Abstract
The global epidemic of obesity is accompanied by an increased prevalence of cardiovascular disease (CVD), in particular stroke and heart attack. Dysfunctional adipose tissue links obesity to CVD by secreting a multitude of bioactive lipids and pro-inflammatory factors (adipokines) with detrimental effects on the cardiovascular system. Adiponectin is one of the few adipokines that possesses multiple salutary effects on insulin sensitivity and cardiovascular health. Clinical investigations have identified adiponectin deficiency (hypoadiponectinaemia) as an independent risk factor for CVD. In animals, elevation of plasma adiponectin by either pharmacological or genetic approaches alleviates obesity-induced endothelial dysfunction and hypertension, and also prevents atherosclerosis, myocardial infarction and diabetic cardiomyopathy. Furthermore, many therapeutic benefits of the peroxisome-proliferator activated receptor gamma agonists, the thiazolidinediones, are mediated by induction of adiponectin. Adiponectin protects cardiovascular health through its vasodilator, anti-apoptotic, anti-inflammatory and anti-oxidative activities in both cardiac and vascular cells. This review summarizes recent findings in the understanding of the physiological role and clinical relevance of adiponectin in cardiovascular health, and in the identification of the receptor and postreceptor signalling events that mediate the cardiovascular actions of adiponectin. It also discusses adiponectin-targeted drug discovery strategies for treating obesity, diabetes and CVD.
LINKED ARTICLES
This article is part of a themed section on Fat and Vascular Responsiveness. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-3
Keywords: adiponectin, adipokine, cardiovascular disease, endothelial dysfunction, inflammation, obesity, vasculature
Introduction
The modern Western diet coupled with a sedentary lifestyle has led to an epidemic of obesity, a consequence of which is a dramatic rise in the incidence of diabetes and cardiovascular disease (CVD), in particular stroke and heart attack. In morbidly obese subjects with a body weight index (BMI) of 40–45 kg·m−2, the median survival rate is reduced by 8 to 10 years compared with those with normal BMI, primarily due to the increased death from CVD (Whitlock et al., 2009). Both animal and clinical investigations suggest that inflammation and dysfunction of adipose tissue (fat) is a key mediator that links obesity with CVD (Mazurek et al., 2003; Xu et al., 2010a).
Both heart and blood vessels are surrounded by adipose tissue. Epicardial adipose tissue is located along the large coronary arteries and on the surface of the ventricles and the apex of the heart, whereas perivascular adipose tissue surrounds the arteries. Both these fat depots are not separated by a fascia from the underlying tissue. Therefore, factors secreted from epicardial and perivascular adipose tissue, including active lipids and adipokines (or adipocytokines), can directly modulate the function of the heart and the vasculature (Karastergiou et al., 2010). The majority of adipokines released from adipose tissue, including tumour necrosis factor α (TNFα), leptin, plasmogen activator inhibitor-1, adipocyte fatty acid binding protein, lipocalin-2, monocyte chemotactic protein 1 and resistin, exert deleterious effects on the cardiovascular system (Xu et al., 2010a). In obesity, the expansion of adipose tissues leads to overproduction of these pro-inflammatory adipokines, thereby contributing to the pathogenesis of CVD. On the other hand, adiponectin, a major adipocyte-secreted adipokine with insulin-sensitizing and anti-inflammatory activities, is down-regulated in obesity and its related pathologies (Zhu et al., 2008).
Adiponectin possesses multiple salutary effects on obesity-related metabolic complications, dyslipidaemia, non-alcoholic fatty liver disease and several types of cancers (Wang et al., 2008). In particular, its role in cardiovascular protection has been extensively studied. This review highlights recent advances in the understanding of the cardiovascular effects of adiponectin in both animals and humans, and explores its potential use as a surrogate marker to develop pharmacological strategies for treating CVD.
The structural features and post-translational modifications of adiponectin
Adiponectin is one of the most abundant adipokines secreted by adipocytes. The circulating level of adiponectin ranges from 5 to 30 µg·mL−2 in humans (Maeda et al., 1996), which represents up to 0.05% of total plasma proteins (Arita et al., 1999; Zhu et al., 2008). The gene that codes for human adiponectin is located on chromosome 3q27, a locus linked with susceptibility to diabetes and CVD (Stumvoll et al., 2002). The protein consists of 247 amino acids and contains an NH2-terminal hyper-variable region, a conserved collagen-like domain comprising 22 Gly-X-Y repeats and a COOH-terminal C1q-like globular domain (Wang et al., 2008). Adiponectin is secreted from adipocytes into the bloodstream as three oligomeric complexes, including trimer, hexamer and high molecular weight (HMW) multimer comprising at least 18 monomers (Magkos and Sidossis, 2007) (Figure 1). The trimeric adiponectin is the basic building block of adiponectin multimers. The trimer is formed via hydrophobic interactions within its globular heads and is stabilized by the non-covalent interactions of the collagen-like domains in a triple-helix stalk. The assembly of hexameric and HMW forms of adiponectin requires the formation of an intermolecular disulfide bond between a highly conserved cysteine residue within the hyper-variable region (Tsao et al., 2003). The post-translational modifications, especially hydroxylation and subsequent glycosylation of several conserved lysine residues within its collagen-like domain are crucial for the intracellular assembly and secretion of HMW oligomeric adiponectin.
Figure 1.
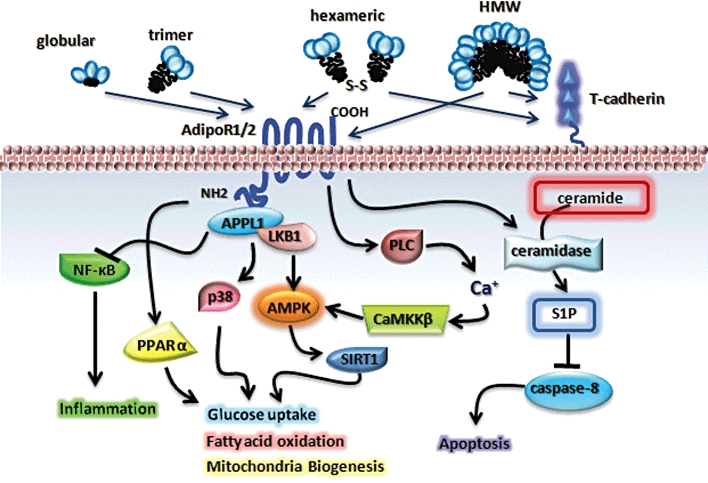
Adiponectin, adiponectin receptors and intracellular signalling. AdipoR, adiponectin receptor; AMPK, AMP kinase; CaMKKβ, Ca2+/calmodulin-dependent protein kinase kinase β; HMW, high molecular weight; PPARα, peroxisome-proliferator activated receptor alpha; S1P, sphingosine-1-phosphate; SIRT1, sirtuin 1.
The biosynthesis and secretion of adiponectin oligomers in adipocytes are tightly controlled by several molecular chaperones in the endoplasmic reticulum, including ERp44 (ER protein of 44 kDa), Ero1-Lα (ER oxidoreductase 1-Lα) and DsbA-L (disulfide-bond A oxidoreductase-like protein). ERp44 inhibits the secretion of adiponectin oligomers by a thiol-mediated retention (Wang et al., 2007). By contrast, Ero1-Lα releases HMW adiponectin trapped by ERp44 (Qiang et al., 2007). DsbA-L promotes the intracellular assembly and secretion of HMW adiponectin (Liu et al., 2008). The three different oligomeric forms of adiponectin possess distinct biological activities. Among them, the HMW oligomer is the major active form mediating the insulin-sensitizing and cardiovascular protective effects of the adipokine (Kobayashi et al., 2004; Pajvani et al., 2004a). In adipose tissue of obese subjects, both the intracellular assembly and the secretion of HMW adiponectin are impaired, which may in turn contribute to insulin resistance and cardiovascular dysfunction.
Adiponectin is also modified by sialic acids through O-linked glycosylation situated on threonine residues within the hyper-variable region (Sato et al., 2001; Richards et al., 2010), which determines the half-life in circulation of the adipokine by modulating its clearance from the bloodstream. Furthermore, the highly conserved cysteine residue (Cys36) within the hyper-variable region of adiponectin is succinylated, thereby blocking its oligomerization through inhibition of disulphide bond formation (Frizzell et al., 2009). The extent of succination of adiponectin is elevated in diabetes, suggesting that this modification contributes to impaired adiponectin secretion in obesity-related disorders. Thus, extensive post-translational modifications are required for efficient maturation, oligomerization and secretion of adiponectin, and are also important for maintaining its stability in the circulation.
Adiponectin receptors and intracellular signalling
Two structurally related seven transmembrane receptors for adiponectin have been identified, adiponectin receptor (AdipoR) 1 and 2 (Yamauchi et al., 2003a). The two are structurally and functionally distinct from classical G-protein coupled receptors (Kadowaki and Yamauchi, 2005). Both AdipoR1 and AdipoR2 have an inverted membrane topology with a cytoplasmic NH2 terminus and a short extracellular COOH terminus of approximately 25 amino acids. AdipoR1 and AdipoR2 mediate adiponectin-evoked activation of AMP kinase (AMPK), peroxisome-proliferator activated receptor alpha (PPARα) and P38 MAP kinase in liver, skeletal muscle and endothelial cells (Kadowaki and Yamauchi, 2005) (Figure 1). Recent characterization of mice lacking AdipoR1 or AdipoR2 confirms the importance of the two receptors in the maintenance of metabolic homeostasis but provides evidence of functional differences between them (Yamauchi et al., 2007). Mice with targeted deletion of AdipoR1 or AdipoR2 are partially or totally defective in adiponectin signalling (Yamauchi et al., 2007). Disruption of AdipoR1 results in the blockade of AMPK activation upon adiponectin administration, whereas the PPARα signalling is largely abolished in AdipoR2-null mice (Yamauchi et al., 2007). Simultaneous disruption of both AdipoR1 and AdipoR2 causes marked glucose intolerance. However, conflicting data have been reported with respect to the phenotypic changes of AdipoR1- and AdipoR2-null mice. In one study, AdipoR1-null mice exhibited increased adiposity with impaired glucose tolerance, while AdipoR2-null mice were lean and resistant to diet-induced glucose intolerance, suggesting that AdipoR1 and AdipoR2 have opposing effects (Bjursell et al., 2007). By contrast in another study, deletion of AdipoR2 rendered mice more resistant to diet-induced insulin resistance, but enhanced their susceptibility to type 2 diabetes mellitus (Liu et al., 2007).
