Abstract
Obesity has become a serious global health issue affecting both adults and children. Recent devolopments in world demographics and declining health status of the world's population indicate that the prevalence of obesity will continue to increase in the next decades. As a disease, obesity has deleterious effects on metabolic homeostasis, and affects numerous organ systems including heart, kidney and the vascular system. Thus, obesity is now regarded as an independent risk factor for atherosclerosis-related diseases such as coronary artery disease, myocardial infarction and stroke. In the arterial system, endothelial cells are both the source and target of factors contributing to atherosclerosis. Endothelial vasoactive factors regulate vascular homeostasis under physiological conditions and maintain basal vascular tone. Obesity results in an imbalance between endothelium-derived vasoactive factors favouring vasoconstriction, cell growth and inflammatory activation. Abnormal regulation of these factors due to endothelial cell dysfunction is both a consequence and a cause of vascular disease processes. Finally, because of the similarities of the vascular pathomechanisms activated, obesity can be considered to cause accelerated, ‘premature’ vascular aging. Here, we will review some of the pathomechanisms involved in obesity-related activation of endothelium-dependent vasoconstriction, the clinical relevance of obesity-associated vascular risk, and therapeutic interventions using ‘endothelial therapy’ aiming at maintaining or restoring vascular endothelial health.
LINKED ARTICLES
This article is part of a themed section on Fat and Vascular Responsiveness. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-3
Keywords: diet, obesity, adipocytes, fat, elderly, hypertension, disease, diabetes
Endothelium-dependent regulation of vascular tone
Endothelial cells form the inner lining of arterial and venous blood vessels and lymphatic vessels which amount to approximately 1.5 kg in a person weighing 70 kg, covering an area of approximately four tennis courts (Cryer, 1983; Luscher and Barton, 1997; Barton, 2006). Under normal conditions, endothelial cells constantly produce vasoactive and trophic substances that control inflammation, vascular smooth muscle cell growth, vasomotion, platelet function, and plasmatic coagulation (Barton and Haudenschild, 2001; Traupe et al., 2003). In the early 1970s, Ross and Glomset reported that endothelial cells protect smooth muscle cells to proliferate, which generated the ‘response-to-injury’ theory of atherosclerosis (Ross and Glomset, 1973). The importance of endothelial cells as both source and target of vasoactive factors, however, was discovered by Robert F. Furchgott around 30 years ago (Furchgott and Zawadzki, 1980; Nilius et al., 2010; Barton, 2011). Since then, physiological roles of these factors have been demonstrated (Furchgott and Vanhoutte, 1989) as well as that these factors both contribute to and interfere with the development of cardiovascular disease (Barton and Haudenschild, 2001; Traupe et al., 2003; Barton, 2010; Vanhoutte, 2011). Finally, the new field of endothelial cell research eventually allowed the development of the first class of drugs specifically targeting an endothelial vasoconstrictor, the endothelin receptor antagonists (Barton, 2011).
Endothelium-dependent vasoconstriction: balancing endogenous vasodilation
Vasoactive factors derived from endothelial cells include the vasodilating gas NO, oxygen-derived free radicals such as ·O2- or ·OH, or peptides such as endothelins and angiotensins (Feletou and Vanhoutte, 2006). Thus, endothelium-derived mediators have either endothelium-derived relaxing factor (EDRF) or endothelium-derived contracting factor (EDCF) functions (Vanhoutte and Tang, 2008; Vanhoutte, 2009b). Endothelial cells also synthesize cyclooxygenase-derived EDCFs and EDRFs, and EDHFs (endothelium-derived hyperpolarizing factors) (Busse et al., 2002; Feletou and Vanhoutte, 2006). Endothelial factors are formed by enzymes such as NO synthase, NADPH oxidases, cyclooxygenases, converting enyzmes and epoxigenases, among others (Feletou and Vanhoutte, 2006). Although termed ‘endothelial’ factors, these mediators are not exclusively formed by endothelial cells, but also synthesized by other cells such as vascular smooth muscle cells, inflammatory cells such as leukocytes, mesangial cells or adipocytes, all of which appear to be centrally involved in obesity-related disease processes (Xu et al., 2003; 2010; Rocha and Libby, 2009; Li et al., 2010). An excessive production or increased activity through specific receptors causes endothelial factors to induce vasoconstriction and vascular cell growth (Luscher and Barton, 1997). The numerous endothelial factors identified so far have been extensively studied under physiological and pathophysiological conditions (reviewed in Vanhoutte et al., 2009; Barton, 2010; 2011).
Endothelium-derived vasoconstrictors: prostanoids, superoxide and endothelin
Arachidonic acid-derived vasoconstrictor prostanoids were the first EDCFs identified by DeMey and Vanhoutte (Vanhoutte and Tang, 2008; Vanhoutte, 2009a; Barton, 2011; Vanhoutte, 2011) shortly after the report of endothelium-dependent dilation (Furchgott and Zawadzki, 1980), demonstrating contractile effects mediated by endothelium-derived cyclooxygenase products (De Mey and Vanhoutte, 1982; Wong and Vanhoutte, 2010). Superoxide anion, a short-lived by-product of oxidative metabolism, was also found to have vasoconstrictor activity again by Vanhoutte's group (Rubanyi and Vanhoutte, 1986) and also by Moncada and associates (Gryglewski et al., 1986). This constrictor effect is largely due to the EDRF/NO-inactivating properties of superoxide anion (Rubanyi and Vanhoutte, 1986). Reactive oxygen species have been studied since the early 1990s and Griendling and coworkers have identified a vascular NADPH oxidase as one of the major sources of vascular reactive oxygen species (Griendling et al., 2000); the nox4 isoenyzme is mainly expressed in endothelial cells (Brandes et al., 2010). Interestingly, EDHF synthase/cytochrome P450 expoxygenase is also a source of superoxide anion (Fleming et al., 2001). In the 1980s, several groups reported the release of a vasoconstrictor substance from cultured endothelial cells (Hickey et al., 1985; Gillespie et al., 1986; O'Brien et al., 1987). Investigators had accidentally detected its peptidergic vasoconstrictor activity in experiments in search of the vasodilator molecule then called EDRF (Rubanyi, 2011 and Dr David M. Pollock, pers. comm.). This ‘EDRF’ was later identified as the gas NO (Ignarro et al., 1987; Furchgott, 1988). The gene and peptide sequence of the vasoconstrictor peptide, named endothelin due to its cellular origin, was ultimately revealed by Masaki's group from Japan and published in 1988 (Yanagisawa et al., 1988; Barton and Yanagisawa, 2008). Subsequently, other members of this peptide family such as endothelin-2 and endothelin-3 were identified (Barton and Yanagisawa, 2008). Through activation of ETA receptors, endothelin-1 (ET-1) causes sustained and potent vasoconstriction and also activates cell proliferation (Barton and Yanagisawa, 2008) and mediates endothelium-dependent contractions via thomboxane A2 (Taddei and Vanhoutte, 1993; Moreau et al., 1996; d'Uscio et al., 1997; Traupe et al., 2002a). As with other vasoconstrictors, NO counterbalances the effects of endothelin (Vanhoutte, 2000). Recently, Yanagisawa's group reported that endothelial cell-derived ET-1 is responsible for the majority of endothelin tissue expression, as endothelial cell-specific prepro-ET-1-deficient mice exhibit a reduction of ET-1 tissue levels in several organs up to 70% compared with wild-type mice (Kisanuki et al., 2010). The hypotension observed in animals with endothelial cell-restricted endothelin deficiency also indicates that the vasoconstrictor activity of endogenous endothelin peptide – via the ETA receptor – outweighs its ETB -mediated dilator activity.
