Abstract
Given the complexity of the respiratory chain structure, assembly and regulation, the diagnostic workout for the identification of defects of oxidative phosphorylation (OXPHOS) is a major challenge. Spectrophotometric assays, that measure the activity of individual respiratory complexes in tissue and cell homogenates or isolated mitochondria, are highly specific, but their utilization is limited by the availability of sufficient biological material and intrinsic sensitivity. A further limitation is tissue specificity, which usually determines attenuation, or disappearance, in cultured fibroblasts, of defects detected in muscle or liver. We used numerous fibroblast cell lines derived from patients with OXPHOS deficiencies to set up experimental protocols required for the direct readout of cellular respiration using the Seahorse XF96 apparatus, which measures oxygen consumption rate (OCR) and extra-cellular acidification rate (ECAR) in 96 well plates. Results demonstrate that first level screening based on microscale oxygraphy is more sensitive, cheaper and rapid than spectrophotometry for the biochemical evaluation of cells from patients with suspected mitochondrial disorders.
Keywords: Micro-oxygraphy, Mitochondrial disorders, OXPHOS, Spectrophotometry
Highlights
► Microscale oxygraphy in human fibroblasts with genetically defined mitochondrial disease. ► This technology is more sensitive, cheaper and rapid than spectrophotometry. ► It can serve as a first-line screening in patients with suspected mitochondrial disease.
1. Introduction
Mitochondrial disorders are clinical conditions characterized by faulty oxidative phosphorylation (OXPHOS) in critical tissues, most frequently muscle and brain. The estimated prevalence is of at least 1 case in 5000 live births (Smeitink et al., 2006). Given the complexity of OXPHOS, the diagnosis of mitochondrial disease, based on the demonstration of the molecular defect and its consequences on energy metabolism, is not trivial. For instance, contrary to virtually all other groups of inborn errors of metabolism, a pathogenic mutation in an OXPHOS-related gene can be associated with mild or sometimes no biochemically detectable defect of the mitochondrial respiratory chain complexes. In addition, defects can also vary widely from tissue to tissue and in cultured cells. Thus, although biochemical assays are often crucial for the diagnosis, they must be evaluated in the context of clinical presentation, metabolic investigation, morphological findings, and molecular genetic screening.
Spectrophotometric assays on mitochondrial-enriched homogenate from bioptic tissue, e.g. skeletal muscle or less frequently liver or heart, is the most widely diagnostic procedure for the biochemical diagnosis of mitochondrial disease, providing valuable information on the functional status of individual components of the mitochondrial energy generating system (Benit et al., 2006; Jannsen et al., 2007; Kirby et al., 2007; Rustin et al., 1994). However, results can be hampered by, for instance, insufficient amount or inadequate storage of bioptic tissue; strict tissue specificity of the defect, for instance liver-restricted mtDNA depletion syndromes, which can give completely normal results in the muscle biopsy; and intrinsic pitfalls of biochemical assays, due to their limited sensitivity. Furthermore, while spectrophotometric assays can measure redox reactions specific to each complex or of parts of the respiratory chain, the proton flow across the inner mitochondrial membrane, associated with the respiratory complexes I, III, IV and V, requires methods that are not established routinely. For example, the routine spectrophotometric assay for oligomycin-sensitive ATPase (complex V) measures ATP hydrolysis, a function of the F1 particle, rather than proton flow, which occurs in the F0 particle. As a result, this assay is very often normal or close to normal in samples carrying the most frequent mutations of complex V, those affecting ATPase 6, a mtDNA-encoded component of the F0 particle. In addition, spectrophotometric assays can be completely negative or display fairly attenuated defects in cultured skin fibroblasts, even for patients with a clear defect in muscle (Rodenburg, 2011). Last but not least, very large amount of cells is needed for standard spectrophotometric or oxygraphic assays, typically > 1 × 106, which requires the use of expensive consumables and plastic ware for a relevant amount of time. Poor cell growth and early senescence are additional hurdles, which not infrequently hamper the analysis of primary fibroblast cultures from mitochondrial disease patients. On the other hand, the use of cultured fibroblasts has several advantages, including minimal invasiveness of the sampling procedure, the possibility to establish a permanent source of biological material, and the option to exploit cells as models for further studies, such as complementation assays for mutation validation, or as a source for the creation of transmitochondrial cybrids or iPS cells.
In an attempt to overcome the limitations due to tissue specificity, phenotypic attenuation, and limited amount of material, we compared the sensitivity of traditional spectrophotometric vs. microscale oxygraphic assays in cell lines from genetically defined mitochondrial disease patients. To this aim, we initially focussed our investigation on isolated defects of the respiratory chain complexes, which is the specific issue considered in the present paper. Several additional experiments are currently underway or have been incorporated in ad hoc projects on other mitochondrial conditions, including those associated with multiple OXPHOS deficiency caused, for instance, by defects of mtDNA translation, e.g. mt-tRNA gene mutations or mutations in mtDNA translation factors.
2. Materials and methods
2.1. Subjects and mutations
We analyzed a total of 19 fibroblast cell lines with different genetically proven, OXPHOS-related defects: six specific to complex I; three to complex III; six to complex IV; four to complex V.