The intracellular signalling events immediately following activation of the two adiponectin receptors are poorly characterized. APPL1, an adaptor protein containing a pleckstrin homology domain, a phosphotyrosine binding domain and a leucine zipper motif, is a direct interacting partner of both AdipoR1 and AdipoR2 (Mao et al., 2006). Upon stimulation by adiponectin, the cytoplasmic domain of AdipoR1 and AdipoR2 bind to APPL1, which in turn promotes the translation of the protein kinase LKB1 from nuclei to cytosol, thereby leading to the activation of AMPK (Zhou et al., 2009) (Figure 1). APPL1 also plays an indispensible role in mediating the insulin-sensitizing effect of adiponectin, by potentiating Akt activation (Cheng et al., 2009). In addition to APPL1, several other signalling molecules, including receptor for activated protein kinase C1 (RACK1) (Xu et al., 2009b), the regulatory subunit of protein kinase CK2 (CK2β) (Heiker et al., 2009), endoplasmic reticulum protein 46 (ERp46) (Charlton et al., 2010) and lymphotoxin-β (Xu et al., 2010b), have been identified as interacting partners of AdipoR1. However, the roles of these proteins in adiponectin signalling remain elusive. The binding of lymphotoxin-β to AdipoR1 appears to be involved in adiponectin-mediated suppression of NF-κB signalling in endothelial cells (Xu et al., 2010b).
Besides the APPL1/LKB1 signalling cascade, adiponectin also activates AMPK by promoting calcium (Ca2+) influx, which in turn stimulates Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) (Zhou et al., 2009; Iwabu et al., 2010). Once AMPK is activated, it enhances the activity of NAD ± dependent type III deacetylase sirtuin 1, resulting in increased mitochondrial oxidative capacity by deacetylation and activation of PPARγ coactivator-1α, a master regulator of mitochondrial biogenesis (Iwabu et al., 2010).
In addition, a recent study by Holland and colleagues demonstrated that the insulin-sensitizing and anti-apoptotic actions of the adipokine in pancreatic beta cells and cardiomyocytes are attributed to its effects on sphingolipid metabolism (Holland et al., 2011). Adiponectin stimulates the activity of ceramidase and the formation of the anti-apoptotic metabolite sphingosine-1-phosphate (S1P). This sphingolipid-associated pathway is activated presumably by either AdipoR1 or AdipoR2, because the ceramidase activity is impaired in cells lacking both adiponectin receptors (Holland et al., 2011), leading to elevated ceramide levels and enhanced susceptibility to palmitate-induced apoptosis. Because a pharmacological inhibitor of ceramidase blocks adiponectin-stimulated activation of AMPK, the latter may be a downstream rather than an upstream event, mediated by a conversion of ceramide to S1P, which in turn triggers an influx of calcium and CaMKK activation (Holland et al., 2011).
In addition to AdipoR1 and AdipoR2, the cell-surface glycoprotein T-cadherin has been shown to specifically bind the hexameric and HMW species of adiponectin (Hug et al., 2004) (Figure 1). A recent study provides compelling evidence demonstrating the cardioprotection exerted by adiponectin depends on T-cadherin (Denzel et al., 2010). In light of the fact that T-cadherin is a glycosylphosphatidylinositol-anchored extracellular protein, it may function as a co-receptor with AdipoR1 and AdipoR2 to facilitate adiponectin signalling in specific tissues or cell types.
Adiponectin as biomarker for CVD
Unlike most other adipokines, the plasma level of adiponectin is decreased in obesity and related pathologies, including type 2 diabetes and CVD (Zhu et al., 2008). Both the mRNA expression of the adiponectin gene and the secretion of HMW oligomeric adiponectin are impaired in adipose tissue of obese subjects (Arita et al., 1999; Bacha et al., 2004; Lara-Castro et al., 2006). Epidemiological studies in different ethnic groups demonstrate that a low serum level of adiponectin, especially of its HMW oligomer, is an independent risk factor for CVD (Koenig et al., 2006; Frystyk et al., 2007).
Hypoadiponectinaemia is independently associated with endothelial dysfunction in diabetic patients, as assessed by flow-mediated vasodilatation (Tan et al., 2004; Torigoe et al., 2007). The plasma level of adiponectin is correlated with the amplitude of the vasodilator response to reactive hyperaemia but not to that to nitroglycerin, indicating that adiponectin modulates endothelium-dependent vasodilatation in peripheral arteries (Ouchi et al., 2003b). In a multiple regression analysis enrolling 36 consecutive non-diabetic patients, the adiponectin concentration [among risk factors including homeostasis model assessment for insulin resistance, body mass index, immunoreactive insulin and triglycerides] was the only independent predictor of coronary endothelial function as evaluated by the coronary vascular response to acetylcholine (Okui et al., 2008).
The carotid intima-media thickness (IMT) is a widely used surrogate marker of subclinical atherosclerosis and is predictive of future myocardial infarction and stroke (Nichols et al., 1999). An inverse correlation between IMT and serum adiponectin has been observed in several clinical cohorts including healthy and diabetic subjects of both genders (Pilz et al., 2005; Lo et al., 2006; Nilsson et al., 2006). In addition, genetic variation within the adiponectin gene promoter is directly associated with carotid IMT in healthy subjects and is independent of circulating adiponectin levels (Patel et al., 2008). Moreover, the leptin to adiponectin ratio is inversely related with IMT (Norata et al., 2007; Kotani et al., 2008), and has been proposed as an atherosclerotic index in patients with type 2 diabetes (Satoh et al., 2004; Kotani et al., 2005).
A strong correlation between hypoadiponectinaemia and coronary heart disease has been documented in a number of cross-sectional and prospective studies (Kumada et al., 2003; Hashimoto et al., 2006). By contrast, high plasma levels of adiponectin are associated with a decreased risk of coronary heart disease, independently of other risk factors (Frystyk et al., 2007). In a nested case–control study over a period of 6 years covering 18 225 male participants, individuals in the highest quintile of adiponectin levels had a significantly reduced risk of myocardial infarction even after adjustment for BMI, history of diabetes and hypertension (Pischon et al., 2004). The association of serum adiponectin with these CVDs might be partly dependent on low- and high-density lipoprotein cholesterol (L/HDL-C), since their association became less significant after adjustment for serum L/HDL-C (Pischon et al., 2004; Koenig et al., 2006).
Two independent longitudinal studies have consistently demonstrated that hypoadiponectinaemia is an independent risk factor for hypertension (Chow et al., 2007; Imatoh et al., 2008). Furthermore, subjects carrying the genetic variants that are related to lower plasma levels of adiponectin have a higher risk of hypertension (Iwashima et al., 2004; Ong et al., 2010). Hyperadiponectinaemia is also an independent risk factor for diabetic cardiomyopathy (Mitsuhashi et al., 2007; Kozakova et al., 2008). In healthy subjects, circulating levels of total and HMW adiponectin are related to left ventricular hypertrophy, independently of age and metabolic factors (Mitsuhashi et al., 2007; Kozakova et al., 2008). A similar association has also been observed in obese individuals (Ebinc et al., 2008).
Although most of the epidemiological studies support the association of hypoadiponectinaemia with CVD, conflicting data have been reported on the prospective association between adiponectin and microvascular disease. In patients with chronic heart failure (Kistorp et al., 2005), angiographic coronary artery disease (Pilz et al., 2006) or chronic kidney disease (Menon et al., 2006), high plasma levels of adiponectin appear to be an independent predictor of mortality. The association of ‘hyperadiponectinaemia’ with increased mortality risk is more pronounced in patients with prevalent CVD than in those without (Kistorp et al., 2005; Laughlin et al., 2007; Maiolino et al., 2007; 2008). These paradoxical observations have been reconciled by the wasting theory (Kistorp et al., 2005) or impaired renal function (Menon et al., 2006). They may also imply the existence of an ‘adiponectin resistance’ (Lin et al., 2007; Van Berendoncks et al., 2010) with ageing and the progression of chronic CVD. Indeed, adiponectin resistance has been documented in both animals and humans (Lin et al., 2007; Kunihiro Matsushita et al., 2007). In obesity, several beneficial effects of adiponectin, including stimulation of fatty acid oxidation in skeletal muscle (Mullen et al., 2007), promotion of endothelial NO production in the vasculature (Li et al., 2010) and protection against ischaemic injury in the heart (Yi et al., 2010), are impaired.
In all of the above studies, the measurement of adiponectin did not distinguish between its different oligomeric isoforms. Therefore, it is still possible that a selective reduction of HMW adiponectin, which is the major bioactive form with close relevance to endothelial function (Kobayashi et al., 2004), occurs in the mentioned hyperadiponectinaemia conditions.
Cardiovascular protection by adiponectin: evidence from in vitro and animal studies
In addition to its insulin-sensitizing and metabolic activities, studies in both rodents and large animals have consistently demonstrated the multiple salutary effects of adiponectin on cardiovascular health, through its direct actions on both the heart and the vasculature (Figure 2).
Figure 2.
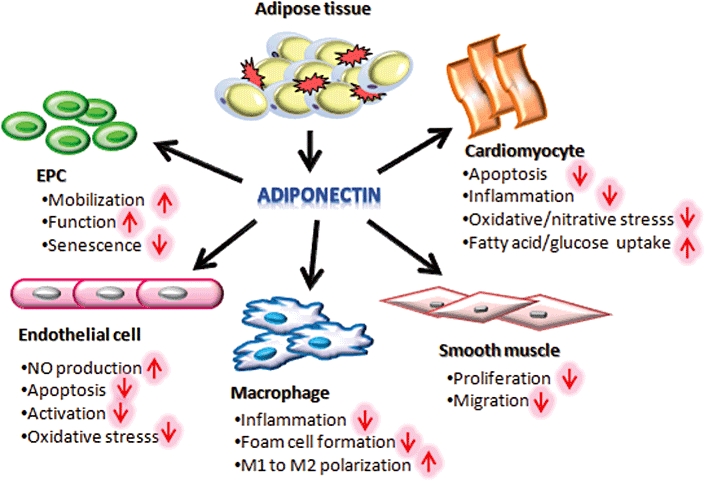
The pleiotropic role of adiponectin in the cardiovascular system. EPC, endothelial progenitor cell.