Obesity, insulin resistance and diabetes: vascular inflammtion as key event
In the 21st century, obesity has become the main cause of diabetes and associated diseases. Already in overweight patients, abnormalities found in obese individuals are present, albeit to a lesser degree. As a direct consequence of the disease, obese patients present with enhanced sympathetic drive, increased vasomotor tone and hypertension; they develop metablic abnormalties such as insulin resistance, dyslipidaemia and diabetes, and organ injury such as fatty-inflammatory degeneration of the liver (non-alcoholic steatohepatitis) and structural injury of the kidney through focal–segmental glomerulosclerosis (Abate et al., 2001; Visscher and Seidell, 2001). Moreover, overweight or obese individuals are at a higher risk to develop left ventricular (Russo et al., 2011) and right ventricular (Wong et al., 2006) diastolic dysfunctions, and to develop heart failure due to obesity cardiomyopathy in the course of the disease (Russo et al., 2011; Wong and Marwick, 2007). Because the metabolic impairments in obesity often deteriorate in overt diabetes, prevention of obesity is of paramount importance. Diabetic complications are now recognized as some of the most frequent causes of organ failure due to cardiovacular causes (myocardial infarction and heart failure), cerebral disease (stroke), renal failure/requirement for dialysis or renal transplant therapy (Farag and Gaballa, 2010; Dunlay et al., 2011; Herman, 2011; Ratner and Sathasivam, 2011), or peripheral vascular disease (Skilton et al., 2011). The mechanisms involved in the disease accelleration by obesity and/or diabetes involve various mechanisms (Visscher and Seidell, 2001), with generalized inflammation being the main unifying principle of disease (Wellen and Hotamisligil, 2003; 2005); importantly, these changes are aggravated in women after menopause where cessation of oestrogen production accelerates the development of obesity, diabetes and hypertension (Barton and Meyer, 2009; Meyer et al., 2011). Impairment of glucose and insulin function are central to the metabolic abnormalities found in obesity (Wellen and Hotamisligil, 2003; 2005). They are, however, not only restricted to the endocrine pancreas and skeletal muscle but also directly involve secretion of proteins from fat tissue that are involved in maintaing adipocyte function, and, if abnormally increased, may directly worsen metabolism, inflammation, endothelial cell dysfunction and organ injury (Ouwens et al., 2010; Zhang et al., 2010; Cui et al., 2011). The so-called adipokines (or adipocytokines), for which disease-modifying roles in obesity have been demonstrated, include adiponectin, leptin and ghrelin (Ouwens et al., 2010; Zhang et al., 2010; Cui et al., 2011). For some of these proteins, direct effects on insulin signalling, fat cell growth and inflammation have been demonstrated (reviewed in Ouwens et al., 2010; Zhang et al., 2010; Cui et al., 2011).
Obesity: a trigger of endothelial cell injury and amplifier of cardiovascular risk
Within only a decade, obesity has become one of the most relevant global health issues (McLellan, 2002; Barton and Furrer, 2003), with the associated health costs exploding (Finucane et al., 2011; Heidenreich et al., 2011). Six years ago, 1.6 billion adults worldwide were diagnosed as overweight, and 400 million were obese. Within only another 4 years, the numbers worldwide will have increased to 2.3 billion adults being overweight and 700 million being obese (Stewart et al., 2008; 2009; Malik et al., 2010), representing an alarming 10-year increases of 44 and 75%, respectively. Most recent studies confirm that the body mass index continues to increase on almost all continents (Finucane et al., 2011). The reasons for this development are economic growth in developing countries as well as changes in nutrition patterns, in combination with the availability of inexpensive and unbalanced diets rich in carbohydrates and fat (Bray and Popkin, 1998; McLellan, 2002; Stewart et al., 2008; 2009; Malik et al., 2010). Excess food intake is further aggravated by an unfavourable lifestyle; lacking physical exercise; and consuming high caloric, non-alcoholic and alcoholic drinks (Barton and Furrer, 2003; Malik et al., 2010). Excessive amounts of visceral fat are now recognized as one of the major contributors of the obesity-associated organ injury, and studies in rodents and in monkeys indicate that either removal of visceral fat or caloric restriction can substantially extend lifespan in mammals (Muzumdar et al., 2008; Colman et al., 2009). Obesity, diabetes and aging share a number of the same etiopathologies that contribute to endothelial and vascular injury (Barton, 2010). One of the most worrisome developments is that obesity now increasingly affects school children (Jolliffe, 2004) (Ludwig, 2007) who – at a young age – present with diseases normally found only in adults of higher age, namely arterial hypertension and diabetes mellitus (Barton and Furrer, 2003). Overweight children prematurely develop abnormal endothelial cell function and thickening of the arterial vascular wall (Woo et al., 2004), as well as myocardial wall thickening (de Jonge et al., 2011), features usually observed only in obese adults or aged individuals (Steinberg et al., 1996). Indeed, a most recent paper concluded that obesity induces premature cardiac aging in younger patients (Niemann et al., 2011). This further underscores that obesity actually mimics (and thus accelerates) normal aging in many aspects, also evident from the increased intima-media thickness found in obese young adults (Berni et al., 2011). This once more illustrates the importance to actively intervene and start obesity prevention as early as possible to interfere with its cardiovascular consequences (Barton and Furrer, 2003).
Evidence for endothelium-derived vascoconstriction in obesity
Endothelium-derived vasoconstrictor prostanoids/EDCF
Enhanced vasoconstriction has been observed in patients with obesity (Sivitz et al., 2007), and both cyclooxygenase and endothelin have been implicated in these responses. In mice with diet-induced obesity, formation of endothelial vasoconstrictor prostanoids is enhanced in both aorta and carotid artery (Traupe et al., 2002b) (Figure 1); these contractions are fully blocked by non-selective COX-inhibition or antagonists of thromboxane receptors (Figure 1C), but not COX-2 selective inhibitors (Traupe et al., 2002b). In an elegant study, Tang et al. subsequently demonstrated using COX-1- and COX-2-deficient mice that COX-1 is indeed the sole enzyme mediating prostanoid-mediated EDCF production in mice (Tang et al., 2005). Results from studies in mice on high-fat diet suggest activation of COX-1-dependent vasoconstrictor pathways in obesity and that these pathways contribute to enhanced vasoconstriction also observed in obese humans (Cardillo et al., 2004; Rask-Madsen and King, 2007) (Figures 1 and 2). Similar to obesity, activation of COX-dependent pathways has been reported to occur with aging (Tang and Vanhoutte, 2008), again suggesting common pathways between both physiopathologies. Recent work comparing functional vascular injury due to obesity in youth and adulthood indeed suggests that obesity causes changes compatible with accelerated, ‘premature’ vascular aging with regard to endothelium-dependent, prostanoid-mediated contractlity (Bhattacharya et al., 2008a). In addition to COX-derived EDCFs activating thromboxane receptors, another endothelium-derived arachidonic acid product, prostacylin (which can also act as an EDCF) (Vanhoutte, 2011), has recently been directly implicated in obesity, by determining the fate for development of fat cells from progenitor cells (Ishibashi and Seale, 2010; Vegiopoulos et al., 2010).
Figure 1.