The clinical presentation, biochemical results, and genetic characterization are summarized in Table 1.
Table 1.
| Muscle |
Fibroblasts |
||||||
|---|---|---|---|---|---|---|---|
| Patient | Affected gene | Mutation | Affected complex | % Residual activity (%heteroplasmy) | % Residual activiy (% heteroplasmy) | MRR% * | Reference |
| P1 | MTND3 | p.A47T | Complex I | 43% (> 95%) | Normal (90%) | 55% | |
| m.10197G>A | |||||||
| P2 | MTND3 | p.S45P | Complex I | 43% (90%) | 42% (50%) | 30% | Bugiani et al., 2004 |
| m.10191T>C | |||||||
| P3 | MTND5 | p.V253A | Complex I | Normal (50%) | Normal (30%) | 65% | Valente et al., 2009 |
| m.13094T>C | |||||||
| P4 | NDUFV1 | p.A341V c.1022C>T | Complex I | 36% | Normal | 55% | Bugiani et al., 2004 |
| p.A341V c.1022C>T | |||||||
| P5 | NDUFA10 | p.G99E c.296G>A | Complex I | 10% | 19% | 30% | |
| p.G99E c.296G>A | |||||||
| P6 | NDUFS1 | p.R557X c.1669C>T | Complex I | 14.5% | 50% | 20% | |
| p.T595A c.1783A>G | |||||||
| P7 | BCS1L | p.R73C c.217C>T | Complex III | n.d. | 30% | 50% | Fernandez-Vizarra et al., 2007 |
| p.F368I c.1102T>A | |||||||
| P8 | BCS1L | p.R183C c.547C>T | Complex III | 30% | Normal | 65% | Fernandez-Vizarra et al., 2007 |
| p.R184C c.550C>T | |||||||
| P9 | BCS1L | p.P407fsX408 c.1220delC | Complex III | 20% | Normal | 55% | |
| 5′UTR-194G>A | |||||||
| P10 | SURF1 | p.R264fsX290 c.790_791delAG | Complex IV | n.d. | 10% | 10% | |
| p.R264fsX290 c.790_791delAG | |||||||
| P11 | SURF1 | p.S252fsX290 c.758_759delCA | Complex IV | n.d. | 15% | 45% | |
| p.S252fsX290 c.758_759delCA | |||||||
| P12 | SURF1 | p.104fsX105 | Complex IV | 10% | 11% | 45% | |
| c.312_321del311_312insAT | |||||||
| IVS7-2A>G | |||||||
| P13 | SCO2 | p.Q146fsX175 c.436insC | Complex IV | 30% | Normal | 40% | |
| p.E140K c.418G>A | |||||||
| P14 | COX15 | p.H152X c.452C>G | Complex IV | 42% | 22% | 70% | Bugiani et al., 2005 |
| p.S344P c.1030T>C | |||||||
| P15 | COX6B1 | p.R19H c.221G>A | Complex IV | 15.0% | 49.0% | 45% | Massa et al., 2008 |
| p.R19H c.221G>A | |||||||
| P16 | MTATP6 | p.L156R | Complex V | n.d. | Normal (80%) | 65% | |
| m.8993T>G | |||||||
| P17 | MTATP6 | p.L156P | Complex V | Normal | Normal (> 90%) | 25% | |
| m.8993T>C | |||||||
| P18 | TMEM70 | IVS2-2A>G | Complex V | 53% | 45% | 75% | |
| p.L177fsX153 | |||||||
| c.348_351delATTG | |||||||
| P19 | TMEM70 | IVS2-2A>G | Complex V | n.d. | 28% | 50% | |
| IVS2-2A>G | |||||||
Complex I: patients P1 to P6
P1, P2 and P3 carried heteroplasmic mutations in ND genes of mtDNA, associated with infantile Leigh syndrome (P1, P2) or juvenile-onset combined progressive external ophthalmoplegia (PEO) and Ataxia (P3). P4, P5 and P6 were affected by infantile macrocystic leukoencephalopathy, caused by autosomal recessive mutations in nuclear-encoded subunits of complex I.
Complex III: patients P7 to P9
These patients presented a fairly homogeneous clinical presentation with lactic acidosis at birth, hypotonia, hypoglycemia, failure to thrive, encephalopathy, growth retardation, and delayed psychomotor development. All three carried mutations in BCS1L, an assembly factor of cIII.
Complex IV: patients P10 to P15
P10, P11 and P12 presented with Leigh syndrome due to Surf1 mutations, a cIV assembly factor. P13 was a compound heterozygous for mutations in SCO2, a protein involved in the formation of the copper centers of cIV, associated with cardiomyopathy and generalized muscle hypotonia. P14 had Leigh disease caused by mutations in COX15, the enzyme that converts inactive heme o into catalytically active heme a. P15 presented a leukodystrophy documented by MRI, with muscle weakness and cognitive decline, due to a homozygous missense mutation in COX6B1, a small nucleus encoded subunit of cIV.
Complex V: patients P16 to P19
P16 and P17 carried the 8993T>G and the 8993T>C mutations respectively, in mt-ATPase 6 gene, both associated with MILS.