Alleviation of endothelial dysfunction and hypertension
Endothelial dysfunction and hypertension are two closely related pathological conditions commonly observed in obesity and diabetes, both of which are major risk factors for CVD. Data obtained from both adiponectin-null mice and gain-of-function experiments demonstrate the protective effects of adiponectin against endothelial dysfunction and hypertension, primarily through its multiple actions on the endothelium (Zhu et al., 2008). Adiponectin knockout mice display impaired endothelium-dependent vasodilatation (Ouchi et al., 2003a), elevated systemic blood pressure (Shimabukuro et al., 2003) and pulmonary hypertension (Summer et al., 2009). Aortic rings isolated from adiponectin-knockout mice exhibit reduced endothelial NOS (eNOS) activation and NO production compared with those from wild-type controls, and these changes are reversed by treatment with recombinant adiponectin (Cao et al., 2009). Likewise, systemic administration of recombinant adiponectin in Sprague-Dawley rats with dietary obesity increases eNOS activity, NO production and relaxation of aortic rings to endothelium-dependent vasodilators (Deng et al., 2010). Furthermore, both globular and full-length adiponectin induces NO-dependent vasodilatation in resistance arteries of Zucker lean rats (Schmid et al., 2011). In both coronary arterioles and aortae of db/db diabetic mice, endothelium-dependent vasodilatations to acetylcholine are blunted compared with preparations of control mice, whereas systemic infusion of adiponectin reverses these changes by suppressing TNFα production (Zhang et al., 2010).
Most beneficial effects of adiponectin on endothelial functions are mediated by its ability to activate AMPK. Indeed, both globular and full-length adiponectin increase eNOS activity and NO production via AMPK-mediated phosphorylation of eNOS at Ser1177 (Chen et al., 2003) and Ser633 (Chen et al., 2009a) (Figure 3). Both subtypes of adiponectin receptors (AdipoR1 and AdipoR2) are expressed in endothelial cells and mediate adiponectin-induced phosphorylation of AMPK and eNOS in a complementary manner (Cheng et al., 2007). Adiponectin also promotes the complex formation between heat shock protein 90 and eNOS, which is required for the maximal activation of the enzyme (Lin et al., 2004; Xi et al., 2005; Cheng et al., 2007).
Figure 3.
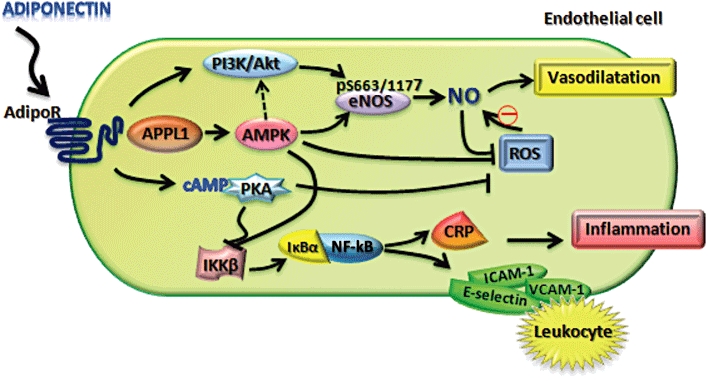
The protective function of adiponectin in endothelial cells. AdipoR, adiponectin receptor; AMPK, AMP kinase; CRP, C-reactive protein; PKA, protein kinase A; ROS, reactive oxygen species.
Although the precise signalling events that couple the two adiponectin receptors and activation of AMPK/eNOS remain poorly understood, the multiple domain protein APPL1 may be a key mediator (Cheng et al., 2007). APPL1 binds to both AdipoR1 and AdipoR2, and mediates adiponectin-induced activation of AMPK possibly by promoting the translocation of its upstream kinase LKB from nuclei to cytosol (Mao et al., 2006; Zhou et al., 2009). Furthermore, adiponectin plays an indispensible role in conferring the insulin-sensitizing effects of adiponectin in skeletal muscle (Wang et al., 2009a). In endothelial cells, suppression of APPL1 expression by RNAi significantly attenuates adiponectin-induced phosphorylation of eNOS at Ser1177, as well as the complex formation between eNOS and heat shock protein 90, resulting in a marked reduction of NO production (Cheng et al., 2007). In both db/db obese mice and Zucker fat rats, the expression of APPL1 in resistance arteries is reduced, and this change is accompanied by impaired vasodilator response of these blood vessels to adiponectin (Cheng et al., 2007; Schmid et al., 2011), suggesting that reduced APPL1 expression may contribute to adiponectin resistance in the vasculature. APPL2, which has the same domain organization with APPL1, also interact with both AdipoR1 and AdipoR2 (Wang et al., 2009a). Interestingly, the binding of APPL2 to the two adiponectin receptors inhibits adiponectin signalling in muscle cells, suggesting that APPL1 and APPL2 may act as a pair of Ying-Yang molecules involved in fine-tuning the adiponectin signalling. However, the physiological relevance of these in vitro observations needs further investigation.
Adiponectin inhibits the production of reactive oxygen species (ROS) induced by high glucose (Ouedraogo et al., 2006), oxidized LDL (Motoshima et al., 2004; Plant et al., 2008) and palmitate (Kim et al., 2010) in cultured endothelial cells. The anti-oxidant activity of adiponectin appears to be mediated by cAMP-dependent protein kinase A (PKA) (Ouedraogo et al., 2006) and AMPK (Kim et al., 2010). Consistent with these observations, aortic rings of adiponectin knockout mice display increased levels of both superoxide anions and peroxynitrite, and these changes are reversed by treatment of mice with recombinant adiponectin (Cao et al., 2009).
In addition to its effects on eNOS activity and ROS production, adiponectin suppresses endothelial activation and monocyte attachment, an early step of the inflammatory reaction leading to atherosclerosis (Zhu et al., 2008). Indeed, adiponectin suppresses TNFα and resistin-induced expression of adhesion molecules as well as interleukin (IL)-8 (Kobashi et al., 2005). This anti-inflammatory effect of adiponectin in endothelial cells appears to be mediated by PKA-dependent suppression of NF-κB activation, through both an AMPK-dependent and -independent mechanism (Ouchi et al., 2000; Wu et al., 2007). In human aortic endothelial cells, adiponectin suppresses high glucose (15 mM)-induced IkappaB (IκB) phosphorylation and NF-κB binding activity, leading to a reduced expression of the pro-inflammatory C-reactive protein (Devaraj et al., 2008). In addition, adiponectin inhibits the interaction between leucocytes and endothelial cells by reducing the expression of E-selectin and vascular cell adhesion molecule-1 (Ouedraogo et al., 2007). Adenovirus-mediated overexpression of AdipoR1 and AdipoR2 potentiates the suppressive effects of adiponectin on endothelial expression of adhesion molecules in both cultured human umbilical vein endothelial cells and in aorta of mice and rats, suggesting that these two receptors play an important role in mediating the anti-inflammatory actions of adiponectin on the endothelium (Zhang et al., 2009).
Promotion of endothelial repair by adiponectin
Impairment in endothelial repair is a hallmark of vascular dysfunction and an early step of the atherosclerotic process. Endothelial progenitor cells (EPCs) are important contributors to endothelial repair following vascular injury (Szmitko et al., 2003). Decreased numbers and/or impaired function of EPCs are causally associated with endothelial dysfunction and CVD (Fadini et al., 2007).
Both animal and clinical investigations suggest that adiponectin promotes endothelial repair and angiogenesis by increasing the number and function of EPCs (Xu et al., 2010a). Angiogenic repair in ischaemic hind limbs, evaluated by laser Doppler flow method and capillary density analysis, is impaired in adiponectin-knockout compared with wild-type mice (Shibata et al., 2004b), and this impairment is reversed by adenovirus-mediated supplementation of adiponectin. In db/db diabetic mice, the lack of adiponectin accelerates diabetes-induced impairment in re-endothelialization after wire-induced carotid denudation (Chang et al., 2010). Furthermore, the stimulatory effect of the PPARγ agonist rosiglitazone on endothelial repair is abrogated in db/db diabetic mice lacking adiponectin.
The endothelial repair mediated by EPCs involves multiple steps, including mobilization of EPCs from the bone marrow or the spleen into the bloodstream, recruitment and adhesion of EPCs to the injured blood vessel wall, followed by differentiation and tubule formation (Zampetaki et al., 2008). Adiponectin modulates almost every step involved in endothelial repair of EPCs (Xu et al., 2010a). Adiponectin-deficient mice exhibit decreased mobilization of EPCs into the circulation in response to hindlimb ischaemia (Shibata et al., 2008), and this is normalized upon adenovirus-mediated adiponectin supplementation. In diabetic rats, the reduction in circulating EPCs and endothelial repair are associated with a reduced serum level of adiponectin (Sambuceti et al., 2009). Treatment of diabetic mice with cobalt protoporphyrin, an inducer of the anti-oxidant haem oxygenase-1, results in up-regulation of adiponectin expression, which in turn facilitates vascular repair by improving the function of EPCs (Li et al., 2008).
Adiponectin potently stimulates survival, proliferation and differentiation of bone marrow-derived EPCs (Eren et al., 2009), and also promotes the migration activities of EPCs through activation of the PI3-kinase/Cdc42/Rac1 signalling cascade (Nakamura et al., 2009). On the other hand, activation of AMPK is required for the vascular recruitment of EPCs by adiponectin (Sambuceti et al., 2009), suggesting that the PI3-kinase/Akt and AMPK pathways may work synergistically in EPC to confer the favourable effects of the adipokine. Indeed, a crosstalk between PI3-Kinase/Akt and AMPK in endothelial cells has been implicated in adiponectin-induced angiogenesis (Ouchi et al., 2004).
