Abstract
Drug screening on sodium currents of native myofibers by means of voltage-clamp recordings is predictive of pre-clinical anti-myotonic activity in vivo and ex vivo. By this approach we identified the N-benzylated beta-proline derivative of tocainide (To10) as the most potent use-dependent blocker of Nav1.4 so far. We tested novel analogs with modifications on the pharmacophore groups of To10. The substitution of the proline cycle with less planar piperidine or piperazine rings disclosed the importance of a two carbon atom distance and/or an additional nitrogen atom for potency. Structural changes on the xylididic group corroborated the role of a proper electronic cloud for hydrophobic interactions with the binding site. The N-benzylated moiety lead to a stereoselective behavior only in the rigid alpha-proline analog To11 vs. To10 and N-benzylated tocainide (To12). The results confirm the strict structural requirements of Nav1.4 blockers and allow to refine the drug design toward novel anti-myotonic drugs.
Keywords: Voltage-gated Na+ channel, Myotonic syndromes, Tocainide, Pharmacophore groups
1. Introduction
The block of voltage-gated Na+ channel by local anesthetic-like (LA) drugs has therapeutic value for numerous disorders characterized by abnormal membrane excitability of central and peripheral nervous system as well as for myotonic syndromes and cardiac arrhythmias [1]. Although a certain overlapping pharmacology exists between channel types of different tissues, structural and biophysical differences represent an important platform for designing selective blockers toward channel isoforms [1–3]. Nowadays, some more isoform-specific blockers are under development, with the aim of obtaining safe and potent anti-nociceptive compounds [4]. A similar approach is of clinical interest for identifying selective blockers of skeletal muscle sodium channels (Nav1.4). The classical LA-like drugs tocainide and mexiletine, formerly used for treating rare myotonic syndromes, have been withdrawn from the market, thus further reducing the few therapeutic options for these patients. In this context, it is of importance to deepen the understanding of the molecular requisites for drugs acting on Nav1.4, in terms of both absolute potency and state-dependent block. This latter mechanism resides in the conformation-dependent change in drug binding site affinity, which increases when the channels open or inactivate [5], then leading to a stronger drug potency in disease-related conditions, such as membrane depolarization or high frequency trains of action potentials. Amino acid residues on the S6 segment of each homologous domain (D1–D4) of the α subunit, are critical for LAs binding and activity on Na+ channels of various excitable tissues. Although the increasing efforts in identifying sub-type specific differences in drug binding regions, the two aromatic residues (Phe and Tyr) on D4–S6 have a recognized role for the interaction with the main pharmacophores of the LA molecule, e.g. the ionizable amino group and the aromatic ring, by means of cation–π and π–π interactions, respectively [6–9]. Also, a third point interaction driven by the chiral carbon atom nearby the pharmacophore amino group can occur at a site in D1–S6 segment and may account for the stereoselective drug action [10–12]. We found that this latter characteristic is enhanced by constraining the stereogenic center in a rigid cycle, as in the α proline-like analog of tocainide, To5 [13]. Furthermore, the increase of the lipophilicity on the nitrogen atom by introducing a benzyl moiety on both tocainide (Benzyl-Toc) and its proline-like analogs (Benzyl-To5 and Benzyl-To9) largely increases drug potency while maintaining a use-dependent behavior, thus adding important evidence about the pharmacophore requirements on tocainide backbone for a strong and therapeutically relevant interaction with high-affinity channel states [12,14]. Interestingly, molecular modeling studies performed to predict docking energies for drug–receptor interaction support the importance in enhancing hydrophobicity and/or molecular surface of pharmacophores for the interaction [15,16]. In parallel the results from in vitro screening on sodium currents is highly predictive for detecting therapeutically relevant effects on skeletal muscle. In fact, in previous studies we have demonstrated that compounds active on frog muscle channels have, in the same concentration range, in vivo and ex vivo anti-myotonic activity in murine models [13,26]. The benzyl-To9 (To10), at the moment the strongest use-dependent blocker of Nav1.4 in our hand, is then a lead compound for obtaining new compounds with an improved pharmacological profile, in term of potency for use-dependent block and possibly tissue selectivity, while enhancing our understanding of the molecular requirements of pharmacophores for drug–channel interactions. To this aim, in the present study we evaluated the effects of further modifications at the level of the pharmacophore groups in the To10 structure on the native Nav1.4. In particular the structure–activity relationship study was aimed at further evaluating the role of rigidity/distance between pharmacophores by constraining the benzylated amino group in the less-planar piperidine/piperazine groups vs. the proline-cycle and testing the effect of substitutions that would influence electron distribution and lipophilicity on the other pharmacophore group, i.e., the xylididic-moiety (Fig. 1). In parallel, due to the proximity of the N-benzyl group to the chiral center and based on preliminary evidences that To10 has a poor, if any, stereoselectivity [17], we tested if the high affinity hydrophobic interaction plays a “dominant” role over the weaker stereoselective disposition of this class of compounds [12]. To this aim, the effects of enantiomeric pairs of To10, benzyl-To5 (To11) and benzyl-tocainide (To12) were also evaluated.
Fig. 1.
Chemical structure of tocainide and its newly synthesized N-benzylated analogs. The boxes show the compounds presently tested as racemates (full line) or enantiomer (dashed lines). ∗The position of the chiral carbon atom.
The results support the potential therapeutic interest of this class of tocainide derivatives, and reinforce our long-term objective to develop a reliable pharmacophore model for N-benzylated compounds which may help to improve potency and selectivity toward Nav1.4 for future application in myotonic patients.
