Highlights
► Latest insights into synaptic mechanisms of hyperalgesia including the recently discovered opioid-withdrawal LTP. ► Depiction of distinct signalling pathways for the induction and for the maintenance of LTP. ► Emerging role of glial cells for LTP at synapses of nociceptive primary afferents. ► Description of newly discovered reversal of LTP by clinically approved drugs. ► We argue that LTP at synapses of nociceptive nerve fibres is an element of a biological cascade amplifier in a nociceptive daisy chain.
Abstract
Long-term potentiation of synaptic strength (LTP) in nociceptive pathways shares principle features with hyperalgesia including induction protocols, pharmacological profile, neuronal and glial cell types involved and means for prevention. LTP at synapses of nociceptive nerve fibres constitutes a contemporary cellular model for pain amplification following trauma, inflammation, nerve injury or withdrawal from opioids. It provides a novel target for pain therapy. This review summarizes recent progress which has been made in unravelling the properties and functions of LTP in the nociceptive system and in identifying means for its prevention and reversal.
Introduction
The vulnerability of tissues rises significantly in case of an injury or an inflammation. The nociceptive systems adapts to this by lowering response thresholds and by increasing response magnitude in order to maintain its protective function [1]. Behaviourally these adaptations manifest as hyperalgesia in experimental animals [2,3], in volunteers [4] and in patients [5]. Pro-nociceptive adaptations may occur at all levels of the neuraxis from nociceptive nerve endings, to spinal dorsal horn and all the way up to cortical neuronal networks. In contrast to sensitization of nociceptive nerve endings, some of the central mechanisms may persist long after the initial cause for pain and the need for special tissue protection has disappeared. Hyperalgesia then becomes maladaptive. The underlying central mechanisms can be grouped into two major categories: Impaired inhibition and enhanced excitation in nociceptive pathways. Multiple mechanisms have been identified so far which relate to the synthesis and/or the release of neurotransmitters, the density, the distribution and the activation of neurotransmitter receptors, the single channel conductance or the open time probability of ion channels and the number and morphology of synapses and dendritic spines. All of which can ultimately modulate the neurons’ intrinsic properties or synaptic strength.
From early on important insights into central components of hyperalgesia have been obtained in humans [6], from reflex measurements in experimental animals, for example [7], as well as from single neuron recordings in ventral- [8] and more importantly in the dorsal horn [9] of the spinal cord. It was only in later studies that synaptic plasticity has been assessed in the nociceptive system [10,11].
Nociceptive neurons are defined by their input (i.e. the excitatory mono- or polysynaptic input from nociceptive nerve fibres), but not by their function. Consequently nociceptive neurons comprise a very heterogeneous group of neurons including excitatory and inhibitory interneurons, projection neurons and motoneurons. Changes in the responsiveness of nociceptive neurons may thus have different and even opposing effects on pain depending upon the neurons’ function. For better understanding, here, the term ‘Principle Pain Neurons’ is used for neurons which, when discharging action potentials, trigger the perception of pain (see discussion in [12]). Here we review recent progress in understanding synaptic plasticity in spinal nociceptive pathways which, when expressed in principle pain neurons, amplify pain. The focus is on most recently published data. Comprehensive reviews on the synaptic mechanisms of hyperalgesia have been published [3,13].
Activity-dependent LTP at the first synapse in nociceptive pathways
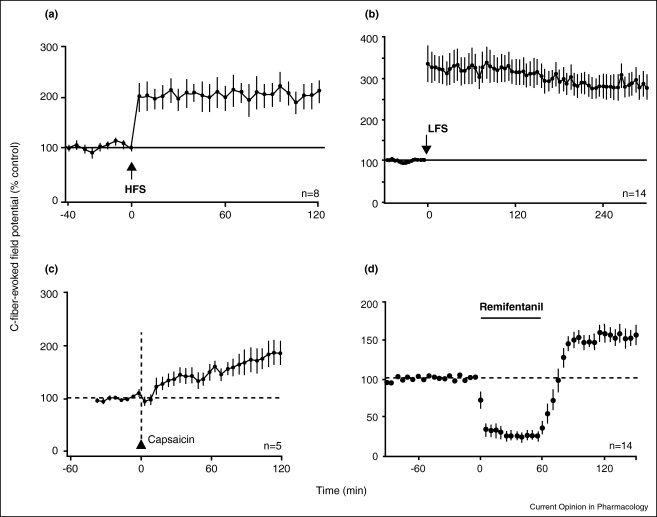
Hyperalgesia and LTP are induced in an activity-dependent manner by strong or lasting discharges in C-fibres generating a central amplification of nociceptive responses. Typically LTP is induced by conditioning high frequency electrical stimulation (∼100 Hz; HFS, Figure 1A) at most synapses in the central nervous system and also at C-fibre synapses in the superficial spinal dorsal horn [14]. At C-fibre synapses LTP can further be induced by conditioning low frequency stimulation (∼2–10 Hz, LFS, Figure 1B) [15], but also by natural noxious stimulation (subcutaneous capsaicin, Figure 1C, formalin, noxious heat or pinching) and by acute nerve injury (sciatic nerve transection or crush) [15–17]. LTP has been demonstrated in vivo and in vitro, mainly in rats (e.g. [18••,19,–21,22•,23,24•,25,26••,27,28•] for recent studies) but also in mice [29]. As a general rule, conditioning stimuli which induce LTP at C-fibre synapses also cause hyperalgesia in behaving animals or human subjects (see below and [3,13] for reviews).
Figure 1.
Induction of LTP at C-fibre synapses.
The figure illustrates different activity-dependent and -independent forms of LTP at C-fibre synapses. The graphs display mean time courses of amplitudes of C-fibre-evoked field potentials measured in the superficial spinal dorsal horn of adult, deeply anaesthetized rats. Field potentials were evoked by stimulation of sciatic nerve fibres at C-fibre intensity. Conditioning stimulation consisted of electrical stimulation of sciatic nerve fibre afferents at a high frequency (A, HFS, 100 Hz given four times for 1 s at 10 s intervals), at a low frequency (B, LFS, 2 Hz for 2 min), or subcutaneous injection of transient receptor potential vanilloid 1 channel agonist capscaicin (C, 1%, 100 μl). In D LTP was induced upon withdrawal from a brief (1 h) intravenous application of a high dose of remifentanil (450 mg kg−1 h−1 for 1 h, black horizontal bar). Modified from [15,18••].
