Abstract
Objective
The aim of the study was to explore the sensitivity and robustness of T2 mapping in the detection and quantification of early degenerative cartilage changes at the patella.
Materials and methods
Forty-two patients (22 women, 20 men) with a mean age of 30.3 years and a symptomatic cartilage defect of ICRS grade ≤2 were examined using a 3 T MRI with an 8-channel knee coil. The cartilage lesion was graded based on high-resolution PD TSE and 3D isotropic TrueFISP images. T2 maps were calculated from a standard MESE-sequence, performed at the beginning and at the end of the scan (40 min in-between). Depending on the defect size, a region-of-interest (ROI) analysis was performed on 1–3 consecutive slices. Mean T2 values for the deep, superficial, and global layer as well as the zonal variation were compared among defect grades (ANOVA, post hoc Duncan-test) and over time (Student's t-test).
Results
T2-measurements directly correlated with the extent of cartilage defect (ICRS grade) at all layers and at both time-points. However, correlations were closer for the second measurement at the end of the scan. In this unloaded state, differences in T2-values became more pronounced and were significant even between cartilage of normal appearance adjacent to the defect and healthy cartilage of control patients (both ICRS grade 0). In contrast, there were no such differences among grades in the zonal variation at any time.
Conclusion
T2 mapping might be a sensitive method for the detection of early cartilage degeneration at the patella in the unloaded joint.
Keywords: Magnetic resonance imaging, Cartilage, T2, Patella, Osteoarthritis
1. Introduction
Osteoarthritis (OA) is a multi-tissue, multi-factorial disease and worldwide a major cause of disability resulting from reduced joint mobility and function [1]. Progressive loss of hyaline cartilage is one of the hallmark features of OA, initiated by a loss of proteoglycans (PG) and an increase in water content, followed by a loss of type II collagen and a change in collagen fiber orientation [2]. OA progression is usually graded based on plain radiographs, using joint space width, continuity of bony contours, and the presence and size of osteophytes as criteria [3]. However, these criteria do not help for the detection of early cartilage changes [4]. As articular cartilage has only limited capability for self-repair an early diagnosis of cartilage degeneration and a sensitive non-invasive diagnostic tool are highly desirable.
Nowadays, magnetic resonance imaging (MRI) has become the method of choice to visualize articular cartilage in vivo. In comparison to radiographs, it provides high soft tissue contrast without ionizing radiation and enables multiplanar examination, however standard MR sequences for joint imaging do not allow for the quantification of early degenerative changes either [5]. Recent MRI studies have included measurements of biomechanical and biochemical properties of cartilage such as the glycosaminoglycan and water content as well as the collagen organization and content [6]. A technique reported to quantify cartilage water content and collagen fiber orientation is quantitative T2 mapping [7]. Focal increase in T2 relaxation time has been associated with cartilage matrix damage, in particular a loss of collagen integrity and an increase in water content [7]. In future, quantitative T2 mapping may aid e.g. in the diagnosis of early cartilage degeneration, as well as to demonstrate the efficacy of disease modifying drugs or successful treatment of the pattelo-femoral pain syndrome.
However, the usefulness of T2 mapping to differentiate stages of cartilage degeneration is not yet clear and T2 values vary considerably [8]. This variability may partly be due to an effect of loading which has been demonstrated for healthy articular cartilage in vitro and in vivo [9,10]. Recently, different T2-values of healthy and repair cartilage [11] as well as a direct correlation of T2 values with cartilage defect grading [12] were shown in the unloaded state of the cartilage at the femoral condyle. Whereas this part of the knee is biomechanical more affected by static loading and weight bearing, the patella cartilage tissue is more loaded by gliding under pressure over the trochlea surface during walking.
The purpose of the current study was to confirm the results of the previous study on the medial femoral condyle [12] at a different cartilage site within the knee joint, the patella, under different loading conditions. Furthermore we investigated the sensitivity of T2 measurements for the detection of early stages of cartilage degeneration by comparison of morphologically intact cartilage areas in healthy patients and in patients with an adjacent focal lesion.