P18 and P19 carried TMEM70 mutations and presented with cardiomyopathy, dysmorphic features and 3-methylglutaconic aciduria.
Control cell lines
As for controls, we used four different fibroblast cell lines derived from healthy donors, two of them being always present in each experiment.
2.2. Cell culture
All cells were investigated at passages 6–8. Cells were grown at 37 °C in a humidified 5% CO2 atmosphere, and were trypsinized once or twice a week. The culture medium, changed once a week, was Dulbecco's modified Eagle's medium (DMEM) containing 4,5 g/L glucose, 10% (V/V) fetal calf serum, 1 mM sodium pyruvate, 200 U/ml Penicillin G, 200 mg/ml streptomycin, and 4 mM glutamine.
2.3. Cell preparation for spectrophotometric assays
A pellet of ≈ 5 × 106 cells was resuspended in 2 ml Buffer A (250 mM sucrose, 20 mM MOPS KOH pH 7,4). Two ml of 0,2 mg/ml digitonin was added to buffer A. After incubation on ice for 5 min, samples were centrifuged at 5000 × g for 3 min, and pellets were resuspended in 3 ml of 1 mM sodium EDTA in buffer A. After incubation on ice for 5 minutes, samples were centrifuged at 10,000 ×g for 3 min. The pellets were resuspended in 1 ml 10 mM potassium phosphate buffer pH 7,4, and snap-frozen and thawed three times before enzyme measurements.
Mitochondrial respiratory chain (MRC) complexes were assayed according to established methods (Bugiani et al., 2004). In particular, we measured complex I as rotenone sensitive NADH oxidase CoQ1 reductase, complex III as antimycin sensitive CoQ1 oxidase cytochrome c reductase, complex IV as cyanide sensitive cytochrome c oxidase, and complex V as oligomycin sensitive ATP hydrolase. Each MRC enzyme specific activity was normalized to that of citrate synthase, a standard marker of cellular mitochondrial content.
2.4. OCR and ECAR measurements
Oxygen consumption rate (OCR) and extra-cellular acidification rate (ECAR) were measured in adherent fibroblasts with a XF96 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA). Each control and mutant fibroblast cell lines were seeded in 12 wells of a XF 96-well cell culture microplate (Seahorse Bioscience) at a density of 15–20 × 103 cells/well in 200 μL of DMEM and incubated for 24 h at 37 °C in 5% CO2 atmosphere. After replacing the growth medium with 180 μL of bicarbonate-free DMEM pre-warmed at 37 °C cells were preincubated for 30 min. before starting the assay procedure.
After baseline measurements of OCR (OCR-B) and ECAR, OCR was measured after sequentially adding to each well 20 μL of oligomycin (OL) and 22 μl of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), to reach working concentrations of 1 μM, and 0,7 μM, respectively.
The detailed protocol was as follows.
-
Basal OCR
4 cycles; each cycle consisting of:- 4′ Mixing
- 5′ Recording
-
Oligomycin addition through port A
4 cycles; each cycle consisting of:- 4′ Mixing
- 5′ Recording
-
FCCP addition through port B
5 cycles; each cycle consisting of:- 4′ Mixing
- 5′ Recording
In a preliminary set of experiments, we evaluated the fraction of non-mitochondrial oxygen consumption (Brandt and Nicholls, 2011), by measuring OCR after complete inhibition of the mitochondrial respiratory chain with either rotenone 0,5 μM, or antimycin 0,3 μM. This value, which was consistently ≈ 5% of total respiration in different cell lines and experiments, was subtracted from the values of other measurements in order to obtain the mitochondrial respiration.
Data were expressed as pmol of O2 per minute and normalized by cell number measured by the CyQUANT Cell proliferation kit (Invitrogen™), which is based on a fluorochrome binding to nucleic acids. Fluorescence was measured in a microplate luminometer with excitation wavelength at 485 ± 10 nm and emission detection wavelength at 530 ± 12.5 nm.
2.5. Data analysis
Four parameters were evaluated (Brandt and Nicholls, 2011).
-
1.
Maximum respiration rate (MRR) corresponds to OCR-F (minus rotenone-insensitive OCR).
-
2.
Respiratory control ratio (RCR) was evaluated from the ratio OCR-F/OCR-O.
-
3.
Oxidative versus glycolytic metabolism was calculated from the ratio between basal OCR (OCR-B)/ECAR.
-
4.
Spare respiratory capacity (SRC) was measured as the difference between OCR-F minus OCR-B.
All determinations were performed in 12 replicates for each sample.
2.6. Statistics
All statistical analyses were performed by using the IBM-SPSS (Statistical Package for Social Science) software package version 17.0 (IBM-SPSS, Chicago, IL).