2. Methods
2.1. Fiber preparation and voltage clamp apparatus
Segments of undamaged single muscle fibers (about 1 cm in length) were obtained by microsurgery (plucking procedure) from the ventral branch of the semitendinosus muscle of Rana Esculenta bathed in normal physiological solution at room temperature. The cut-end fiber was then perfused with an internal solution and mounted across three chamber partitions, which delineated the four pools. Three strips of vaseline were applied over the fiber and carefully sealed to the fiber to reduce leakage. The width of the gaps of the central pools (A and B) had been previously set to 70–100 μm and 200 μm, respectively. Four KCl/agar bridges electrodes connected the recording chamber to the voltage clamp amplifier based on methods described by Hille and Campbell (1976) and detailed elsewhere [11,12,18]. For recordings, the solution in the pool A was replaced with the external solution and after about 10 min of equilibration the recordings were performed at 10 °C. The usual holding potential (h.p.) was −100 mV, unless otherwise specified.
Sodium currents were recorded using an amplifier connected via an A/D and D/A Digidata 1200 Interface (Axon) to a 486 DX2/66 personal computer and stored on the hard disk. The stimulation protocols and data acquisition was driven by the Clampex program (pClamp6 software package; Axon Instruments, Foster City, CA). The currents flowing in response to depolarizing command voltages were low-pass filtered at 10 kHz (Frequency Devices, USA), visualized on an oscilloscope and sampled at 20 kHz. The acquired traces were analyzed later using Clampfit program (pClamp6 software package; Axon Instruments).
2.2. Drugs and solutions
The following solutions (mM) were used: normal physiological solution: NaCl 115, KCl 2.5, CaCl2 1.8, Na2HPO4 2.15, NaH2PO4 0.85; external solution: NaCl 77, choline–Cl 38, KCl 2.5, CaCl2 1.8, Na2HPO4 2.15, NaH2PO4 0.85; internal solution: CsF 105, MOPS 5, MgSO4 2, EGTA 5, Na2ATP 0.55. The pH was adjusted at 7.2 with a standard NaOH concentrated (5 N) solution.
The compounds tested and shown in Fig. 1 were tocainide (Toc), N-benzyl-tocainide (To12); the α-proline derivatives: N-(2,6-dimethylphenyl)pyrrolidine-2-carboxamide (To5), and its benzyl derivative 1-benzyl-N-(2,6-dimethylphenyl)pyrrolidine-2-carboxamide (To11); the β-proline derivatives: N-(2,6-dimethylphenyl)pyrrolidine-3-carboxamide (To9), and its benzyl derivative, 1-benzyl-N-(2,6-dimethylphenyl)pyrrolidine-3-carboxamide (To10); the α and β N-benzylated piperidine derivatives: 1-benzyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide (To35) and 1-benzyl-N-(2,6-dimethylphenyl)piperidine-3-carboxamide (To31); the N-benzylated β piperazine-derivative: 4-benzyl-N-(2,6-dimethylphenyl)piperazine-2-carboxamide (To32); the derivatives of To10 with substitutions at the xylidide moiety: 1-benzyl-N-phenylpyrrolidine-3-carboxamide (To13); 1-benzyl-N-(4-chlorophenyl)pyrrolidine-3-carboxamide (To14); 1-benzyl-N-(2,3,5,6-tetramethylphenyl)pyrrolidine-3-carboxamide (To15); 1-benzyl-N-(4-chloro-2,3,5,6-tetramethylphenyl)pyrrolidine-3-carboxamide (To16) (Fig. 1).
All the compounds were prepared in our laboratories as salts, according to procedures described in details elsewhere [19,20]. The compounds were synthesized as enantiomers (To10, To11 and To12) or racemates (piperidine and piperazine derivatives and analogs substituted at the xylididic moiety).
Stock solutions were prepared by dissolving the compounds in external solution containing DMSO (<1%). DMSO, at the highest concentration used for dilution (<0.2%), was without effect on the parameters recorded. All other chemicals used were of analytical grade and obtained from Sigma Chemical Company (St. Louis, MO, USA).
2.3. Pulse protocols and statistical analysis
The tonic and the use-dependent blocks exerted by each compound were evaluated as the block of INa at the beginning and the end of a high frequency (10 Hz) stimulation. For this protocol, the membrane potential was held at −100 mV, a h.p. closer to physiological values at which almost all the channels are still in the resting state [12], unless otherwise specified for specific experimental purposes. Thirty seconds trains of depolarizing 10 ms pulses to −20 mV from the h.p. were applied at the frequency of 2 and 10 Hz. At both frequencies, the drug-induced reduction of peak INa at the first pulse was considered as tonic block (block of the channel in the resting state). The use-dependent behavior was estimated by the further reduction of the current observed in the presence, but not in the absence, of use-dependent compounds, that progressively cumulated over the tonic block in a frequency-dependent manner until a new equilibrium was reached. The value of the current at the equilibrium normalized with respect to the current in the absence of drug was used to calculate the potency of the drug for blocking the channels under conditions of excessive stimulation (e.g. high-frequency firing).
Steady-state inactivation (h∞) curves were determined by cyclic protocol of pulse sequences. Each sequence consisted of a conditioning pulse to −140 mV for 500 ms (to have most of the sodium channels in the “activatable” state), a prepulse of variable potential of 1000 ms duration and the 10 ms test pulse to −20 mV; after a pause of 1 s the sequence was cyclically repeated 18–20 times with the prepulse potential value increased each time by 5 mV steps [11,12].
