Abstract
The variability of shoot architecture in plants is striking and one of the most extreme examples of adaptive growth in higher organisms. Mediated by the differential activity of apical and lateral meristems, flexibility in stem growth essentially contributes to this variability. In spite of this importance, the regulation of major events in stem development is largely unexplored. Recently, however, novel approaches exploiting knowledge from root and leaf development are starting to shed light on molecular mechanisms that regulate this essential plant organ. In this review, we summarize our understanding of initial patterning events in stems, discuss prerequisites for the initiation of lateral stem growth and highlight the burning questions in this context.
Stems are central organs of plants
Plant growth is flexible, especially for shoots, differing extremely in size, architecture and function. Classically, shoots of seed plants have been divided into repetitive units called phytomers, each of which consists of a leaf, a leaf attachment site including an axillary bud (nodium) and an associated piece of stem (internodium) (Figure 1a). The modification of this unit, in phylogenetic and ontogenetic terms, is fundamental for establishment of the large diversity of plant growth forms and the adaptability of plants to various environmental conditions. Modification of stem development is essential for this flexibility, because stems are the central part of the plant, connect various body parts and provide, or transport, substances important for long-distance cell-to-cell communication. Interestingly, in spite of its fundamental role in plant growth, our knowledge of how stem development is regulated is limited, and attempts to change this situation are rare. Moreover, in a large repertoire of species, stems have the capacity to grow laterally. Mediated by stem cell-like (meristematic) tissues, in this case predominantly the cambium (Figure 1b, c), lateral growth of stems and roots is essential for generating large plant bodies. Thereby, it substantially contributes to the dominance of seed plants in terrestrial ecosystems, to wood formation and thus to carbon immobilization. The initiation of lateral meristems has not been studied in depth at the cellular level; this is another underexplored patch in our knowledge of the regulation of stem anatomy and growth dynamics.
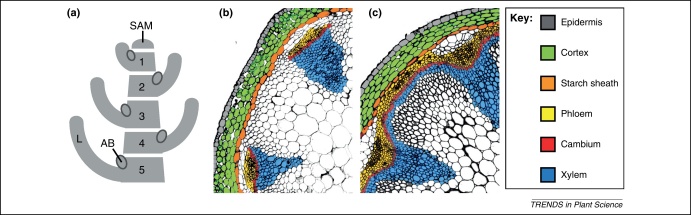
Figure 1.
(a) Schematic representation of the phytomer concept proposed for plant shoots. Phytomers along the shoot are sequentially labeled from young to old and include one leaf (L), one axillary bud (AB) and one associated stem fragment each. SAM, Shoot apical meristem. (b, c) Comparison of cross-sections from a primary (b) and secondary (c)Arabidopsis stem. Pictures taken and adapted from [71].
In this review, we describe the establishment of primary stem anatomy (Figure 1b) as a derivative of the shoot apical meristem (SAM) and highlight developmental parallels to other organs such as roots and leaves. We then turn to lateral stem growth and the ontogenetic relationship between apical and lateral stem meristems. Among lateral meristems, the cambium has a long history of investigation in woody species [1] and there are numerous excellent reviews addressing the regulation of cambium activity emphasizing hormonal control and the cross-fertilization of Arabidopsis (Arabidopsis thaliana) research and research into tree species [2–4]. Here, we focus on the early stages of stem development and discuss properties of the primary stem (Figure 1b) possibly required for the induction of cambium-based lateral growth (Figure 1c).
Establishment of the primary stem
Plant organs diversified during evolution
Apical growth mediated by one or several stem cells leading to the formation of cylindrical tip-grown organs is a characteristic feature of land plants (Figure 2a). Based on fossil records, it is assumed that instead of forming distinct shoot and root systems, early land plants were built of simple leafless growth axes (telomes), growing predominantly horizontally and containing (pro)vascular tissue organized as a protostele [5] (Figure 2b). According to this view, an important adaptation to a land-based lifestyle was the diversification of telomic structures into organs specialized for above- and underground growth. Ancient organ diversification is supported by anatomical similarities in stems and roots and, for instance, the observation that in extant species similar molecular mechanisms regulate the dynamics of both shoot and root apical meristems [6,7]. In addition, shoot poles can be transformed into root poles and vice versa by ectopic expression of PLETHORA and CLASS III HOMEODOMAIN-LEUCINE ZIPPER (HD-ZIPIII) genes, respectively [8,9], indicating that polar growth does not depend on the establishment of two different organ identities and that growth axis identities are transformable by the activity of selected key regulators.
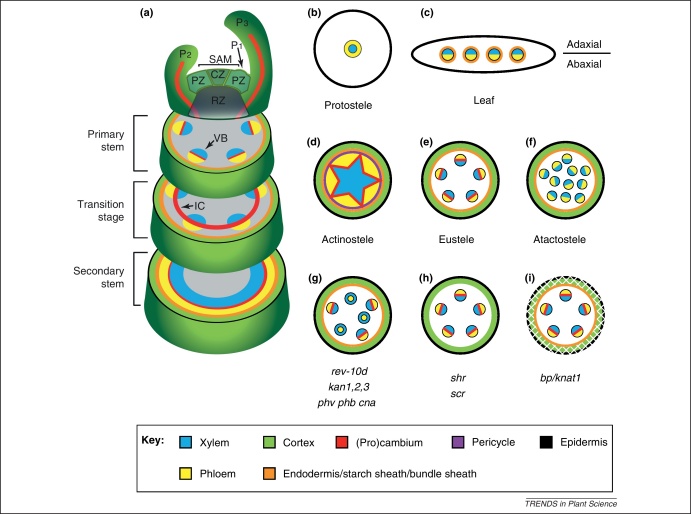
Figure 2.