For individual experiments, data referred to individual cell lines are presented as the mean of the replicates ± standard deviation (SD). Although the measurements obtained from the 12 replicates for each sample were relatively consistent in individual experiments, the values varied widely among different experiments, carried out in different days. In order to make the data collected in different experiments comparable to each other, and perform statistical analysis on cumulative measurements of the entire collection of experimental values, the measures of OCR-B, OCR-O, and OCR-F from each experiment were transformed into standard (z) scores. This transformation was made before experiment-to-experiment comparison. Altogether, we used 217 measures for control, and 350 measures for mutant cells. In practice, for each sample the overall mean was subtracted from each measure, and the result was divided by the overall SD of the experiment. The resulting values indicate how many SD's separate a given observation from the reference value of the experiment. Transformed data were then compared across samples and across experiments by univariate or repeated measures ANOVA (with Tamhane's T2 post-hoc test and with Welch statistics in the event of inequality of variances). MRR and SRC were calculated as z differences, while for RCR and oxidative metabolism we used raw data, assuming that the ratio itself allows standardization among experiments. Results with p-values < 0.05 were considered as statistically significant.
Two-step hierarchical cluster analysis was used to identify groups of subjects with similar values in OCR-B, OCR-O, and OCR-F independently of their status (pts or controls). The method calculates the contribution of each measure to the likelihood of cluster assignment. Clustering was performed adopting the Bayesian information criterion of Schwarz with log-likelihood measures for probability distribution of variables. The number of grouping clusters was not pre-determined.
3. Results
3.1. Spectrophotometric activities in fibroblasts
As reported in Table 1 a specific and isolated OXPHOS defect was identified in 11 mutant fibroblast cell lines, whereas the remaining 8 mutant cell lines showed normal spectrophotometric activities despite that pathogenic mutations in OXPHOS-related genes were found in all cases.
3.2. Microscale oxygraphic profile
Microscale oxygraphy simultaneously measures oxygen consumption rate (OCR) in different conditions, and extra-cellular acidification rate (ECAR) in 96-well plates, on as little as 1,5–2,0 × 104 cells/well. As an example we show a typical result obtained in a single experiment (Fig. 1) comprising one control and two mutant cell lines.
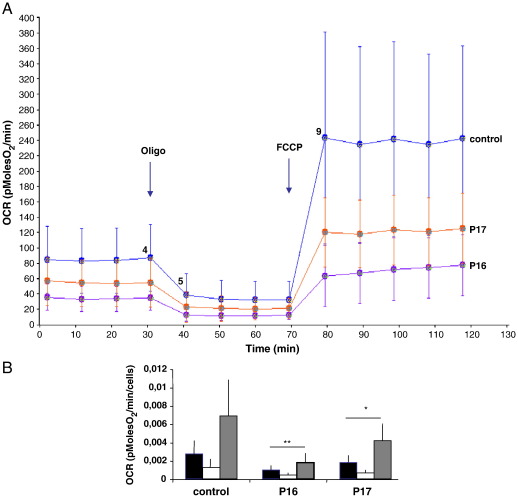
Fig. 1.
OCR profile in control and mutant cell lines. A: OCR Traces, expressed as pMolesO2/min in control and mutant cell lines P16 and P17. Vertical lines indicate the time of addition of Oligomycin and FCCP. Values at points 4, 5, 9 reflect OCR-B, -O, F, respectively. B: OCR normalization to cell number. Black, white and gray histograms indicate OCR-B, -O, -F, respectively. Bars indicate the standard deviation (SD). * p < 0.05; ** p < 0.01 (unpaired, two-tail Student's t test).
Panel A shows the graph of oxygen consumption (OCR) in different conditions. As expected, OCR in basal conditions (OCR-B) is inhibited when ATP synthesis is blocked by oligomycin (OCR-O), whereas is stimulated when oxidation is uncoupled from phosphorylation by FCCP (OCR-F). All three values are higher in the control cell line compared to mutant cell lines. The histogram in panel B reports the normalization of these data to the number of cells/well, expressed as mean values ± SD of 12 replicates/cell line. Unpaired two-tailed Student's t-test gave consistently significant p values (< 0.05) for each OCR measurement.
3.3. Analysis of OCR-B, OCR-O, and OCR-F in mutant vs. control cells
To overcome the high variability of numerical results in different experiments, individual values were used to obtain z-scores of OCR-B, -O, F.
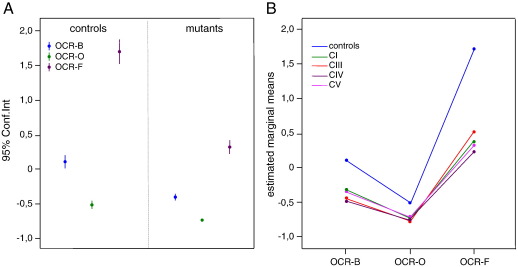
For statistical analysis, we compared the z-scores obtained in the control cell group vs. mutant cells, considered as a single group (Fig. 2A) or divided into subgroups according to the specific RC complex involved by the genetic defect (Fig. 2B). In both cases the values obtained in the control group were consistently and significantly higher than those obtained in the mutant groups, for each of the three mitochondrial respiratory conditions.
Fig. 2.