While LTP can be induced at most, if not all synapses in the central nervous system, the susceptibility for LTP induction and suitable parameters for LTP induction vary, however, considerably. A good example are synapses of nociceptive skin afferents which are apparently less prone to express LTP as compared to synapses from muscle afferents [26••]. Conditioning stimulation of C-fibre afferents which innervate the skin may fail to induce LTP while identical conditioning stimulation of afferents in a mixed nerve or in a muscle nerve induces robust LTP [26••]. This difference disappears when brain-derived neurotrophic factor (BDNF) is applied directly onto the spinal cord at a low concentration suggesting that lack of this neurotrophic factor in cutaneous afferents renders them less prone to express LTP. The differential susceptibility of skin versus muscle afferents to express LTP correlates well with their respective ability to trigger prolonged facilitation of nociceptive reflexes [30].
Activity-independent forms of LTP
Opioid withdrawal LTP Hyperalgesia and spinal LTP can also be induced in the absence of any activity in nociceptive nerve fibres. A clinically relevant example is hyperalgesia which develops after abrupt withdrawal from opioids. This form of hyperalgesia may also involve expression of LTP at C-fibre synapses [18••]. A brief application of the ultra-short acting μ-opioid receptor (MOR) agonist remifentanil in vivo or d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) in vitro leads to an acute depression of synaptic strength in C-fibres (Figure 1D). Upon withdrawal synaptic strength not only quickly returns to normal but becomes potentiated for prolonged periods of time (Figure 1D). The induction of withdrawal LTP at C-fibre synapses in vitro [18••] requires activation of postsynaptic G-proteins, postsynaptic NMDA-receptors and a rise in postsynaptic Ca2+ levels [18••]. Some MOR agonists activate additional pro-nociceptive mechanisms. For example, withdrawal from fentanyl or morphine not only causes opioid withdrawal LTP but in addition activates descending, facilitatory, serotonergic pathways acting on spinal 5-HT3 receptors [31•]. Hyperalgesia results when descending facilitation and/or opioid withdrawal LTP are expressed at synapses between nociceptive C-fibres and principle pain neurons.
Other activity-independent forms of LTP are induced at C-fibre synapses by spinal application of BDNF [20], adenosine triphosphate (ATP) [27] or reactive oxygen species donors [24] and in nerve injured rats also by tumour necrosis factor-α (TNFα) [32].
Distinct signalling pathways for LTP induction versus LTP maintenance
The signalling pathways which are involved in the induction of LTP are different from those which are required for its maintenance. They further differ between different induction protocols for spinal LTP which are expressed at C-fibre synapses, see Figures 2 and 3.
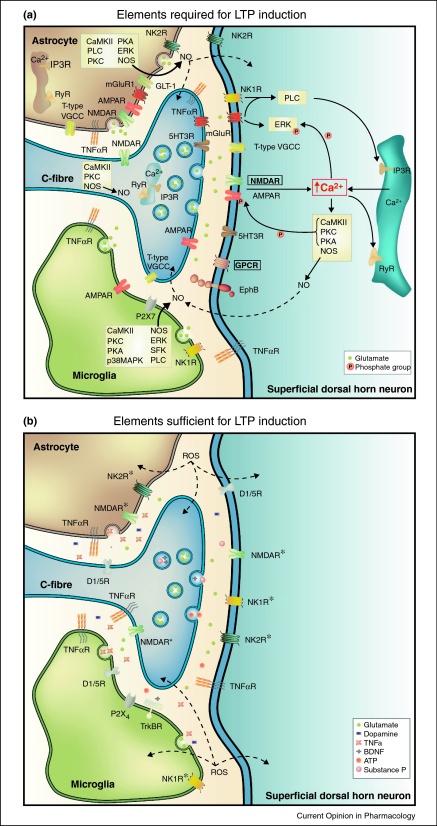
Figure 2.
Signalling pathways of LTP induction at C-fibre synapses.
The schemes summarize elements of the signalling pathways which are required (A) or sufficient (B) for the induction of LTP at spinal C-fibre synapses. The elements involved in LTP induction are typically identified by the respective blockers (required elements) or activators (sufficient elements) which were applied topically to the spinal cord. Many of the involved signalling elements are expressed at more than one cellular site as shown in the figure. The cellular site(s) of action is/are thus in most cases not known, except when substances were applied directly into the postsynaptic neuron as shown for Ca2+, NMDAR, and GPCR (which are in boxes here) in A. Suggested signalling pathways are indicated by arrows. Diffusion of elements is illustrated by dotted lines. * indicates that activation of this element induces LTP in spinalised animals only.
Abbreviations and literature:
AMPAR: α-amino-3-3hydroxy-5-5methyl-4-4isoxazoleproprionic acid receptor [72]; ATP: Adenosine triphosphate [22•,27]; BDNF: Brain derived neurotrophic factor [20,34]; CaMKII: calcium/calmodulin-dependent protein kinase II [15,18••,19,73]; D1,5R: Dopamine receptor D1,5 [74]; EphB: Ephrin B receptor [29,75•]; ERK: Extracellular signal-regulated kinase [76]; GLT-1: Glutamate transporter 1 [77]; GPCR: G-protein coupled receptor [18••]; IP3R: Inositol triphosphate receptor [14,15]; mGluR1: Metabotropic glutamate receptor group 1 [78,79]; NK1R: Neurokinin 1 receptor [14,15,19,80,81]; NK2R: Neurokinin 2 receptor [80,81]; NMDAR: N-methyl d-aspartate receptor [11,14–16,18••,19,81]; NO: Nitric oxide [15,65]; NOS: Nitric oxide synthase [15,65]; PKA: Protein kinase A [73]; PKC: Protein kinase C [15,18••,19,73]; PLC: Phospholipase C [14,15,19]; P2X7, P2X4: Ionotropic purinergic receptor [22•,27]; p38MAPK: p38 mitogen-activated protein kinases [20,27]; ROS: Reactive oxygen species [24•]; RyR: Ryanodine receptor [18••,19,21]; SFK: Src family kinases [25]; TNFα: Tumour necrosis factor α [25,32]; TNFαR: Tumour necrosis factor α receptor [25,32]; TrkBR: Neurotrophic tyrosine kinase receptor type 2 [20]; T-type VGCC: T-type voltage gated calcium channel [14,15,19]; 5HT3R: Serotonin type 3 receptor [82].
