., Storlien L. Muscle triglyceride and insulin resistance. Annu. Rev. Nutr. 2002;22:325–346. doi: 10.1146/annurev.nutr.22.010402.102912. [DOI] [PubMed] [Google Scholar]
- Kershaw E.E., Hamm J.K., Verhagen L.A., Peroni O., Katic M., Flier J.S. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- Kienesberger P.C., Lee D., Pulinilkunnil T., Brenner D.S., Cai L., Magnes C., Koefeler H.C., Streith I.E., Rechberger G.N., Haemmerle G. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J. Biol. Chem. 2009;284:30218–30229. doi: 10.1074/jbc.M109.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienesberger P.C., Oberer M., Lass A., Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J. Lipid Res. 2009;50(Suppl):S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralisch S., Klein J., Lossner U., Bluher M., Paschke R., Stumvoll M., Fasshauer M. Isoproterenol, TNFalpha, and insulin downregulate adipose triglyceride lipase in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2005;240:43–49. doi: 10.1016/j.mce.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Krawczyk M., Grünhage F., Zimmer V., Lammert F. Variant adiponutrin (PNPLA3) represents a common fibrosis risk gene: non-invasive elastography-based study in chronic liver disease. J. Hepatol. 2011;55:299–306. doi: 10.1016/j.jhep.2010.10.042. [DOI] [PubMed] [Google Scholar]
- Kurat C.F., Natter K., Petschnigg J., Wolinski H., Scheuringer K., Scholz H., Zimmermann R., Leber R., Zechner R., Kohlwein S.D. Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 2006;281:491–500. doi: 10.1074/jbc.M508414200. [DOI] [PubMed] [Google Scholar]
- Kurat C.F., Wolinski H., Petschnigg J., Kaluarachchi S., Andrews B., Natter K., Kohlwein S.D. Cdk1/Cdc28-dependent activation of the major triacylglycerol lipase Tgl4 in yeast links lipolysis to cell-cycle progression. Mol. Cell. 2009;33:53–63. doi: 10.1016/j.molcel.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Labar G., Bauvois C., Borel F., Ferrer J.L., Wouters J., Lambert D.M. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. ChemBioChem. 2010;11:218–227. doi: 10.1002/cbic.200900621. [DOI] [PubMed] [Google Scholar]
- Lake A.C., Sun Y., Li J.L., Kim J.E., Johnson J.W., Li D., Revett T., Shih H.H., Liu W., Paulsen J.E., Gimeno R.E. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J. Lipid Res. 2005;46:2477–2487. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- Langerhans R. Über multiple Fettgewebsnekrose. Virchows Arch. 1890;122:252–270. [Google Scholar]
- Langin D., Laurell H., Holst L.S., Belfrage P., Holm C. Gene organization and primary structure of human hormone-sensitive lipase: possible significance of a sequence homology with a lipase of Moraxella TA144, an antarctic bacterium. Proc. Natl. Acad. Sci. USA. 1993;90:4897–4901. doi: 10.1073/pnas.90.11.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J.G., Gorkiewicz G., Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lass A., Zimmermann R., Oberer M., Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J.L., Weissenbach J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman A.H., Martin B.R. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005;168:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- Listenberger L.L., Ostermeyer-Fay A.G., Goldberg E.B., Brown W.J., Brown D.A. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- Long J.Z., Li W., Booker L., Burston J.J., Kinsey S.G., Schlosburg J.E., Pavón F.J., Serrano A.M., Selley D.E., Parsons L.H. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Yang X., Liu J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle. 2010;9:2719–2725. doi: 10.4161/cc.9.14.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Perfield J.W., 2nd, Souza S.C., Shen W.J., Zhang H.H., Stancheva Z.S., Kraemer F.B., Obin M.S., Greenberg A.S. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- Montero-Moran G., Caviglia J.M., McMahon D., Rothenberg A., Subramanian V., Xu Z., Lara-Gonzalez S., Storch J., Carman G.M., Brasaemle D.L. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J. Lipid Res. 2010;51:709–719. doi: 10.1194/jlr.M001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder H., Sörhede-Winzell M., Contreras J.A., Fex M., Ström K., Ploug T., Galbo H., Arner P., Lundberg C., Sundler F. Hormone-sensitive lipase null mice exhibit signs of impaired insulin sensitivity whereas insulin secretion is intact. J. Biol. Chem. 2003;278:36380–36388. doi: 10.1074/jbc.M213032200. [DOI] [PubMed] [Google Scholar]