In diabetic patients, both the circulating number and function of EPCs are impaired, partly due to hyperglycaemia-induced oxidative stress (Seeger et al., 2005; Sorrentino et al., 2007). Adiponectin counteracts diabetes-induced damage of EPC function by decreasing high glucose-induced intracellular ROS accumulation (Chang et al., 2010). In db/db diabetic mice, the lack of adiponectin exacerbates hyperglycaemia-induced decreases in circulating number of EPCs, whereas this change is reversed by chronic treatment with recombinant adiponectin. Adiponectin prevents high glucose-induced premature senescence of EPCs derived from both human peripheral blood and mouse bone marrow (Chang et al., 2010). At the molecular level, adiponectin decreases high glucose-induced accumulation of intracellular ROS by activation of AMPK and consequently suppresses activation of p38 MAP kinase and expression of the senescence marker p16INK4A (Chang et al., 2010). Because eNOS plays an indispensible role in endothelial repair by promoting mobilization of EPCs (Wegiel et al., 2010) and because adiponectin is a potent stimulator of the production of endothelial NO, the adipokine may also protect against diabetes-associated EPC dysfunction by favouring eNOS signalling.
Anti-atherosclerotic and anti-inflammatory properties of adiponectin
The protective effects of adiponectin against atherogenesis have been demonstrated in mice (Okamoto et al., 2002; Yamauchi et al., 2003b) and rabbits (Li et al., 2007). In apoE-deficient mice, elevation of circulating adiponectin by either transgenesis or an adenovirus delivery system attenuates atherosclerotic plaque formation (Okamoto et al., 2002; Yamauchi et al., 2003b), whereas the suppressive effects of the PPARγ agonists on atherogenesis are abrogated in the absence of adiponectin (Hiuge-Shimizu et al., 2011). Adiponectin exerts its anti-atherosclerotic effects through multiple actions on almost each vascular cell type (Figure 2). In addition to its beneficial effects on endothelial function and EPCs-mediated endothelial repair, adiponectin inhibits neointimal formation by suppressing proliferation and migration of vascular smooth muscle cells (Matsuda et al., 2002; Wang et al., 2005; Motobayashi et al., 2009), and blocks inflammation and foam cell formation from macrophages (Ouchi et al., 2001; Yamaguchi et al., 2005; Tsubakio-Yamamoto et al., 2008).
Adiponectin in physiological concentrations suppresses the proliferation and migration of human vascular smooth muscle cells induced by platelet-derived growth factor-BB (Arita et al., 2002), basic fibroblast growth factor, and heparin-binding epidermal growth factor-like growth factor (Wang et al., 2005). This effect of adiponectin is attributed to its ability of interacting with these atherogenic growth factors in an oligomerization-dependent manner, thereby blocking binding to their respective cell membrane receptors for further activation of the mitogenic pathways (Wang et al., 2005). In addition, adiponectin inhibits insulin-like growth factor-1 induced migration and proliferation of vascular smooth muscle cells through AMPK-dependent suppression of P44/P42 MAP kinase (Motobayashi et al., 2009). Consistent with these in vitro findings, mechanically injured arteries of adiponectin-deficient mice exhibit severe neointimal thickening and increased proliferation of vascular smooth muscle cells (Matsuda et al., 2002), and this change can be prevented by supplementation of recombinant adiponectin (Okamoto et al., 2002).
In macrophages, chronic treatment with both globular and full-length adiponectin inhibits the production of pro-inflammatory cytokines induced by several stimuli, including lipopolysaccharide, leptin and resistin (Rae and Graham, 2006; Yamaguchi et al., 2005). The anti-inflammatory effect of adiponectin is associated with its ability to suppress NF-κB and p42/p44 MAP kinase-dependent signalling (Wulster-Radcliffe et al., 2004; Yamaguchi et al., 2005). In human monocyte-derived macrophages, adiponectin also induces a sequential up-regulation of the anti-inflammatory cytokine IL-10 (an anti-inflammatory cytokine that renders macrophages tolerant to further stimulation by endotoxin or other pro-inflammatory cytokines) and tissue inhibitor of metalloproteinase-1 (Kumada et al., 2004). Acute treatment with adiponectin triggers the release of TNFα and IL-6 via NF-κB and ERK1/2 activation, which subsequently causes an induction of IL-10 (Park et al., 2007). Globular adiponectin induces IL-10 production by stimulating the phosphorylation of cAMP-response element binding protein, thereby transactivating the IL-10 promoter in macrophages (Park et al., 2008). In addition, adiponectin may exert its anti-inflammatory activity by regulating macrophage polarization (Lovren et al., 2010). Indeed, upon stimulation with adiponectin, human monocytes are primed into anti-inflammatory M2 macrophages as opposed to the classically activated M1 phenotype. Incubation of M1 macrophages with adiponectin-treated M2-derived culture supernatant results in a pronounced inhibition in secretion of the pro-inflammatory factors such as TNFα and monocyte chemotactic protein 1. Furthermore, macrophages isolated from adiponectin knockout mice exhibits diminished levels of markers for M2 macrophages, and this phenomenon can be prevented by adiponectin treatment (Lovren et al., 2010).
Several independent mechanisms have been proposed to explain the inhibitory effects of adiponectin on the conversion of macrophage to foam cells. First, adiponectin suppress class A scavenger receptor expression, thereby reducing uptake of acetylated LDL particles into macrophages (Ouchi et al., 2001). Second, it decreases the activity of acyl-coenzyme A: cholesterol acyltransferase, a key enzyme that catalyses cholesteryl ester formation (Furukawa et al., 2004). Third, it increases cholesterol efflux by enhancing the expression of the ATP-binding cassette transporter ABCA1 in macrophages (Tsubakio-Yamamoto et al., 2008).
In addition to its direct actions on blood vessels, adiponectin acts in an autocrine manner to inhibit obesity-induced macrophage infiltration and production of pro-inflammatory cytokines in adipose tissue (Kim et al., 2007; Ohashi et al., 2010). This may also contribute to its anti-atherosclerotic properties by preventing the ‘inflammatory signal’ from adipose tissue to the vasculature. In ob/ob obese mice with transgenic expression of adiponectin, the degree of macrophage infiltration in adipose tissue and systemic inflammation is markedly attenuated in comparison with wild-type obese mice, in spite of a marked expansion of adipose tissue (Kim et al., 2007).
Cardio-protective effects of adiponectin
In addition to its effects on the vasculature, both in vitro studies and animal experiments demonstrate that adiponectin acts directly on cardiomyocytes to protect the heart from ischaemic injury, hypertrophy, cardiomyopathy and systolic dysfunction (Goldstein et al., 2009). In adiponectin-deficient mice, pressure overload results in enhanced concentric cardiac hypertrophy and increases mortality compared with wild-type mice (Shibata et al., 2004a). During ischaemia-reperfusion (I/R) injury, the lack of adiponectin exacerbates myocardial infarct size and myocardial apoptosis and decreases cardiac functions (Shibata et al., 2005; Tao et al., 2007). Furthermore, adiponectin-deficient mice display more severe angiotensin II-induced cardiac fibrosis and left ventricular dysfunction as well as doxorubicin-induced cardiomyopathy compared with wild-type mice (Fujita et al., 2008; Konishi et al., 2011). All these pathological changes can be reversed by adenovirus-mediated supplementation of recombinant adiponectin. Adiponectin has been identified as a key mediator conferring the beneficial effects of caloric restriction in improving left ventricular function and limiting infarction size after I/R injury (Shinmura et al., 2007). In db/db diabetic obese mice, adiponectin improves cardiomyocyte contractile function possibly by alleviating endoplasmic reticulum stress (Dong and Ren, 2009). Consistent with aforementioned findings in rodents, the protective effects of adiponectin against myocardial I/R injury has also been observed in pigs (Kondo et al., 2010).
The cardio-protective effects of adiponectin are attributed to its ability in suppressing apoptosis, oxidative/nitrative stress and inflammation in cardiomyocytes (Figure 4). In both cultured cardiomyocytes and animals, adiponectin inhibits apoptosis and promotes cell survival (Tao et al., 2007). The anti-apoptotic activity of adiponectin is dependent on the activation of AMPK (Kadowaki and Yamauchi, 2005; Shibata et al., 2005). Adenovirus-mediated expression of dominant negative AMPK prevents the suppressive effects of adiponectin on I/R-induced apoptosis in mice as well as in cultured cardiomyocytes (Wang et al., 2009b). In addition, a recent study demonstrated that adiponectin protects against palmitate-induced cardiomyocyte apoptosis by activation of ceramidase which in turn promotes the formation of an anti-apoptotic metabolite S1P (Holland et al., 2011). In rat neonatal left ventricular cardiomyocytes, adiponectin increases cell survival and prevents stress-induced cell apoptosis in an Akt-dependent manner (Skurk et al., 2008). Thus, adiponectin appears to exert its anti-apoptotic effects through distinct mechanisms under different pathological conditions.
Figure 4.
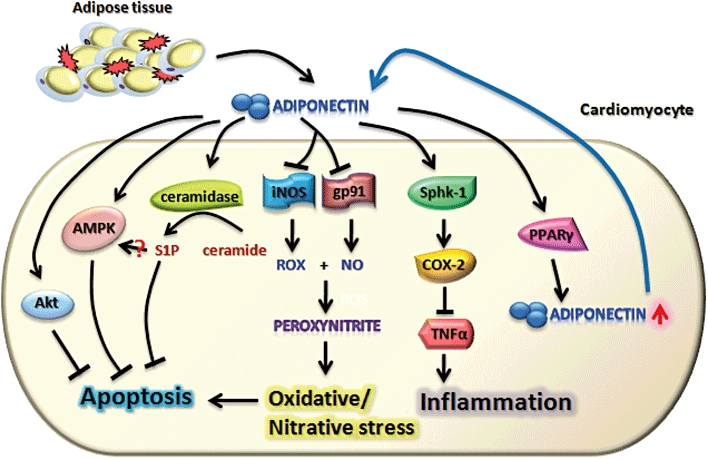
Major signalling pathways underlying the cardio-protective effects of adiponectin. AMPK, AMP kinase; PPARγ, peroxisome-proliferator activated receptor gamma; S1P, sphingosine-1-phosphate; Sphk-1, sphingosine kinase-1.