Figure 3.
Signalling pathways of LTP maintenance and LTP reversal at C-fibre synapses.
The schemes summarize elements of signalling pathways which are required for the maintenance of LTP at spinal C-fibre synapses. Thus, when any of these elements is blocked established LTP diminishes or disappears (required elements for LTP maintenance, A). The diagram in B summarizes elements which, when activated reverse established LTP. These sufficient elements for the reversal of LTP are underlined. Elements which are not underlined are required for the reversal of LTP. When blocked these elements prevent the reversal of LTP by at least one of the sufficient elements. Blockers and activators of the respective elements were usually applied topically to the spinal cord. Many of the known signalling elements are expressed at more than one cellular site as shown in the figure. The cellular site(s) of action is/are thus not known in most cases. Suggested signalling pathways are indicated by arrows. Diffusion of elements is illustrated by dotted lines.
Abbreviations and literature:
AMPAR: α-amino-3-3hydroxy-5-5methyl-4-4isoxazoleproprionic acid receptor (unpubl.); A1R: Adenosine 1 receptor [83]; α2-AR: α2-adrenergic receptor [84]; α2δ VGCC: Voltage gated calcium channel [85]; CaMKII: Calcium/calmodulin-dependent protein kinase II [73]; cGMP: Cyclic guanosine monophosphate [84]; D1,5R: Dopamine receptor D1,5 [74]; ERK: Extracellular signal-regulated kinase [76,86]; GABAAR: γ-aminobutyric acid A receptor [57]; mAChR: Muscarinic acetylcholine receptor [84]; mGluR1: Metabotropic glutamate receptor group 1 (unpubl.); MOR: μ-opioid-receptor [85]; NMDAR: N-methyl d-aspartate receptor (unpubl.); NO: Nitric oxide [84]; NOS: Nitric oxide synthase [84] PKA: Protein kinase A [73]; PKC: Protein kinase [73]; PP1: Protein phosphatase 1 (unpubl.); RyR: Ryanodine receptor (unpubl.); TrkBR: Neurotrophic tyrosine kinase receptor type 2 [34].
Postsynaptic signalling for LTP induction
Virtually all known forms of LTP induction at spinal C-fibre synapses require a rise in postsynaptic Ca2+ concentration [14,15,18••]. Postsynaptic Ca2+ rises by opening of postsynaptic NMDA receptors [14], T-type voltage-gated calcium channels [15], Ca2+-permeable AMPA receptors [33] and by Ca2+ release from intracellular Ca2+ stores triggered by activation of metabotropic glutamate receptors or neurokinin 1 receptors [19]. Metabotropic receptors mobilise intracellular Ca2+ by activation of ryanodine and inositol-1,4,5 trisphosphate (IP3) receptors via phospholipase C [19], see Figure 2A. The rise in postsynaptic Ca2+ then activates Ca2+-dependent signalling pathways involving protein kinase C (PKC), calcium-calmodulin-dependent protein kinase II (CaMKII) and nitric oxide synthase (NOS) [15]. Other enzymes involved are extracellular signal-regulated kinase (ERK) which induces a lasting phosphorylation and activation of the transcription factor cAMP responsive element-binding protein (CREB), see Figure 2A [34].
Not all forms of spinal LTP require the activation of all of these signalling elements for induction. For example, LTP induced by spinal application of ATP or BDNF [27,34], but not high frequency stimulation-induced LTP [32] depends upon p38 mitogen-activated protein kinase (p38 MAPK). Likewise, high- and low frequency stimulation-induced LTP [15,19] but not opioid-withdrawal LTP [18] requires activation of CaMKII.
Pre- and postsynaptic signalling for LTP maintenance
While the induction of all known forms of LTP at spinal C-fibre synapses requires postsynaptic signalling, recent studies suggest that the maintenance of LTP may involve both, post- as well as presynaptic signalling, see Figure 3A. The early phase of LTP consists of the first few hours of LTP expression. Early phase LTP involves posttranslational modifications of synaptic proteins, such as phosphorylation of synaptic AMPA receptors [35]. Conditioning LFS of primary afferent C-fibres induces phosphorylation of the GluR1 subunit of spinal AMPA receptor channels at Ser831 [36••] which increases their unitary single channel conductance. AMPA receptor-mediated currents in spinal nociceptive neurons are further elevated by enhanced AMPA receptor expression and by modified trafficking [35]. AMPA receptors are largely located postsynaptically where they mediate neuronal excitation. AMPA receptors may also be expressed at or near the terminals of a subset of dorsal root ganglion cells where they, by contrast, mediate presynaptic inhibition but not facilitation [37]. Taken together these findings suggest a postsynaptic component to LTP maintenance via enhanced AMPA receptor function.
By contrast, H.-L. Pan and his colleagues concluded from their data [38] that both, the induction and the maintenance of opioid withdrawal LTP at unidentified synapses in spinal dorsal horn is presynaptic. We provided, however, evidence that the induction of withdrawal LTP is postsynaptic at C-fibre synapses [18••], see paragraph above and our eLetter to their report. After any postsynaptic induction the expression of LTP may, nonetheless, involve presynaptic mechanisms. And indeed upon withdrawal from fentanyl or morphine but not from remifentanil the paired-pulse ratio of C-fibre-evoked field potentials decreases suggesting an increased neurotransmitter release [31•]. At synapses in the brain protein kinase M ζ (PKMζ) is one of the key factors responsible for the maintenance of LTP [39]. Recent studies suggest that this kinase is also required for plasticity in nociceptive pathways in the spinal cord [40] and in the anterior cingulate cortex [41••]. It is presently unknown whether PKMζ is also involved in the maintenance of LTP at C-fibre synapses.
Late phase LTP develops slowly over the first hours after LTP induction and persists for days, weeks or even longer. Expression of late phase LTP requires synapse-to-nucleus signalling via signalling molecules such as ERK1/2 and cAMP all of them may trigger the activation of CREB. The transcription factor CREB controls the expression of a myriad of proteins, many of which are relevant for synaptic transmission. Late phase LTP can consequently be blocked by protein synthesis inhibitors [34] and may involve incorporation of new AMPA receptors into the postsynaptic membrane [42], see Figure 2B and [13,43,44] for recent reviews.