2.4. Data analysis and statistic
The data were expressed as mean ± standard error of the mean (SEM). The estimates of SEM of normalized INa values have been obtained as described previously [11,12]. Molar concentrations of the drugs tested producing a 50% block of INa (IC50) in the various experimental conditions were determined by using a non-linear least squares fit of the concentration–response curves to the following logistic equation: Effect = −100/{1 + (K/[drug])n}, where Effect = percent change of INa; −100 = maximal percent block of INa; K = IC50; n = logistic slope factor; [drug] = molar concentration of the compound. The h∞ curves have been fitted with a single Boltzmann distribution and the potential at which 50% of the sodium channels were inactivated (Vh1/2) was calculated at the inflection point of the curves [21,14]. The left shift of the h∞ curve produced by the drug was a function of the concentration used and of the relative affinity for the channel in the inactivated state.
Statistical significance of differences between couples of mean values has been estimated by unpaired Student’s t-test and considered significant for p < 0.05. The statistical significance between IC50 values ± SE obtained from the fit was also evaluated by a Student’s t distribution using a number of degrees of freedom equal to the total number of preparations determining each point of the curve minus the number of means determining the curve minus two for the free parameters [12].
pKa values were calculated using Advanced Chemistry Development (ACD) Software Solaris V. 4.76 [19].
3. Results
3.1. Piperidine and piperazine analogs of To10 and To11: potency for tonic and use-dependent block
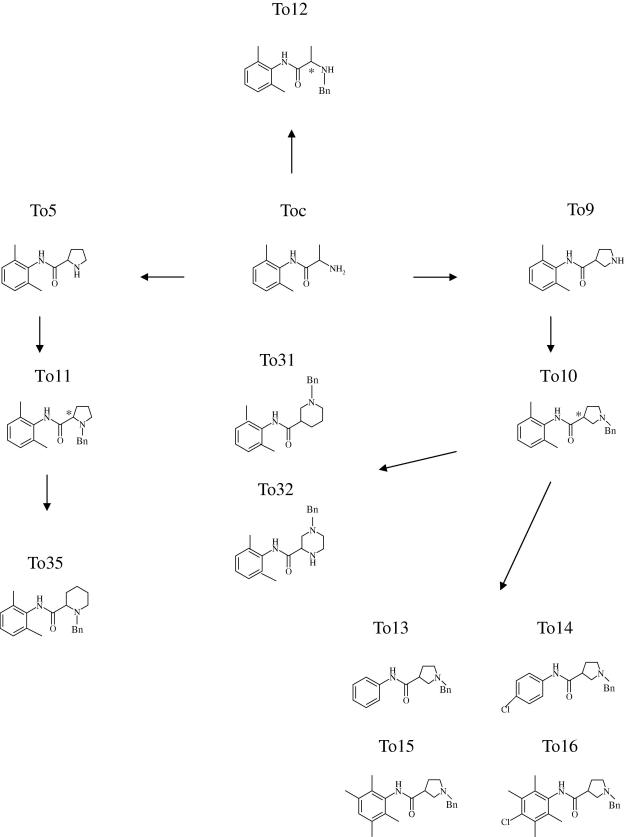
As first change, the proline cycle has been replaced with a piperidine one having the benzyl group on the aminic nitrogen atom in beta (To31) or in alpha (To35) with respect to the stereogenic center (Fig. 1). Thus To31 and To35 are piperidine analogs of To10 and To11, respectively. This substitution allows to further investigating the role of distance between pharmacophores, as the piperidine ring has a less planar configuration vs. the proline one. To31 and To35 behaved as classical use-dependent channel blockers (Fig. 2 – current traces). However, they showed a decrease in potency for tonic and use-dependent block (at either 2 or 10 Hz stimulation frequencies) vs. the corresponding proline analogs (Fig. 2B). In addition, To35 was 4- and 8-fold less potent that To31, in tonic and use-dependent block, respectively, confirming that the presence of the N-benzyl moiety at a greater distance allows a better interaction with the binding site (Table 1). Interestingly, both To31 and To35 had lower pKa values (pKa values: To31: 7.86 ± 0.40; To35: 7.83 ± 0.40) with respect to To10 and To11 (pKa values: To10: 8.60 ± 0.40; To11: 10.01 ± 0.40) that may account for the decrease of use-dependent behavior [14]. As opposite, the piperazine analog of To10, To32, had a potency and a use-dependent behavior almost comparable to that of To10 (ratio IC50TB/IC50 UDB around 12) (Fig. 2, Table 1). The convenient profile vs. piperidine compounds might be related to the presence of an additional protonable amino group, although the potential increase in basicity is not supported by the calculation of the two individual pKa values (pKa value: To32 pKa alpha 7.77 ± 0.60, pKa beta 5.86 ± 0.70). Other mechanisms related to the presence of an additional primary amine, such as the establishment of hydrogen bound with the binding site, can favorably influence the state-dependent interaction underlying the use-dependent behavior.
Fig. 2.
Evaluation of the tonic and use-dependent block exerted by piperidine (To31 and To35) and piperazine (To32) derivatives in comparison with their parent compounds To10 and To11. In the upper panel are shown traces of sodium current transients recorded by the three vaseline gap voltage-clamp method from single fibers of frog semitendinosus muscle in the absence and presence of the test compounds. In each group of traces the greatest one has been recorded in the absence of drug, with depolarizing step from the h.p. of −100 mV to −20 mV for 10 ms. The application of the same depolarizing stimulus 10 min after the application of the test compound allowed to estimate the tonic block exerted. Afterwards, similar depolarizing steps were repetitively applied at a 10-Hz frequency for 20–30 s that produced an additional cumulative block due to a use-dependent behavior, until a new equilibrium was reached. The sodium current reduction exerted by increasing drug concentrations and evaluated on the first-pulse trace and on the residual steady-state current allowed to construct the dose–response curves for tonic (lower panel A) and use-dependent block at 10 Hz (lower panel B). The curves fitting the experimental points were obtained using the logistic function described in Section 2. Each value is the mean ± SEM from 3 to 5 fibers of the percent block of INa observed in the presence of each concentration of drugs versus INa in the absence of the drug in the same fiber.