2. Materials and methods
2.1. Patient population
In this retrospective study, we reviewed data of 169 consecutive patients, referred to our institution by the local trauma surgery for standard MRI examination between April 2007 and May 2009 and suspected of a cartilage lesion at the patella. We included all patients who had (a) ≥18 years of age, (b) a maximum cartilage defect comprising ≤50% of the full thickness at written radiological report (at our institution all knee MRI's are evaluated by the senior author S.T.), and (c) two standard T2-measurements performed, one at the beginning and one at the end of the MRI scan. Based on patient history and clinical reports, patients with previous knee surgery, history of patella fracture or luxation, or misalignment of the lower extremity were excluded. In total, 42 (22 female and 20 male) patients with a mean age of 30.3 (range 18–55) years entered the analysis. All eligible patients had given written informed consent to use their anonymised data for scientific purposes prior to the MRI scan. The protocol was acknowledged by the Ethics Committee of the Medical University of Vienna.
2.2. Magnetic resonance imaging
MRI had routinely been performed on a 3.0 T whole body Magnetom TimTrio scanner (Siemens Medical Solutions, Erlangen, Germany), using a gradient strength of 40 mT/m and an 8-channel knee array coil (IN vivo, Gainesville, FL, USA). Patients had been positioned in supine position with the extended knee tightly fixed and the joint space in the middle of the coil.
The MRI protocol consisted of two axial multi-echo spin-echo (MESE) T2 acquisitions (at the beginning and at the end of the scan) using a standard sequence as previously described [12]: repetition time (TR) 1200 ms; echo time (TE) 13.8, 27.6, 41.4, 55.2, 69, and 82.8 ms; field of view (FoV) 160 mm × 160 mm; pixel matrix 384 × 384; voxel size 0.4 mm × 0.4 mm × 3 mm; bandwidth 228 Hz/pixel; 12 slices; scan time 4:09 min. Measurements were run in the morning and patients advised to perform normal daily activities before and to come to the MRI site by foot. The time elapsing from lying down for the scan until the start of the first T2 map sequence (early unloading) was limited to ≤5 min. At this point in time, the cartilage layer was believed to be unloaded for the first time. The identical second T2 map at the end of the examination (late unloading) started about 40 min later to evaluate possible unloading effects over time.
For the morphological evaluation, the MRI protocol consisted of an isotropic three dimensional (3D) true fast imaging with steady-state precession (TrueFISP) sequence [13]: TR 7.69 ms; TE 3.85 ms; flip angle 30°; FoV 192 mm × 192 mm; pixel matrix 384 × 384; voxel size 0.5 mm × 0.5 mm × 0.5 mm; scan time 6:21 min.
The TrueFISP-sequence was obtained for the whole knee in the coronal plane and reformatted in the axial and sagittal plane through multiplanar reconstruction (MPR). Additionally, a high-resolution proton-density (PD) turbo-spin-echo (TSE) sequence was obtained for the patella in the axial plane: TR 2400 ms; TE: 36 ms; FoV 120 mm × 120 mm; matrix size 512 × 512; voxel size 0.2 mm × 0.2 mm × 2 mm; 24 slices; interslice gap 10%; flip angle 160°; bandwidth 244 Hz/pixel; scan time 6:11 min. The pixel size of 200 μm for the morphological PD TSE sequence remained below the level that is deemed required to reliably show superficial alterations of articular cartilage [14].
Morphological and T2 sequences were planed on the same set of localizers. Acquired images were perpendicular to the patella surface in the axial plane.
Additional sequences performed during the scans were not used for this analysis.