Role of glial cells for LTP induction
In the central nervous system neurons and glial cell heavily interact and mutually influence their functions [45]. This also applies to the nociceptive system where excitation of nociceptive nerve fibres not only activates spinal neurons but also spinal microglia and astrocytes which, in turn, release neuroactive substances [46]. The release of these gliotransmitters contributes to the induction and perhaps also to the maintenance of LTP at C-fibres, see Figures 2A, B, 3A, and B. For example, HFS-induced LTP can be prevented by blocking or silencing spinal P2X7 receptors which are largely expressed on microglia [22•]. Activated glial cells, Src-family kinases and p38 MAPK all contribute to the induction of LTP at C-fibre synapses via release of TNFα and activation of TNF receptor-1 [25]. In the absence of any C-fibre activation, spinal application of BDNF [20] induces late-phase LTP which requires activation of spinal microglia, Src-family kinases and p38 MAPK, see Figure 2A, B. This will consequently induce the release of TNFα, interleukin-1 and interleukin-6, among others [47,48].
Is spinal LTP homo- or heterosynaptic in nature?
Activity-dependent LTP may not only affect synapses which were activated by the conditioning stimulus. LTP may also ‘spread’ to inactive synapses converging onto the same postsynaptic neuron. It is still unknown if LTP in nociceptive pathways is homosynaptic in nature. Homosynaptic LTP at nociceptive synapses with principle pain neurons leads to primary hyperalgesia. Heterosynaptic LTP at synapses between nociceptive afferents and principle pain neurons would cause pain amplification outside but close to the area of injury or inflammation, that is, secondary hyperalgesia. A recent study by Carole Torsney [49••] suggests that hindpaw inflammation by complete Freund's adjuvant leads to a heterosynaptic facilitation of monosynaptic Aδ-fibre input to spinal lamina I neurons expressing the neurokinin-1 receptor. This finding could well explain heterosynaptic mechanisms underlying mechanical hyperalgesia. Ongoing studies in our laboratory further suggest that in superficial spinal dorsal horn homo- and heterosynaptic forms of LTP are expressed at C-fibre- and GABAergic synapses, respectively [50•].
Prevention of LTP induction in nociceptive pathways
Previous studies have identified a growing number of targets for preventing LTP induction [13]. Clinically useful tools for preventing LTP induction include NMDA receptor antagonists, for example, [11,15,51], opioids [52,53] and the noble gas xenon [54]. NMDA receptor antagonists proved effective also in volunteers [55]. The inducibility of spinal LTP is further modulated by descending systems originating from various brain sites. When descending pathways are interrupted conditioning stimuli which are normally ineffective may now induce LTP [16] indicating a pre-emptive function of endogenous antihyperalgesic systems. These include descending oxytocinergic hypothalamic pathways from the paraventricular nucleus [56]. The induction of LTP thus not only depends upon the parameters of conditioning stimulation and the type of afferent fibres involved, but also upon the modulation by endogenous pro- and antinociceptive systems.
Reversal of established LTP in nociceptive pathways
LTP at synapses between nociceptive nerve fibres and principle pain neurons causes hyperalgesia. Reversal of LTP, that is, ‘depotentiation’ thus constitutes a potential means to erase a memory trace of pain. When benzodiazepines are applied directly onto the spinal cord during early phase LTP its consolidation is impaired [57], see Figure 3B. A brief (1 h), systemic application of a high dose of the ultra-short acting MOR agonist remifentanil reverses LTP induced by low- or high-frequency conditioning stimulation of C-fibre afferents or by subcutaneous capsaicin [36••]. The opioid-induced depotentiation involves activation of NMDA receptors, metabotropic glutamate receptors, Ca2+ release from ryanodine-sensitive intracellular stores and activation of protein phosphatase 1, see Figure 3B. AMPA receptor channels are phosphorylated at Ser831 by LTP-inducing stimuli. This leads to enhanced single channel conductance and thus synaptic strength. AMPA receptors are dephosphorylated at Ser831 by protein phosphatase 1 after high dose opioid administration. This probably constitutes a key mechanism for opioid-induced depotentiation [36]. Thus, in contrast to current believes opioids may not only temporarily dampen pain, they may also eliminate an important cause for hyperalgesia.
Hyperalgesia resulting from a biological cascade amplifier in a nociceptive daisy chain
LTP is a form of synaptic plasticity which can be induced at many different, it not all types of excitatory and inhibitory synapses in the central nervous system. It is thus not surprising that LTP is not only expressed at the first synaptic relays in nociceptive pathways [14,15,18••]. LTP has also been observed at synapses of glutamatergic [28] and GABAergic [50] interneurons in superficial spinal dorsal horn and at excitatory synapses between neurons in the spinal trigeminal subnucleus caudalis and -oralis [58]. Furthermore, LTP can be elicited at synapses in putatively nociceptive relays in the anterior cingulate cortex [59–61]. When LTP is simultaneously expressed at multiple sites connected serially along excitatory nociceptive pathways it will boost nociception exponentially. In the first instance trauma or inflammation trigger sensitization of nociceptors [1]. The resulting enhanced, ongoing discharges in nociceptive nerve fibres induce LTP at the first synaptic relays [3,13]. An obvious outcome of LTP at excitatory synapses is the increased firing of action potentials of the postsynaptic neuron in response to presynaptic activity. LTP-inducing stimuli indeed lead to elevated C-fibre-evoked discharges in dorsal horn neurons, see [56,62,63] for recent studies. This in turn will probably facilitate LTP induction at synapses further downstream in nociceptive pathways [61]. And indeed, conditioning, LTP-inducing stimulation of sciatic nerve fibres causes enhanced positron-emission tomography signals in the primary somatosensory cortex and delayed responses in the amygdala, the periaqueductal grey, the rostral ventromedial medulla, and the dorsolateral pontomesencephalic tegmentum [64]. Such a sequence of events constitutes a biological cascade amplifier in a nociceptive daisy chain.
Behavioural correlates of LTP in nociceptive pathways
Conditioning stimuli which induce LTP at spinal C-fibre synapses lead to hyperalgesia in behaving animals. Under local muscular paralysis with lidocaine, brief conditioning HFS of sciatic nerve fibre afferents at C-fibre strength leads to thermal [65] and to mechanical [66] hyperalgesia for 6–9 days at the ipsilateral but not at the contralateral hindpaw.
