Abstract
Resistin, originally described as an adipocyte-specific hormone, has been suggested to be an important link between obesity, insulin resistance and diabetes. Although its expression was initially defined in adipocytes, significant levels of resistin expression in humans are mainly found in mononuclear leukocytes, macrophages, spleen and bone marrow cells. Increasing evidence indicates that resistin plays important regulatory roles apart from its role in insulin resistance and diabetes in a variety of biological processes: atherosclerosis and cardiovascular disease (CVD), non-alcoholic fatty liver disease, autoimmune disease, malignancy, asthma, inflammatory bowel disease and chronic kidney disease. As CVD accounts for a significant amount of morbidity and mortality in patients with diabetes and without diabetes, it is important to understand the role that adipokines such as resistin play in the cardiovascular system. Evidence suggests that resistin is involved in pathological processes leading to CVD including inflammation, endothelial dysfunction, thrombosis, angiogenesis and smooth muscle cell dysfunction. The modes of action and signalling pathways whereby resistin interacts with its target cells are beginning to be understood. In this review, the current knowledge about the functions and pathophysiological implications of resistin in CVD development is summarized; clinical translations, therapeutic considerations and future directions in the field of resistin research are discussed.
LINKED ARTICLES
This article is part of a themed section on Fat and Vascular Responsiveness. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-3
Keywords: resistin, cardiovascular disease, atherosclerosis, endothelial dysfunction, inflammation, human peripheral blood monocytes, thrombosis, angiogenesis
Introduction
Resistin (or ‘resistance to insulin’) was originally discovered in mice in 2001 and named for its ability to resist (interfere with) insulin action (Steppan et al., 2001a); at that time, it was proposed as a link between obesity and diabetes. Resistin is also known as found in inflammatory zone 3 and adipocyte-secreted factor (Banerjee and Lazar, 2001; Steppan et al., 2001a; Schinke et al., 2004). It belongs to a family of resistin-like molecules (RLM) with distinct expression patterns and biological effects (Steppan et al., 2001b). Several cell types known to express resistin include adipocytes (Steppan et al., 2001a; Rajala et al., 2002), intestinal epithelium and skeletal muscle cells (Nogueiras et al., 2003), and possibly astrocytes (Morash et al., 2002). The main source of mouse resistin is white adipose tissue. Mouse resistin is an 11 kDa cysteine-rich polypeptide, and its gene is located on chromosome 8. It is synthesized as a 114 amino acid (aa) precursor, with a 20 aa signal sequence and a 94 aa mature segment. It contains five intramolecular disulfide bonds and multiple β-turns (Juan et al., 2003). Resistin itself can form homodimers or multimers of varying sizes through disulfide and non-disulfide-linkage (Banerjee and Lazar, 2001; Chen et al., 2002). However, formation of these dimers or multimers may be not required for its bioactivity (Juan et al., 2003). Between mice and rats, there is 72% aa identity in the mature segment (Steppan et al., 2001a; Del Arco et al., 2003). In rats, resistin has also been observed to interfere with insulin-stimulated glucose uptake by skeletal muscle (Pravenec et al., 2003).
There is debate about the functional role of resistin in the mouse and human. Human resistin is a 12.5 kDa cysteine-rich peptide with a mature sequence consisting of 108 aa. The human resistin gene is located on chromosome 19. While the mature segments are 55% aa identical between mice and humans (Steppan et al., 2001a), the genes have markedly divergent promoter regions, indicating different mechanisms of regulation, tissue distribution and functions (Ghosh et al., 2003; Yang et al., 2003). The mature protein has a tendency to form oligomers, thus circulating in human serum in several different low molecular weight and high molecular weight isoforms (Gerber et al., 2005). Normally, the serum concentration of resistin in humans ranges from 7 to 22 ng·mL−1. In humans, resistin is primarily produced by cell populations other than adipocytes, which include peripheral blood mononuclear cells (PBMCs), macrophages and bone marrow cells (Fain et al., 2003; Patel et al., 2003). Some studies have reported that mature human adipocytes lack resistin expression, while preadipocytes can express resistin (Janke et al., 2002; Fain et al., 2003). Other studies showed that mature human adipocytes do produce resistin (Degawa-Yamauchi et al., 2003). These different observations may be due to the timing of secretion and the disconnection between mRNA expression and protein secretion (McTernan et al., 2003). It has been reported that in obese and diabetic patients, the serum concentration of resistin is significantly increased (Steppan et al., 2001a; Gerber et al., 2005). Likewise, increased resistin expression has been correlated with inflammatory markers, coronary artery disease and cardiovascular disease (CVD) in patients with the metabolic syndrome (MetS) (Ohmori et al., 2005; Reilly et al., 2005).