Schematic representation of typical tissue patterns in different organs of vascular plants. (a) Typical stages and organization of a shoot of a dicotyledonous plant. After establishment of the primary pattern, a tube-like domain of meristematic activity is established that transforms the primary into a secondary stem. (b) The protostele contains a central and concentric vascular system. It is regarded as being the most ancient tissue organization in vascular plant stems and is found in juvenile plants of many extant ferns. (c) General organization of a typical leaf. (d) Actinostele as usually found in roots of vascular plants. (e) The eustele is typical for stems of Magnoliids and Eudicotyledons and is characterized by a parenchymatous pith in the stem center and collateral bundles. Individual bundles are separated to various degrees by parenchymatous rays. (f) The atactostele as usually found in stems of monocotyledons. A common endodermis encompassing the whole stele is sometimes present. (g) In stems of rev-10d gain-of-function and in phv phb cna or kan1,2,3 triple mutants, vascular bundles are radialized and shifted towards the stem center. (h)shr and scr mutants fail to establish a starch sheath. (i) Differentiation of peripheral tissues is disturbed in bp/knat1 mutants. Abbreviations: CZ, central zone; IC, interfascicular cambium; Px, leaf primordial; PZ, peripheral zone; RZ, rib zone; SAM, shoot apical meristem; VB, vascular bundle.
A strong phylogenetic relationship also seems to exist between stems and leaves. According to Zimmermann's telome theory, leaves are derived from shoot-like precursors in euphyllophytes (ferns and seed plants) [10]. Starting with overtopping by the main shoot, lateral stem clusters are thought to have undergone planation and subsequent fusion by photosynthetically active tissue (webbing) [11]. In fact, the presence of the same signaling modules regulating, for instance, lateral organ formation at the flanks of the SAM and the formation of leaflets in growing leaves supports a strong evolutionary link between both organs [12,13] and provides scenarios for the molecular bases for the distinct transformation steps during the evolution of leaves (reviewed in [11,14]). Thus, postulating a tight phylogenetic relationship between stems and other organs, the analysis of stem development can be expected to be rewarding for an understanding of the development and origin of various plant body structures.
There are major anatomical parallels between stems and other organs
The anatomy of the primary stem is established immediately below the SAM in a region designated the rib zone [15] (Figure 2a). Owing to the lack of stereotypic cell divisions in the Arabidopsis SAM and obstacles in microscopic accessibility, a detailed analysis of these events is challenging and has rarely been undertaken. These obstacles might be overcome in part by extrapolating observations from other organs to the stem. Endodermis specification is an example of regulatory parallels between stems and roots. In both organs, endodermis formation depends on the SHORTROOT–SCARECROW (SHR–SCR) signaling module, consisting of two members of the GRAS family of transcription factors [16–18] (Figure 2). In the root apical meristem (RAM), SHR is expressed in procambial cells of the stele, whereas SCR is expressed in the adjacent endodermis and the quiescent center [18]. Endodermis specification and stable SCR expression depend on the movement of the SHR protein out of the stele into the neighboring cell layer. Here, it translocates to the nucleus after it has dimerized with the SCR protein and regulates the transcription of downstream targets [18,19]. SHR is similarly expressed in (pre)procambial cells in leaves and the SHR protein also moves to the nucleus of the surrounding cell layer [20], which potentially represents the developing bundle sheath expressing SCR [16]. Recently, a reciprocal interaction between SHR–SCR and HD-ZIPIII genes has been characterized in roots. After traveling to the endodermis, SHR activates the transcription of miRNA165a and 166b genes together with SCR and the respective miRNAs move back to the stele periphery where they repress HD-ZIPIII activity [21]. The spatial and molecular interaction of these gene activities is elusive in stems. In this case, analysis of the SCR–SHR interaction in the rib zone would be revealing with respect to mechanisms of endodermis specification in a cellular environment without extensive predictability of cell division patterns and nonradialized anatomy (Figures 2 and 3c). Careful inspection of stem anatomy of respective mutants and high-resolution imaging of expression domains in the rib zone might reveal similar interactions in this case.
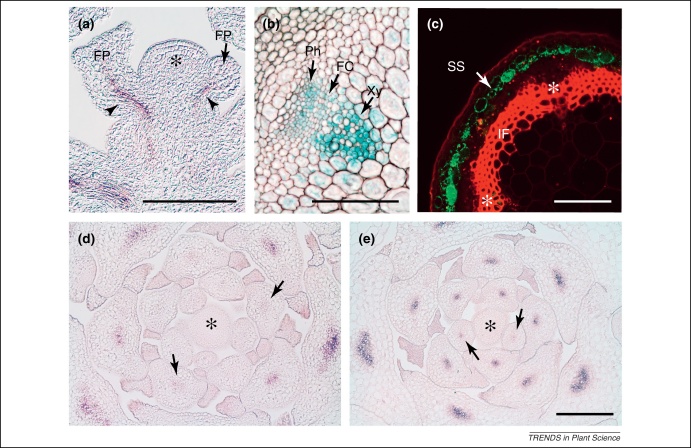
Figure 3.