Table 1.
Concentrations for half-maximal tonic and use-dependent block of sodium currents by tocainide derivatives.
| Compound | Tonic block (IC50, μM) | Use-dependent block (IC50, μM) |
|
|---|---|---|---|
| 2 Hz | 10 Hz | ||
| To10 | 27.5 ± 1.1 | 5.7 ± 0.1 | 2.2 ± 0.1 |
| To11 | 82.2 ± 4.3 | 49.7 ± 7.6 | 19.8 ± 2.1 |
| N-Benzylated derivatives with the chiral center constrained in a piperadine or piperazine cycle | |||
| To31 | 38.5 ± 1.5 | 12.5 ± 0.8 | 4.5 ± 0.3 |
| To32 | 31.5 ± 0.8 | 8.9 ± 1.3 | 2.5 ± 0.4 |
| To35 | 146.0 ± 14.9 | 98.4 ± 7.5 | 36.0 ± 5.2 |
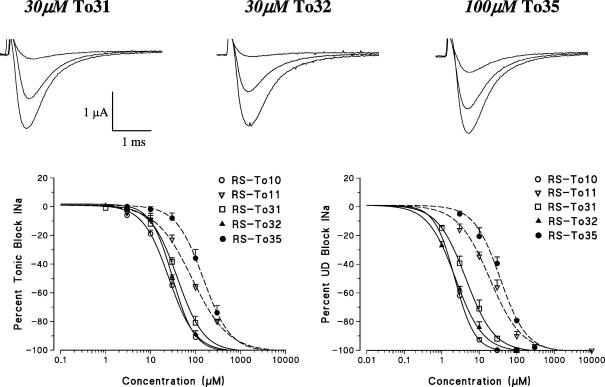
| N-Benzylated derivatives with structural modifications on the xylidide moiety | |||
| To13 | 30.2 ± 5.3 | 17.6 ± 2.4 | 8.4 ± 0.4 |
| To14 | 35.7 ± 5.6 | 20.2 ± 2.1 | 11.9 ± 1.0 |
| To15 | 17.2 ± 1.2 | 4.2 ± 0.3 | 2.2 ± 0.1 |
| To16 | 35.8 ± 4.1 | 16.2 ± 1.6 | 11.1 ± 1.3 |
Concentrations able to produce half-maximal response (IC50, in μM) in producing a tonic block (calculated during infrequent depolarizing stimulation from −100 to −20 mV) and a use-dependent block calculated by using trains of depolarizing pulses at the frequency of 2 Hz and 10 Hz. The IC50 values have been obtained during non-linear least squares fit of the concentration–response data to logistic equation described in Section 2.
All the newly synthesized piperidine and piperazine derivatives acted as inactivated sodium channel blockers, shifting the steady state inactivation curves (h∞) towards more negative potentials, in relation to their potency as sodium channel blockers (data not shown).
3.2. Analogs of To10 with substitutions at the level of the pharmacophore xylididic moiety
Structural modifications on the aromatic ring of the pharmacophore 2,6-xylididic moiety were introduced to evaluate the role of both electronic cloud and steric hindrance for the interaction, based on previous data showing that the potency of phenyl-derivatives of mexiletine is further modulated by the removal of methyl groups of the aryloxy ring [12]. The removal of the two ortho-methyl groups with (To14) or without (To13) the concomitant insertion of a chlorine atom in para position of the aromatic ring were detrimental for drug potency, as both compounds were 4–5-fold less potent than To10 during use-dependent blockade (Fig. 3, Table 1). As opposite, the increase of the electronic cloud with the insertion of two additional methyl groups in meta-position of the aromatic ring (To15) slightly increased the potency for tonic block with respect to To10, while maintaining the use-dependent profile; then To10 and To15 were equieffective in condition of high frequency stimulation (Fig. 3, Table 1). Interestingly, the addition of chlorine atom in para position of To15 (as in To16), exerting an electron withdrawal effect, reduced back the potency and use-dependent behavior to those of To13 and To14. All the newly synthesized compounds with substitution at the xylididic pharmacophore moiety acted as inactivated sodium channel blockers, shifting the steady state inactivation curves (h∞) towards more negative potentials, in relation to their potency as sodium channel blockers (data not shown).
Fig. 3.
Evaluation of the tonic and use-dependent block exerted by To10 analogs with substitution on the xylidide moiety obtained with removal of the two methyl groups with or without the insertion of a chlorine atom in para position (To14 and To13, respectively) or by adding two methyl groups with or without the insertion of a chlorine atom in para position (To16 and To15, respectively). In the upper panel are shown traces of sodium current transients recorded by the three vaseline gap voltage-clamp method from single fibers of frog semitendinosus muscle in the absence and presence of the test compounds. In each group of traces the greatest one has been recorded in the absence of drug, with depolarizing step from the h.p. of −100 mV to −20 mV for 10 ms. The application of the same depolarizing stimulus 10 min after the application of the test compound allowed to estimate the tonic block exerted. Afterwards, similar depolarizing steps were repetitively applied at a 10-Hz frequency for 20–30 s that produced an additional cumulative block due to a use-dependent behavior, until a new equilibrium was reached. The sodium current reduction exerted by increasing drug concentrations and evaluated on the first-pulse trace and on the residual steady-state current allowed to construct the dose–response curves for tonic (lower panel A) and use-dependent block at 10 Hz (lower panel B). The curves fitting the experimental points were obtained using the logistic function described in Section 2. Each value is the mean ± SEM from 3 to 6 fibers of the percent block of INa observed in the presence of each concentration of drugs versus INa in the absence of the drug in the same fiber.