Although resistin was first described as a factor contributing to the development of insulin resistance and diabetes mellitus in humans, debate is still ongoing regarding the exact role it plays in obesity, insulin sensitivity and the development of type 2 diabetes mellitus (DM2). Meanwhile, resistin has also been linked to the development of atherosclerosis and CVD, non-alcoholic fatty liver disease, rheumatic disease, malignancy, asthma, inflammatory bowel disease and chronic kidney disease (Filkova et al., 2009; Gnacinska et al., 2009). As such, resistin may modulate molecular pathways involved in metabolic, inflammatory and autoimmune diseases, in addition to its cardiovascular targets. Although a great deal of controversy surrounds its exact biological functions in humans, recent studies suggest that resistin directly causes endothelial dysfunction. In clinical studies, resistin has been shown to be a predictive factor for coronary artery disease and CVD-related mortality. Furthermore, resistin appears to be involved in angiogenesis, thrombosis and vascular smooth muscle cell (VSMC) migration and proliferation, all of which contribute to atherosclerosis. In this review, we discuss the current advances towards understanding the role of resistin in CVD development and the known molecular mechanisms behind this action. Resistin is emerging as an important biomarker and potentially useful therapeutic target for coronary artery disease, as well as other diseases.
Correlation of resistin with insulin sensitivity, diabetes and metabolic syndrome
When resistin was first described in 2001 (Steppan et al., 2001b), several major discoveries were reported: plasma resistin levels were increased in diet-induced and genetic forms of the obese mouse model; administration of an anti-resistin antibody increased insulin sensitivity in obese and insulin-resistant animals; treatment of healthy mice with recombinant resistin impaired glucose tolerance and insulin action; and, resistin administration impaired insulin-induced glucose uptake in adipocytes. From these observations, it was concluded that resistin plays an important role in insulin resistance and obesity in the diabetic mouse model.
The applicability of these findings to human studies, however, has been difficult to determine. In mice, resistin is secreted mainly from white adipose tissue. In contrast, resistin in humans is mainly secreted from circulating blood monocytes, with low levels coming from white adipose tissue (Savage et al., 2001). Also, human resistin is only 59% homologous to the mouse resistin at the aa level (Ghosh et al., 2003), which again highlights the limitations of using a mouse model to study human metabolism. Ultimately, controversy persists regarding the pathogenic role of resistin in the development of insulin resistance and obesity in humans.
Several studies support a positive correlation between obesity, insulin resistance and elevated serum resistin in humans. For one, it has been described that resistin is expressed in human hepatocytes and induces insulin resistance (Sheng et al., 2008). Also, resistin mRNA levels have been found to be easily detectable in human PBMCs and higher in female patients with DM2 compared with healthy women, suggesting a role for resistin in the pathogenesis of human DM2 (Tsiotra et al., 2008). Similarly, in an investigation of the relationship between serum resistin levels, obesity and insulin resistance among 125 Jordanian patients with DM2, it was determined that serum resistin levels were higher in obese patients with DM2 (body mass index ≥ 30 kg·m−2) compared with non-diabetic obese controls; this correlation was not statistically significant between diabetics and controls that were normal weight or overweight (Gharibeh et al., 2010). This evidence suggests that resistin plays a role in the pathogenesis of obesity and insulin resistance in humans, both of which appear to contribute to the development of DM2.