Analysis of various gene expression patterns in different Arabidopsis organs. (a)ATHB8[98,99] transcript accumulation in procambial strands (arrowheads) as visualized by RNA in situ hybridization indicates the early initiation of vascular tissue in lateral organs. A longitudinal section through a generative apex is shown. The asterisk denotes the SAM. (b) As indicated by the activity of a STM:GUS reporter [100], the STM promoter is active in differentiated tissues of vascular bundles in stems. (c) The SCR:GFP marker visualizes the starch sheath at the bottom of stems of 15-cm-tall plants (green signal). The rough hand section was counterstained with propidium iodide (red signal). Asterisks label the position of primary vascular bundles. (d, e) RNA in situ hybridizations of vegetative shoot apices reveal an early activation of WOX4 (d) in vascular bundles of leaf primordia which is, however, later than the activation of the ‘preprocambial’ marker ATHB8(e). Arrows indicate the youngest primordia in which respective transcript accumulation is detectable. Asterisks label the SAM. Scale bars: 100 μm. Same magnification in (d) and (e). RNA in situ hybridizations were performed as described previously [71]. Abbreviations: FC, Fascicular cambium; FP, flower primordium; IF, interfascicular fibers; Ph, phloem; SS, starch sheath; Xy, xylem.
In addition to peripheral tissues, such as the endodermis and cortex, primary stem anatomy mainly differs from root anatomy in the organization of their central part, the stele. Stems often display a eustelic (most dicots, Figure 2e) or an atactostelic (monocots, Figure 2f) organization, whereas roots usually develop an actinostele (Figure 2d). Interestingly, genes known to regulate adaxial–abaxial polarity of leaves are also important for stele development in stems and roots [21–23]. In this context, the class III homeodomain-leucine zipper (HD-ZIPIII) family of transcription factors is well characterized. It is hypothesized that in telomes of early land plants, HD-ZIPIII transcription factors mediated central cell fate including vascular tissue identity and that the promotion of ‘central’ cell fate has evolved into the promotion of ‘adaxial’ cell fate and xylem differentiation during the evolution of stems and leaves in higher plants [14,23,24]. A role of HD-ZIPIIIs in the formation of central vasculature is suggested by expression patterns in lycophytes [23]. In this regard, a function of the HD-ZIPIII genes in regulating vascular programs in the stem center predates any roles in leaves.
Clarification of the role of the five HD-ZIPIII genes found in Arabidopsis [REVOLUTA (REV/IFL), PHABULOSA (PHB/ATHB14), PHAVOLUTA (PHV/ATHB9), CORONA (CNA/ATHB15) and ATHB8] in determining the overall tissue composition in stems is not straightforward, because redundant and antagonistic interactions in various combinations exist [25]. In rev mutants, for example, interfascicular fibers are missing and xylem differentiation is disturbed [26,27] and these defects are more prominent in rev phb and rev phv double mutants [25]. By contrast, rev-specific defects are slightly suppressed in rev cna athb8 triple mutants [25]. Consistent with opposite roles of REV and CNA, in CNA-deficient plants the formation of xylem is elevated [25,28]. The same effect is observed in stems of plants with increased activity of the HD-ZIPIII-repressing miR166 in which predominantly CNA transcript abundance is reduced [28,29]. Importantly, collateral bundle organization is partly transformed into amphivasal organization (see Glossary) in phv phb cna triple mutants and in rev gain-of-function mutants [22,25,30] (Figure 2g). Similar pattern alterations are observed in mutants defective for multiple members of the KANADI (KAN) gene family belonging to the group of GARP transcription factors [22]. KAN genes function antagonistically to HD-ZIPIII genes in determining leaf polarity by mediating abaxial leaf fate [22,31,32]. In addition to defects in adaxial–abaxial polarity, the position of vascular bundles is partly shifted towards the center of the stem in hd-zipIII and kan multiple mutants [22,25] (Figure 2g). This suggests that not only internal bundle organization but also the overall radial organization of the stele depend on the proper establishment of adaxial–abaxial polarity. It remains to be elucidated whether other components identified to regulate leaf polarity [33] are also involved in the regulation of stem anatomy.
Consistent with the idea that eustelic organization is a derived form of stem anatomy, the parenchymatous character of Arabidopsis pith cells is actively maintained by the action of the WRKY12 transcription factor (Figure 2). WRKY12 binds and represses the promoter of the NAC SECONDARY WALL THICKENING PROMOTING FACTOR2 (NST2) gene encoding a NAC domain transcription factor, which belongs to a transcription factor cascade that promotes secondary cell wall formation [34,35]. Consequently, wrky12 mutants display enhanced secondary cell wall thickening and ectopic lignin deposition in pith cells [36]. This indicates that, by default, differentiation processes in the stem center resemble those in fiber or xylem cells present in adaxial tissues of vascular bundles.
Regulation of meristematic attributes during the establishment of stem tissues
The reticulate and plastic nature of the plant vascular system is achieved by the self-reinforcing formation of routes of polar auxin transport along narrow arrays of cells, which subsequently gain procambium identity and differentiate into vascular bundles [37–39]. A local increase in the expression of the PIN-FORMED1 (PIN1) auxin exporter represents one of the earliest markers for procambium initiation in various organs [38,39]. Auxin and PIN1 distributions have, again, not been characterized in the rib zone. However, computational modeling supports the idea that vascular bundle distribution in the stem is controlled by auxin maxima derived from polar transport [40]. It is important to note that in seed plants the differentiation of large parts of the stem vasculature starts from lateral organ primordia, which are main sources of auxin and thus determine sites of vascularization in stems (Figures 2a and 3a). The initiation of vascular strands does not require the vascularization of lateral organs or PIN1 itself, however, as in PIN1-defective Arabidopsis plants, whose elongated shoots are devoid of lateral organs, and patterning of vascular bundles is only mildly affected. The enhancement of xylem formation below cauline leaves and slightly changed bundle positions are the most obvious alterations [41]. Thus, it can be assumed that a complex regulation exists that involves various members of the family of PIN auxin exporters, which are active in a predefined cylindrical domain in the rib zone. Importantly, auxin flow along procambium cells seems to be essential for maintaining the meristematic attributes important for the formation of secondary vascular tissues (see below). Therefore, it is possible that early establishment of auxin transport routes in the rib zone generates a continuum of meristematic cell identity during the transition of cells from the SAM to the procambium.