3.3. Evaluation of stereoselective use-dependent activity of To10, To11 and To12
A further issue for increasing potency is the potential enhancement of stereoselectivity of these rigid structures. In fact, previous studies have shown that To5, the alpha-proline derivative of tocainide and parent compound of To11 (Fig. 1), has an enhanced stereoselective behavior during use-dependent block, with eudismic ratio (IC50 distomer/IC50 eutomer) > 3 at 10 Hz frequency [13]. However, preliminary data suggest that To10 is poorly stereoselective [17]. Thus, we evaluated if stereoselectivity was related to the position of the chiral carbon atom with respect to the amino group in the rigid proline cycle or rather if the presence of the benzyl moiety decreases stereoselectivity as a result of a pivotal role of the hydrophobic interaction.
To this aim we tested the potency in use-dependent block of INa of the enantiomers of the two N-benzylated proline analogs To10 and To11. In parallel, the enantiomers of the N-benzyl derivative of Toc, To12 were also tested, as the both the chiral center and the benzylated amino group were not constrained in the rigid cycle.
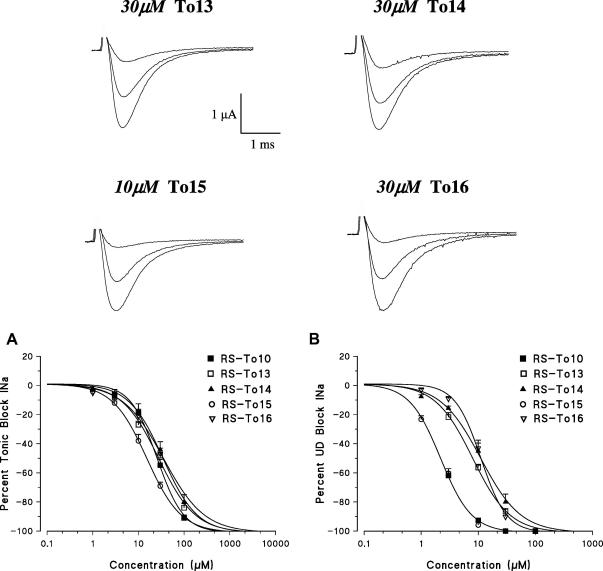
To12 did not show any stereoselective behavior, in fact the IC50 values were 7.9 ± 0.7 μM and 9.2 ± 0.7 μM for R and S enantiomers, respectively (Fig. 4). However, as expected, both enantiomers were about 30-fold more potent than Toc ones (IC50 > 200 μM) in producing the 10 Hz use-dependent block. Similarly and in agreement with preliminary findings, To10 was almost devoid of stereoselectivity during use-dependent blockade, the effect of the two enantiomers being clearly overlapping (Fig. 4). Then the eudismic ratio [IC50 distomer/IC50 eutomer] was 1.3, the IC50 values being 1.8 ± 0.1 μM and 2.3 ± 0.1 μM for R and S enantiomers, respectively. Interestingly, a lack of stereoselectivity was also observed with non-benzylated parent compound (data not shown).
Fig. 4.
Use dependent block exerted by enantiomers of To12, To11 and To10. In the upper panel are shown representative traces of the sodium currents in the absence and presence of the test enantiomers. The current in the presence of the compounds have been evaluated at the end of a 10 Hz stimulation frequency. The sodium current reduction exerted by increasing drug concentrations and evaluated on the first-pulse trace and on the residual steady-state current allowed to construct the dose–response curves for each couple of enantiomers. The curves fitting the experimental points were obtained using the logistic function described in Section 2. Each value is the mean ± SEM from 3 to 6 fibers of the percent block of INa observed in the presence of each concentration of drugs versus INa in the absence of the drug in the same fiber.
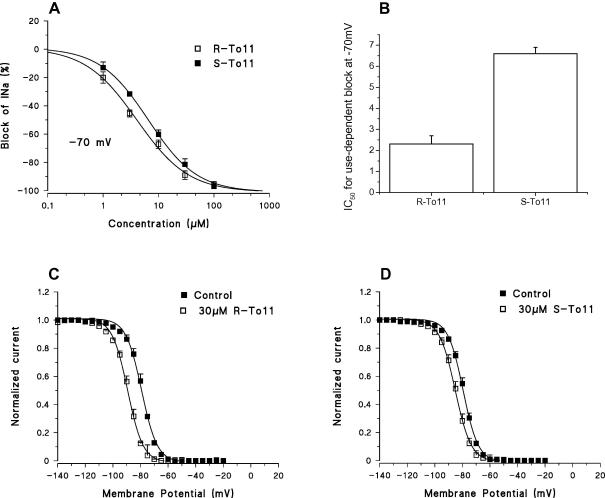
In contrast, the introduction of the benzyl group on the α-proline cycle, as in To11, maintained the stereoselectivity of the parent compound (To5) during high frequency stimulation, with the R enantiomer being the eutomer. This can be clearly appreciated by the left shift of the concentration response curve of R-To11 vs. that of S-To11 as well as by the IC50 values that were 10.1 ± 1.8 μM and 31.3 ± 5.7 μM for R and S, respectively (Fig. 4). Then the eudismic ratio [IC50 distomer/IC50 eutomer] was greater than 3 for 10 Hz use-dependent block. To verify the possible stereoselective interaction of To11 with inactivated sodium channels [12,13] we evaluated the block produced by the two enantiomers when the holding potential was held at −70 mV, at which about half of the sodium channels directly transit from the closed to the inactivated state. Again, we found a greater potency of the R enantiomer vs. the S one, with a ratio between IC50 values similar to that found during use-dependent blockade (Fig. 5A and B). Also To11 produced a stereoselective shift of the h∞, in fact the eutomer R-To11 at 30 μM produced a shift of 14.4 ± 2.7 mV (n = 3) which was significantly greater than that observed with the same concentration of S-To11 (5.7 ± 0.8 mV; p < 0.01 by Student’s t-test; Fig. 5C and D).