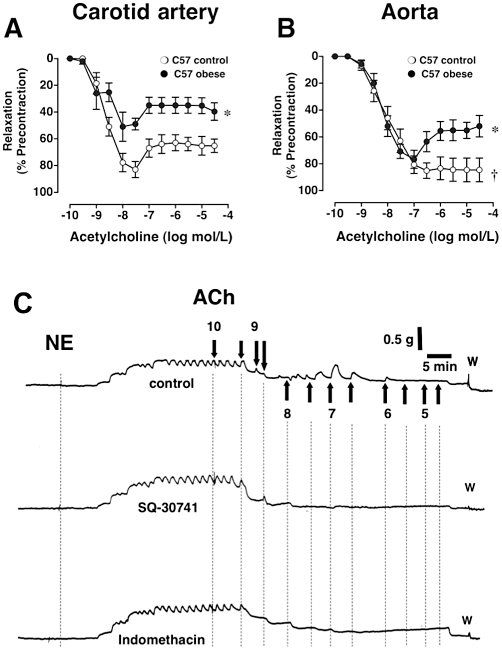
Effect of diet-induced obesity (•) on acetylcholine-mediated, endothelium-dependent vasoreactivity in the carotid artery (A, C) and thoracic aorta (B) of C57 mice. Controls (○) were fed a normal chow diet. In the carotid artery, diet-induced obesity impairs NO-mediated endothelium-dependent relaxation while at the same time enhancing endothelium-dependent contractions in the carotid artery (A). In the aorta, the larger conduit vessel, NO-dependent dilation is preserved during obesity; however, endothelium-dependent contractions now become visible (B). *P < 0.05 vs. C57 control. Panel C shows three original recordings of responses to acetylcholine in norepinephrine-precontracted carotid artery rings from the same obese C57 animal after 30 weeks on high-fat diet in the absence of inhibitors (upper tracing), in the presence of the thromboxane receptor antagonist SQ-30741 (middle tracing) or the cyclooxygenase inhibitor indomethacin (bottom tracing). Transient, endothelium-dependent contraction responses to acetylcholine are visible beginning at concentrations of 30 nmol·L−1 in the untreated carotid artery ring, whereas inhibition of either thromboxane receptors (SQ-30741) or cyclooxygenase (indomethacin) completely abrogates endothelium-dependent contractions. NE indicates norepinephrine, arrows indicate administration of increasing cumulative concentrations of acetylcholine (mol·L−1), ‘w’ indicates wash-out. Figure panels A an B are adapted from Traupe et al., 2002b and reproduced with permission of the publisher.
Figure 2.
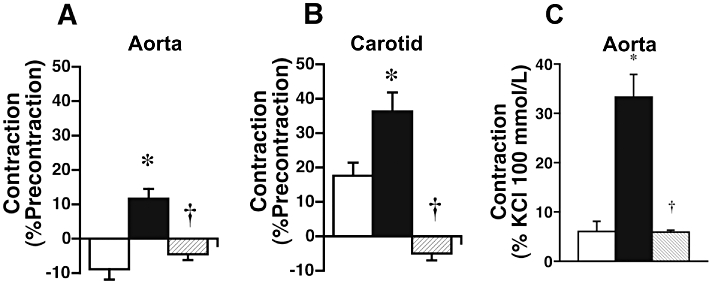
Effect of 30 weeks of diet-induced obesity in placebo-treated ( ) or endothelin ETA-receptor antagonist-treated (
) or endothelin ETA-receptor antagonist-treated ( ) C57 mice on endothelium-dependent contractions to acetylcholine (30 µmol·L−1) in NO-depleted vascular rings of aorta (A) and carotid artery (B). Contractions to angiotensin II in the aorta are depicted on the right (C). Depletion of endothelium-derived NO was achieved by acute treatment with L-NAME (300 µmol·L−1), a non-selective inhibitor of NO synthases. In NO-depleted arteries of control animals on chow diet (□), EDCF were only present in the carotid artery. In mice with diet-induced obesity (
) C57 mice on endothelium-dependent contractions to acetylcholine (30 µmol·L−1) in NO-depleted vascular rings of aorta (A) and carotid artery (B). Contractions to angiotensin II in the aorta are depicted on the right (C). Depletion of endothelium-derived NO was achieved by acute treatment with L-NAME (300 µmol·L−1), a non-selective inhibitor of NO synthases. In NO-depleted arteries of control animals on chow diet (□), EDCF were only present in the carotid artery. In mice with diet-induced obesity ( ), the residual relaxation to acetylcholine is converted into a contraction in the aorta, and the magnitude of EDCF-mediated contractions was doubled in the carotid artery. Chronic treatment with the orally active endothelin ETA receptor antagonist darusentan (LU135252) (
), the residual relaxation to acetylcholine is converted into a contraction in the aorta, and the magnitude of EDCF-mediated contractions was doubled in the carotid artery. Chronic treatment with the orally active endothelin ETA receptor antagonist darusentan (LU135252) ( ) – without affecting body weight – not only completely prevented enhanced EDCF-mediated contractions, but also caused acetylcholine to elicit a small relaxation instead (B). Similarly, in NO-depleted aortic rings, contractions to angiotensin II (0.1 µmol·L−1) were markedly enhanced by obesity (
) – without affecting body weight – not only completely prevented enhanced EDCF-mediated contractions, but also caused acetylcholine to elicit a small relaxation instead (B). Similarly, in NO-depleted aortic rings, contractions to angiotensin II (0.1 µmol·L−1) were markedly enhanced by obesity ( ), an effect again completely abrogated after chronic endothelin receptor antagonist treatment which had no effect on obesity (
), an effect again completely abrogated after chronic endothelin receptor antagonist treatment which had no effect on obesity ( ). *P < 0.05 versus control; †P < 0.05 versus obesity. Panels A and B: This research was originally published in Clinical Science. Traupe et al., 2002a. © Portland Press Limited. Panel C is from Barton et al., 2000b, and reproduced with permission of the American Heart Association and the publisher.
). *P < 0.05 versus control; †P < 0.05 versus obesity. Panels A and B: This research was originally published in Clinical Science. Traupe et al., 2002a. © Portland Press Limited. Panel C is from Barton et al., 2000b, and reproduced with permission of the American Heart Association and the publisher.
Endothelium-derived angiotensin II
Obesity activates the renin-angiotensin-aldosterone system (RAAS) (reviewed in Barton et al., 2003a). Similar to what occurs during aging (Barton et al., 1997), obesity does not equally affect all vascular beds to the same degree. In the C57 mouse model of diet-induced obesity (Surwit et al., 1988), contractions to angiotensin II markedly increase only in the aorta (Figure 3C, filled bar) but not in the carotid artery (Barton et al., 2000b). Chronic treatment with an orally active endothelin ETA receptor antagonist (darusentan) completely abrogated the increased contractility (Barton et al., 2000b) (Figure 3C, hatched bar), indicating a molecular interaction between these two vasoactive systems and their cellular targets. These effects were independent of body weight and arterial blood pressure, compatible with the notion that endogenous endothelin becomes activated during obesity and that endothelin – at least partially – contributes to angiotensin-mediated vasoconstriction in certain vascular beds. Contractility to angiotensin in this model was also blocked by cyclooxygenase inhibition in vitro to a large degree, suggesting that – unlike in other species – in the mouse vasculature, endothelial EDCFs formed from vasoconstrictor prostanoids largely contribute to responses elicited by other vasoconstrictors (Barton et al., 2000b). This effect appears to develop with age (Kretz et al., 2006). Obesity also increases protein expression of the main cellular target of angiotensin II, the AT1 receptor, which is up-regulated only if the diet contained high amounts of fat (Mundy et al., 2007b).
Figure 3.