Along the same line, the family of KNOX1 transcription factors [SHOOT MERISTEMLESS (STM), BREVIPEDICELLUS (BP/KNAT1), KNOTTED-LIKE HOMEOBOX PROTEIN2 (KNAT2) and KNAT6] is associated with the maintenance of meristematic identity in the Arabidopsis SAM [42]. This is reflected by the expression of STM and BP in the SAM and their immediate downregulation in cells initiating the leaf formation program [43,44]. By contrast, the expression of both genes is maintained in the rib zone [43,44]. In fact, a BP:GUS transcriptional marker has been used to show that the subapical zone is extended in plants defective for the ATH1 homeobox transcription factor [45]. Interestingly, KNOX1 genes continue to be expressed in highly differentiated stem tissues [43,44] (Figure 3b) and are, at least partly, involved in the regulation of differentiation processes. In bp mutants, cortex and epidermis differentiation is affected [46,47] (Figure 2i), spacing of vascular bundles is disturbed [48], lignin is deposited ectopically, and interfascicular fiber and xylem formation appears earlier than in the wild type [49]. In addition to the base of the SAM and the rib zone, BP is expressed in vascular tissues and in the cortex [44,47,50]. BP functions in concert with the BEL1-like transcription factor PENNYWISE (PNY) to repress KNAT2 and KNAT6 activities in pedicels and stems, respectively [48,51]. An antagonistic interaction between BP/PNY and KNAT6 in stem development is supported by the attenuation of bp and pny-specific alterations in shoot morphology in KNAT6-deficient backgrounds [51]. Considering the essential role of KNOX1 genes in SAM maintenance, it is tempting to speculate that they also positively influence the activity of lateral meristems in the stem by preserving or stimulating meristematic cell properties. Such an influence was demonstrated for STM and BP orthologs in hybrid aspen (Populus tremula × Populus alba), ARK1 and ARK2, which, among other tissues, are active in the cambium [52,53]. Consistent with a positive role of KNOX1 genes in the regulation of lateral growth, plants overexpressing either of both genes display delayed differentiation of vascular tissues and an enlarged cambium zone [4,52,53].
Initiation of lateral growth
Stems of gymnosperms and most angiosperms grow in diameter by the cambium-based production of secondary vascular tissue. Fossil records suggest that the bifacial vascular cambium, as known from extant species, evolved approximately 400 million years ago in early seed plants, which thereby overcame hydraulic constraints [54] and subsequently achieved a dramatic increase in growth-form plasticity and increased adaptability to different environments [55].
Auxin is essential for lateral growth
In ontogenetic terms, cambium-based lateral growth is closely connected to the establishment of primary vascular bundles in an ‘open’ conformation, meaning that procambium attributes are maintained in the bundle center. The established view is that after determination of procambial strands, xylem and phloem differentiation starts from adaxial and abaxial bundle poles, respectively, progressing towards the bundle center [56]. Which factors are important for the maintenance of (pro)cambium characteristics in the bundle center, that is, for the distinction between open and closed bundles, is unclear. Although other hormones partly have a strong influence on cambium activity [57–59], one strong candidate for the maintenance of procambium characteristics is the differential regulation of auxin transport, perception and/or production during bundle differentiation. Auxin has been shown in numerous studies to be essential for inducing and maintaining fascicular and interfascicular cambium activity. Snow demonstrated 75 years ago that decapitated sunflower seedlings activated vascular cambium in the stem only when treated apically with auxin [60]. The same type of experiment has confirmed the necessity of apex-derived auxin for secondary growth in other species such as Arabidopsis and Populus [61–63]. Indeed, measurements in the stem of Pinus sylvestris and Populus along the radial sequence of tissues show that auxin concentration peaks in the vascular cambium [64–66].
In Arabidopsis, the size of the domain in the bundle center displaying high levels of auxin signaling and cambium activity itself is defined by the interplay between KAN and HD-ZIPIII genes [24]. Removal of genes from either group increases the (pro)cambium domain [24], indicating that cambium characteristics are suppressed in adaxial and abaxial bundle domains to allow cell differentiation. Consistently, REV-deficient stems display enhanced cambium activity [27] and ectopic expression of KAN1 inhibits (pro)cambium formation [24,31]. By contrast, overexpression of a REV ortholog from Populus (PRE) leads to pleiotropic defects, one of them being ectopic cambium formation in the cortex, also suggesting a positive effect of the gene on cambium activity [67]. However, considering the complex interaction among the HD-ZIPIII family members [25], this effect could be due to the repression of other family members, for example the Populus CNA ortholog, which is believed to promote cell differentiation [68].
Why is there no maintenance of an active cambium in leaves even though initial events of bundle formation are very similar to those in stems? The production of secondary vascular tissue in leaves is only rudimentary in most species, including Arabidopsis [69]. However, the two cambium markers PHLOEM INTERCALATED WITH XYLEM (PXY) and WUSCHEL-RELATED HOMEOBOX4 (WOX4) are expressed in leaf bundles [70–72] (see below, Figure 3d), suggesting that the cambium-specific stem cell niche is established during leaf development and that leaf bundles keep their open character. One possible explanation for the missing cambium activity is the attenuation of auxin production in older leaves [73]. Auxin treatments of leaf explants in tissue culture lead to plant regeneration, often starting with proliferation of cells from the vasculature [69,74], suggesting that, even when they are not actively dividing, cells within the vasculature harbor a particular meristematic potential, which can be activated by elevated auxin levels. Thus, establishment and maintenance of the cambium-specific stem cell niche throughout different plant organs might be one important prerequisite for the high degree of plant growth plasticity.