Analysis of OCR-B, -O, -F. The z score values (dots) for OCR-B, -O, -F are shown for control vs. mutant cells. In panel A, the control and mutant cells were considered as single groups and expressed as the means (dots) and 95% confidence intervals, Conf.Int. (bars). ANOVA test for controls vs. mutants: OCR-B p < 0,0001; OCR-O p < 0,0001; OCR-F p < 0,0001. In panel B the mutant cells were divided into subgroups according to the specific RC complex involved by the genetic defect. ANOVA test for interaction through repeated measures: mutants vs. controls, p < 0.0001; between mutants, p = 0.384. See text for details.
3.4. Integrated bioenergetics parameters in mutant vs. control cells
Values of OCR-B, -O, -F were used to calculate the bioenergetic parameters, i.e. MRR, RCR and SRC in the control vs. mutant cells, again considered as either a single group (Fig. 3A) or divided by subgroups each affecting a specific RC complex (Fig. 3B).
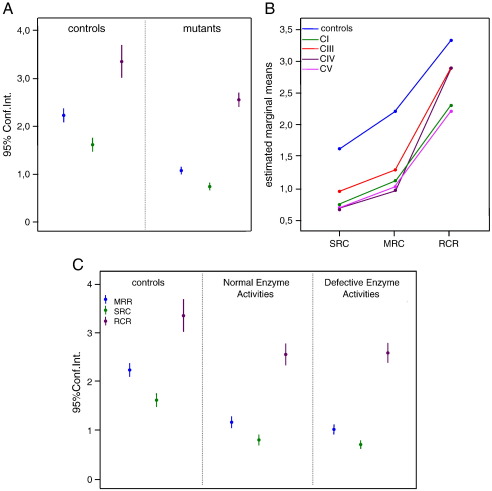
Fig. 3.
Analysis of integrated bioenergetic parameters MRR, SRC and RCR. In panel A, the control and mutant cells were considered as single groups and MRR, SRC, and RCR values are expressed as the means (dots) and 95% confidence intervals (Conf.Int., bars). ANOVA test: MRR (F = 241,61 p < 0,0001); SRC (F = 168,13 p < 0,0001) and RCR (F = 22,56 p < 0,0001). In panel B the mutant cells were divided into subgroups according to the specific RC complex involved by the genetic defect. Values are expressed as estimated marginal means. ANOVA test for interaction through repeated measures, mutant vs. controls: p < 0.0001. Panel C reports the results obtained in controls and in two series of mutant cell lines: spectrophotometrically normal cell lines (n. 8, Normal Enzyme Activities); and spectrophotometrically defective cell lines (n. 11, Defective Enzyme Activities). ANOVA test for controls vs. mutants, p < 0.0001. See text for details.
MRR, RCR, and SRC were all significantly lower in the mutant vs control groups. Importantly, significantly reduced values were obtained also in a subgroup composed of the 8 mutant cell lines displaying normal activity of individual respiratory chain complexes (Fig. 3C), indicating higher sensitivity of the oxygraphic relative to the spectrophotometric assay in detecting impaired mitochondrial RC function. Interestingly, defective values were also detected in cell lines with the 8993T>G mutation in mt-ATPase, that usually display normal or very mild reduction by the spectrophotometric assay for complex V, based on ATP hydrolysis.
MRR or maximal respiration rate is the difference between maximal mitochondrial respiration measured as OCR in mitochondria uncoupled by a ΔΨ dissipator, FCCP, and the non-mitochondrial OCR measured in mitochondria exposed to rotenone, a strong complex I inhibitor. A reduction of MRR indicates reduced electron flow through the respiratory chain from complexes I and II, to complexes III and IV, and eventually to molecular oxygen. SRC, or spare respiratory capacity, is the difference between OCR-F and OCR-B, and stands as an indicator of the bioenergetic reserve. A reduction of SRC indicates that in basal conditions cells work already at sub-maximal capacity, being unable to respond to further energetic demand. Likewise, the respiratory control ratio, RCR, is an index of mitochondrial coupling. In isolated mitochondria or permeabilized cells, RCR is calculated as the ratio between the OCR in the presence vs. absence of ADP (respiratory states 3 and 4, respectively). Since intact cells are impermeable to ADP, we simulated states 3 and 4 by measuring OCR in the presence of the uncoupler FCCP (OCR-F) or of the complex V inhibitor oligomycin (OCR-O), respectively (Brandt and Nicholls, 2011). Thus, a decrease of RCR can be due to either reduced inhibitory effect of oligomycin on complex V, reflected by increased OCR-O, or decreased maximal respiratory capacity, reflected by reduced OCR-F.
As expected, reduced RCR in complex I, III and IV defects was largely due to reduced OCR-F. The reduction of RCR observed in complex V mutant cells was partly due to high OCR-O, reflecting reduced sensitivity to oligomycin, as previously suggested in NARP mutant cybrids (Mattiazzi et al., 2004) and in a NARP yeast model (Kucharczyk et al., 2009). However, OCR-F was also reduced, which suggests an effect of impaired complex V on respiration. This data, which is in agreement with previous results on 8993T>G and 9176>T>G cybrids (D'Aurelio et al., 2010), could be related to the recent observation that complex V dimers play a structural role in the formation of mitochondrial cristae (Paumard et al., 2002).