2.3. Image and data analysis
The cartilage surface of the patella was morphologically graded based on a modified ICRS classification system (grade 0: normal cartilage without notable defects; grade 1: cartilage without tissue-loss, but with fibrillation and superficial fissures at the superficial surface; grade 2: defects that extend to less than 50% in depth) [15].
T2 relaxation times were derived from online reconstructed T2 maps using a pixel-wise, mono-exponential, non-negative least square (NNLS) fit analysis (MapIt, Siemens Medical Solutions, Erlangen, Germany). To verify the localization and size of cartilage defects, we used their 3D spatial presentation on the high-resolution TrueFISP images. With the help of header information and anatomical landmarks, the defects were reproduced on the T2 maps using the first echo images (ET = 13.8 ms), as these have the best SNR. T2 values from the subchondral bone to the cartilage surface (global T2) (Fig. 1) were manually assessed on one to three consecutive slices of the T2 map, depending on defect size (mean ROI size: 488 pixels, range 149–1495 pixels). In addition, a control area of morphologically normal appearance on TrueFISP and PD TSE images, i.e. with preserved cartilage thickness, intact surface, and without intrachondral signal alterations, was identified in the same patient, either on the contralateral facet of the patella or at least two slices away from the defect in the cranio-caudal direction (control patient). If possible, again corresponding T2 relaxation times were manually assessed as for the defect site on one to three slices at similar size of the ROI (mean 497 pixels, range 165–1523). Patients without any morphological sign of cartilage defect on high-resolution images served as control group (ROI mean 624 pixels, range 314–982) (control healthy) (Fig. 2).
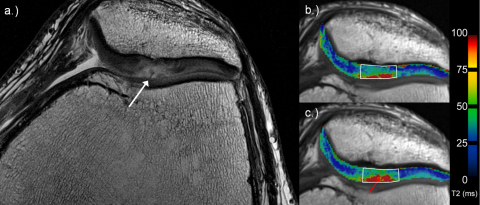
Fig. 1.
36-Year-old man with a cartilage defect according to ICRS grade 2 (white arrow) at the apex of the patella on high-resolution PD TSE image (a). (b and c) Examples for global assessment of T2 values on corresponding T2 maps. Note the increase of T2-values from early (b) to late unloading (c; red arrow).
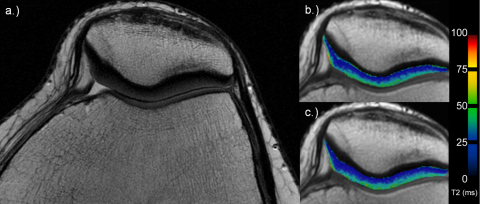
Fig. 2.
27-Year-old woman with morphological normal cartilage on axial PD TSE image (a). Corresponding T2 maps for the early (b) and late unloading (c). Note no visible differences over time.
Subsequently, to evaluate the impact of cartilage degeneration on the zonal variation of T2, ROIs were divided into two layers of equal thickness, a deep and a superficial one (Fig. 3), and zonal variation was calculated as the difference between T2 values measured in both.
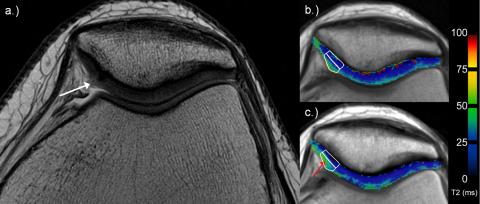
Fig. 3.
35-Year-old woman with superficial fibrillation (ICRS grade 1; white arrow) of the cartilage on the medial facet of the patella on high-resolution PD TSE image (a; white arrow). Examples for deep and superficial ROI analysis in corresponding T2 maps (b and c; white rectangles). Note the increasing T2 values from early (b) to late unloading (c), especially in the superficial layer (red arrow).
To assess the reproducibility of general T2 measurements in comparison to the hypothesized changes of T2 due to unloading in the patellar cartilage, we also draw ROIs in the muscle of 10 randomly selected patients on 3 consecutive slices at both time points. Mean T2 values of the muscle were included in the analysis.