Activation of the cambium-specific stem cell niche
Subsequent to fascicular cambium activation, meristematic characteristics of the fascicular cambium are extended to interfascicular regions [59], thereby establishing a continuous tube-like domain of meristematic activity (Figures 1b, c and 2a). It has been a matter of debate whether the interfascicular cambium is established ‘de novo’ or whether there is a precambial domain, a group of cells derived from the apical meristem, whose cell fate is predetermined to produce the interfascicular cambium [56,75]. However, the stochastic pattern of procambial strands during leaf formation [38], the ectopic inducibility of vascular tissues in stems by auxin [39] and cases where the interfascicular cambium originates from different cell types at different growth stages [59] show that procambium identity can be induced in a wide range of cell types and that ‘de novo’ initiation is more probable.
Are there cell types in the stem other than the procambium predetermined for initiating meristematic activity? There are indications that a tissue with these properties exists in Arabidopsis roots. Here, lateral root meristems originate from the outermost cell layer of the stele, the pericycle [76]. Also, root cambial activity is partly established in pericycle cells [77] and shoot regeneration starts predominantly from this tissue type in root tissue culture [78]. Thus, the pericycle seems to harbor a special meristematic potential, which depends, at least in part, on auxin signaling events initiated very close to the root apical meristem [79]. In contrast to the endodermis-like characteristics that are found in both organs, there is no pericycle-like tissue in the stem (Figures 2 and 3) and sites of shoot branching and vascular cambium formation are spatially separated. Thus, with the exception of the procambium, there is no indication for the establishment of a tissue with an exceptional meristematic potential in the rib zone essential for vascular cambium initiation.
Direct screens for mutants with altered stem patterning have identified a limited number of mutants with enhanced formation of vascular tissue in interfascicular regions. One example is continuous vascular ring1 (cov1), which is defective for a predicted transmembrane protein of unknown function [80]. A similar phenotype is observed in hypermorphic mutants of the transcription factor DOF5.6/HCA2 [81] and in high cambial activity (hca), the molecular basis of which has not yet been identified but is not allelic to HCA2 or COV1 [82]. Although tissue pattern alterations are not absolutely identical, all three mutants display stunted growth of stems and leaves, suggesting that these processes are linked. Overall, it seems as if all three genes are involved in regulating the initiation of secondary growth in the Arabidopsis stem.
The transition to secondary growth is also repressed by genes promoting flowering [83]. SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1) and FRUITFULL (FUL) are two MADS box transcription factors expressed in the inflorescence meristem and immediately below in the rib zone [84,85]. soc1 ful double mutants display delayed flowering, but also increased secondary growth [83]. This shows that secondary growth in flowering and elongating Arabidopsis shoots is actively repressed. By contrast, floral transition and the activity of the floral stimulator CONSTANS (CO) have been positively associated with secondary growth in the hypocotyl [86]. This shows that the initiation of flowering and stem elongation has different effects at different positions along the main plant growth axis.
The cambium shares properties with the procambium and the SAM
Despite the large anatomical differences between apical meristems and the vascular cambium, it has been shown that both meristem types are controlled by similar players. PXY, a leucine-rich repeat receptor-like kinase (LRR-RLK) also known as TDR [72], is expressed in Arabidopsis cambium and was first identified by a forward mutagenesis screen for mutants affected in primary stem anatomy [87]. In pxy mutants, collateral bundle organization is disturbed as phloem tissue is mixed with xylem, possibly because of defects in the orientation of (pro)cambial cell divisions [87,88]. CLAVATA3/ESR-RELATED (CLE41 and CLE44) signaling peptides are produced in the phloem and bind and activate PXY [72,88], providing positional information similar to LRR-RLK/CLE-dependent cell-to-cell communication events found in shoot and root apical meristems [7,89]. In contrast to known signaling modules in apical meristems, which inhibit meristematic activity, the PXY/CLE41 module stimulates cambium proliferation and represses xylem differentiation [70,72]. Thus, PXY/CLE-based communication between (pro)cambium-derived tissues and the cambium itself seems to be important for controlling both the direction and the level of cell production during lateral growth. As a possible downstream target of PXY in cambium cells, the WOX4 transcription factor has been identified [70]. As for other members of the WOX gene family in apical meristems [6,89], WOX4 activity in the cambium is essential for maintaining meristematic cell fate [70,90]. CLE41 treatment causes a rapid, and PXY-dependent, increase in WOX4 mRNA accumulation and enhanced cambium activity, an effect that depends on both PXY and WOX4 [70]. Interestingly, auxin accumulation in the stem also enhances WOX4 mRNA levels independently of PXY [90]. Thus, as an essential factor of the cambium-specific stem cell niche, WOX4 seems to integrate several pathways acting on the cambium. Recently, more cambium-specific LRR-RLKs have been identified [71,91] and one of them, MORE LATERAL GROWTH1 (MOL1), fulfills a negative role in cambium regulation [71], resembling the role of the LRR-RLKs CLV1 and ACR4 in apical meristems [7,89]. Another one is XYLEM INTERMIXED WITH PHLOEM1 (XIP1), which represses ectopic formation of secondary cell walls and lignin deposition [91]. Because lateral growth is a highly dynamic process, integrating various endogenous and external cues [57], a complex molecular integration of these inputs at the level of cambium cells can be expected and cambium-expressed LRR-RLKs seem to contribute heavily to this integration.