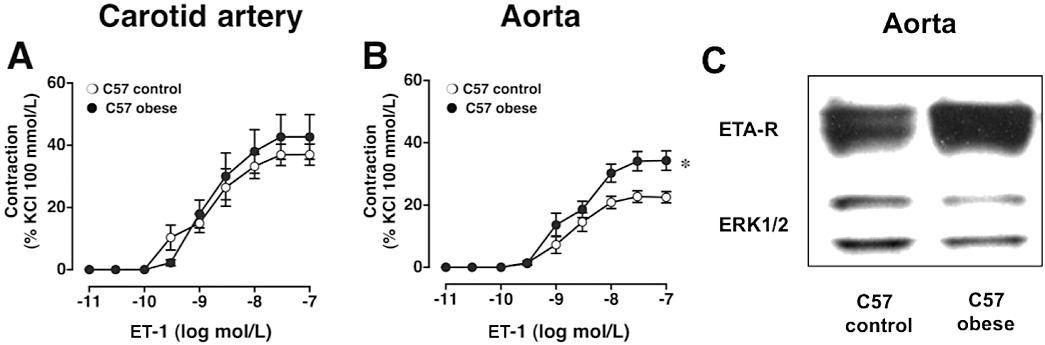
Anatomic heterogeneity of ET-1 mediated vascular contractility in C57 mice. The magnitude of contractions to ET-1 in the carotid artery (A) was twice that of the aorta (B), yet diet-induced obesity augmented ET-1-induced contractions only in aorta (B) but not the carotid artery (A). Western blot experiments of aortic expression of ETA receptor; total ERK1/2 protein was used as loading control. Diet-induced obesity substantially increases aortic endothelin ETA receptor expression, whereas ERK1/2 protein remains unafffected (C). *P < 0.05 versus control. Figure panels are in part adapted from Traupe et al., 2002b (Panels A and B) and Mundy et al., 2007b; 73:368–375 (Panel C). Figures are reproduced with permission of the publishers.
Endothelium-derived ET-1
As recently shown by Yanagisawa and co-workers, endothelium-derived endothelin contributes to the majority of endothelin found in different organs and in plasma, and also is important for maintaining basal blood pressure (Kisanuki et al., 2010). Endothelin production not only is regulated by angiotensin II both in vitro and in vivo (reviewed in Lüscher and Barton, 2000), but diet-induced obesity also up-regulates renal (but not pulmonary) ACE activity in an ETA receptor-dependent manner (Barton et al., 2000b). Again, this suggests that both the RAAS and the endothelin system interact with each other in a positive feedback loop (Barton et al., 2000b). The data also indicate – that under certain conditions such as obesity – endothelin receptor antagonists exert ACE inhibitor-like activity (Barton et al., 2000a). One of the most important factors responsible for the high prevalence of obesity is an increased intake of high-calorie food rich in carbohydrates and fat (Bhattacharya et al., 2008a). Several experimental models of diet-induced obesity are available (Surwit et al., 1988; Tschop and Heiman, 2001; Collins et al., 2004) in which changes in the vasculature and kidney have been studied. Experimental studies suggest that these animal models exhibit many of the changes seen with obesity in humans, including inflammation, dyslipidaemia and abnormalities of vasomotor tone (Surwit et al., 1988; Tschop and Heiman, 2001; Traupe et al., 2002b; Collins et al., 2004). Indeed, like in humans (Cardillo et al., 2004), vascular responses to endothelin are enhanced in both models of diet-induced (Figure 3) and monogenetic leptin-deficient obesity (Traupe et al., 2002a,b; Mundy et al., 2007a,b; Bhattacharya et al., 2008b). Importantly, the susceptibility to the obesity-enhanced responsivness to ET-1 varies between vascular beds (Figure 3), an anatomic heterogeneity that could also be of relevance for the arterial circulation of obese humans. In addition to its vasoconstrictor function ET-1 is a potent pro-atherogenic peptide (Barton et al., 2003b), which likely plays a role in the increased vascular risk seen with obesity (Barton, 2010). Indeed, with obesity vascular ET-1 expression increases at the mRNA level in the vasculature (Traupe et al., 2002b) and at the peptide level in the kidney (Barton et al., 2000b). Obesity-induced increases of vascular protein levels of the main target of ET-1, the ETA receptor (Mundy et al., 2007b), have also been reported (Figure 3C). Thus, the experimental studies provide some mechanistic explanation (Figure 2) why diet-induced obesity exerts specific changes promoting enhanced vasoconstriction similar to what can be seen in obese humans with regard to an activated endothelin pathway (Cardillo et al., 2004). Clinical studies using endothelin receptor antagonists (ERAs) also indicate beneficial metabolic effects (Shemyakin et al., 2006; 2010; Ahlborg et al., 2007) and suggest possible therapeutic potential for endothelin receptor antagonists in patients with obesity (Barton et al., 2003a). Recent studies also suggest therapeutic potential for obesity-related renal complications such as proteinuria (Barton, 2008; Thoenes et al., 2009). In fact, three clinical studies in obese patients with kidney disease (Weber et al., 2009; Kohan et al., 2010; 2011; Mann et al., 2010) have been recently published, showing a reversal of functional renal injury after endothelin blockade.
Endothelium-derived free radicals and inactivation of NO
Several studies in experimental animals and humans have shown that in obesity, the bioactivity of NO is reduced (Bender et al., 2007; Rask-Madsen and King, 2007; Bourgoin et al., 2008; Damjanovic and Barton, 2008). The mechanistic concept that has been mostly propagated is the inactivation of NO by superoxide anion (O2-), leading to formation of peroxynitrite. The source of increased O2-production is not only enzymes such as NADPH oxidase, but also uncoupled NO synthase (Forstermann and Munzel, 2006; Martins et al., 2010). Increased nitrotyrosine formation as a consequence of peroxynitrite production has been described in obese animal models (Brodsky et al., 2004; Galili et al., 2007; Bourgoin et al., 2008). More recently, other pathways such as guanylate cyclase, the intracellular target of NO, have also been shown to be affected by obesity and have been directly linked to inflammation (Rizzo et al., 2010). Due to the fact that NO is formed by the multi-enzyme complex NO synthase (Förstermann et al., 1994), which concomitantly produces reactive oxygen species such as superoxide anion through its NADPH oxidase domain, increasing NO bioactivity has been complicated by NO synthase uncoupling (Wever et al., 1997; Stroes et al., 1998; Landmesser et al., 2003). As the reaction between NO and superoxide anion is essentially diffusion limited, substantial amounts of peroxynitrite (ONOO-) are formed (Barton, 2010). ONOO- causes cell injury through the nitrosylation of proteins which partially or completely inactivates them (Abello et al., 2009). Nitrosylation of proteins, which will cause relatively stable nitrotyrosine to be formed, will change the function, structure, and thus the ability of these proteins to interact with other proteins (Musci et al., 2006). In addition to superoxide anion and peroxynitrite, vascular formation and activity of other oxygen derived radicals are altered in obesity. In lean control mice and mice with monogenetic obesity (Mundy et al., 2007a), ET-1 stimulates hydroxyl radical formation, an effect that is more or less abolished by obesity (Mundy et al., 2007a). However, relaxant responses to hydroxyl radical are enhanced in animals with monogenetic obesity (Mundy et al., 2007a). Similar observations were made in models of diet-induced obesity, where vascular responses to hydroxyl radical changed from contraction in lean animals into relaxation upon obesity induction, again effects being specific to certain vascular beds (Bhattacharya et al., 2008b).
Endothelium-derived peptides neuropeptide y and atrial natriuretic peptides
Neuropeptide Y, a centrally acting peptide involved in appetite regulation (Achike et al., 2011; Kim et al., 2010), has been recently shown to stimulate adipogenesis (Baker et al., 2009). Neuropeptide Y binds to endothelial cells, causes NO-dependent dilation, stimulates endothelial cell growth and affects endothelial cell macromolecule permeability (Sanabria and Silva, 1994; Noll et al., 1996; Marion-Audibert et al., 2000; Nilsson et al., 2000). Although a role for neuropeptide Y in adipogenesis and endothelial cell function – including enhanced thromboxane/EDCF-mediated vascular tone (Fabi et al., 1998) – has been demontrated, no data showing its involvement in obesity-associated vascular dysfunction have been published. Another group of peptides are the atrial natriuretic peptides ANP, BNP, and CNP, which have been recently implicated in obesity and lipid mobilization (Bartels et al., 2010; Chen-Tournoux et al., 2010; Koppo et al., 2010; Saritas et al., 2010). Atrial natriuretic peptides are also formed and metabolized by endothelial cells (Johnson et al., 1990; Lew and Baertschi, 1992; Sugiyama et al., 1995; Yamada and Yokota, 1996). CNP causes endothelium-independent and endothelium-dependent relaxation (Evans et al., 1993; Barton et al., 1998; Chauhan et al., 2003; Villar et al., 2007; Leuranguer et al., 2008; Liang et al., 2010), yet direct effects of obesity on endothelial-cell dependent responses or activities of atrial natriuretic peptides have not been reported.