Fig. 5.
(A) Concentration–response curves for the enantiomers of To11 obtained with infrequent depolarizing stimulation form the holding potential of −70 mV. Each point is the mean ± SEM from 3 to 6 fibers. In (B) values of the calculated IC50 values. In (C) and (D) are shown the steady-state inactivation curves obtained in the absence and in the presence of 30 μM R-To11 and S-To11, respectively. In point is the mean ± SEM (from 3 to 6 fibers).
4. Discussion
Our study was aimed at further characterizing the molecular determinants of LA-like drugs, in order to refine the drug design of potent and selective blockers of skeletal muscle sodium channels to be safely used in the treatment of excitability disturbance of patients affected by channelopaties, such as myotonic syndromes and periodic paralyses [22,23]. Tocainide and mexiletine are in fact among the few drugs clinically effective to symptomatically solve myotonic-like hyperexcitability [24,25]. The use of tocainide has been long questioned and limited for the relevant side effects, while recently also mexiletine has been withdrawn from the market. Then the identification of new potent use-dependent blockers of Nav1.4 is of pivotal importance to ensure a proper therapy of the orphan myotonic diseases. In support to this view, we have previously found that use-dependent analogs identified on screening on frog native myofibers, exert an efficacious control of myotonic symptoms in ADR myotonic mice, in the same concentration range [13,26]. The discovery of potent use dependent blockers is important as first step toward the increase of therapeutic index as well as for pharmacogenomic approaches and for the necessary addressed tissue-selectivity tests. In fact, despite the increasing information obtained by mutagenesis, biophysical and SAR studies on various channel sub-types, it is still difficult to predict a cardiac or CNS safety only from drug structure.
Based on these considerations, To10 is a highly promising compound, combining an increased potency with a remarkable use-dependent behavior, the main feature toward therapeutically relevant channel blockers. Interestingly, To10 (renamed NeP1) has been confirmed to be a potent use-dependent blocker of heterologously expressed hNav1.4 and hNav1.7 and to be more potent than tocainide in reverting the reduction of the pain threshold in a rat model of oxaliplatin-induced neuropathic pain [27]. The result of the present study allowed to further understand the structural requirements necessary for optimal use-dependent blockade. First of all we tested the effect of the substitution of the proline cycle with the less planar piperidine ring. The substitution is of interest as several local anesthetics used in therapy such as mepivacaine, bupivacaine and ropivacaine are alkyl-substituted 2-piperidine carboxanilides, structurally related to our compounds [28]. Furthermore a piperidine ring is also present in many natural occurring alkaloids and synthetic drugs, which reinforced the interest in establishing its potential role in modulating pharmacophore requirement for further search of potential candidates in existing pharmacopeia. Our results confirmed the pivotal role of the proline cycle for optimal rigidity and planarity required for proper physico-chemical properties and receptor interaction. In fact the slight modification of both features by mean of a substitution with a 6 atom ring, is detrimental for potency, unless in the presence of an additional primary amine group, as in the piperazine ring. In fact the piperazine analog To32 maintains both potency and use-dependent behavior of To10, likely in relation to the possibility to establish relevant interaction with the binding site, via an additional hydrogen bound. Then To32 may represent an interesting homolog for future studies. However the possible formation of metabolites with the benzyl piperazine moiety, described for other piperazine-containing drugs, has to be considered for in vivo studies due to the well know psychoactive effects of this structure due to the potential interaction with various receptors and channels [29]. Then, the advantage of a piperazine vs. a proline cycle in vivo, in terms of metabolic stability and toxicity is an open issue.
The 2,6-xylidide moiety also plays a key role in drug interaction with the binding site on Nav1.4 channel and it is essential for the in vivo anti-nociceptive effect; then modifications at this level can help to gain insight in the pharmacological sensitivity of different channel isoforms [14,30]. In this respect we found that a proper electronic cloud on the aromatic xylidide portion is of importance for further tuning of drug potency, a slight increase being observed with the introduction of two additional electron donor methyl groups, as in To31. Then, the presence of methyl groups that ensure the proper density of the electronic cloud of the pharmacophore xylididic moiety of potent N-benzyl derivatives is important for tuning the interaction with the binding site during high affinity conformation transition. The To10-like profile of To15 is also of interest as it shows a high binding activity to human serum albumin, due to the enhanced lipophilicity [20]. This allows to predict a longer half-life with respect to the parent compounds in vivo, while maintaining a strong sodium channel blocking activity.