- Narbonne P., Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari L., Baladron V., Aroca-Aguilar J.D., Balko N., Heredia R., Meyer C., Notario P.M., Saravanamuthu S., Nueda M.L., Sanchez-Sanchez F. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J. Biol. Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- Ong K.T., Mashek M.T., Bu S.Y., Greenberg A.S., Mashek D.G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuga J., Ishibashi S., Oka T., Yagyu H., Tozawa R., Fujimoto A., Shionoiri F., Yahagi N., Kraemer F.B., Tsutsumi O., Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl. Acad. Sci. USA. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Kim H.J., Wang S., Higashimori T., Dong J., Kim Y.J., Cline G., Li H., Prentki M., Shulman G.I. Hormone-sensitive lipase knockout mice have increased hepatic insulin sensitivity and are protected from short-term diet-induced insulin resistance in skeletal muscle and heart. Am. J. Physiol. Endocrinol. Metab. 2005;289:E30–E39. doi: 10.1152/ajpendo.00251.2004. [DOI] [PubMed] [Google Scholar]
- Perkins J.M., Davis S.N. Endocannabinoid system overactivity and the metabolic syndrome: prospects for treatment. Curr. Diab. Rep. 2008;8:12–19. doi: 10.1007/s11892-008-0004-3. [DOI] [PubMed] [Google Scholar]
- Peyot M.L., Guay C., Latour M.G., Lamontagne J., Lussier R., Pineda M., Ruderman N.B., Haemmerle G., Zechner R., Joly E. Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion. J. Biol. Chem. 2009;284:16848–16859. doi: 10.1074/jbc.M109.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner F.P., Streith I.E., Schoiswohl G., Schweiger M., Kumari M., Eichmann T.O., Rechberger G., Koefeler H.C., Eder S., Schauer S. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J. Biol. Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B.N., Ables G.P., Otlivanchik O.A., Schoiswohl G., Zechner R., Blaner W.S., Goldberg I.J., Schwabe R.F., Chua S.C., Jr., Huang L.S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.A., Ben Ali Y., Abdelkafi S., Mendoza L.D., Leclaire J., Fotiadu F., Buono G., Carrière F., Abousalham A. In vitro stereoselective hydrolysis of diacylglycerols by hormone-sensitive lipase. Biochim. Biophys. Acta. 2010;1801:77–83. doi: 10.1016/j.bbalip.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel T.J., Williams J.M., Krieger E., Moshiri F., Stallings W.C., Brown S.M., Pershing J.C., Purcell J.P., Alibhai M.F. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry. 2003;42:6696–6708. doi: 10.1021/bi027156r. [DOI] [PubMed] [Google Scholar]
- Rydén M., Jocken J., van Harmelen V., Dicker A., Hoffstedt J., Wirén M., Blomqvist L., Mairal A., Langin D., Blaak E., Arner P. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1847–E1855. doi: 10.1152/ajpendo.00040.2007. [DOI] [PubMed] [Google Scholar]
- Rydén M., Agustsson T., Laurencikiene J., Britton T., Sjölin E., Isaksson B., Permert J., Arner P. Lipolysis—not inflammation, cell death, or lipogenesis—is involved in adipose tissue loss in cancer cachexia. Cancer. 2008;113:1695–1704. doi: 10.1002/cncr.23802. [DOI] [PubMed] [Google Scholar]
- Sakurada T., Noma A. Subcellular localization and some properties of monoacylglycerol lipase in rat adipocytes. J. Biochem. 1981;90:1413–1419. doi: 10.1093/oxfordjournals.jbchem.a133607. [DOI] [PubMed] [Google Scholar]
- Samuel V.T., Petersen K.F., Shulman G.I. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer T., O'Hare J., Diggs-Andrews K., Schweiger M., Cheng B., Lindtner C., Zielinski E., Vempati P., Su K., Dighe S. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg J.E., Blankman J.L., Long J.Z., Nomura D.K., Pan B., Kinsey S.G., Nguyen P.T., Ramesh D., Booker L., Burston J.J. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Schoiswohl G., Lass A., Radner F.P., Haemmerle G., Malli R., Graier W., Cornaciu I., Oberer M., Salvayre R. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J. Biol. Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- Shen W.J., Patel S., Miyoshi H., Greenberg A.S., Kraemer F.B. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J. Lipid Res. 2009;50:2306–2313. doi: 10.1194/jlr.M900176-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W.J., Yu Z., Patel S., Jue D., Liu L.F., Kraemer F.B. Hormone-sensitive lipase modulates adipose metabolism through PPARγ. Biochim. Biophys. Acta. 2011;1811:9–16. doi: 10.1016/j.bbalip.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Yoshimura K., Furuya N., Koike M., Ueno T., Komatsu M., Arai H., Tanaka K., Kominami E., Uchiyama Y. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem. Biophys. Res. Commun. 2009;382:419–423. doi: 10.1016/j.bbrc.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Shibata M., Yoshimura K., Tamura H., Ueno T., Nishimura T., Inoue T., Sasaki M., Koike M., Arai H., Kominami E., Uchiyama Y. LC3, a microtubule-associated protein1A/B light chain3, is involved in cytoplasmic lipid droplet formation. Biochem. Biophys. Res. Commun. 2010;393:274–279. doi: 10.1016/j.bbrc.2010.01.121. [DOI] [PubMed] [Google Scholar]
- Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Xiang Y., Wang Y., Baikati K., Cuervo A.M., Luu Y.K., Tang Y., Pessin J.E., Schwartz G.J., Czaja M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni K.G., Mardones G.A., Sougrat R., Smirnova E., Jackson C.L., Bonifacino J.S. Coatomer-dependent protein delivery to lipid droplets. J. Cell Sci. 2009;122:1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strålfors P., Belfrage P. Phosphorylation of hormone-sensitive lipase by cyclic AMP-dependent protein kinase. J. Biol. Chem. 1983;258:15146–15152. [PubMed] [Google Scholar]