3.5. OCR/ECAR ratio
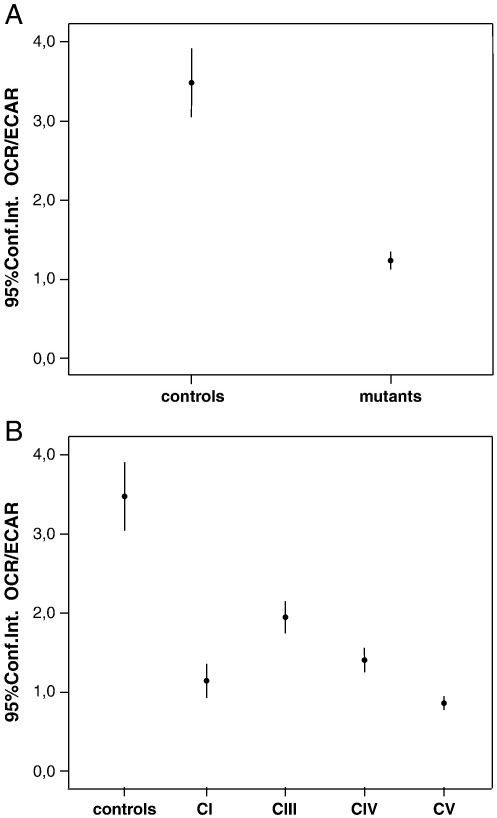
ECAR, or extra-cellular acidification rate, is proportional to the production of lactic acid, therefore reflecting the rate of glucose utilization via anaerobic glycolysis (in basal conditions). OCR-B and ECAR are indicators of mitochondrial respiration and glycolysis respectively. Defects of the former can be associated with compensatory stimulation of the latter. Accordingly, the OCR/ECAR ratio, which is around 3.5 in normal cells, was significantly decreased in the mutant cell group, ranging from 1.0 to 1,5, (Fig. 4A). The lower OCR/ECAR ratio was observed in complex V deficient patients (Fig. 4B).
Fig. 4.
Analysis of OCR/ECAR in basal conditions. A. OCR/ECAR in the control vs mutant cell lines considered as single groups. Bars indicate the confidence intervals (Conf.Int.). ANOVA test for controls vs. mutants, p < 0.0001. B. OCR/ECAR ratio evaluated into subgroups according to the specific RC complex involved by the genetic defect. Bars indicate the confidence intervals (Conf.Int.). ANOVA test of controls vs. mutants, p < 0.0001.
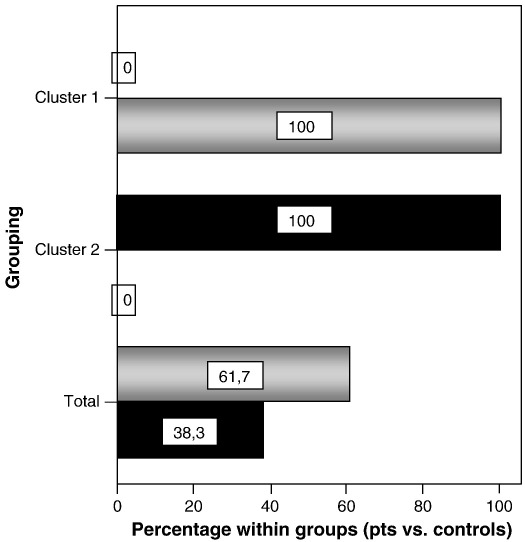
3.6. Cluster analysis
Clusterization of individual samples based on experimental values of OCR-B, OCR-O, and OCR-F, resulted in the formation of two widely separate clusters, one comprising all the mutant, the other all the control cell lines. Of the 567 total measurements analyzed in this work and assigned to clusters, 217 derived from controls and were assigned to cluster 1, while the remaining 350 derived from mutant cells and were assigned to cluster 2. This result, shown as integrated clustering (Fig. 5), indicates high specificity of microscale oxygraphy in discriminating OXPHOS defective from OXPHOS proficient cells. In fact, cluster 1 contained the 100% of the control cell lines, whereas cluster 2 contained the 100% of mutant cell lines without any overlap.
Fig. 5.
Clustering of individual samples based on experimental values of OCR-B, OCR-O, and OCR-F. The histogram shows the distribution and frequency of each cluster. Of the 567 cases assigned to clusters, 217 were assigned to the first (38,3%), 350 to the second (61,7%). Cluster 1 contains all of the control cell lines (100%), whereas cluster 2 contains all of the mutant cell lines (100%), with no overlap (0). Gray bars: mutant cell lines; Black bars: control cell lines.
4. Discussion
The aim of this study was threefold.
-
a)
to set-up biochemical protocols on fibroblasts with genetically proven defects in OXPHOS-related genes, predicting impaired function of isolated RC complexes;
-
b)
to test whether OCR and ECAR abnormalities can be detected in fibroblasts with normal spectrophotometric activities derived from patients with well established OXPHOS defects in muscle or with specific mutations associated with mitochondrial disease;
-
c)
to scale-down the amount of material, and speed up the procedure for the biochemical screening of OXPHOS disease in cultured cells.