For comparison of corresponding early and late unloading measurements, T2 maps were assessed side by side with a Leonardo workstation (Siemens Medical Solutions).
Morphological and ROI analyses were performed by an experienced senior musculoskeletal radiologist (S.T., 23 years of experience), in consensus with an orthopaedic surgeon having a special interest in musculoskeletal MRI (G.H.W., 8 years of experience).
2.4. Statistical analysis
A one-way analysis of variance (ANOVA) along with a post hoc Duncan test was used to compare T2 values and their zonal variation among ICRS grades. The association between T2 values and cartilage defect grade was investigated using a Spearman's rank correlation analysis. Differences in cartilage T2 values over time were tested for significance using a paired t-test. T2 values of the muscle were compared using the same analysis. Statistical analysis was performed using SPSS for Windows (V17.0, SPSS, Chicago, IL, USA) and a level of significance of 0.05.
3. Results
In 11 patients (26.2%) no cartilage defect was identified at the patella, 10 patients (23.8%) had a patellar cartilage defect of ICRS grade 1, and 21 patients (50%) of ICRS grade 2. Out of the 31 patients with a focal cartilage defect, 26 patients also had a region of apparently healthy cartilage in the surrounding tissue assessed. In the remaining 5 patients, we were unable to identify any cartilage region of normal appearance.
With increasing defect size (=ICRS grade) patients had consistently higher T2 values regardless of layer (Table 1). In patients with a focal defect, adjacent cartilage regions of morphologically normal appearance also showed consistently higher mean T2 values than control patients without defect. However, levels in these regions still remained lower than at corresponding defect sites (Table 1).
Table 1.
Mean T2 values (ms) wit–h 95%CIs for various layers of the patellar cartilage and variation over time.
| A: ICRS 0 Control healthy (n = 11) |
B: ICRS 1 (n = 10) |
C: ICRS 2 (n = 21) |
Pgrade | BC: ICRS 0, area adjacent to defect Control patient (n = 26) |
PA vs BC | ||
|---|---|---|---|---|---|---|---|
| Global | 1st | 33.1 (29.0–37.2) | 38.3 (28.6–48.0) | 42.3 (37.7–46.9) | 0.063 | 36.8 (34.0–39.6) | 0.132 |
| 2nd | 33.4 (28.9–37.9) | 42.1 (34.8–49.4) | 44.8 (39.6–50.0) | 0.016 | 38.3 (35.5–41.0) | 0.053 | |
| Δ | 0.3 (−0.6–1.3) | 3.8 (0.1–7.5) | 2.5 (1.1–3.9) | 0.069 | 1.4 (0.6–2.7) | 0.104 | |
| Ptime | 0.483 | 0.045 | 0.001 | 0.0001 | |||
| Superficial | 1st | 39.1 (35.5–42.7) | 44.8 (33.0–56.6) | 48.9 (43.5–54.4) | 0.100 | 43.6 (40.3–46.8) | 0.099 |
| 2nd | 39.6 (35.4–43.8) | 49.5 (40.3–58.7) | 52.2 (45.8–58.6) | 0.029 | 45.0 (42.0–47.9) | 0.041 | |
| Δ | 0.5 (−0.8–1.7) | 4.6 (0.2–9.1) | 3.3 (1.3–5.2) | 0.092 | 1.4 (0.2–2.6) | 0.355 | |
| Ptime | 0.709 | 0.138 | 0.005 | 0.026 | |||
| Deep | 1st | 27.1 (22.0–32.2) | 31.8 (23.9–39.6) | 35.7 (30.9–40.5) | 0.079 | 30.1 (27.4–32.7) | 0.238 |
| 2nd | 27.3 (21.9–32.6) | 34.7 (28.3–41.1) | 37.4 (32.4–42.5) | 0.030 | 31.5 (28.7–34.3) | 0.110 | |
| Δ | 0.2 (−0.7–1.0) | 2.9 (−1.1–6.9) | 1.7 (0.6–2.9) | 0.172 | 1.5 (0.5–2.4) | 0.082 | |
| Ptime | 0.411 | 0.042 | 0.002 | 0.003 | |||
| Zonal | 1st | 12.0 (8.8–15.1) | 13.1 (7.8–18.3) | 13.2 (8.6–17.7) | 0.919 | 13.5 (11.7–15.4) | 0.360 |
| 2nd | 12.3 (9.1–15.6) | 14.8 (8.5–21.1) | 14.8 (9.6–19.8) | 0.757 | 13.4 (11.4–15.5) | 0.537 | |
| Δ | 0.3 (−0.6–1.3) | 1.7 (−2.5–6.0) | 1.5 (0.1–3.1) | 0.639 | −0.1 (−1.5–1.3) | 0.701 | |
| Ptime | 0.457 | 0.392 | 0.048 | 0.886 | |||