Therapeutic interventions for patients with obesity: ‘endothelial therapy’
A decade ago, we coined the concept of ‘endothelial therapy’ as a means to preserve and/or improve vascular function by reducing production of deleterious endothelium-derived mediators in order to attenuate atherosclerosis progression (Barton and Haudenschild, 2001). Generally, either increasing cellular antioxidant capacity or reducing oxidative stress will have similar beneficial effects on the vasculature. Beneficial effects of interventions to reduce oxidative stress and inflammation (Figure 4) have been shown, among others, for diseases such as atherosclerosis, myocardial infarction, stroke, peripheral vascular disease, arterial hypertension, chronic renal failure, pulmonary arterial hypertension (Vanhoutte et al., 2009), and for a number of disease conditions mainly associated with chronic inflammation such as connective tissue diseases and metabolic conditions such as insulin resistance and diabetes (Libby, 2005; Rocha and Libby, 2008; 2009; Agouni et al., 2009). A number of modalities are available to interfere with obesity-related changes in endothelial cell function (Jensen-Urstad et al., 1999). Preventive measures, which must be applied already to children and adolescents, should include maintaining normal body weight (or weight reduction, if required) and avoiding unbalanced diets rich in fat and sugars and low in fibres (Chen et al., 2010). Equally important appears to be the ‘therapeutic’ role of regular physical activity, which reduces the incidence and prevalence of the obesity-related co-morbidities diabetes, hypertension, dyslipidaemia and depression (Colditz, 1999; O'Brien and Dixon, 2002; Barton, 2010). Regular intense exercise in humans has beneficial effects on cardiovascular health showing a dramatic risk reduction (Manson et al., 2002), which appears to be maintained even in the presence of obesity. Similarly, weight loss has been shown to improve the vascular risk profile, including a reduction of aortic pulse wave velocity (Rider et al., 2010). In humans, endothelium-dependent vasoreactivity can be preserved by exercise even at a high age (Jensen-Urstad et al., 1999). Obesity is highly prevalent among elderly individuals (Bramlage et al., 2004), as is arterial hypertension, dyslipidemia and atherosclerosis (Barton and Furrer, 2003; Bramlage et al., 2004). Unfortunately, these conditions are no longer restricted to elderly individuals but already present to a considerable degree in children (Barton and Furrer, 2003; Ludwig, 2007). It will thus require immediate action and intervention to avoid future disease in adulthood. This is of particular importance in view of the fact that childhood obesity – even if normal body weight is achieved later in life – has been linked to an increased likelihood of adult coronary artery disease (Baker et al., 2007; Bibbins-Domingo et al., 2007; Ludwig, 2007).
Figure 4.
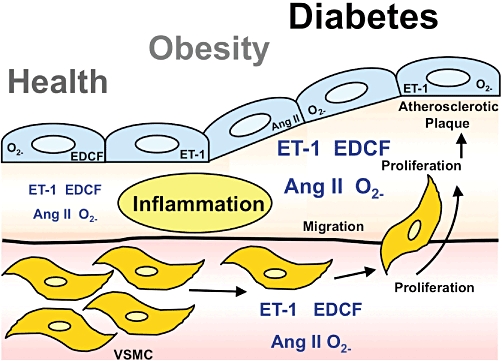
Role of endothelium-derived vasoconstrictors for atherogenesis. Shown are levels and localization of the endothelial vasoconstrictors and growth factors ET-1, prostanoid endothelium-derived vasoconstricting factor/thromboxane A2/prostaglandin H2 (EDCF), angiotensin II (Ang II) and supoxide anion (O2−) in health (left), obesity/pre-diabetes (middle) and overt diabetes (right). With prolonged exposure to moderate metabolic risk (obesity) or severe metabolic risk (diabetes) involving inflammatory activation, production of endothelium-derived vasocontrictors in endothelial cells (blue), intima and subintimal space (orange), and media with its vascular smooth muscle cells (pink/yellow) increases and stimulates to vasoconstriction, cell proliferation and atherosclerotic plaque formation. Part of the figure was adapted from Barton et al., 2007 and reproduced with permission of the publishers. VSMC, vascular smooth muscle cells.
Perspective and implications for therapeutic interventions
It is currently unclear if and how certain drugs, which specifically target obesity and despite drug-related improvements in lipid profile and vascular function, can affect overall morbidity, life expectancy, and quality of life and well-being. One of several unsuccessful recent drug candidates is the cannabinoid antagonist rimonabant, for which clinical trials have been recently terminated due to serious health risks (Kwatra, 2010; Roberfroid et al., 2010; Topol et al., 2010). Whether or not drug therapy can provide the solution to reduce the risk related to obesity (a complex neurophysiological problem with metabolic and physical consequences) remains yet to be shown. However, the underuse of free and readily available, non-pharmacological (i.e. physical) interventions clearly require dramatic behavioural changes to reduce body weight and improve physical fitness and health around the world. Recent studies unfortunately show that the trend towards increases of obesity prevalence continues around the world (Finucane et al., 2011; Heidenreich et al., 2011). Should interventions fail, it appears likely that – for the first time and regardless of all pharmaceutical advances made – mankind could experience a decline in the overall longevity (Olshansky et al., 2005; Stewart et al., 2009) that has increased continously since the beginning of time. Therefore, the preventive power of ‘endothelial therapy’ will hopefully be recognized and put to work where needed.
Acknowledgments
This work was supported by the Swiss National Science Foundation (3200–108258, K33KO-122504 and PBZHP3-135874).
Glossary
- COX
cyclooxygenase
- EDCF
endothelium-derived contracting factor
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- ET-1
endothelin-1
- ETA
endothelin subtype A receptor
- L-NAME
L-nitro arginine methyl ester
- LU135252
ETA-selective endothelin antagonist, darusentan
- NADPH
nicotineamide adenine dinucleotide phosphate
- NO
nitric oxide
- O2-
superoxide anion
- ONOO-
peroxynitrite
Conflict of Interest
There are no conflicts of interest involved for any of the authors.