Another important and open issue is the possibility to further enhance drug potency of LA-like compounds Nav1.4 channel by the use of enantiomers. LAs drugs exert a modest stereoselective block of sodium channels of various tissues, including skeletal muscle [13,31–33]. However, the use of pure enantiomers might be clinically useful for the enantioselective metabolism and the possible presence in vivo of active stereoselective metabolites [34,35]. Thus, we evaluated if stereoselectivity was related to the position of the chiral carbon atom with respect to the amino group in the rigid proline cycle or rather if the presence of the benzyl moiety decreases stereoselectivity as a result of a pivotal role of the hydrophobic interaction. We found that the constrained position and the proper distance between the chiral center and the terminal amino group is pivotal for the stereoselective interaction while the group present on the amino groups are main determinant of affinity. Thus it is possible to separately modulate the two properties upon specific structural modifications. Interestingly the eutomer of To11, the less potent N-benzylated derivative in the racemic form, has a potency approaching that of To10, then disclosing another potential strategies to further ameliorate drug affinity [36–38]. Then the results corroborate the interest of cyclic N-benzylated derivatives of tocainide as a therapeutically promising series of new sodium channel blockers. The results also provide clear indications about the structural requirement of pharmacophores for further increasing potency and state-dependent block, with particular attention to the nature of the lipophilic group linked to the amino group combined with a proper constrained distance of this latter from the chiral carbon atom. Additional addressed experiments will help to establish the necessary tissue selectivity.
Policy and ethics
The work described has been carried out in accordance with EC Directive 86/609/EEC for animal experiments.
Acknowledgements
This work has been supported by Telethon-Italy, Project No. GGP10101.
The contribution of Association Française contre les Myopathies (AFM), Project MYOTONIADRUGS is also acknowledged.
References
- 1.Clare J.J., Tate S.N., Nobbs M., Romanos M. A voltage-gated sodium channels as therapeutic targets. Drug Discov Today. 2000;5:506–520. doi: 10.1016/s1359-6446(00)01570-1. [DOI] [PubMed] [Google Scholar]
- 2.Wang D.W., Nie L., George A.L., Jr., Bennett P.B. Distinct local anesthetic affinities in Na+ channel subtypes. J Biophys. 1996;70:1700–1708. doi: 10.1016/S0006-3495(96)79732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R.A., Ennis I.L., Tomaselli G.F., Marban E. Structural basis of differences in isoform-specific gating and lidocaine block between cardiac and skeletal muscle sodium channels. Mol Pharmacol. 2002;61:136–141. doi: 10.1124/mol.61.1.136. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X.F., Shieh C.C., Chapman M.L. A-887826 is a structurally novel, potent and voltage-dependent Na(v)1.8 sodium channel blocker that attenuates neuropathic tactile allodynia in rats. Neuropharmacology. 2010;59(3):201–207. doi: 10.1016/j.neuropharm.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Catterall W.A. Molecular mechanisms of gating and drug block of sodium channels. Novartis Found Symp. 2002;241:206–218. [PubMed] [Google Scholar]
- 6.Ahern C.A., Eastwood A.L., Dougherty D.A., Horn R. New insights into the therapeutic inhibition of voltage-gated sodium channels. Channels (Austin) 2008;2(1):1–3. doi: 10.4161/chan.2.1.6007. [DOI] [PubMed] [Google Scholar]
- 7.Sheets M.F., Fozzard H.A., Lipkind G.M., Hanck D. Sodium channel molecular conformations and antiarrhythmic drug affinity. Trends Cardiovasc Med. 2010;20(1):16–20. doi: 10.1016/j.tcm.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarov-Yarovoy V., Brown J., Sharp E.M., Clare J.J., Scheuer T., Catterall W.A. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel alpha subunit. J Biol Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- 9.Yarov-Yarovoy V., McPhee J.C., Idsvoog D., Pate C., Scheuer T., Catterall W.A. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel alpha subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- 10.Nau C., Wang S.Y., Strichartz G.R., Wang G.K. Point Mutations at N434 in D1-S6 of Mu1 Na+ channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol Pharmacol. 1999;56:404–413. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- 11.De Luca A., Natuzzi F., Falcone G. Inhibition of frog skeletal muscle sodium channels by newly synthesized chiral derivatives of mexiletine and tocainide. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:777–787. doi: 10.1007/pl00005118. [DOI] [PubMed] [Google Scholar]
- 12.De Luca A., Natuzzi F., Desaphy J.F. Molecular determinants of mexiletine structure for potent and use-dependent block of skeletal muscle sodium channels. Mol Pharmacol. 2000;57:268–277. [PubMed] [Google Scholar]
- 13.Talon S., De Luca A., De Bellis M. Increased rigidity of the chiral centre of tocainide favours stereoselectivity and use-dependent block of skeletal muscle Na+ channels enhancing the antimyotonic activity in vivo. Br J Pharmacol. 2001;134:1523–1531. doi: 10.1038/sj.bjp.0704366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Luca A., Talon S., De Bellis M. Optimal requirements for high affinity and use-dependent block of skeletal muscle sodium channel by N-benzyl analogs of tocainide-like compounds. Mol Pharmacol. 2003;64:932–945. doi: 10.1124/mol.64.4.932. [DOI] [PubMed] [Google Scholar]