1st: first measurement (early unloading); 2nd: second measurement (late unloading); Δ: difference over time; Pgrade: ANOVA; Ptime: t-Test.
Bold values indicate p < 0.05.
By contrast, there were no significant differences among ICRS grades in the zonal variation of T2 values although it appeared to be larger in patients with as compared to those without focal defect (Table 1).
Unloading of the knee did hardly alter T2 relaxation times in subjects without defect (control healthy) (Fig. 2), whereas it resulted in significant increases in patients having such a defect (Figs. 1 and 3). As a consequence, after unloading differences became more pronounced and significant, not only among grades, but also between cartilage areas of apparently morphologically normal cartilage adjacent to the defect (control patient) and cartilage of subjects without defect (control healthy) (Table 1). Correlations between T2 values and defect size also became stronger after unloading (Table 2). Unloading also increased the zonal variation of T2-values although this increase was significant in patients with ICRS grade 2 defects only (Table 1). However, T2 values of the muscle were not affected by loading and remained constant over time (means ± SD, early unloading, 30.8 ± 2.5 ms versus late unloading 30.7 ± 2.6 ms; P = 0.814).
Table 2.
Spearman rank correlation analysis between T2 values and ICRS grade in different layers at both time points.
| Early unloading |
Late unloading |
|||
|---|---|---|---|---|
| rS | P | rS | P | |
| Global layer | 0.313 | 0.009 | 0.389 | 0.0001 |
| Deep layer | 0.288 | 0.017 | 0.323 | 0.007 |
| Superficial layer | 0.288 | 0.018 | 0.378 | 0.002 |
| Zonal variation | 0.034 | 0.786 | 0.134 | 0.277 |
4. Discussion
In this study, we compared T2 relaxation times at different cartilage layers, defect grades, and loading states with routine high-spatial-resolution MRI of the patella. The design of the study was similar to the one that we have recently published on cartilage defects at the femoral condyle [12]. The study confirmed a major impact of loading on T2 values of degenerated patella cartilage, even though the femoro-patellar joint is more affected by sliding movements during walking rather than static loading. Whereas this effect appeared not to depend on the presence of any cartilage defect at the femoral condyle [12], in this study, T2-values for the patellar cartilage of healthy patients without defects were hardly affected by the loading status.
This is in contrast to results published by Liess et al. [9] who described a significant increase in T2-values of patellar cartilage in 20 healthy volunteers after performing 60 knee bends. They hypothesized that compression after the knee bends is reversed and water reabsorbed by the cartilage, which in combination with a decrease in proteoglycans and collagen density may have entailed the T2 increase. As our measurements derive from clinical routine, there was no specific exercise program prior to the MRI scan and therefore the initial loading and thus molecular changes might have been much lower. This may explain the only minimal and statistically insignificant increase observed in patients without cartilage lesion. However, normal daily activities prior to the MR scan and walking into the magnet room apparently were sufficient to alter water content and molecular cartilage composition of injured cartilage, particularly in the superficial layer. Thus, sensitivity to loading effects might be linked to the location and integrity of cartilage.