References
- Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, et al. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001;38:379–383. doi: 10.1161/01.hyp.38.3.379. [DOI] [PubMed] [Google Scholar]
- Abello N, Kerstjens HA, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J Proteome Res. 2009;8:3222–3238. doi: 10.1021/pr900039c. [DOI] [PubMed] [Google Scholar]
- Achike FI, To NH, Wang H, Kwan CY. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38:1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- Agouni A, Lagrue-Lak-Hal AH, Mostefai HA, Tesse A, Mulder P, Rouet P, et al. Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in Zucker fatty rats (Fa/Fa) PLoS ONE. 2009;4:e5557. doi: 10.1371/journal.pone.0005557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlborg G, Shemyakin A, Bohm F, Gonon A, Pernow J. Dual endothelin receptor blockade acutely improves insulin sensitivity in obese patients with insulin resistance and coronary artery disease. Diabetes Care. 2007;30:591–596. doi: 10.2337/dc06-1978. [DOI] [PubMed] [Google Scholar]
- Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SB, Cohen M, Kuo L, Johnson M, Al-Attar A, Zukowska Z. The role of the neuropeptide Y2 receptor in liporemodeling: neuropeptide Y-mediated adipogenesis and adipose graft maintenance. Plast Reconstr Surg. 2009;123:486–492. doi: 10.1097/PRS.0b013e3181954c80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels ED, Nielsen JM, Bisgaard LS, Goetze JP, Nielsen LB. Decreased expression of natriuretic peptides associated with lipid accumulation in cardiac ventricle of obese mice. Endocrinology. 2010;151:5218–5225. doi: 10.1210/en.2010-0355. [DOI] [PubMed] [Google Scholar]
- Barton M. Endothelin in the microvasculature. In: Shepro D, editor. Microvascular Research: Biology and Pathology. San Diego, CA: Elsevier Academic Press; 2006. pp. 5–12. [Google Scholar]
- Barton M. Reversal of proteinuric renal disease and the emerging role of endothelin. Nat Clin Pract Nephrol. 2008;4:490–501. doi: 10.1038/ncpneph0891. [DOI] [PubMed] [Google Scholar]
- Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflugers Arch. 2010;460:825–837. doi: 10.1007/s00424-010-0860-y. [DOI] [PubMed] [Google Scholar]
- Barton M. The discovery of endothelium-dependent contraction: the legacy of Paul M. Vanhoutte. Pharmacol Res. 2011;63:455–462. doi: 10.1016/j.phrs.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Barton M, Haudenschild C. Endothelium and atherogenesis: endothelial therapy revisited. J Cardiovasc Pharmacol. 2001;38(Suppl. 2):S23–S25. doi: 10.1097/00005344-200111002-00007. [DOI] [PubMed] [Google Scholar]
- Barton M, Furrer J. Cardiovascular consequences of the obesity pandemic: need for action. Expert Opin Investig Drugs. 2003;12:1757–1759. doi: 10.1517/13543784.12.11.1757. [DOI] [PubMed] [Google Scholar]
- Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Lüscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- Barton M, Beny JL, d'Uscio LV, Wyss T, Noll G, Luscher TF. Endothelium-independent relaxation and hyperpolarization to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol. 1998;31:377–383. doi: 10.1097/00005344-199803000-00008. [DOI] [PubMed] [Google Scholar]
- Barton M, Carmona R, Krieger JE, Goettsch W, Morawietz H, d'Uscio LV, et al. Endothelin regulates angiotensin-converting enzyme in the mouse kidney. J Cardiovasc Pharmacol. 2000a;36(Suppl. 1):S244–S247. doi: 10.1097/00005344-200036051-00072. [DOI] [PubMed] [Google Scholar]
- Barton M, Carmona R, Morawietz H, d'Uscio LV, Goettsch W, Hillen H, et al. Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension. 2000b;35:329–336. doi: 10.1161/01.hyp.35.1.329. [DOI] [PubMed] [Google Scholar]
- Barton M, Carmona R, Ortmann J, Krieger JE, Traupe T. Obesity-associated activation of angiotensin and endothelin in the cardiovascular system. Int J Biochem Cell Biol. 2003a;35:826–837. doi: 10.1016/s1357-2725(02)00307-2. [DOI] [PubMed] [Google Scholar]
- Barton M, Traupe T, Haudenschild CC. Endothelin, hypercholesterolemia and atherosclerosis. Coron Artery Dis. 2003b;14:477–490. doi: 10.1097/00019501-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Barton M, Minotti R, Haas E. Inflammation and atherosclerosis. Circ Res. 2007;104:288–291. doi: 10.1161/CIRCRESAHA.107.162487. [DOI] [PubMed] [Google Scholar]
- Bender SB, Herrick EK, Lott ND, Klabunde RE. Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes Metab. 2007;9:688–696. doi: 10.1111/j.1463-1326.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- Berni A, Giuliani A, Tartaglia F, Tromba L, Sgueglia M, Blasi S, et al. Effect of vascular risk factors on increase in carotid and femoral intima-media thickness. Identification of a risk scale. Atherosclerosis. 2011;216:109–114. doi: 10.1016/j.atherosclerosis.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Bhattacharya I, Damjanovic M, Gut A, Hager S, Perez-Dominguez A, Minotti R, et al. Childhood obesity induced by a high-fat diet causes premature vascular aging involving endothelium-dependent mechanisms. Hypertension. 2008a;52:e89. [Google Scholar]
- Bhattacharya I, Mundy AL, Widmer CC, Kretz M, Barton M. Regional heterogeneity of functional changes in conduit arteries after high-fat diet. Obesity (Silver Spring) 2008b;16:743–748. doi: 10.1038/oby.2007.111. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- Bourgoin F, Bachelard H, Badeau M, Melancon S, Pitre M, Lariviere R, et al. Endothelial and vascular dysfunctions and insulin resistance in rats fed a high-fat, high-sucrose diet. Am J Physiol Heart Circ Physiol. 2008;295:H1044–H1055. doi: 10.1152/ajpheart.00516.2008. [DOI] [PubMed] [Google Scholar]
- Bramlage P, Wittchen HU, Pittrow D, Kirch W, Krause P, Lehnert H, et al. Recognition and management of overweight and obesity in primary care in Germany. Int J Obes Relat Metab Disord. 2004;28:1299–1308. doi: 10.1038/sj.ijo.0802752. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res. 2004;94:377–384. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Campia U, Iantorno M, Panza JA. Enhanced vascular activity of endogenous endothelin-1 in obese hypertensive patients. Hypertension. 2004;43:36–40. doi: 10.1161/01.HYP.0000103868.45064.81. [DOI] [PubMed] [Google Scholar]
- Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Caballero B, Mitchell DC, Loria C, Lin PH, Champagne CM, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation. 2010;121:2398–2406. doi: 10.1161/CIRCULATIONAHA.109.911164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Tournoux A, Khan AM, Baggish AL, Castro VM, Semigran MJ, McCabe EL, et al. Effect of weight loss after weight loss surgery on plasma N-terminal pro-B-type natriuretic peptide levels. Am J Cardiol. 2010;106:1450–1455. doi: 10.1016/j.amjcard.2010.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31(Suppl.):S663–S667. doi: 10.1097/00005768-199911001-00026. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer A. Scale and diversity of interaction at the vascular endothelium. In: Cryer A, editor. Biochemical Interactions at the Endothelium. Amsterdam: Elsevier; 1983. pp. 1–3. [Google Scholar]
- Cui J, Panse S, Falkner B. The role of adiponectin in metabolic and vascular disease: a review. Clin Nephrol. 2011;75:26–33. [PubMed] [Google Scholar]
- Damjanovic M, Barton M. Fat intake and cardiovascular response. Curr Hypertens Rep. 2008;10:25–31. doi: 10.1007/s11906-008-0007-0. [DOI] [PubMed] [Google Scholar]
- De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982;51:439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DH, Toop T, Donald J, Forrest JN., Jr C-type natriuretic peptides are potent dilators of shark vascular smooth muscle. J Exp Zool. 1993;265:84–87. doi: 10.1002/jez.1402650112. [DOI] [PubMed] [Google Scholar]