The final proof for any pain mechanism is a perceptual correlate in the human subject. In volunteers, transcutaneous conditioning HFS of cutaneous nerve fibres induces a long-lasting increase in pain sensitivity at the stimulation site (homotopic facilitation) as well as in the immediately surrounding skin area (heterotopic facilitation) [67•]. A number of recent studies have confirmed and extended the initial reports of perceptual [55,68–70] and electrophysiological [71•] correlates of spinal LTP in volunteers, see [3,13] for reviews. At present a direct comparison between the results obtained from experimental animals with those from human subjects is, however, hampered by the fact that in humans conditioning stimulation was always applied to a small set of cutaneous afferents, while in most previous animal experiments conditioning electrical nerve stimulation recruited virtually all fibres in large mixed nerves, thus including muscle afferents. This probably makes a major difference as muscle and skin afferents differ substantially in their ability to express synaptic LTP [26]. Nonetheless the volunteer studies importantly demonstrate that perceptual correlates of LTP can be demonstrated in humans and they are probably also relevant for pain in patients.
Concluding remarks
LTP is a feature of most, if not all synapses in the central nervous system but its properties much depend on the type of synapse involved, the induction protocols used and the context of its induction. LTP at C-fibre synapses constitutes a powerful model system for the prolonged amplification of nociception. LTP probably contributes to enhanced pain-related behaviour in experimental animals and to the amplification of pain perception in human subjects. Understanding LTP in nociceptive pathways appears to be promising for developing better strategies for the prevention and the treatment of some types of chronic pain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Supported by grants from the Austrian Science Fund (FWF) grant # P22306-B019, the Vienna Science and Technology Fund grant # LS 07-040 and the Oesterreichische Nationalbank (OeNB) Jubiläumsfonds 14420.
References
- 1.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogil J.S. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 3.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 4.Schmelz M. Translating nociceptive processing into human pain models. Exp Brain Res. 2009;196:173–178. doi: 10.1007/s00221-009-1809-2. [DOI] [PubMed] [Google Scholar]
- 5.Baron R. Handb Exp Pharmacol. 2009. Neuropathic pain: a clinical perspective. 3–30. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J.D., Wolff H.G., Goodell H. Experimental evidence on the nature of cutaneous hyperalgesia. J Clin Invest. 1950;29:115–140. doi: 10.1172/JCI102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennard A.M. Sensitization of the spinal cord of the cat to pain-inducing stimuli. J Neurosurg. 1953;10:169–177. doi: 10.3171/jns.1953.10.2.0169. [DOI] [PubMed] [Google Scholar]
- 8.Woolf C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 9.McMahon S.B., Wall P.D. Receptive fields of rat lamina 1 projection cells move to incorporate a nearby region of injury. Pain. 1984;19:235–247. doi: 10.1016/0304-3959(84)90002-2. [DOI] [PubMed] [Google Scholar]
- 10.Randic M., Jiang M.C., Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X-G., Sandkühler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-d-aspartic acid receptor blockage. Neurosci Lett. 1995;191:43–46. doi: 10.1016/0304-3940(95)11553-0. [DOI] [PubMed] [Google Scholar]
- 12.Sandkühler J: Spinal plasticity and pain. In Wall and Melzack's Textbook of Pain, edn 6. Edited by Koltzenburg M, McMahon SB. Elsevier Churchill Livingstone, Philadelphia; forthcoming 2012, ISBN: 9780702040597.
- 13.Ruscheweyh R., Wilder-Smith O., Drdla R., Liu X-G., Sandkühler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda H., Heinke B., Ruscheweyh R., Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda H., Stark J., Fischer H., Wagner M., Drdla R., Jäger T., Sandkühler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 16.Sandkühler J., Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H-M., Zhou L-J., Hu X-D., Hu N-W., Zhang T., Liu X-G. Acute nerve injury induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn of intact rat. Sheng Li Xue Bao. 2004;56:591–596. [PubMed] [Google Scholar]
- 18••.Drdla R., Gassner M., Gingl E., Sandkühler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]; A novel form of synaptic plasticity was discovered which is induced at synapses of primary afferent C-fibers upon withdrawal from μ-opioid receptor agonists in vitro and in vivo. This ‘opioid withdrawal LTP’ is a potential cellular mechanism leading to opioid-induced hyperalgesia.
- 19.Drdla R., Sandkühler J. Long-term potentiation at C-fibre synapses by low-level presynaptic activity in vivo. Mol Pain. 2008;4:18. doi: 10.1186/1744-8069-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L-J., Yang T., Wei X., Liu Y., Xin W-J., Chen Y., Pang R-P., Zang Y., Li Y-Y., Liu X-G. Brain-derived neurotrophic factor contributes to spinal long-term potentiation and mechanical hypersensitivity by activation of spinal microglia in rat. Brain Behav Immun. 2011;25:322–334. doi: 10.1016/j.bbi.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L-Z., Lu N., Zhang Y-Q., Zhao Z-Q. Ryanodine receptors contribute to the induction of nociceptive input-evoked long-term potentiation in the rat spinal cord slice. Mol Pain. 2010;6:1–11. doi: 10.1186/1744-8069-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Chu Y.-X., Zhang Y., Zhang Y.-Q., Zhao Z.-Q. Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun. 2010;24:1176–1189. doi: 10.1016/j.bbi.2010.06.001. [DOI] [PubMed] [Google Scholar]; This study demonstrates that activation of spinal P2X7 receptors, which are mainly expressed on microglia, is necessary for the induction of LTP at C-fiber synapsese and for mechanical hyperalgesia. The data further strengthen the crucial role of microglia for pronociceptive mechanisms in spinal dorsal horn.
- 23.Hjornevik T., Schoultz B.W., Marton J., Gjerstad J., Drzezga A., Henriksen G., Willoch F. Spinal long-term potentiation is associated with reduced opioid neurotransmission in the rat brain. Clin Physiol Funct Imaging. 2010;30:285–293. doi: 10.1111/j.1475-097X.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- 24•.Lee K.Y., Chung K., Chung J.M. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J Neurophysiol. 2010;103:382–391. doi: 10.1152/jn.90906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that reactive oxygen species are both, necessary and sufficient for the induction of LTP at C-fiber synapses in a spinal cord slice preparation.