Importantly, even though they are not as early as the preprocambial marker ATHB8 (Figure 3d, e), cambium genes such as PXY and WOX4 are expressed from the procambium stage onwards and focus their expression gradually in the bundle center [71,88,92] (Figure 3d, e). This underlines the specialization of cells during procambium formation and, moreover, supports a continuity of cell fate characteristics during the procambium–cambium transition. Recently, transcriptome remodeling during interfascicular cambium formation in Arabidopsis was characterized in a tissue-specific manner [71]. Comparison of the repertoire of genes induced in cells gaining cambium identity and those active in the SAM did not reveal a large overlap, thus questioning the concept of a continuity of major cell attributes between both meristems.
Concluding remarks and outlook
The importance of the stem as a central structure of the plant makes it crucial for researchers to face the challenges of studying stem development and take advantage of improvements in microscopy techniques, targeted forward and reverse mutagenesis screens and the establishment of high-resolution expression maps. In part, this has already been done for selected tissue types and various stem positions [59,71,93–95] but needs to be pursued in a more comprehensive way, including different developmental stages, as done previously for roots [96,97]. These advances will reveal parallels with and differences to other organs in terms of tissue formation, cell-to-cell communication and radial polarity, and will contribute to filling the large ‘white patches’ still present on the map of tissue patterning and plant organ formation. In particular, the initiation of secondary growth, and its regulation by long- and short-distance signaling [59,71,72,83,86,90], represents an attractive subject to learn more about how different growth processes are coordinated and integrated.
Acknowledgments
We thank John Bowman (Monash University, Australia) and members of the Greb laboratory for helpful comments on the manuscript, Wolfgang Werr (University of Cologne, Germany) for donation of the STM:GUS reporter line and Philip Benfey (Duke University, Durham, NC, USA) for the SCR:GFP line. Eva Maria Sehr (currently at Roche, Vienna, Austria) kindly provided the picture shown in Figure 3c. This work was supported by a grant from the Austrian Science Fund (FWF, grant number P23781-B16 to P.S.).
Glossary
- Amphivasal
describes a concentric vascular bundle in which the xylem surrounds the phloem.
- Collateral
describes a vascular bundle having phloem only on one side of the xylem.
- Fascicular cambium
the stem cell niche present in open vascular bundles producing secondary vascular tissues.
- Interfascicular cambium
the vascular tissue-producing stem cell niche established between vascular bundles and connecting the fascicular cambium of neighboring bundles.
- Procambium
group of cells that form the primary vascular bundle.
- Vascular cambium
the whole of fascicular and interfascicular cambia producing secondary vascular tissues by periclinal cell divisions.
References
- 1.Larson P.R. Springer-Verlag; 1994. The Vascular Cambium: Development and Structure. [Google Scholar]
- 2.Zhang J. Arabidopsis as a model for wood formation. Curr. Opin. Biotechnol. 2011;22:293–299. doi: 10.1016/j.copbio.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Matte Risopatron J.P. The vascular cambium: molecular control of cellular structure. Protoplasma. 2010;247:145–161. doi: 10.1007/s00709-010-0211-z. [DOI] [PubMed] [Google Scholar]
- 4.Du J., Groover A. Transcriptional regulation of secondary growth and wood formation. J. Integr. Plant Biol. 2010;52:17–27. doi: 10.1111/j.1744-7909.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 5.Friedman W.E. The evolution of plant development. Am. J. Bot. 2004;91:1726–1741. doi: 10.3732/ajb.91.10.1726. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar A.K. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 7.Stahl Y. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr. Biol. 2009;19:909–914. doi: 10.1016/j.cub.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Smith Z.R., Long J.A. Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature. 2010;464:423–426. doi: 10.1038/nature08843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aida M. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann W. G. Fischer; 1930. Die Phylogenie der Pflanzen. [Google Scholar]
- 11.Beerling D.J., Fleming A.J. Zimmermann's telome theory of megaphyll leaf evolution: a molecular and cellular critique. Curr. Opin. Plant Biol. 2007;10:4–12. doi: 10.1016/j.pbi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Blein T. A conserved molecular framework for compound leaf development. Science. 2008;322:1835–1839. doi: 10.1126/science.1166168. [DOI] [PubMed] [Google Scholar]
- 13.Busch B.L. Shoot branching and leaf dissection in tomato are regulated by homologous gene modules. Plant Cell. 2011;23:3595–3609. doi: 10.1105/tpc.111.087981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floyd S.K., Bowman J.L. Gene expression patterns in seed plant shoot meristems and leaves: homoplasy or homology? J. Plant Res. 2010;123:43–55. doi: 10.1007/s10265-009-0256-2. [DOI] [PubMed] [Google Scholar]
- 15.Ruonala R. CENL1 expression in the rib meristem affects stem elongation and the transition to dormancy in Populus. Plant Cell. 2008;20:59–74. doi: 10.1105/tpc.107.056721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wysocka-Diller J.W. Molecular analysis of Scarecrow function reveals a radial patterning mechanism common to root and shoot. Development. 2000;127:595–603. doi: 10.1242/dev.127.3.595. [DOI] [PubMed] [Google Scholar]
- 17.Fukaki H. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 1998;14:425–430. doi: 10.1046/j.1365-313x.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima K. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- 19.Sozzani R. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466:128–132. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardiner J. Simultaneous activation of SHR and ATHB8 expression defines switch to preprocambial cell state in Arabidopsis leaf development. Dev. Dyn. 2011;240:261–270. doi: 10.1002/dvdy.22516. [DOI] [PubMed] [Google Scholar]