Several studies have linked the cardio-protective effects of adiponectin to its anti oxidative/nitrative stress activities (Tao et al., 2007; Gonon et al., 2008; Wang et al., 2009b). Formation of NO, superoxide anions and their cytotoxic product (peroxynitrite) are all higher in cardiac tissue of adiponectin-null mice than in that of wild-type mice (Tao et al., 2007). Moreover, administration of recombinant adiponectin prior to reperfusion reduces I/R-induced protein expression of iNOS/gp91phox, decreases NO and superoxide anion production, blocks peroxynitrite formation, and reverses the pro-apoptotic phenotype in adiponectin-null mice. In isolated rat hearts, adiponectin improves left ventricular function and increases coronary flow during reperfusion whereas administration of the NOS inhibitor nitro-l-arginine (L-NNA) abrogates the improvement in myocardial function induced by the adipokine (Gonon et al., 2008). Although transgenic mice with cardiomyocyte-specific overexpression of a mutant AMPKα2 subunit (AMPK-DN) suffer from greater cardiac injury after myocardial I/R (Wang et al., 2009b), adiponectin still evokes cardioprotection against myocardial I/R and the resultant cardiac oxidative and nitrative stress in those mice, implying that the anti-oxidative/anti-nitrative effects of adiponectin are independent of AMPK (Wang et al., 2009b). The findings of this study may indicate that adiponectin differentially regulates NO production by eNOS and iNOS. Adiponectin stimulates NO production via eNOS activation, thereby contributing to its vasodilator and vascular protective effects. However, under pathological conditions such as myocardial I/R injury, adiponectin exerts its anti-nitrative actions in cardiomyocytes by preventing the induction of iNOS expression and the resulting excess NO generation (Wang et al., 2009b).
The suppressive effects of adiponectin against myocardial I/R-induced inflammation appear to be mediated by cyclooxygenase-2 (COX-2), a rate-limiting enzyme for prostanoid synthesis (Salvado et al., 2009). In cardiomyocytes, adiponectin induces COX-2 expression and increases its activity through sphingosine kinase-1 (Ikeda et al., 2008). Pharmacological inhibition of COX-2 reverses the suppressive effects of adiponectin on myocardial I/R-induced TNFα production and infarct size (Shibata et al., 2005).
Both AdipoR1 and AdipoR2 are expressed in cardiac cells (Fujioka et al., 2006; Ding et al., 2007). However, the physiological roles of these two adiponectin receptors in cardiac functions have never been explored. Instead, T-cadherin, an adiponectin-interacting partner anchored at cell surface by glycosyl phosphatidylinositol, plays an indispensible role in adiponectin-mediated cardioprotection in mice (Denzel et al., 2010). There is an extensive colocalization between adiponectin and T-cadherin in cardiomyocytes in vivo. In T-cadherin deficient mice, adiponectin fails to associate with cardiac tissue. Furthermore, the protective effects of adiponectin against pressure overload-induced cardiac hypertrophy and I/R-induced myocardial infarction are lost in mice lacking T-cadherin (Denzel et al., 2010). These findings provide compelling evidence supporting the role of T-cadherin as a physiological adiponectin-binding receptor that enables the association of adiponectin with cardiac tissue.
As a metabolic regulator, adiponectin may preserve cardiac functions by exerting its beneficial effects on glucose and lipid metabolism. In adult cardiomyocytes, adiponectin increases CD36 translocation and fatty acid uptake by activation of AMPK, enhances insulin-stimulated glucose transport through Akt (Fang et al., 2010), and also stimulates lipoprotein lipase activity via RhoA/Rho-associated protein kinase-mediated actin remodelling (Ganguly et al., 2011). In addition, adiponectin stimulates vascular endothelial growth factor production in an AMPK-dependent manner, which may also contribute to its cardio-protective effects by enhancing angiogenesis (Shimano et al., 2010).
Although circulating adiponectin is produced predominantly from adipose tissue, this adipokine is also expressed and secreted in cardiomyocytes (Ding et al., 2007; Amin et al., 2010a,b). Moreover, cardiomyocyte-derived adiponectin is biologically active in protecting cells against I/R injury by paracrine/autocrine activation of adiponectin receptors (Wang et al., 2010). Exogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARγ-dependent autocrine mechanism that leads to increased expression and secretion of endogenous adiponectin (Amin et al., 2010a). These in vitro observations are corroborated by a clinical study showing the expression of adiponectin in human cardiac cells (Skurk et al., 2008). In patients with dilated cardiomyopathy, the cardiac adiponectin protein expression is down-regulated, independently of the serum adiponectin level. Taken together, these findings support a key role of the autocrine function of adiponectin in conferring its cardio-protective activities.
Adiponectin-targeted strategies to combat CVD
As adiponectin possesses multiple beneficial effects on cardiovascular health, increasing circulating adiponectin levels and/or enhance adiponectin signalling may represent a promising strategy for the treatment of CVD. Although supplementation with exogenous adiponectin is effective in alleviating CVD in animals, it is practically difficult to use recombinant adiponectin as a therapeutic agent, due to the high circulating levels required, the extensive post-translational modifications needed for its activity and its relatively short half-life. An alternative approach is to increase the secretion of endogenous adiponectin by the adipocytes, a process that is impaired in obesity-related pathologies. In fact, the beneficial effects of several therapeutic interventions for CVD, including lifestyle modification and several anti-diabetic and cardiovascular drugs, are associated with elevation of circulating adiponectin (Zhu et al., 2008).
Adiponectin and lifestyle modifications
Prolonged weight reduction by either gastric bypass surgery or caloric restriction increase circulating levels of adiponectin in obese subjects (Yang et al., 2001). Exercise is another effective way to elevate adiponectin levels, possibly by improving oxidative capacity. Overweight males exhibit higher level of adiponectin after a 10 week aerobic training programme (Kriketos et al., 2004). Restriction of calorie intake in combination with moderate physical activity significantly induces adiponectin expression, especially among obese or diabetic subjects (Esposito et al., 2003). Furthermore, weight loss selectively increases the circulating levels of the HMW oligomeric adiponectin (Martos-Moreno et al., 2010). The positive metabolic outcomes after lifestyle intervention in overweight/obese children are also accompanied by an increase in circulating adiponectin (Cambuli et al., 2008). In mice, the protective effects of short-term caloric restriction against myocardial infarction are abrogated in the absence of adiponectin (Shinmura et al., 2007). Furthermore, adiponectin plays an obligatory role in mediating the stimulatory effects of adiponectin on revascularization in response to ischaemia (Kondo et al., 2009). These findings suggest that the benefits of lifestyle interventions on cardiovascular health are mediated at least in part by adiponectin.
Adiponectin and PPAR agonists
The PPARγ agonists thiazolidinediones (TZDs), including pioglitazone and rosiglitazone, are a class of insulin-sensitizing drugs that also possess protective effects against endothelial dysfunction and atherosclerosis (Mazzone et al., 2006; Lincoff et al., 2007). TZDs elevate circulating levels of adiponectin in both humans and rodents (Tao et al., 2010; Hiuge-Shimizu et al., 2011), by increasing its gene expression (Matsuoka et al., 2001) as well as by augmenting the secretion of HMW adiponectin from adipocytes (Phillips et al., 2009). In diabetic patients, TZDs-mediated increases in adiponectin, especially its HMW oligomeric complexes, correlate with the improvement in insulin sensitivity (Pajvani et al., 2004b). Several therapeutic effects of TZDs, including their insulin-sensitizing activity (Nawrocki et al., 2006), protection against I/R-induced myocardial infarction (Tao et al., 2010) and angiotensin II-induced cardiac hypertrophy (Li et al., 2010), alleviation of endothelial dysfunction and improvement in EPCs-mediated endothelial repair (Chang et al., 2010), as well as amelioration of atherosclerosis (Hiuge-Shimizu et al., 2011), are all absent in mice lacking adiponectin. Taken in conjunction, these data support an indispensible role of adiponectin in conferring the therapeutic benefits of TZDs.
Both experimental and clinical studies have demonstrated that the PPARα agonists bezafibrate and fenofibrate augment circulating levels of adiponectin (Koh et al., 2006; Hiuge et al., 2007; Rosenson, 2009). However, the PPARα agonists-mediated increase in circulating adiponectin is modest compared with that of the TZDs. Whether or not adiponectin is required for the lipid-lowering and endothelium-protective effects of the PPARα agonists is unclear.
In addition to raising the circulating levels of adiponectin, both PPARγ and PPARα agonists increase the expression of AdipoR2 in primary and THP-1 macrophages (Chinetti et al., 2004), suggesting that these agents may also potentiate adiponectin's actions by enhancing its signal transduction.
Adiponectin and inhibitors of the renin-angiotensin system (RAS)
The RAS is a critical regulatory system for blood pressure. The overproduction of angiotensin II in obesity plays an important role in the pathogenesis of insulin resistance, hypertension and CVD. Pharmacological inhibitors of RAS, such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), decrease blood pressure and also possess anti-diabetic and cardio-protective activities (Wang and Scherer, 2008). Clinical studies demonstrate that these agents increase plasma adiponectin levels in humans (Furuhashi et al., 2003; Koh et al., 2004; 2005; Clasen et al., 2005). Administration of losartan only or in combination with simvastatin leads to a significant elevation of plasma levels of adiponectin in hypertensive patients (Koh et al., 2004). In a study comparing five antihypertensive drugs for their effect on adiponectin, ACEIs and ARBs, but not the other drugs, were found to increase circulating levels of adiponectin (Yilmaz et al., 2007). The ARBs-mediated elevation of adiponectin may contribute to the additional beneficial effects of these drugs in hypertensive patients (Furuhashi et al., 2003). However, further studies in adiponectin knockout mice are needed to evaluate whether or not the therapeutic benefits of ARBs and ACEIs are mediated partly by adiponectin.