Recent observations indicate that mitochondrial respiration is largely carried out by the fraction of respiratory chain complexes that assemble together in supercomplexes forming functionally active units, the respirasomes (Acín-Pérez et al., 2008; Wittig and Schägger, 2009). Thus, the measurement of activities of single mitochondrial complexes does not necessarily reflect their functional capacity in vivo. We reasoned that microscale oxygraphy could overcome the limited sensitivity of spectrophotometric measurements, improve efficiency by scaling down the amount of required material, and increase throughput by simultaneously screening numerous cell lines. In order to test this approach, we used several fibroblast cell lines derived from patients with genetically defined OXPHOS deficiency. The quantitative output of the analysis included the maximal respiration rate, the respiratory control ratio, the spare respiratory capacity and the bioenergetic partitioning between respiration and glycolysis.
An isolated defect of complex I, complex III, complex IV or complex V was spectrophotometrically documented in 11 cell lines, whereas the remaining 8 had no detectable defect in either MRC complex, although the genetic abnormality associated with each of these lines predicted functional impairment of one specific complex.
Variable but consistent reduction of MRR was indeed measured in each and every mutant cell line, irrespective of the affected complex and type of mutation (Table 1). Likewise, highly significant differences of OCR-B, -O, and –F, and derived bioenergetic parameters, MRR, SRC and RCR, were also obtained by ANOVA analysis of the overall values of control vs mutant cell lines, expressed as z-scores to allow comparison of results obtained in different experiments. Together, these results indicate that microscale oxygraphy is much more sensitive than spectrophotometric assays, at least in fibroblast cells. An instructive example is that of P3 cells, carrying 30% heteroplasmic V253A mutation in the ND5 subunit of complex I. Although we previously showed that a complex I defect can spectrophotometrically be detected for this mutation only when the mutation load is > 80% (Valente et al., 2009), and our P3 cells were in fact completely normal by this assay, the MRR was as low as 65% of the control mean obtained in the same experiment (Table 1). In each and every experiment, the MRR percentages of mutant vs control cell lines were all variably but consistently reduced (Table 1).
In all cases this result was accompanied by reduced OCR/ECAR ratio, suggesting the occurrence of a glycolytic shift, and, in many cases, by concordant reduction of the OXPHOS RCR and spare respiratory capacity.
In conclusion, increased sensitivity, higher discriminative capacity, and reduced sample size, make microscale oxygraphy suitable and indeed worthwhile for the primary screening of OXPHOS defects in cells. In addition, this system can be used to validate the biochemical consequences of new OXPHOS related mutant genes in cultured cells, making them exploitable for further investigation, for instance rescue and complementation assays, or biochemical characterization of cybrid derivatives. Accordingly, we recently applied Seahorse technology to evaluating global respiration in individuals affected by a progressive encephalopathy associated with cIII deficiency detected in muscle but not in fibroblasts that were in fact normal. Patients had mutations in a gene encoding a putative CIII assembly factor, i.e. tetratricopeptide 19 (TTC19). However, the oxygen consumption rate was significantly defective in naïve mutant fibroblasts and myoblasts, and returned to normal after re-expression of the wild-type TTC19 cDNA (Ghezzi et al., 2011). The same technology was used to evaluate respiration in fibroblasts derived from a LHON patient carrying the 3460G>A mtDNA mutation (Giorgio et al., 2012). The OCR of mutant cells was lower than that of wild-type cells in basal conditions, and was virtually insensitive to FCCP, indicating hardly any reserve respiratory capacity (Giorgio et al., 2012). Thus, like the TTC19 mutant fibroblasts, microscale oxygraphy unraveled a significant respiratory defect also in LHON fibroblasts, unlike traditional spectrophotometric assays performed in the same cells (Giorgio et al., 2012). Most importantly, the detection of a defective bioenergetic profile in cells can further orient the diagnostic investigation and serve as a preliminary screening for, or even an approach alternative to, more invasive procedures, e.g. muscle biopsy, especially in pediatric cases. Last but not least, microscale oxygraphy can be extended to a number of other biological samples, including fresh tissue homogenates, isolated mitochondria, and even small experimental organisms such as flies, fish embryos and worms. Accordingly, protocols to evaluate global respiration in isolated mitochondria have recently been published using an XF24 instrument (Rogers et al., 2011). We have successfully adapted this protocol on the XF96 instrument and are setting up the measurement of OCR on the 800x g supernatant from 10% crude muscle homogenate.
The main limitation of the current protocol is the impossibility to identify which of the respiratory chain complexes is defective. Dedicated protocols based on the use of specific respiratory substrates in permeabilized cells could eventually overcome this limitation.
Competing interests
PB Jensen is a current employee of Seahorse Bioscience. There are no patents, products in development or marketed products to declare. However, Seahorse Bioscience produces a product, the XF96 analyzer, which is used for development of the approach described in this manuscript. This does not alter the authors' adherence to all the Mitochondrion policies on sharing data and materials.
Acknowledgments
The financial support of the Pierfranco and Luisa Mariani Foundation (grant n. R-10-84 to V.T.), Telethon GGP 11088 to V.T., Telethon GPP 10005 and Cariplo 2011-05-26 to M.Z. grants are gratefully acknowledged. I.D. is supported by the Italian Foundation Mitocon ONLUS.