- Fabi F, Argiolas L, Ruvolo G, del Basso P. Neuropeptide Y-induced potentiation of noradrenergic vasoconstriction in the human saphenous vein: involvement of endothelium generated thromboxane. Br J Pharmacol. 1998;124:101–110. doi: 10.1038/sj.bjp.0701808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2010;26:28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, et al. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Furchgott RF. Studies on relaxation of rabbit aorta by sodium nitrite: the basis for the proposal that acid-activable inhibitory factor from bovine retractor penis is inorganic nitrite and the endothelium-derived relaxing factor is nitric oxide. In: Vanhoutte PM, editor. Vasodilation: Vascular Smooth Muscle, Peptides, Autonomic Nerves and Endothelium. New York, NY: Raven Press; 1988. pp. 401–414. [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, et al. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- Gillespie MN, Owasoyo JO, McMurtry IF, O'Brien RF. Sustained coronary vasoconstriction provoked by a peptidergic substance released from endothelial cells in culture. J Pharmacol Exp Ther. 1986;236:339–343. [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Herman WH. The economics of diabetes prevention. Med Clin North Am. 2011;95:373–384. doi: 10.1016/j.mcna.2010.11.010. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248((Pt 1)):C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Urstad K, Bouvier F, Jensen-Urstad M. Preserved vascular reactivity in elderly male athletes. Scand J Med Sci Sports. 1999;9:88–91. doi: 10.1111/j.1600-0838.1999.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Arik L, Pitts BJ, Foster CJ. Rapid receptor-mediated catabolism of 125I-atrial natriuretic factor by vascular endothelial cells. Biochem J. 1990;268:771–776. doi: 10.1042/bj2680771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe D. Extent of overweight among US children and adolescents from 1971 to 2000. Int J Obes Relat Metab Disord. 2004;28:4–9. doi: 10.1038/sj.ijo.0802421. [DOI] [PubMed] [Google Scholar]
- de Jonge LL, van Osch-Gevers L, Willemsen SP, Steegers EA, Hofman A, Helbing WA, et al. Growth, obesity, and cardiac structures in early childhood: the generation R study. Hypertension. 2011;57:934–940. doi: 10.1161/HYPERTENSIONAHA.110.163303. [DOI] [PubMed] [Google Scholar]
- Kim DH, Woods SC, Seeley RJ. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes. 2010;59:907–915. doi: 10.2337/db09-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Emoto N, Ohuchi T, Widyantoro B, Yagi K, Nakayama K, et al. Low blood pressure in endothelial cell-specific endothelin 1 knockout mice. Hypertension. 2010;56:121–128. doi: 10.1161/HYPERTENSIONAHA.109.138701. [DOI] [PubMed] [Google Scholar]
- Kohan DE, Molitch ME, Pritchett Y, Audhya U, Wen S, Yan B. Low dose atrasentan safely reduces albuminuria in subjects with type 2 diabetic nephropathy (EN) on renin-angiotensin system (RAS) inhibitors. J Am Soc Nephrol. 2010;21:41A. doi: 10.1681/ASN.2010080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, et al. Addition of atrasentan to Renin-Angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22:763–772. doi: 10.1681/ASN.2010080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppo K, Larrouy D, Marques MA, Berlan M, Bajzova M, Polak J, et al. Lipid mobilization in subcutaneous adipose tissue during exercise in lean and obese humans. Roles of insulin and natriuretic peptides. Am J Physiol Endocrinol Metab. 2010;299:E258–E265. doi: 10.1152/ajpendo.00767.2009. [DOI] [PubMed] [Google Scholar]
- Kretz M, Mundy AL, Widmer CC, Barton M. Early aging and anatomic heterogeneity determine cyclooxygenase-mediated vasoconstriction to angiotensin II in mice. J Cardiovasc Pharmacol. 2006;48:30–33. doi: 10.1097/01.fjc.0000242061.18981.d3. [DOI] [PubMed] [Google Scholar]
- Kwatra SG. Termination of the CRESCENDO trial. Lancet. 2010;376:1984. doi: 10.1016/S0140-6736(10)62255-6. author reply 1984–1985. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuranguer V, Vanhoutte PM, Verbeuren T, Feletou M. C-type natriuretic peptide and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br J Pharmacol. 2008;153:57–65. doi: 10.1038/sj.bjp.0707476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RA, Baertschi AJ. Endothelium-dependent ANF secretion in vitro. Am J Physiol. 1992;263((Pt 2)):H1071–H1077. doi: 10.1152/ajpheart.1992.263.4.H1071. [DOI] [PubMed] [Google Scholar]
- Li FY, Cheng KK, Lam KS, Vanhoutte PM, Xu A. Cross-talk between adipose tissue and vasculature: role of adiponectin. Acta Physiol (Oxf) 2010 doi: 10.1111/j.1748-1716.2010.02216.x. doi: 10.1111/j.1748-1716.2010.02216.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Liang CF, Au AL, Leung SW, Ng KF, Feletou M, Kwan YW, et al. Endothelium-derived nitric oxide inhibits the relaxation of the porcine coronary artery to natriuretic peptides by desensitizing big conductance calcium-activated potassium channels of vascular smooth muscle. J Pharmacol Exp Ther. 2010;334:223–231. doi: 10.1124/jpet.110.166652. [DOI] [PubMed] [Google Scholar]
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–1228. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. Childhood obesity–the shape of things to come. N Engl J Med. 2007;357:2325–2327. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20(Suppl. 2):II-3-10. [PubMed] [Google Scholar]
- Lüscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- McLellan F. Obesity rising to alarming levels around the world. Lancet. 2002;359:1412. doi: 10.1016/S0140-6736(02)08397-6. [DOI] [PubMed] [Google Scholar]
- Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–724. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- Marion-Audibert AM, Nejjari M, Pourreyron C, Anderson W, Gouysse G, Jacquier MF, et al. [Effects of endocrine peptides on proliferation, migration and differentiation of human endothelial cells] Gastroenterol Clin Biol. 2000;24:644–648. [PubMed] [Google Scholar]
- Martins MA, Catta-Preta M, Mandarim-de-Lacerda CA, Aguila MB, Brunini TC, Mendes-Ribeiro AC. High fat diets modulate nitric oxide biosynthesis and antioxidant defence in red blood cells from C57BL/6 mice. Arch Biochem Biophys. 2010;499:56–61. doi: 10.1016/j.abb.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2010.02237.x. doi: 10.1111/j.1748-1716.2010.02237.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Takase H, Luscher TF. Effect of endothelin antagonists on the responses to prostanoid endothelium-derived contracting factor. Br J Pharmacol. 1996;118:1429–1432. doi: 10.1111/j.1476-5381.1996.tb15556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Baumann K, et al. Endothelin stimulates vascular hydroxyl radical formation: effect of obesity. Am J Physiol Regul Integr Comp Physiol. 2007a;293:R2218–R2224. doi: 10.1152/ajpregu.00295.2007. [DOI] [PubMed] [Google Scholar]
- Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, et al. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res. 2007b;73:368–375. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Musci G, Persichini T, Casadei M, Mazzone V, Venturini G, Polticelli F, et al. Nitrosative/oxidative modifications and ageing. Mech Ageing Dev. 2006;127:544–551. doi: 10.1016/j.mad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann B, Chen Y, Teschner M, Li L, Silber RE, Rohrbach S. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J Am Coll Cardiol. 2011;57:577–585. doi: 10.1016/j.jacc.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Nilius B, Serban DN, Vanhoutte PM. Robert F. Furchgott and his heritage: endothelial vasomotor control. Pflugers Arch. 2010;459:785–786. doi: 10.1007/s00424-010-0813-5. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Lind H, Brunkvall J, Edvinsson L. Vasodilation in human subcutaneous arteries induced by neuropeptide Y is mediated by neuropeptide Y Y1 receptors and is nitric oxide dependent. Can J Physiol Pharmacol. 2000;78:251–255. [PubMed] [Google Scholar]
- Noll T, Hempel A, Piper HM. Neuropeptide Y reduces macromolecule permeability of coronary endothelial monolayers. Am J Physiol. 1996;271((Pt 2)):H1878–H1883. doi: 10.1152/ajpheart.1996.271.5.H1878. [DOI] [PubMed] [Google Scholar]
- O'Brien PE, Dixon JB. The extent of the problem of obesity. Am J Surg. 2002;184:4S–8S. doi: 10.1016/s0002-9610(02)01172-8. [DOI] [PubMed] [Google Scholar]