Adiponectin and nutraceuticals
A large number of nutraceutical products with beneficial effects on cardiovascular health, including fish oil (Rossi et al., 2005), safflower oil (Sekine et al., 2008), conjugated linoleic acid (Nagao et al., 2003), omega-3 polyunsaturated acids (Flachs et al., 2006), grape-seed extract (Terra et al., 2009), green tea extract (Hsu et al., 2008), taurine (Chen et al., 2009b), dietary docosahexaenoic acid (Lefils et al., 2010), and the polyphenol resveratrol (Wang et al., 2011), increase adiponectin production in either animals or humans. There is also a growing interest to use adiponectin as a surrogate marker for screening lead compounds from nutraceutical products with anti-diabetic and cardio-protective activities (Xu et al., 2009a). Such a strategy has been used successfully for identification of astragaloside II and isoastragaloside I from the medicinal herb Radix Astragali (Huang Qi in Chinese). Astragaloside II and isoastragaloside I specifically increase adiponectin secretion in primary adipocytes, and raise plasma levels of adiponectin in mice with dietary or genetic obesity (Xu et al., 2009a). Moreover, long-term treatment with these two compounds improves obesity-related metabolic complications in an adiponectin-dependent manner (Xu et al., 2009a). Noticeably, Radix Astragali has been used as a herb medicine to treat CVD for several centuries in many Asia countries. Further studies are warranted to investigate whether or not its beneficial effect is mediated by astragaloside II and isoastragaloside I-induced adiponectin secretion.
Concluding remarks
Both clinical data and experimental evidence obtained in the past decade have demonstrated the multiple salutary effects of adiponectin in obesity-related metabolic and cardiovascular complications. Due to the close proximity between adipose and cardiovascular tissues, adiponectin acts in both an endocrine and paracrine manner to modulate circulatory function. Adiponectin is a key component that mediates the cross-talk between adipose tissue, cardiac cells and the vasculature (Li et al., 2010).
As epidemiological studies have identified adiponectin deficiency as an etiological factor of CVD, elevation of plasma adiponectin by either pharmaceutical or lifestyle interventions represent a promising therapeutic strategy for CVD. Indeed, numerous drugs and nutraceuticals with anti-diabetic and cardiovascular protective activities increase plasma adiponectin in humans and/or animals. Notable among them are TZDs, which bear striking similarity to adiponectin with respect to their beneficial effects on insulin-sensitization, vascular protection and anti-inflammation. Several independent studies on adiponectin knockout mice provide compelling evidence that indeed almost all the beneficial effects of TZDs are mediated by induction of adiponectin. However, TZDs have several side effects including body weight gain, fluid retention, and anaemia (Semenkovich, 2005). Furthermore, the use of rosiglitazone, one of the most widely used TZD drugs, is compromised by an increased cardiac risk. A meta-analysis of controlled clinical trials found a significant increase in the risk of myocardial infarction and a near-significant increased risk of death from cardiovascular causes when rosiglitazone was compared with placebo or with standard diabetes drugs (Nissen and Wolski, 2007). In contrast to TZDs, adiponectin possesses cardio-protective activities. Therefore, pharmacological agents that selectively increase plasma adiponectin may be able to avoid the detrimental effects of TZDs. To develop lead compounds that selectively induce adiponectin production in adipocytes, it is of critical importance to further elucidate the detailed molecular events involved in regulating the expression, post-translational modifications, oligomerization and secretion of the adipokine, and to dissect the pathological pathways that lead its impaired secretion in obesity-related disorders.
An alternative adiponectin-targeted drug development strategy is to design chemical agonists that can mimic adiponectin to activate its receptors and/or postreceptor signalling pathways. Such an approach may also be able to reverse ‘adiponectin resistance’, which has been observed in both animals and humans (Lin et al., 2007; Kunihiro Matsushita et al., 2007). However, since the discovery of the two adiponectin receptors (AdipoR1 and AdipoR2) in 2003, little progress has been made in the understanding of the structural basis underlying their ligand-receptor interactions. The molecular events whereby the binding of adiponectin to its receptors results in the activation of its downstream signalling pathways remain poorly characterized. Despite the fact that AdipoR1 and AdipoR2 are expressed in both cardiac cells and blood vessels, their physiological roles in modulating cardiovascular function have not been explored so far. Although T-cadherin has been identified as a physiological adiponectin-interacting receptor required for conferring the cardio-protective effects of this adipokine, the relationship between T-cadherin, AdipoR1 and AdipoR2 in the cardiovascular system requires further clarification. Further studies in this exciting field should provide important molecular and structural information for the rational design of adiponectin agonists that can potentially be used to combat the escalating epidemic of obesity, diabetes and CVD.
Acknowledgments
This work was supported by collaborative research fund (HKU 2/07C and HKU4/CRF/10) from the Research Grants Council of Hong Kong.
Glossary
- AdipoR
adiponectin receptor
- AMPK
AMP kinase
- CVD
cardiovascular disease
- EPC
endothelial progenitor cell
- HMW
high molecular weight
- I/R
ischaemia-reperfusion
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- S1P
sphingosine-1-phosphate
- TNFα
tumour necrosis factor α
- TZD
thiazolidinedione
Conflict of interest
None.
References
- Amin RH, Mathews ST, Alli A, Leff T. Endogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARgamma-dependent autocrine mechanism. Am J Physiol Heart Circ Physiol. 2010a;299:H690–H698. doi: 10.1152/ajpheart.01032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPARgamma in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2010b;298:E28–E37. doi: 10.1152/ajpendo.00446.2009. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–593. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- Cambuli VM, Musiu MC, Incani M, Paderi M, Serpe R, Marras V, et al. Assessment of adiponectin and leptin as biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. J Clin Endocrinol Metab. 2008;93:3051–3057. doi: 10.1210/jc.2008-0476. [DOI] [PubMed] [Google Scholar]
- Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, et al. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–419. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Li Y, Huang Y, Lam KS, Hoo RL, Wong WT, et al. Adiponectin prevents diabetic premature senescence of endothelial progenitor cells and promotes endothelial repair by suppressing the p38 MAP kinase/p16INK4A signaling pathway. Diabetes. 2010;59:2949–2959. doi: 10.2337/db10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton HK, Webster J, Kruger S, Simpson F, Richards AA, Whitehead JP. ERp46 binds to AdipoR1, but not AdipoR2, and modulates adiponectin signalling. Biochem Biophys Res Commun. 2010;392:234–239. doi: 10.1016/j.bbrc.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Cir Res. 2009a;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, et al. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009b;49:1554–1562. doi: 10.1002/hep.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- Cheng KK, Iglesias MA, Lam KS, Wang Y, Sweeney G, Zhu W, et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417–427. doi: 10.1016/j.cmet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004;314:151–158. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, et al. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49:1455–1461. doi: 10.1161/HYPERTENSIONAHA.107.086835. [DOI] [PubMed] [Google Scholar]
- Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, et al. PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes (Lond) 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Torok N, Dasu MR, Samols D, Jialal I. Adiponectin decreases C-reactive protein synthesis and secretion from endothelial cells: evidence for an adipose tissue-tissue-vascular loop. Arterioscler Thromb Vasc Biol. 2008;28:1368–1374. doi: 10.1161/ATVBAHA.108.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Qin Q, He N, Francis-David SC, Hou J, Liu J, et al. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma. J Mol Cell Cardiol. 2007;43:73–84. doi: 10.1016/j.yjmcc.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Ren J. Adiponectin improves cardiomyocyte contractile function in db/db diabetic obese mice. Obesity (Silver Spring) 2009;17:262–268. doi: 10.1038/oby.2008.545. [DOI] [PubMed] [Google Scholar]
- Ebinc H, Ebinc FA, Ozkurt ZN, Dogru MT, Tulmac M, Yilmaz M, et al. Impact of adiponectin on left ventricular mass index in non-complicated obese subjects. Endocr J. 2008;55:523–528. doi: 10.1507/endocrj.k07e-098. [DOI] [PubMed] [Google Scholar]
- Eren P, Camus S, Matrone G, Ebrahimian TG, Francois D, Tedgui A, et al. Adiponectinemia controls pro-angiogenic cell therapy. Stem Cells. 2009;27:2712–2721. doi: 10.1002/stem.219. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care. 2007;30:1305–1313. doi: 10.2337/dc06-2305. [DOI] [PubMed] [Google Scholar]
- Fang X, Palanivel R, Cresser J, Schram K, Ganguly R, Thong FS, et al. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab. 2010;299:E721–E729. doi: 10.1152/ajpendo.00086.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- Frizzell N, Rajesh M, Jepson MJ, Nagai R, Carson JA, Thorpe SR, et al. Succination of thiol groups in adipose tissue proteins in diabetes: succination inhibits polymerization and secretion of adiponectin. J Biol Chem. 2009;284:25772–25781. doi: 10.1074/jbc.M109.019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- Fujioka D, Kawabata K, Saito Y, Kobayashi T, Nakamura T, Kodama Y, et al. Role of adiponectin receptors in endothelin-induced cellular hypertrophy in cultured cardiomyocytes and their expression in infarcted heart. Am J Physiol Heart Circ Physiol. 2006;290:H2409–H2416. doi: 10.1152/ajpheart.00987.2005. [DOI] [PubMed] [Google Scholar]
- Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2008;28:863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, et al. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Hori M, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, et al. Adiponectin down-regulates acyl-coenzyme A: cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochem Biophys Res Commun. 2004;317:831–836. doi: 10.1016/j.bbrc.2004.03.123. [DOI] [PubMed] [Google Scholar]