References
- Acín-Pérez R., Fernández-Silva P., Peleato M.L., Pérez-Martos A., Enriquez J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Benit P., Goncalves S., Philippe D.E., Briere J.J., Martin G., Rustin P. Three spectrophotometric assays for the measurements of the five respiratory chain complexes in minuscule biological samples. Clin. Chim. Acta. 2006;34:283–292. doi: 10.1016/j.cca.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M., Invernizzi F., Alberio S., Briem E., Lamantea E., Carrara F., Moroni I., Farina L., Spada M., Donati M.A., Uziel G., Zeviani M. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta. 2004;1659:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bugiani M., Tiranti V., Farina L., Uziel G., Zeviani M. Novel mutations in COX15 in a long surviving Leigh syndrome patient with cytochrome c oxidase deficiency. J. Med. Genet. 2005;42(5):e28. doi: 10.1136/jmg.2004.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aurelio M., Vives-Bauza C., Davidson M.M., Manfredi G. Mitochondrial DNA background modifies the bioenergetics of NARP/MILS ATP6 mutant cells. Hum. Mol. Genet. 2010;19:374–386. doi: 10.1093/hmg/ddp503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Vizarra E., Bugiani M., Goffrini P., Carrara F., Farina L., Procopio E., Donati A., Uziel G., Ferrero I., Zeviani M. Impaired complex III assembly associated with BCS1L gene mutations in isolated mitochondrial encephalopathy. Hum. Mol. Genet. 2007;16:1241–1252. doi: 10.1093/hmg/ddm072. [DOI] [PubMed] [Google Scholar]
- Ghezzi D., Arzuffi P., Zordan M., Da Re C., Lamperti C., Benna C., D'Adamo P., Diodato D., Costa R., Mariotti C., Uziel G., Smiderle C., Zeviani M. Mutations in TTC19 cause mitochondrial complex III deficiency and neurological impairment in humans and flies. Nat. Genet. 2011;43:259–263. doi: 10.1038/ng.761. [DOI] [PubMed] [Google Scholar]
- Giorgio V., Petronilli V., Ghelli A., Carelli V., Rugolo M., Lenaz G., Bernardi P. The effects of idebenone on mitochondrial bioenergetics. Biochim. Biophys. Acta. 2012;1817(2):363–369. doi: 10.1016/j.bbabio.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A.J., Trijbels F.J., Sengers R.C., Smeitink J.A., van den Heuvel L.P., Wintjes L.T., Stoltenborg-Hogenkamp B.J., Rodenburg R.J. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin. Chem. 2007;53(4):729–734. doi: 10.1373/clinchem.2006.078873. [DOI] [PubMed] [Google Scholar]
- Kirby D.M., Thorburn D.R., Turnbull D.M., Taylor R.W. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:93–119. doi: 10.1016/S0091-679X(06)80004-X. [DOI] [PubMed] [Google Scholar]
- Kucharczyk R., Rak M., di Rago J.-P. Biochemical consequences in yeast of the human mitochondrial DNA 8993T > C mutation in the ATPase6 gene found in NARP/MILS patients. Biochim. Biophys. Acta. 2009;1793:817–824. doi: 10.1016/j.bbamcr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Massa V., Fernandez-Vizarra E., Alshahwan S., Bakhsh E., Goffrini P., Ferrero I., Mereghetti P., D'Adamo P., Gasparini P., Zeviani M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am. J. Hum. Genet. 2008;82:1281–1289. doi: 10.1016/j.ajhg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M., Vijayvergiya C., Gajewski C.D., DeVivo D.C., Lenaz G., Wiedmann M., Manfredi G. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum. Mol. Genet. 2004;13(8) doi: 10.1093/hmg/ddh103. [DOI] [PubMed] [Google Scholar]
- Paumard P., Vaillier J., Coulary B., Schaeffer J., Soubannier V., Mueller D.M., Brèthes D., di Rago J.P., Velours J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg R.J. Biochemical diagnosis of mitochondrial disorders. J. Inherit. Metab. Dis. 2011;34:283–292. doi: 10.1007/s10545-010-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G.W., Brand M.D., Petrosyan S., Ashok D., Elorza A.A., Ferrick D.A., Murphy A.N. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One. 2011;6(7):e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P., Chretien D., Bourgeron T., Gérard B., Rötig A., Saudubray J.M., Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Smeitink J.A., Zeviani M., Turnbull D.M., Jacobs H.T. Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 2006;3:9–13. doi: 10.1016/j.cmet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Valente L., Piga D., Lamantea E., Carrara F., Uziel G., Cudia P., Zani A., Farina L., Morandi L., Mora M., Spinazzola A., Zeviani M., Tiranti V. Identification of novel mutations in five patients with mitochondrial encephalomyopathy. Biochim. Biophys. Acta. 2009;1787:491–501. doi: 10.1016/j.bbabio.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Wittig I., Schägger H. Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochim. Biophys. Acta. 2009;1787:672–680. doi: 10.1016/j.bbabio.2008.12.016. [DOI] [PubMed] [Google Scholar]