- O'Brien RF, Robbins RJ, McMurtry IF. Endothelial cells in culture produce a vasoconstrictor substance. J Cell Physiol. 1987;132:263–270. doi: 10.1002/jcp.1041320210. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- Ratner RE, Sathasivam A. Treatment recommendations for prediabetes. Med Clin North Am. 2011;95:385–395. doi: 10.1016/j.mcna.2010.11.007. viii-ix. [DOI] [PubMed] [Google Scholar]
- Rider OJ, Tayal U, Francis JM, Ali MK, Robinson MR, Byrne JP, et al. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 2010;18:2311–2316. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- Rizzo NO, Maloney E, Pham M, Luttrell I, Wessells H, Tateya S, et al. Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding. Arterioscler Thromb Vasc Biol. 2010;30:758–765. doi: 10.1161/ATVBAHA.109.199893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid D, Lachat C, Lucet C. Termination of the CRESCENDO trial. Lancet. 2010;376:1983–1984. doi: 10.1016/S0140-6736(10)62254-4. author reply 1984–1985. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Libby P. The multiple facets of the fat tissue. Thyroid. 2008;18:175–183. doi: 10.1089/thy.2007.0296. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM. The discovery of endothelin: the power of bioassay and the role of serendipity in the discovery of endothelium-derived vasocative substances. Pharmacol Res. 2011;63:448–454. doi: 10.1016/j.phrs.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Effect of obesity and overweight on left ventricular diastolic function a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria P, Silva WI. Specific 125I neuropeptide Y binding to intact cultured bovine adrenal medulla capillary endothelial cells. Microcirculation. 1994;1:267–273. doi: 10.3109/10739689409146753. [DOI] [PubMed] [Google Scholar]
- Saritas T, Tascilar E, Abaci A, Yozgat Y, Dogan M, Dundaroz R, et al. Importance of plasma N-terminal pro B-type natriuretic peptide, epicardial adipose tissue, and carotid intima-media thicknesses in asymptomatic obese children. Pediatr Cardiol. 2010;31:792–799. doi: 10.1007/s00246-010-9705-x. [DOI] [PubMed] [Google Scholar]
- Shemyakin A, Bohm F, Wagner H, Efendic S, Bavenholm P, Pernow J. Enhanced endothelium-dependent vasodilatation by dual endothelin receptor blockade in individuals with insulin resistance. J Cardiovasc Pharmacol. 2006;47:385–390. doi: 10.1097/01.fjc.0000210070.47205.16. [DOI] [PubMed] [Google Scholar]
- Shemyakin A, Salehzadeh F, Bohm F, Al-Khalili L, Gonon A, Wagner H, et al. Regulation of glucose uptake by endothelin-1 in human skeletal muscle in vivo and in vitro. J Clin Endocrinol Metab. 2010;95:2359–2366. doi: 10.1210/jc.2009-1506. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications. 2007;21:149–157. doi: 10.1016/j.jdiacomp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Skilton MR, Chin-Dusting JP, Dart AM, Brazionis L, Lantieri O, O'Dea K, et al. Metabolic health, obesity and 9-year incidence of peripheral arterial disease: the D.E.S.I.R. study. Atherosclerosis. 2011;216:471–476. doi: 10.1016/j.atherosclerosis.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ST, Woodward RM, Rosen AB, Cutler DM. The impact of symptoms and impairments on overall health in US national health data. Med Care. 2008;46:954–962. doi: 10.1097/MLR.0b013e318179199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes E, Hijmering M, van Zandvoort M, Wever R, Rabelink TJ, van Faassen EE. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett. 1998;438:161–164. doi: 10.1016/s0014-5793(98)01292-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Kugiyama K, Matsumura T, Suga S, Itoh H, Nakao K, et al. Lipoproteins regulate C-type natriuretic peptide secretion from cultured vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:1968–1974. doi: 10.1161/01.atv.15.11.1968. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Taddei S, Vanhoutte PM. Endothelium-dependent contractions to endothelin in the rat aorta are mediated by thromboxane A2. J Cardiovasc Pharmacol. 1993;22(Suppl. 8):S328–S331. doi: 10.1097/00005344-199322008-00086. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2-/- knockout but not COX1-/- knockout mice. J Cardiovasc Pharmacol. 2005;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- Thoenes M, Reil JC, Khan BV, Bramlage P, Volpe M, Kirch W, et al. Abdominal obesity is associated with microalbuminuria and an elevated cardiovascular risk profile in patients with hypertension. Vasc Health Risk Manag. 2009;5:577–585. doi: 10.2147/vhrm.s5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet. 2010;376:517–523. doi: 10.1016/S0140-6736(10)60935-X. [DOI] [PubMed] [Google Scholar]
- Traupe T, D'Uscio L, Muenter K, Morawietz H, Vetter W, Barton M. Effects of obesity on endothelium-dependent reactivity during acute nitric oxide synthase inhibition: modulatory role of endothelin. Clin Sci (Lond) 2002a;103(Suppl. 1):13S–15S. doi: 10.1042/CS103S013S. [DOI] [PubMed] [Google Scholar]
- Traupe T, Lang M, Goettsch W, Munter K, Morawietz H, Vetter W, et al. Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens. 2002b;20:2239–2245. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- Traupe T, Ortmann J, Munter K, Barton M. Endothelial therapy of atherosclerosis and its risk factors. Curr Vasc Pharmacol. 2003;1:111–121. doi: 10.2174/1570161033476763. [DOI] [PubMed] [Google Scholar]
- Tschop M, Heiman ML. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes. 2001;109:307–319. doi: 10.1055/s-2001-17297. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Moreau P, Shaw S, Takase H, Barton M, Luscher TF. Effects of chronic ETA-receptor blockade in angiotensin II-induced hypertension. Hypertension. 1997;29((Pt 2)):435–441. doi: 10.1161/01.hyp.29.1.435. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Say NO to ET. J Auton Nerv Syst. 2000;81:271–277. doi: 10.1016/s0165-1838(00)00126-0. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009a;73:595–601. doi: 10.1253/circj.cj-08-1169. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. How we learned to say NO. Arterioscler Thromb Vasc Biol. 2009b;29:1156–1160. doi: 10.1161/ATVBAHA.109.190215. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension. 2011;57:526–531. doi: 10.1161/HYPERTENSIONAHA.110.165100. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! J Physiol. 2008;586((Pt 22)):5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- Villar IC, Panayiotou CM, Sheraz A, Madhani M, Scotland RS, Nobles M, et al. Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res. 2007;74:515–525. doi: 10.1016/j.cardiores.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–375. doi: 10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, et al. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1423–1431. doi: 10.1016/S0140-6736(09)61500-2. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun. 1997;237:340–344. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- Wong C, Marwick TH. Obesity cardiomyopathy: diagnosis and therapeutic implications. Nat Clin Pract Cardiovasc Med. 2007;4:480–490. doi: 10.1038/ncpcardio0964. [DOI] [PubMed] [Google Scholar]
- Wong MS, Vanhoutte PM. COX-mediated endothelium-dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin. 2010;31:1095–1102. doi: 10.1038/aps.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CY, O'Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol. 2006;47:611–616. doi: 10.1016/j.jacc.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wang Y, Lam KS, Vanhoutte PM. Vascular actions of adipokines molecular mechanisms and therapeutic implications. Adv Pharmacol. 2010;60:229–255. doi: 10.1016/B978-0-12-385061-4.00008-8. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Yokota M. Production of C-type natriuretic peptide in human aortic endothelial cells induced by activation of protein kinase C. Am J Hypertens. 1996;9:924–929. doi: 10.1016/s0895-7061(96)00107-0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cui J, Zhang C. Emerging role of adipokines as mediators in atherosclerosis. World J Cardiol. 2010;2:370–376. doi: 10.4330/wjc.v2.i11.370. [DOI] [PMC free article] [PubMed] [Google Scholar]