- Ganguly R, Schram K, Fang X, Kim M, Rodrigues B, Thong FS, et al. Adiponectin increases LPL activity via RhoA/ROCK-mediated actin remodelling in adult rat cardiomyocytes. Endocrinology. 2011;152:247–254. doi: 10.1210/en.2010-0530. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjoquist PO, et al. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kanda J, Nakamura T, Horie A, Kurosawa H, Hashimoto T, et al. Association of hypoadiponectinemia in men with early onset of coronary heart disease and multiple coronary artery stenoses. Metabolism. 2006;55:1653–1657. doi: 10.1016/j.metabol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Heiker JT, Wottawah CM, Juhl C, Kosel D, Morl K, Beck-Sickinger AG. Protein kinase CK2 interacts with adiponectin receptor 1 and participates in adiponectin signaling. Cell Signal. 2009;21:936–942. doi: 10.1016/j.cellsig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Hiuge A, Tenenbaum A, Maeda N, Benderly M, Kumada M, Fisman EZ, et al. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler Thromb Vasc Biol. 2007;27:635–641. doi: 10.1161/01.ATV.0000256469.06782.d5. [DOI] [PubMed] [Google Scholar]
- Hiuge-Shimizu A, Maeda N, Hirata A, Nakatsuji H, Nakamura K, Okuno A, et al. Dynamic Changes of Adiponectin and S100A8 Levels by the Selective Peroxisome Proliferator-Activated Receptor-{gamma} Agonist Rivoglitazone. Arterioscler Thromb Vasc Biol. 2011;31:792–799. doi: 10.1161/ATVBAHA.110.221747. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. 2008;27:363–370. doi: 10.1016/j.clnu.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Ohashi K, Shibata R, Pimentel DR, Kihara S, Ouchi N, et al. Cyclooxygenase-2 induction by adiponectin is regulated by a sphingosine kinase-1 dependent mechanism in cardiac myocytes. FEBS Lett. 2008;582:1147–1150. doi: 10.1016/j.febslet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imatoh T, Miyazaki M, Momose Y, Tanihara S, Une H. Adiponectin levels associated with the development of hypertension: a prospective study. Hypertens Res. 2008;31:229–233. doi: 10.1291/hypres.31.229. [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Karastergiou K, Evans I, Ogston N, Miheisi N, Nair D, Kaski JC, et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Song SE, Kim YW, Kim JY, Park SC, Park YK, et al. Adiponectin inhibits palmitate-induced apoptosis through suppression of reactive oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase. J Endocrinol. 2010;207:35–44. doi: 10.1677/JOE-10-0093. [DOI] [PubMed] [Google Scholar]
- Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, et al. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245–1252. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. J Am Coll Cardiol. 2006;48:1369–1377. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, et al. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687–3692. doi: 10.1161/01.CIR.0000143085.86697.13. [DOI] [PubMed] [Google Scholar]
- Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol. 2005;45:1649–1653. doi: 10.1016/j.jacc.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Kim JA, et al. Additive beneficial effects of fenofibrate combined with candesartan in the treatment of hypertriglyceridemic hypertensive patients. Diabetes Care. 2006;29:195–201. doi: 10.2337/diacare.29.02.06.dc05-1418. [DOI] [PubMed] [Google Scholar]
- Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Shibata R, Unno K, Shimano M, Ishii M, Kito T, et al. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3:166–173. doi: 10.1161/CIRCINTERVENTIONS.109.872044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Haraguchi G, Ohigashi H, Ishihara T, Saito K, Nakano Y, et al. Adiponectin protects against doxorubicin-induced cardiomyopathy by anti-apoptotic effects through AMPK up-regulation. Cardiovasc Res. 2011;89:309–319. doi: 10.1093/cvr/cvq335. [DOI] [PubMed] [Google Scholar]
- Kotani K, Sakane N, Saiga K, Kurozawa Y. Leptin : adiponectin ratio as an atherosclerotic index in patients with type 2 diabetes : relationship of the index to carotid intima-media thickness. Diabetologia. 2005;48:2684–2686. doi: 10.1007/s00125-005-0015-4. [DOI] [PubMed] [Google Scholar]
- Kotani K, Shimohiro H, Sakane N. The relationship between leptin: adiponectin ratio and carotid intima-media thickness in asymptomatic females. Stroke. 2008;39:e32–e33. doi: 10.1161/STROKEAHA.107.505669. Author reply e34. [DOI] [PubMed] [Google Scholar]
- Kozakova M, Muscelli E, Flyvbjerg A, Frystyk J, Morizzo C, Palombo C, et al. Adiponectin and left ventricular structure and function in healthy adults. J Clin Endocrinol Metab. 2008;93:2811–2818. doi: 10.1210/jc.2007-2580. [DOI] [PubMed] [Google Scholar]
- Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27:629–630. doi: 10.2337/diacare.27.2.629. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefils J, Geloen A, Vidal H, Lagarde M, Bernoud-Hubac N. Dietary DHA: time course of tissue uptake and effects on cytokine secretion in mice. Br J Nutr. 2010;104:1304–1312. doi: 10.1017/S0007114510002102. [DOI] [PubMed] [Google Scholar]
- Li CJ, Sun HW, Zhu FL, Chen L, Rong YY, Zhang Y, et al. Local adiponectin treatment reduces atherosclerotic plaque size in rabbits. J Endocrinol. 2007;193:137–145. doi: 10.1677/JOE-06-0173. [DOI] [PubMed] [Google Scholar]
- Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- Li P, Shibata R, Unno K, Shimano M, Furukawa M, Ohashi T, et al. Evidence for the importance of adiponectin in the cardioprotective effects of pioglitazone. Hypertension. 2010;55:69–75. doi: 10.1161/HYPERTENSIONAHA.109.141655. [DOI] [PubMed] [Google Scholar]
- Lin LY, Lin CY, Su TC, Liau CS. Angiotensin II-induced apoptosis in human endothelial cells is inhibited by adiponectin through restoration of the association between endothelial nitric oxide synthase and heat shock protein 90. FEBS Lett. 2004;574:106–110. doi: 10.1016/j.febslet.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE, et al. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes. 2007;56:1969–1976. doi: 10.2337/db07-0127. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, et al. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683–692. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, et al. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci U S A. 2008;105:18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo J, Dolan SE, Kanter JR, Hemphill LC, Connelly JM, Lees RS, et al. Effects of obesity, body composition, and adiponectin on carotid intima-media thickness in healthy women. J Clin Endocrinol Metab. 2006;91:1677–1682. doi: 10.1210/jc.2005-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–H663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Magkos F, Sidossis LS. Recent advances in the measurement of adiponectin isoform distribution. Curr Opin Clin Nutr Metab Care. 2007;10:571–575. doi: 10.1097/MCO.0b013e3282bf6ea8. [DOI] [PubMed] [Google Scholar]
- Maiolino G, Cesari M, Antezza K, Zanchetta M, Pedon L, Pessina AC, et al. Prognostic effect of plasma adiponectin (ADPN) in non diabetic high-risk patients. Hypertension. 2007;50:818–818. [Google Scholar]
- Maiolino G, Cesari M, Sticchi D, Zanchetta M, Pedon L, Antezza K, et al. Plasma adiponectin for prediction of cardiovascular events and mortality in high-risk patients. J Clin Endocrinol Metab. 2008;93:3333–3340. doi: 10.1210/jc.2007-2405. [DOI] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Martos-Moreno GA, Barrios V, Martinez G, Hawkins F, Argente J. Effect of weight loss on high-molecular weight adiponectin in obese children. Obesity (Silver Spring) 2010;18:2288–2294. doi: 10.1038/oby.2010.68. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Ogawa Y, Masuzaki H, Ebihara K, Aizawa-Abe M, Satoh N, et al. Decreased triglyceride-rich lipoproteins in transgenic skinny mice overexpressing leptin. Am J Physiol Endocrinol Metab. 2001;280:E334–E339. doi: 10.1152/ajpendo.2001.280.2.E334. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Yatsuya H, Tamakoshi K, Muramatsu T, Mitsuhashi H, Shibata R, et al. Inverse association of adiponectin with insulin resistance is attenuated with the escalation of insulin resistance: a possible finding of adiponectin resistance. Circulation. 2007;116:814. [Google Scholar]
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D'Agostino RB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296:2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, et al. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi H, Yatsuya H, Tamakoshi K, Matsushita K, Otsuka R, Wada K, et al. Adiponectin level and left ventricular hypertrophy in Japanese men. Hypertension. 2007;49:1448–1454. doi: 10.1161/HYPERTENSIONAHA.106.079509. [DOI] [PubMed] [Google Scholar]
- Motobayashi Y, Izawa-Ishizawa Y, Ishizawa K, Orino S, Yamaguchi K, Kawazoe K, et al. Adiponectin inhibits insulin-like growth factor-1-induced cell migration by the suppression of extracellular signal-regulated kinase 1/2 activation, but not Akt in vascular smooth muscle cells. Hypertens Res. 2009;32:188–193. doi: 10.1038/hr.2008.19. [DOI] [PubMed] [Google Scholar]
- Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Mullen KL, Smith AC, Junkin KA, Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007;293:E83–E90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- Nagao K, Inoue N, Wang YM, Yanagita T. Conjugated linoleic acid enhances plasma adiponectin level and alleviates hyperinsulinemia and hypertension in Zucker diabetic fatty (fa/fa) rats. Biochem Biophys Res Commun. 2003;310:562–566. doi: 10.1016/j.bbrc.2003.09.044. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Naruse K, Matsuki T, Hamada Y, Nakashima E, Kamiya H, et al. Adiponectin promotes migration activities of endothelial progenitor cells via Cdc42/Rac1. FEBS Lett. 2009;583:2457–2463. doi: 10.1016/j.febslet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Nichols WW, Pepine CJ, O'Rourke MF. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke. N Engl J Med. 1999;340:1762–1763. [PubMed] [Google Scholar]
- Nilsson PM, Engstrom G, Hedblad B, Frystyk J, Persson MM, Berglund G, et al. Plasma adiponectin levels in relation to carotid intima media thickness and markers of insulin resistance. Arterioscler Thromb Vasc Biol. 2006;26:2758–2762. doi: 10.1161/01.ATV.0000249638.01416.4b. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Norata GD, Raselli S, Grigore L, Garlaschelli K, Dozio E, Magni P, et al. Leptin: adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke. 2007;38:2844–2846. doi: 10.1161/STROKEAHA.107.485540. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- Okui H, Hamasaki S, Ishida S, Kataoka T, Orihara K, Fukudome T, et al. Adiponectin is a better predictor of endothelial function of the coronary artery than HOMA-R, body mass index, immunoreactive insulin, or triglycerides. Int J Cardiol. 2008;126:53–61. doi: 10.1016/j.ijcard.2007.03.116. [DOI] [PubMed] [Google Scholar]