Our study also confirmed previous studies showing an increase in T2 relaxation times with progressing OA, at the femoral condyle [5] as well as at the patella [16]. Several in vivo and in vitro studies [7] proposed a breakdown of collagen fibers and an increase of water content to be responsible for such an increase. In the present study as in the previous one on the cartilage layer above the posterior horn of the meniscus [12], we found increasing T2 values also with increasing cartilage defect grade. However at the patella, differences became significant only for T2 values obtained after unloading. Also the correlation analysis revealed a stronger association of T2 values with cartilage defect grade after unloading, but generally smaller correlation coefficients as compared to the femoral condyle [12]. Again, this difference might be due to local cartilage characteristics within the knee joint and the different loading patterns between the femoral condyle cartilage and the retropatellar cartilage.
Whereas our study was rather confirmatory with respect to the impact of loading and the correlation of T2 values with defect grades, the observation of significantly increased T2-values in areas adjacent to focal defects despite normal morphology on high-resolution images is relatively new. Again, differences versus controls without cartilage defects were most pronounced and statistically significant in the superficial layer after unloading. Hannila et al. [16] also evaluated cartilage defects at the patella through T2 mapping and highlighted that standard MRI may underestimate lesion sizes. They already concluded on a possible higher sensitivity of T2 mapping for the detection of early cartilage degeneration. The findings of both studies may indicate an impact of focal defects on the surrounding cartilage and the whole patello-femoral joint. It is conceivable that abrasion of small cartilage particles followed by inflammation within the joint leads to cartilage wear starting at the superficial layer with a disruption of the strict perpendicular alignment of collagen fibers at the surface. Thus, the significant increase in T2 relaxation times in the superficial layer after unloading observed in our study might reflect an early onset of cartilage degeneration in the surrounding tissue.
By contrast, we did not find differences in the zonal variation of T2 values between healthy cartilage and areas of cartilage defects regardless of loading. Previously, the zonal variation was used to differentiate between healthy cartilage and repair cartilage tissue [17]. However, for the assessment of early cartilage defects, the zonal variation seems to be useless as the normal T2 gradient of hyaline cartilage from the subchondral bone to the articular surface [7] seems to be unaffected by the presence or the size of a focal defect in our study.
A limitation of the present study is the lack of surgical or histological confirmation of the cartilage state or a cross-comparison with delayed gadolinium enhanced magnet resonance imaging of the cartilage (dGEMRIC). Both would be essential to qualify and validate the method and to control for specific error sources such as the magic angle effect [7]. However, the relatively flat surface of the patellar cartilage may be protective as this effect has not been reported at the patella yet. We did also not perform thickness measurements on the T2 maps at the two time points, however the impact of loading on cartilage thickness is well known [9,18]. The lack of a standardized activity protocol prior to the MRI may certainly have increased variability in the loading effect. On the other hand, it has to be pointed out that this analysis was based on MRIs acquired during clinical routine and hence our observations are likely to be robust. Reproducibility for T2 values was not shown within this study, however another recent study on T2 mapping at 3 Tesla revealed high inter- and intraobserver reproducibility as well as a low coefficient of variation [19].
In conclusion, T2 mapping appears to be sensitive to early cartilage changes, but also to the loading state of the joint. Even changes not yet visible in high resolution MRI might be detectable with quantitative T2 mapping. These findings need to be confirmed by prospective studies with larger patient cohorts comparing T2 results under standardized loading conditions with histological outcomes. Based on our results, we recommend for the clinical routine to measure T2 values of patellar cartilage at the end of the MRI-protocol.