- Summers S.A. Sphingolipids and insulin resistance: the five Ws. Curr. Opin. Lipidol. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- Tang Y., Chen Y., Jiang H., Nie D. Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ. 2011;18:602–618. doi: 10.1038/cdd.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschler U., Radner F.P., Heier C., Schreiber R., Schweiger M., Schoiswohl G., Preiss-Landl K., Jaeger D., Reiter B., Koefeler H.C. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J. Biol. Chem. 2011;286:17467–17477. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C., Stokowski R.P., Kershenobich D., Ballinger D.G., Hinds D.A. Variant in PNPLA3 is associated with alcoholic liver disease. Nat. Genet. 2010;42:21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- Turban S., Hajduch E. Protein kinase C isoforms: mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett. 2011;585:269–274. doi: 10.1016/j.febslet.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Unger R.H., Clark G.O., Scherer P.E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Vallerie S.N., Hotamisligil G.S. The role of JNK proteins in metabolism. Sci. Transl. Med. 2010;2:60rv65. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Berger J.E., Steinberg D. Hormone-Sensitive Lipase and Monoglyceride Lipase Activities in Adipose Tissue. J. Biol. Chem. 1964;239:401–409. [PubMed] [Google Scholar]
- Villena J.A., Roy S., Sarkadi-Nagy E., Kim K.H., Sul H.S. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- Voshol P.J., Haemmerle G., Ouwens D.M., Zimmermann R., Zechner R., Teusink B., Maassen J.A., Havekes L.M., Romijn J.A. Increased hepatic insulin sensitivity together with decreased hepatic triglyceride stores in hormone-sensitive lipase-deficient mice. Endocrinology. 2003;144:3456–3462. doi: 10.1210/en.2002-0036. [DOI] [PubMed] [Google Scholar]
- Wang H., Hu L., Dalen K., Dorward H., Marcinkiewicz A., Russell D., Gong D., Londos C., Yamaguchi T., Holm C. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J. Biol. Chem. 2009;284:32116–32125. doi: 10.1074/jbc.M109.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Bell M., Sreenevasan U., Hu H., Liu J., Dalen K., Londos C., Yamaguchi T., Rizzo M.A., Coleman R. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Holmes A.G., Pinnamaneni S.K., Garnham A.P., Steinberg G.R., Kemp B.E., Febbraio M.A. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- Wei E., Ben Ali Y., Lyon J., Wang H., Nelson R., Dolinsky V.W., Dyck J.R., Mitchell G., Korbutt G.S., Lehner R. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11:183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Welch C., Santra M.K., El-Assaad W., Zhu X., Huber W.E., Keys R.A., Teodoro J.G., Green M.R. Identification of a protein, G0S2, that lacks Bcl-2 homology domains and interacts with and antagonizes Bcl-2. Cancer Res. 2009;69:6782–6789. doi: 10.1158/0008-5472.CAN-09-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead R.H. A note on the absorption of fat. Am. J. Physiol. 1909;24:294–296. [Google Scholar]
- Wilson P.A., Gardner S.D., Lambie N.M., Commans S.A., Crowther D.J. Characterization of the human patatin-like phospholipase family. J. Lipid Res. 2006;47:1940–1949. doi: 10.1194/jlr.M600185-JLR200. [DOI] [PubMed] [Google Scholar]
- Wu J.W., Wang S.P., Alvarez F., Casavant S., Gauthier N., Abed L., Soni K.G., Yang G., Mitchell G.A. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- Yang L., Li P., Fu S., Calay E.S., Hotamisligil G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Lu X., Lombès M., Rha G.B., Chi Y.I., Guerin T.M., Smart E.J., Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Mu J., Birnbaum M.J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J. Biol. Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]
- Yuan X., Waterworth D., Perry J.R., Lim N., Song K., Chambers J.C., Zhang W., Vollenweider P., Stirnadel H., Johnson T. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am. J. Hum. Genet. 2008;83:520–528. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbergen F., Mandard S., Escher P., Tan N.S., Patsouris D., Jatkoe T., Rojas-Caro S., Madore S., Wahli W., Tafuri S. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem. J. 2005;392:313–324. doi: 10.1042/BJ20050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R., Madeo F. Cell biology: Another way to get rid of fat. Nature. 2009;458:1118–1119. doi: 10.1038/4581118a. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Haemmerle G., Wagner E.M., Strauss J.G., Kratky D., Zechner R. Decreased fatty acid esterification compensates for the reduced lipolytic activity in hormone-sensitive lipase-deficient white adipose tissue. J. Lipid Res. 2003;44:2089–2099. doi: 10.1194/jlr.M300190-JLR200. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]