- 25.Zhong Y., Zhou L-J., Ren W-J., Xin W-J., Li Y-Y., Zhang T., Liu X-G. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-α. Brain Behav Immun. 2010;24:874–880. doi: 10.1016/j.bbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 26••.Zhou L.-J., Ren W.-J., Zhong Y., Yang T., Wei X.-H., Xin W.-J., Liu C.-C., Zhou L.-H., Li Y.-Y., Liu X.-G. Limited BDNF contributes to the failure of injury to skin afferents to produce a neuropathic pain condition. Pain. 2010;148:148–157. doi: 10.1016/j.pain.2009.10.032. [DOI] [PubMed] [Google Scholar]; This study provides a plausible cause for the different capacitiy of skin- versus muscle afferents to induce pronociceptive plasticity in the spinal dorsal horn. Conditioning stimulation of fine skin afferents induces LTP at C-fiber synapses only if BDNF is additionally applied. Upon conditioning stimulation of muscle afferents BDNF is appartently released in spinal cord at sufficient concentrations for inducing LTP.
- 27.Gong Q-J., Li Y-Y., Xin W-J., Zang Y., Ren W-J., Wei X-H., Li Y-Y., Zhang T., Liu X-G. ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: The roles of P2X4 receptors and p38 MAPK in microglia. Glia. 2009;57:583–591. doi: 10.1002/glia.20786. [DOI] [PubMed] [Google Scholar]
- 28•.Santos S.F., Luz L.L., Szucs P., Lima D., Derkach V.A., Safronov B.V. Transmission efficacy and plasticity in glutamatergic synapses formed by excitatory interneurons of the substantia gelatinosa in the rat spinal cord. PLoS ONE. 2009;4:e8047. doi: 10.1371/journal.pone.0008047. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrates that LTP can be elicited not only at the first synaptic relays in nociceptive pathways as shown previously but in addition also at synapses between interneurons in lamina II of spinal dorsal horn.
- 29.Liu W-T., Han Y., Li H-C., Adams B., Zheng J-H., Wu Y-P., Henkemeyer M., Song X-J. An in vivo mouse model of long-term potentiation at synapses between primary afferent C-fibers and spinal dorsal horn neurons: essential role of EphB1 receptor. Mol Pain. 2009;5:29. doi: 10.1186/1744-8069-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wall P.D., Woolf C.J. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Heinl C., Drdla R., Xanthos D.N., Sandkühler J. Distinct mechanisms underlying pronociceptive effects of opioids. J Neurosci. 2011;31:16748–16756. doi: 10.1523/JNEUROSCI.3491-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a novel pronoceptive effect of opioids, the immediate onset, descending facilitation of synaptic strength at C-fibers. The immediate onset, descending facilitation requires activation of extraspinal μ-opioid receptors and spinal 5-HT3 receptors and is mechanistically independent of opioid withdrawal LTP.
- 32.Liu Y.-L., Zhou L.-J., Hu N.-W., Xu J.-T., Wu C.-Y., Zhang T., Li Y.-Y., Liu X.-G. Tumor necrosis factor-α induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52:708–715. doi: 10.1016/j.neuropharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann B., Ahmadi S., Heppenstall P.A., Lewin G.R., Schott C., Borchardt T., Seeburg P.H., Zeilhofer H.U., Sprengel R., Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44:637–650. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L-J., Zhong Y., Ren W-J., Li Y-Y., Zhang T., Liu X-G. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp Neurol. 2008;212:507–514. doi: 10.1016/j.expneurol.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Wu J., Wu Z., Lin Q., Yue Y., Fang L. Regulation of AMPA receptors in spinal nociception. Mol Pain. 2010;6:5–12. doi: 10.1186/1744-8069-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Drdla-Schutting R, Benrath J, Wunderbaldinger G, Sandkühler J: Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration, Science 2012, in press. [DOI] [PubMed]; First study to demonstrate that opioids may reverse LTP at C-fiber synapse and hyperalgesia in behaving animals. Reversal of LTP involved activation of protein phosphatase 1 and was associated with the dephosphorylation of AMPA receptors at Ser831 in spinal dorsal horn.
- 37.Lee C.J., Bardoni R., Tong C-K., Engelman H.S., Joseph D.J., Magherini P.C., MacDermott A.B. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35:135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H-Y., Chen S-R., Chen H., Pan H-L. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30:4460–4466. doi: 10.1523/JNEUROSCI.5857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacktor T.C. How does PKMζ maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- 40.Asiedu M.N., Tillu D.V., Melemedjian O.K., Shy A., Sanoja R., Bodell B., Ghosh S., Porreca F., Price T.J. Spinal protein kinase M ζ underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Li X.-Y., Ko H.-G., Chen T., Descalzi G., Koga K., Wang H., Kim S.S., Shang Y., Kwak C., Park S.W. Alleviating neuropathic pain hypersensitivity by inhibiting PKMζ in the anterior cingulate cortex. Science. 2010;330:1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]; This study demonstrates that maintenance of neuropathic pain requires activity of atypical protein kinase Mζ in the anterior cingulate cortex. This important finding strongly suggests that maintenance of LTP at synapses in this brain region is essential for hyperalgesia after nerve injury.
- 42.Galan A., Laird J.M., Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain. 2004;112:315–323. doi: 10.1016/j.pain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 44.Li X-Y., Ko H-G., Chen T., Collingridge G.L., Kaang B-K., Zhuo M. Erasing injury-related cortical synaptic potentiation as a new treatment for chronic pain. J Mol Med (Berl) 2011;89:847–855. doi: 10.1007/s00109-011-0768-9. [DOI] [PubMed] [Google Scholar]
- 45.Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 46.McMahon S.B., Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 47.Milligan E.D., Watkins L.R. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhuo M., Wu G., Wu L-J. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain. 2011;4:31. doi: 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Torsney C. Inflammatory pain unmasks heterosynaptic facilitation in lamina I neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2011;31:5158–5168. doi: 10.1523/JNEUROSCI.6241-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that a peripheral inflammation (CFA) leads to an increased incidence of monosynaptic Aδ-fiber input to lamina I neurons, possibly by converting ‘silent’ Aδ-fiber synapses into synapses expressing functional AMPARs. This heterosynaptic strengthening of synaptic transmission may underlie mechanical hyperalgesia in inflammatory pain.