- 21.Carlsbecker A. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465:316–321. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery J.F. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Floyd S.K., Bowman J.L. Distinct developmental mechanisms reflect the independent origins of leaves in vascular plants. Curr. Biol. 2006;16:1911–1917. doi: 10.1016/j.cub.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 24.Ilegems M. Interplay of auxin, KANADI and class III HD-ZIP transcription factors in vascular tissue formation. Development. 2010;137:975–984. doi: 10.1242/dev.047662. [DOI] [PubMed] [Google Scholar]
- 25.Prigge M.J. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong R., Ye Z.H. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbert P.B. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development. 1995;121:2723–2735. doi: 10.1242/dev.121.9.2723. [DOI] [PubMed] [Google Scholar]
- 28.Kim J. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005;42:84–94. doi: 10.1111/j.1365-313X.2005.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams L. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development. 2005;132:3657–3668. doi: 10.1242/dev.01942. [DOI] [PubMed] [Google Scholar]
- 30.Zhong R., Ye Z.H. Amphivasal vascular bundle 1, a gain-of-function mutation of the IFL1/REV gene, is associated with alterations in the polarity of leaves, stems and carpels. Plant Cell Physiol. 2004;45:369–385. doi: 10.1093/pcp/pch051. [DOI] [PubMed] [Google Scholar]
- 31.Eshed Y. Establishment of polarity in lateral organs of plants. Curr. Biol. 2001;11:1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- 32.Kerstetter R.A. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi: 10.1038/35079629. [DOI] [PubMed] [Google Scholar]
- 33.Kidner C.A., Timmermans M.C. Signaling sides adaxial-abaxial patterning in leaves. Curr. Top. Dev. Biol. 2010;91:141–168. doi: 10.1016/S0070-2153(10)91005-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhong R. Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol. Plant. 2010;3:1087–1103. doi: 10.1093/mp/ssq062. [DOI] [PubMed] [Google Scholar]
- 35.Mitsuda N. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell. 2007;19:270–280. doi: 10.1105/tpc.106.047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H. Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22338–22343. doi: 10.1073/pnas.1016436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachs T. The control of the patterned differentiation of vascular tissues. Adv. Bot. Res. 1981;9:151–162. [Google Scholar]
- 38.Scarpella E. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauer M. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006;20:2902–2911. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibanes M. Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13630–13635. doi: 10.1073/pnas.0906416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gälweiler L. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 42.Ha C.M. Shoot apical meristem form and function. Curr. Top. Dev. Biol. 2010;91:103–140. doi: 10.1016/S0070-2153(10)91004-1. [DOI] [PubMed] [Google Scholar]
- 43.Long J.A. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 44.Lincoln C. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell. 1994;6:1859–1876. doi: 10.1105/tpc.6.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Mena C., Sablowski R. Arabidopsis thaliana homeobox gene1 establishes the basal boundaries of shoot organs and controls stem growth. Plant Cell. 2008;20:2059–2072. doi: 10.1105/tpc.108.059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venglat S.P. The homeobox gene Brevipedicellus is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4730–4735. doi: 10.1073/pnas.072626099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Douglas S.J. KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell. 2002;14:547–558. doi: 10.1105/tpc.010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith H.M., Hake S. The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell. 2003;15:1717–1727. doi: 10.1105/tpc.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mele G. The knotted1-like homeobox gene Brevipedicellus regulates cell differentiation by modulating metabolic pathways. Genes Dev. 2003;17:2088–2093. doi: 10.1101/gad.1120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Douglas S.J., Riggs C.D. Pedicel development in Arabidopsis thaliana: contribution of vascular positioning and the role of the BREVIPEDICELLUS and ERECTA genes. Dev. Biol. 2005;284:451–463. doi: 10.1016/j.ydbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Ragni L. Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell. 2008;20:888–900. doi: 10.1105/tpc.108.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groover A.T. The Populus homeobox gene ARBORKNOX1 reveals overlapping mechanisms regulating the shoot apical meristem and the vascular cambium. Plant Mol. Biol. 2006;61:917–932. doi: 10.1007/s11103-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 53.Du J. The Populus homeobox gene Arborknox2 regulates cell differentiation during secondary growth. Plant J. 2009;60:1000–1014. doi: 10.1111/j.1365-313X.2009.04017.x. [DOI] [PubMed] [Google Scholar]
- 54.Gerrienne P. A simple type of wood in two early Devonian plants. Science. 2011;333:837. doi: 10.1126/science.1208882. [DOI] [PubMed] [Google Scholar]
- 55.Rowe N., Speck T. Plant growth forms: an ecological and evolutionary perspective. New Phytol. 2005;166:61–72. doi: 10.1111/j.1469-8137.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 56.Steeve T.A., Sussex I.M. Cambridge University Press; 1989. Patterns in Plant Development. [Google Scholar]
- 57.Elo A. Stem cell function during plant vascular development. Semin. Cell Dev. Biol. 2009;20:1097–1106. doi: 10.1016/j.semcdb.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Agusti J. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20242–20247. doi: 10.1073/pnas.1111902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sehr E.M. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J. 2010;63:811–822. doi: 10.1111/j.1365-313X.2010.04283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snow R. Activation of cambial growth by pure hormones. New Phytol. 1935;34:347–360. [Google Scholar]
- 61.Ko J.H. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol. 2004;135:1069–1083. doi: 10.1104/pp.104.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Björklund S. Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J. 2007;52:499–511. doi: 10.1111/j.1365-313X.2007.03250.x. [DOI] [PubMed] [Google Scholar]
- 63.Little C.H.A. Involvement of indole-3-acetic acid in fascicular and interfascicular cambial growth and interfascicular extraxylary fiber differentiation in Arabidopsis thaliana inflorescence stems. Int. J. Plant Sci. 2002;163:519–529. [Google Scholar]