- 15.Lipkind G.M., Fozzard H.A. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68(6):1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- 16.Carrieri A., Muraglia M., Corbo F., Pacifico C. 2D- and 3D-QSAR of tocainide and mexiletine analogues acting as Na(v)1.4 channel blockers. Eur J Med Chem. 2009;44(4):1477–1485. doi: 10.1016/j.ejmech.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Muraglia M., Franchini C., Corbo F. Synthesis of beta-proline like derivatives and their evaluation as sodium channel blockers. J Heterocycl Chem. 2007;44:1099–1103. [Google Scholar]
- 18.Hille B., Campbell D.T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976;67:265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catalano A., Carocci A., Corbo F. Contrained analogues of tocainide as potent skeletal muscle sodium channel blockers toward the development of antimyotonic agents. Eur J Med Chem. 2008;43:2535–2540. doi: 10.1016/j.ejmech.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Pistolozzi M., Franchini C., Corbo F. Tocainine analogues binding to human serum albumin: a HPLAC and circular dichroism study. J Pharm Biomed Anal. 2010;53:179–185. doi: 10.1016/j.jpba.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 21.De Luca A., Pröbstle T., Brinkmeier H., Rüdel R. The different use dependences of tocainide and benzocaine are correlated with different effects on sodium channel inactivation. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:596–601. doi: 10.1007/BF00170658. [DOI] [PubMed] [Google Scholar]
- 22.Cannon S.C. Ion-channel defects and aberrant excitability in myotonia and periodic paralysis. Trends Neurosci. 1996;19:3–10. doi: 10.1016/0166-2236(96)81859-5. [DOI] [PubMed] [Google Scholar]
- 23.Jurkat-Rott K., Lehmann-Horn F. Human muscle voltage-gated ion channels and hereditary disease. Curr Opin Pharmacol. 2001;1:280–287. doi: 10.1016/s1471-4892(01)00050-9. [DOI] [PubMed] [Google Scholar]
- 24.Meola G., Sansone V. Therapy in myotonic disorders and in muscle channelopathies. Neurol Sci. 2000;21:S953–S961. doi: 10.1007/s100720070009. [DOI] [PubMed] [Google Scholar]
- 25.Conte Camerino D., Tricarico D., Desaphy J.F. Ion channel pharmacology. Neurotherapeutics. 2007;4(2):184–198. doi: 10.1016/j.nurt.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 26.De Luca A., Pierno S., Liantonio A. New potent mexiletine and tocainide analogues evaluated in vivo and in vitro as antimyotonic agents on the myotonic ADR mouse. Neuromuscul Disord. 2004;14(7):405–416. doi: 10.1016/j.nmd.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Ghelardini C., Desaphy J.F., Muraglia M. Effects of a new potent analog of tocainide on hNav1.7 sodium channels and in vivo neuropathic pain models. Neuroscience. 2010;169(2):863–873. doi: 10.1016/j.neuroscience.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Ho B., Michael Crider A., Stables J.P. Synthesis and structure–activity relationships of potential anticonvulsants based on 2-piperidinecarboxylic acid and related pharmacophores. Eur J Med Chem. 2001;36(3):265–286. doi: 10.1016/s0223-5234(00)01206-x. [DOI] [PubMed] [Google Scholar]
- 29.de Boer D., Bosman I.J., Hidvegi E. Piperazine-like compounds: a new group of designer drugs-of-abuse on the European market. For Sci Int. 2001;121:47–56. doi: 10.1016/s0379-0738(01)00452-2. [DOI] [PubMed] [Google Scholar]
- 30.Corbo F., Franchini C., Lentini G. Synthesis and biological evaluation of chiral alpha-aminoanilides with central antinociceptive activity. J Med Chem. 2007;50:1907–1915. doi: 10.1021/jm061078e. [DOI] [PubMed] [Google Scholar]
- 31.Sheldon R.S., Hill R.J., Touis M., Wilson L.M. Aminoalkyl structural requirements for interaction of lidocaine with class I antiarrhythmic drug receptor on rat cardiac myocytes. Mol Pharmacol. 1991;39:609–614. [PubMed] [Google Scholar]
- 32.Hill R.J., Duff H.J., Sheldon R.S. Determinants of stereospecific binding of type I antiarrhythmic drugs to cardiac sodium channels. Mol Pharmacol. 1988;34:659–663. [PubMed] [Google Scholar]
- 33.De Luca A., Natuzzi F., Lentini G., Franchini C., Tortorella V., Conte Camerino D. Stereoselective effects of mexiletine enantiomers on sodium currents and excitability characteristics of adult skeletal muscle fibers. Naunyn Schmiedebergs Arch Pharmacol. 1995;352(6):653–661. doi: 10.1007/BF00171325. [DOI] [PubMed] [Google Scholar]
- 34.Knoche B., Gehrcke B., König W.A., Wainer I.W. Determination of the enantiomeric composition of mexiletine and its four hydroxylated metabolites in urine by enantioselective capillary gas chromatography. Chirality. 1996;8:30–34. doi: 10.1002/(SICI)1520-636X(1996)8:1<30::AID-CHIR7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.De Bellis M., De Luca A., Rana F. Evaluation of the pharmacological activity of the major mexiletine metabolites on skeletal muscle sodium currents. Br J Pharmacol. 2006;149(3):300–310. doi: 10.1038/sj.bjp.0706867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desaphy J.F., De Luca A., Tortorella P., De Vito D., George A.L., Jr., Conte Camerino D. Gating of myotonic Na+ channel mutants defines the response to mexiletine and a potent derivative. Neurology. 2001;57:1849–1857. doi: 10.1212/wnl.57.10.1849. [DOI] [PubMed] [Google Scholar]
- 37.Kuo C.C., Huang R.C., Lou B.S. Inhibition of Na+ current by diphenhydramine and other diphenyl compounds: molecular determinants of selective binding to the inactivated channels. Mol Pharmacol. 2000;57:135–143. [PubMed] [Google Scholar]
- 38.Yang Y.C., Kuo C.C. Inhibition of Na+ current by imipramine and related compounds: different binding kinetics as an inactivation stabilizer and as open channel blocker. Mol Pharmacol. 2002;62:1228–1237. doi: 10.1124/mol.62.5.1228. [DOI] [PubMed] [Google Scholar]