- Ong KL, Li M, Tso AW, Xu A, Cherny SS, Sham PC, et al. Association of genetic variants in the adiponectin gene with adiponectin level and hypertension in Hong Kong Chinese. Eur J Endocrinol. 2010;163:251–257. doi: 10.1530/EJE-10-0251. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003a;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003b;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, et al. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004a;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004b;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem. 2007;282:21695–21703. doi: 10.1074/jbc.M701419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PH, Huang H, McMullen MR, Bryan K, Nagy LE. Activation of cyclic-AMP response element binding protein contributes to adiponectin-stimulated interleukin-10 expression in RAW 264.7 macrophages. J Leukoc Biol. 2008;83:1258–1266. doi: 10.1189/jlb.0907631. [DOI] [PubMed] [Google Scholar]
- Patel S, Flyvbjerg A, Kozakova M, Frystyk J, Ibrahim IM, Petrie JR, et al. Variation in the ADIPOQ gene promoter is associated with carotid intima media thickness independent of plasma adiponectin levels in healthy subjects. Eur Heart J. 2008;29:386–393. doi: 10.1093/eurheartj/ehm526. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Kung J, Ciaraldi TP, Choe C, Christiansen L, Mudaliar S, et al. Selective regulation of cellular and secreted multimeric adiponectin by antidiabetic therapies in humans. Am J Physiol Endocrinol Metab. 2009;297:E767–E773. doi: 10.1152/ajpendo.00378.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Horejsi R, Moller R, Almer G, Scharnagl H, Stojakovic T, et al. Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J Clin Endocrinol Metab. 2005;90:4792–4796. doi: 10.1210/jc.2005-0167. [DOI] [PubMed] [Google Scholar]
- Pilz S, Mangge H, Wellnitz B, Seelhorst U, Winkelmann BR, Tiran B, et al. Adiponectin and mortality in patients undergoing coronary angiography. J Clin Endocrinol Metab. 2006;91:4277–4286. doi: 10.1210/jc.2006-0836. [DOI] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- Plant S, Shand B, Elder P, Scott R. Adiponectin attenuates endothelial dysfunction induced by oxidised low-density lipoproteins. Diab Vasc Dis Res. 2008;5:102–108. doi: 10.3132/dvdr.2008.017. [DOI] [PubMed] [Google Scholar]
- Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Graham A. Human resistin promotes macrophage lipid accumulation. Diabetologia. 2006;49:1112–1114. doi: 10.1007/s00125-006-0187-6. [DOI] [PubMed] [Google Scholar]
- Richards AA, Colgrave ML, Zhang J, Webster J, Simpson F, Preston E, et al. Sialic acid modification of adiponectin is not required for multimerization or secretion but determines half-life in circulation. Mol Endocrinol. 2010;24:229–239. doi: 10.1210/me.2009-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS. Effect of fenofibrate on adiponectin and inflammatory biomarkers in metabolic syndrome patients. Obesity (Silver Spring) 2009;17:504–509. doi: 10.1038/oby.2008.530. [DOI] [PubMed] [Google Scholar]
- Rossi AS, Lombardo YB, Lacorte JM, Chicco AG, Rouault C, Slama G, et al. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R486–R494. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]
- Salvado MD, Alfranca A, Escolano A, Haeggstrom JZ, Redondo JM. COX-2 limits prostanoid production in activated HUVECs and is a source of PGH2 for transcellular metabolism to PGE2 by tumor cells. Arterioscler Thromb Vasc Biol. 2009;29:1131–1137. doi: 10.1161/ATVBAHA.109.188540. [DOI] [PubMed] [Google Scholar]
- Sambuceti G, Morbelli S, Vanella L, Kusmic C, Marini C, Massollo M, et al. Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage, reversed by increases in pAMPK, heme oxygenase-1, and adiponectin. Stem Cells. 2009;27:399–407. doi: 10.1634/stemcells.2008-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J Biol Chem. 2001;276:28849–28856. doi: 10.1074/jbc.M104148200. [DOI] [PubMed] [Google Scholar]
- Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, et al. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- Schmid PM, Resch M, Steege A, Fredersdorf-Hahn S, Stoelcker B, Birner C, et al. Globular and Full-Length Adiponectin Induce NO-Dependent Vasodilation in Resistance Arteries of Zucker Lean but Not Zucker Diabetic Fatty Rats. Am J Hypertens. 2011;243:270–277. doi: 10.1038/ajh.2010.239. [DOI] [PubMed] [Google Scholar]
- Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, et al. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- Sekine S, Sasanuki S, Murano Y, Aoyama T, Takeuchi H. Alpha-linolenic acid-rich flaxseed oil ingestion increases plasma adiponectin level in rats. Int J Vitam Nutr Res. 2008;78:223–229. doi: 10.1024/0300-9831.78.45.223. [DOI] [PubMed] [Google Scholar]
- Semenkovich CF. TZDs and diabetes: testing the waters. Nat Med. 2005;11:822–824. doi: 10.1038/nm0805-822. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004a;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004b;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Skurk C, Ouchi N, Galasso G, Kondo K, Ohashi T, et al. Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett. 2008;582:1607–1612. doi: 10.1016/j.febslet.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236–3240. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210–220. doi: 10.1016/j.yjmcc.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- Skurk C, Wittchen F, Suckau L, Witt H, Noutsias M, Fechner H, et al. Description of a local cardiac adiponectin system and its deregulation in dilated cardiomyopathy. Eur Heart J. 2008;29:1168–1180. doi: 10.1093/eurheartj/ehn136. [DOI] [PubMed] [Google Scholar]
- Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Tschritter O, Fritsche A, Staiger H, Renn W, Weisser M, et al. Association of the T-G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes. 2002;51:37–41. doi: 10.2337/diabetes.51.1.37. [DOI] [PubMed] [Google Scholar]
- Summer R, Fiack CA, Ikeda Y, Sato K, Dwyer D, Ouchi N, et al. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol. 2009;297:L432–L438. doi: 10.1152/ajplung.90599.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S. Endothelial progenitor cells: new hope for a broken heart. Circulation. 2003;107:3093–3100. doi: 10.1161/01.CIR.0000074242.66719.4A. [DOI] [PubMed] [Google Scholar]
- Tan KC, Xu A, Chow WS, Lam MC, Ai VH, Tam SC, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- Tao L, Wang Y, Gao E, Zhang H, Yuan Y, Lau WB, et al. Adiponectin: an indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circ Res. 2010;106:409–417. doi: 10.1161/CIRCRESAHA.109.211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra X, Montagut G, Bustos M, Llopiz N, Ardevol A, Blade C, et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Torigoe M, Matsui H, Ogawa Y, Murakami H, Murakami R, Cheng XW, et al. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clin Endocrinol (Oxf) 2007;67:276–281. doi: 10.1111/j.1365-2265.2007.02876.x. [DOI] [PubMed] [Google Scholar]
- Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- Tsubakio-Yamamoto K, Matsuura F, Koseki M, Oku H, Sandoval JC, Inagaki M, et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2008;375:390–394. doi: 10.1016/j.bbrc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, et al. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010;3:185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, et al. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009a;284:31608–31615. doi: 10.1074/jbc.M109.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009b;119:835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298:E663–E670. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Liu M, Liu X, Dong LQ, Glickman RD, Slaga TJ, et al. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J Biol Chem. 2011;286:60–66. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B, Gallo DJ, Raman KG, Karlsson JM, Ozanich B, Chin BY, et al. Nitric oxide-dependent bone marrow progenitor mobilization by carbon monoxide enhances endothelial repair after vascular injury. Circulation. 2010;121:537–548. doi: 10.1161/CIRCULATIONAHA.109.887695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Mahadev K, Fuchsel L, Ouedraogo R, Xu SQ, Goldstein BJ. Adiponectin suppresses IkappaB kinase activation induced by tumor necrosis factor-alpha or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293:E1836–E1844. doi: 10.1152/ajpendo.00115.2007. [DOI] [PubMed] [Google Scholar]
- Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun. 2005;332:200–205. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte PM, Wang Y, et al. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology. 2009a;150:625–633. doi: 10.1210/en.2008-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang N, Ling F, Li P, Gao Y. Receptor for activated C-kinase 1, a novel binding partner of adiponectin receptor 1. Biochem Biophys Res Commun. 2009b;378:95–98. doi: 10.1016/j.bbrc.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang Y, Lam KS, Vanhoutte PM. Vascular actions of adipokines molecular mechanisms and therapeutic implications. Adv Pharmacol. 2010a;60:229–255. doi: 10.1016/B978-0-12-385061-4.00008-8. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang C, Wang N, Ling F, Li P, Gao Y, et al. Adiponectin inhibits lymphotoxin-beta receptor-mediated NF-KappaB signaling in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2010b;404:1060–1064. doi: 10.1016/j.bbrc.2010.12.110. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003a;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003b;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- Yi W, Sun Y, Gao E, Wei X, Lau W, Zheng Q, et al. Reduced cardioprotective action of adiponectin in high fat diet induced type II diabetic mice and its underlying mechanisms. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3722. Epub 2010 Nov 21 doi: 10.1089/ars.2010.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz MI, Sonmez A, Caglar K, Celik T, Yenicesu M, Eyileten T, et al. Effect of antihypertensive agents on plasma adiponectin levels in hypertensive patients with metabolic syndrome. Nephrology (Carlton) 2007;12:147–153. doi: 10.1111/j.1440-1797.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wang Y, Fan Y, Tang Z, Wang N. Overexpression of adiponectin receptors potentiates the antiinflammatory action of subeffective dose of globular adiponectin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:67–74. doi: 10.1161/ATVBAHA.108.178061. [DOI] [PubMed] [Google Scholar]
- Zhang H, Park Y, Zhang C. Coronary and aortic endothelial function affected by feedback between adiponectin and tumor necrosis factor alpha in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2010;30:2156–2163. doi: 10.1161/ATVBAHA.110.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009;284:22426–22435. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]