- 50•.Fenselau H., Heinke B., Sandkühler J. Heterosynaptic long-term potentiation at GABAergic synapses of spinal lamina I neurons. J Neurosci. 2011;31:17383–17391. doi: 10.1523/JNEUROSCI.3076-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration of heterosynaptic LTP in the spinal cord. In this study conditioning stimulation of primary afferent C-fibers not only caused homosynaptic LTP at C-fiber synapses but in addition also LTP at GABAergic synapses converging onto the same lamina I neuron.
- 51.Benrath J., Brechtel C., Stark J., Sandkühler J. Low dose of S(+)-ketamine prevents long-term potentiation in pain pathways under strong opioid analgesia in the rat spinal cord in vivo. Br J Anaesth. 2005;95:518–523. doi: 10.1093/bja/aei215. [DOI] [PubMed] [Google Scholar]
- 52.Terman G.W., Eastman C.L., Chavkin C. Mu opiates inhibit long-term potentiation induction in the spinal cord slice. J Neurophysiol. 2001;85:485–494. doi: 10.1152/jn.2001.85.2.485. [DOI] [PubMed] [Google Scholar]
- 53.Benrath J., Brechtel C., Martin E., Sandkühler J. Low doses of fentanyl block central sensitization in the rat spinal cord in vivo. Anesthesiology. 2004;100:1545–1551. doi: 10.1097/00000542-200406000-00030. [DOI] [PubMed] [Google Scholar]
- 54.Benrath J., Kempf C., Georgieff M., Sandkühler J. Xenon blocks the induction of synaptic long-term potentiation in pain pathways in the rat spinal cord in vivo. Anesth Analg. 2007;104:106–111. doi: 10.1213/01.ane.0000250368.27822.31. [DOI] [PubMed] [Google Scholar]
- 55.Klein T., Magerl W., Nickel U., Hopf H-C., Sandkühler J., Treede R-D. Effects of the NMDA-receptor antagonist ketamine on perceptual correlates of long-term potentiation within the nociceptive system. Neuropharmacology. 2007;52:655–661. doi: 10.1016/j.neuropharm.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 56.DeLaTorre S., Rojas-Piloni G., Martínez-Lorenzana G., Rodríguez-Jiménez J., Villanueva L., Condés-Lara M. Paraventricular oxytocinergic hypothalamic prevention or interruption of long-term potentiation in dorsal horn nociceptive neurons: electrophysiological and behavioral evidence. Pain. 2009;144:320–328. doi: 10.1016/j.pain.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Hu X-D., Ge Y-X., Hu N-W., Zhang H-M., Zhou L-J., Zhang T., Li W-M., Han Y-F., Liu X-G. Diazepam inhibits the induction and maintenance of LTP of C-fiber evoked field potentials in spinal dorsal horn of rats. Neuropharmacology. 2006;50:238–244. doi: 10.1016/j.neuropharm.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Youn D-H. N-methyl-d-aspartate-dependent long-term potentiation of excitatory transmission in trigeminal subnucleus oralis. Neuroreport. 2008;19:733–738. doi: 10.1097/WNR.0b013e3282fd695b. [DOI] [PubMed] [Google Scholar]
- 59.Toyoda H., Zhao M.G., Mercaldo V., Chen T., Descalzi G., Kida S., Zhuo M. Calcium/calmodulin-dependent kinase IV contributes to translation-dependent early synaptic potentiation in the anterior cingulate cortex of adult mice. Mol Brain. 2010;3:27. doi: 10.1186/1756-6606-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhuo M. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells. 2007;23:259–271. [PubMed] [Google Scholar]
- 61.Zhuo M. Cortical plasticity as a new endpoint measurement for chronic pain. Mol Pain. 2011;7:54. doi: 10.1186/1744-8069-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qu X-X., Cai J., Li M-J., Chi Y-N., Liao F-F., Liu F-Y., Wan Y., Han J-S., Xing G-G. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009;215:298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Haugan F., Rygh L.J., Tjølsen A. Ketamine blocks enhancement of spinal long-term potentiation in chronic opioid treated rats. Acta Anaesthesiol Scand. 2008;52:681–687. doi: 10.1111/j.1399-6576.2008.01637.x. [DOI] [PubMed] [Google Scholar]
- 64.Hjornevik T., Jacobsen L.M., Qu H., Bjaalie J.G., Gjerstad J., Willoch F. Metabolic plasticity in the supraspinal pain modulating circuitry after noxious stimulus-induced spinal cord LTP. Pain. 2008;140:456–464. doi: 10.1016/j.pain.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X-C., Zhang Y-Q., Zhao Z-Q. Involvement of nitric oxide in long-term potentiation of spinal nociceptive responses in rats. Neuroreport. 2005;16:1197–1201. doi: 10.1097/00001756-200508010-00013. [DOI] [PubMed] [Google Scholar]
- 66.Ying B., Lü N., Zhang Y-Q., Zhao Z-Q. Involvement of spinal glia in tetanically sciatic stimulation-induced bilateral mechanical allodynia in rats. Biochem Biophys Res Commun. 2006;340:1264–1272. doi: 10.1016/j.bbrc.2005.12.139. [DOI] [PubMed] [Google Scholar]
- 67•.Pfau D.B., Klein T., Putzer D., Pogatzki-Zahn E.M., Treede R.-D., Magerl W. Analysis of hyperalgesia time courses in humans after painful electrical high-frequency stimulation identifies a possible transition from early to late LTP-like pain plasticity. Pain. 2011;152:1532–1539. doi: 10.1016/j.pain.2011.02.037. [DOI] [PubMed] [Google Scholar]; This study evaluated the time courses of perceptual correlates of LTP in human volunteers. Conditioning high frequency stimulation of skin afferents lead to primary and secondary hyperalgesia with time courses compatible with the development of early-phase LTP. In some volunteers delayed time course suggestive of development of late-phase LTP were observed, possibly indicating a transition to persistent pain disorders.