Source of funding
Funding for the study was provided by the Austrian Science Funds: FWF-Project P18110-B15 and by the Vienna Spots of Excellence Program (Vienna Advances Clinical Imaging Centre-VIACLIC).
Conflict of interest
T.C. Mamisch is a medical consultant of Siemens Health Care. All other authors have no conflict of interest.
Contributor Information
S. Apprich, Email: sebastian.apprich@meduniwien.ac.at.
T.C. Mamisch, Email: mamisch@bwh.harvard.edu.
G.H. Welsch, Email: welsch@bwh.harvard.edu.
D. Stelzeneder, Email: david.stelzeneder@meduniwien.ac.at.
C. Albers, Email: christoph.albers@insel.ch.
U. Totzke, Email: uwe.totzke@merckserono.net.
S. Trattnig, Email: siegfried.trattnig@akhwien.at.
References
- 1.Michael J.W., Schluter-Brust K.U., Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenkrantz G., Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 3.Altman R.D., Fries J.F., Bloch D.A. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30(11):1214–1225. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- 4.Eckstein F., Burstein D., Link T.M. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19(7):822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Benjamin Ma C., Link T.M. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trattnig S., Domayer S., Welsch G.W., Mosher T., Eckstein F. MR imaging of cartilage and its repair in the knee—a review. Eur Radiol. 2009;19(7):1582–1594. doi: 10.1007/s00330-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 7.Mosher T.J., Dardzinski B.J. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 8.Burstein D., Gray M.L. Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis? Osteoarthritis Cartilage. 2006;14(11):1087–1090. doi: 10.1016/j.joca.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Liess C., Lusse S., Karger N., Heller M., Gluer C.C. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 10.Nishii T., Kuroda K., Matsuoka Y., Sahara T., Yoshikawa H. Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging. 2008;28(1):175–180. doi: 10.1002/jmri.21418. [DOI] [PubMed] [Google Scholar]
- 11.Mamisch T.C., Trattnig S., Quirbach S., Marlovits S., White L.M., Welsch G.H. Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading—initial results. Radiology. 2010;254(3):818–826. doi: 10.1148/radiol.09090335. [DOI] [PubMed] [Google Scholar]
- 12.Apprich S., Welsch G.H., Mamisch T.C. Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthritis Cartilage. 2010;18(9):1211–1217. doi: 10.1016/j.joca.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Duc S.R., Pfirrmann C.W., Schmid M.R. Articular cartilage defects detected with 3D water-excitation true FISP: prospective comparison with sequences commonly used for knee imaging. Radiology. 2007;245(1):216–223. doi: 10.1148/radiol.2451060990. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein J.D., Li J.G., Majumdar S., Henkelman R.M. Image resolution and signal-to-noise ratio requirements for MR imaging of degenerative cartilage. AJR Am J Roentgenol. 1997;169(4):1089–1096. doi: 10.2214/ajr.169.4.9308470. [DOI] [PubMed] [Google Scholar]
- 15.Brittberg M., Winalski C.S. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl. 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hannila I., Nieminen M.T., Rauvala E., Tervonen O., Ojala R. Patellar cartilage lesions: comparison of magnetic resonance imaging and T2 relaxation-time mapping. Acta Radiol. 2007;48(4):444–448. doi: 10.1080/02841850701280817. [DOI] [PubMed] [Google Scholar]
- 17.Welsch G.H., Mamisch T.C., Domayer S.E. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology. 2008;247(1):154–161. doi: 10.1148/radiol.2471070688. [DOI] [PubMed] [Google Scholar]
- 18.Mosher T.J., Liu Y., Torok C.M. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18(3):358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsch G.H., Apprich S., Zbyn S. Biochemical (T2, T2* and magnetisation transfer ratio) MRI of knee cartilage: feasibility at ultra-high field (7 T) compared with high field (3 T) strength. Eur Radiol. 2010 doi: 10.1007/s00330-010-2029-7. [DOI] [PubMed] [Google Scholar]