- 64.Schrader J. Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J. 2004;40:173–187. doi: 10.1111/j.1365-313X.2004.02199.x. [DOI] [PubMed] [Google Scholar]
- 65.Uggla C. Indole-3-acetic acid controls cambial growth in scots pine by positional signaling. Plant Physiol. 1998;117:113–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uggla C. Auxin as a positional signal in pattern formation in plants. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robischon M. The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiol. 2011;155:1214–1225. doi: 10.1104/pp.110.167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Du J. The Populus class III HD ZIP transcription factor POPCORONA affects cell differentiation during secondary growth of woody stems. PLoS ONE. 2011;6:e17458. doi: 10.1371/journal.pone.0017458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y. Initiation of dedifferentiation and structural changes in in vitro cultured petiole of Arabidopsis thaliana. Protoplasma. 2010;241:75–81. doi: 10.1007/s00709-010-0108-x. [DOI] [PubMed] [Google Scholar]
- 70.Hirakawa Y. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell. 2010;22:2618–2629. doi: 10.1105/tpc.110.076083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agusti J. Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genet. 2011;7:e1001312. doi: 10.1371/journal.pgen.1001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirakawa Y. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15208–15213. doi: 10.1073/pnas.0808444105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006;20:1790–1799. doi: 10.1101/gad.1415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rose R.J. Root meristems in Medicago truncatula tissue culture arise from vascular-derived procambial-like cells in a process regulated by ethylene. J. Exp. Bot. 2006;57:2227–2235. doi: 10.1093/jxb/erj187. [DOI] [PubMed] [Google Scholar]
- 75.Siebers A.M. Initiation of radial polarity in the interfascicular cambium of Ricinus communis L. Acta Botanica Neerlandica. 1971;20:211–220. [Google Scholar]
- 76.Parizot B. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol. 2008;146:140–148. doi: 10.1104/pp.107.107870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Busse J.S., Evert R.F. Vascular differentiation and transition in the seedling of Arabidopsis thaliana (Brassicaceae) Int. J. Plant Sci. 1999;160:241–251. [Google Scholar]
- 78.Atta R. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009;57:626–644. doi: 10.1111/j.1365-313X.2008.03715.x. [DOI] [PubMed] [Google Scholar]
- 79.De Rybel B. A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 2010;20:1697–1706. doi: 10.1016/j.cub.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Parker G. Isolation of COV1, a gene involved in the regulation of vascular patterning in the stem of Arabidopsis. Development. 2003;130:2139–2148. doi: 10.1242/dev.00441. [DOI] [PubMed] [Google Scholar]
- 81.Guo Y. Dof5.6/HCA2, a Dof transcription factor gene, regulates interfascicular cambium formation and vascular tissue development in Arabidopsis. Plant Cell. 2009;21:3518–3534. doi: 10.1105/tpc.108.064139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pineau C. hca: an Arabidopsis mutant exhibiting unusual cambial activity and altered vascular patterning. Plant J. 2005;44:271–289. doi: 10.1111/j.1365-313X.2005.02526.x. [DOI] [PubMed] [Google Scholar]
- 83.Melzer S. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 2008;40:1489–1492. doi: 10.1038/ng.253. [DOI] [PubMed] [Google Scholar]
- 84.Samach A. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- 85.Hempel F.D. Floral determination and expression of floral regulatory genes in Arabidopsis. Development. 1997;124:3845–3853. doi: 10.1242/dev.124.19.3845. [DOI] [PubMed] [Google Scholar]
- 86.Sibout R. Flowering as a condition for xylem expansion in Arabidopsis hypocotyl and root. Curr. Biol. 2008;18:458–463. doi: 10.1016/j.cub.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 87.Fisher K., Turner S. PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr. Biol. 2007;17:1061–1066. doi: 10.1016/j.cub.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 88.Etchells J.P., Turner S.R. The PXY–CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development. 2010;137:767–774. doi: 10.1242/dev.044941. [DOI] [PubMed] [Google Scholar]
- 89.Schoof H. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 90.Suer S. WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. Plant Cell. 2011;23:3247–3259. doi: 10.1105/tpc.111.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bryan A.C. XYLEM INTERMIXED WITH PHLOEM1, a leucine-rich repeat receptor-like kinase required for stem growth and vascular development in Arabidopsis thaliana. Planta. 2011;235:111–122. doi: 10.1007/s00425-011-1489-6. [DOI] [PubMed] [Google Scholar]
- 92.Ji J. WOX4 promotes procambial development. Plant Physiol. 2009;152:1346–1356. doi: 10.1104/pp.109.149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang C. Global characterization of cell-specific gene expression through fluorescence-activated sorting of nuclei. Plant Physiol. 2008;147:30–40. doi: 10.1104/pp.107.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ehlting J. Global transcript profiling of primary stems from Arabidopsis thaliana identifies candidate genes for missing links in lignin biosynthesis and transcriptional regulators of fiber differentiation. Plant J. 2005;42:618–640. doi: 10.1111/j.1365-313X.2005.02403.x. [DOI] [PubMed] [Google Scholar]
- 95.Suh M.C. Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol. 2005;139:1649–1665. doi: 10.1104/pp.105.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brady S.M. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–806. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 97.Birnbaum K. A gene expression map of the Arabidopsis root. Science. 2003;302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- 98.Donner T.J. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development. 2009;136:3235–3246. doi: 10.1242/dev.037028. [DOI] [PubMed] [Google Scholar]
- 99.Scarpella E. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development. 2004;131:3445–3455. doi: 10.1242/dev.01182. [DOI] [PubMed] [Google Scholar]
- 100.Kirch T. The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell. 2003;15:694–705. doi: 10.1105/tpc.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]