- 68.Klein T., Stahn S., Magerl W., Treede R-D. The role of heterosynaptic facilitation in long-term potentiation (LTP) of human pain sensation. Pain. 2008;139:507–519. doi: 10.1016/j.pain.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Hansen N., Klein T., Magerl W., Treede R-D. Psychophysical evidence for long-term potentiation of C-fiber and Aδ-fiber pathways in humans by analysis of pain descriptors. J Neurophysiol. 2007;97:2559–2563. doi: 10.1152/jn.01125.2006. [DOI] [PubMed] [Google Scholar]
- 70.Lang S., Klein T., Magerl W., Treede R-D. Modality-specific sensory changes in humans after the induction of long-term potentiation (LTP) in cutaneous nociceptive pathways. Pain. 2007;128:254–263. doi: 10.1016/j.pain.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 71•.van den Broeke E.N., van Rijn C.M., Biurrun Manresa J.A., Andersen O.K., Arendt-Nielsen L., Wilder-Smith O.H. Neurophysiological correlates of nociceptive heterosynaptic long-term potentiation in humans. J Neurophysiol. 2010;103:2107–2113. doi: 10.1152/jn.00979.2009. [DOI] [PubMed] [Google Scholar]; This study provides evidence that in human volunteers LTP-inducing stimuli trigger heterotopic effects on mechanically evoked event-related cortical potentials. The data suggest that conditioning stimulation of nociceptive afferents triggers heterotopic and heterosynaptic facilitation in low threshold mechanosensitive pathways to the somatosensory cortex.
- 72.Svendsen F., Tjølsen A., Hole K. AMPA and NMDA receptor-dependent spinal LTP after nociceptive tetanic stimulation. Neuroreport. 1998;9:1185–1190. doi: 10.1097/00001756-199804200-00041. [DOI] [PubMed] [Google Scholar]
- 73.Yang H-W., Hu X-D., Zhang H-M., Xin W-J., Li M-T., Zhang T., Zhou L-J., Liu X-G. Roles of CaMKII, PKA and PKC in the induction and maintenance of LTP of C-fiber evoked field potentials in rat spinal dorsal horn. J Neurophysiol. 2004;91:1122–1133. doi: 10.1152/jn.00735.2003. [DOI] [PubMed] [Google Scholar]
- 74.Yang H-W., Zhou L-J., Hu N-W., Xin W-J., Liu X-G. Activation of spinal D1/D5 receptors induces late-phase LTP of c-fiber evoked field potentials in rat spinal dorsal horn. J Neurophysiol. 2005;94:961–967. doi: 10.1152/jn.01324.2004. [DOI] [PubMed] [Google Scholar]
- 75•.Song X.-J., Zheng J.-H., Cao J.-L., Liu W.-T., Song X.-S., Huang Z.-J. EphrinB-EphB receptor signaling contributes to neuropathic pain by regulating neural excitability and spinal synaptic plasticity in rats. Pain. 2008;139:168–180. doi: 10.1016/j.pain.2008.03.019. [DOI] [PubMed] [Google Scholar]; This study provides evidence that interactions between ephrins and Eph receptor tyrosine kinase in spinal dorsal horn lead to LTP at C-fiber synapses and contribute to neuropathic pain.
- 76.Xin W-J., Gong Q-J., Xu J-T., Yang H-W., Zang Y., Zhang T., Li Y-Y., Liu X-G. Role of phosphorylation of ERK in induction and maintenance of LTP of the C-fiber evoked field potentials in spinal dorsal horn. J Neurosci Res. 2006;84:934–943. doi: 10.1002/jnr.21013. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z-Y., Zhang Y-Q., Zhao Z-Q. Inhibition of tetanically sciatic stimulation-induced LTP of spinal neurons and Fos expression by disrupting glutamate transporter GLT-1. Neuropharmacology. 2006;51:764–772. doi: 10.1016/j.neuropharm.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 78.Azkue J.J., Liu X-G., Zimmermann M., Sandkühler J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain. 2003;106:373–379. doi: 10.1016/j.pain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Jung S.J., Kim S.J., Park Y.K., Oh S.B., Cho K., Kim J. Group I mGluR regulates the polarity of spike-timing dependent plasticity in substantia gelatinosa neurons. Biochem Biophys Res Commun. 2006;347:509–516. doi: 10.1016/j.bbrc.2006.06.134. [DOI] [PubMed] [Google Scholar]
- 80.Liu X-G., Sandkühler J. Characterization of long-term potentiation of C-fiber-evoked potentials in spinal dorsal horn of adult rat: essential role of NK1 and NK2 receptors. J Neurophysiol. 1997;78:1973–1982. doi: 10.1152/jn.1997.78.4.1973. [DOI] [PubMed] [Google Scholar]
- 81.Liu X-G., Sandkühler J. Activation of spinal N-methyl-d-aspartate or neurokinin receptors induces long-term potentiation of spinal C-fibre-evoked potentials. Neuroscience. 1998;86:1209–1216. doi: 10.1016/s0306-4522(98)00107-9. [DOI] [PubMed] [Google Scholar]
- 82.Rygh L.J., Suzuki R., Rahman W., Wong Y., Vonsy J.L., Sandhu H., Webber M., Hunt S., Dickenson A.H. Local and descending circuits regulate long-term potentiation and zif268 expression in spinal neurons. Eur J Neurosci. 2006;24:761–772. doi: 10.1111/j.1460-9568.2006.04968.x. [DOI] [PubMed] [Google Scholar]
- 83.Gong Q-J., Li Y-Y., Xin W-J., Wei X-H., Cui Y., Wang J., Liu Y., Liu C-C., Li Y-Y., Liu X-G. Differential effects of adenosine A1 receptor on pain-related behavior in normal and nerve-injured rats. Brain Res. 2010;1361:23–30. doi: 10.1016/j.brainres.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 84.Ge Y-X., Xin W-J., Hu N-W., Zhang T., Xu J-T., Liu X-G. Clonidine depresses LTP of C-fiber evoked field potentials in spinal dorsal horn via NO-cGMP pathway. Brain Res. 2006;1118:58–65. doi: 10.1016/j.brainres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 85.Tanabe M., Murakami H., Honda M., Ono H. Gabapentin depresses C-fiber-evoked field potentials in rat spinal dorsal horn only after induction of long-term potentiation. Exp Neurol. 2006;202:280–286. doi: 10.1016/j.expneurol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 86.Hu N-W., Zhang H-M., Hu X-D., Li M-T., Zhang T., Zhou L-J., Liu X-G. Protein synthesis inhibition blocks the late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. J Neurophysiol. 2003;89:2354–2359. doi: 10.1152/jn.01027.2002. [DOI] [PubMed] [Google Scholar]