Resistin concentrations have also been found to be higher on average in patients with gestational diabetes (GDM) (21.9 ng·mL−1) than in pregnant women with normal glucose tolerance (19.03 ng·mL−1) and non-pregnant women (14.8 ng·mL−1, P < 0.0001). The elevations in serum resistin observed in the patients with GDM, meanwhile, were correlated with serum IL-6 levels, not insulin levels, suggesting that changes in insulin sensitivity in patients with GDM were mediated by inflammatory pathways which may involve resistin (Kuzmicki et al., 2009). Similarly, it has been described that in patients with DM2 and a ‘diabetic foot’– namely, full-thickness foot ulceration in diabetic patients requiring >14 days for healing – plasma resistin and IL-6 levels were elevated in comparison with patients with DM2 and no foot ulceration, again linking resistin, DM2 and inflammation (Tuttolomondo et al., 2010).
Discrepancies exist in the data regarding the relationship between resistin and obesity and/or diabetes. In some rodent models, it has been demonstrated that resistin mRNA expression in the adipose tissue of obese animals does not correlate with serum resistin levels, which in turn do not correlate with serum insulin or glucose (Lee et al., 2005); in other studies, resistin has actually been found to be down-regulated in the adipose tissue of obese animals (Le Lay et al., 2001; Milan et al., 2002). In human studies, circulating levels of resistin and resistin gene expression have been reported as being both increased and unchanged in obesity and/or insulin resistance (Lee et al., 2003; Filippidis et al., 2005; Hasegawa et al., 2005; Iqbal et al., 2005). Meanwhile, another study (Laudes et al., 2010) found that resistin expression was significantly increased in obese subjects compared with controls, but with no correlation with DM2. Clearly, resistin's involvement (or lack thereof) in the pathogenesis of obesity-related insulin resistance and DM2 warrants further investigation; it is likely that resistin is a biomarker for and/or contributes to insulin resistance in specific populations.
Association of resistin with cardiovascular disease
Cardiovascular disease, including heart disease, vascular disease and atherosclerosis, is a critical global health threat, contributing to more than one-third of global morbidity. Emerging evidence suggests that CVD is accompanied by changes in serum resistin levels. For example, one recent study tested 220 patients with chest pain and found that patients who were having acute coronary syndrome (ACS) had significantly higher serum resistin levels (1.18 ± 0.48 µg·L−1) than those patients who were subsequently classified as normal control and stable angina pectoris groups (0.49 ± 0.40 and 0.66 ± 0.40 µg·L−1 respectively; P < 0.01). Within the ACS group, the increased serum resistin level was significantly correlated with serum high-sensitive C-reactive protein (hs-CRP) and white blood cell count; it likewise correlated with the number of coronary vessels demonstrating >50% stenosis. Overall, serum resistin was concluded to be a strong risk factor for ACS (Wang et al., 2009). A similar study demonstrated a significant increase in plasma resistin levels in patients with unstable angina when compared with patients with stable angina or control patients; again, plasma resistin was positively correlated with indicators of inflammation and endothelial activation such as leukocyte counts, hs-CRP and endothelin-1 (ET-1) levels in blood (Hu et al., 2007a). Accordingly, in a study of 39 patients with ACS, it was found that plasma resistin levels were markedly increased 24 h after onset when compared with controls. This significant increase persisted for a week, and again, the increase was higher in patients with more severe, acute disease (Chu et al., 2008). These findings all suggest that resistin plays a role in the pathogenesis of CVD, and a determination of its role in atherogenesis and ACS is currently underway.
Resistin and macrophages
A key step in the formation of chronic inflammatory atherosclerotic disease is the migration of circulating monocytes into the subendothelial space, where they differentiate into macrophages. Macrophages then take up cholesterol-rich atherogenic Apo-B lipoproteins (VLDL, IDL and LDL), forming foam cells (Glass and Witztum, 2001). In humans, resistin is mainly expressed in monocytes/macrophages (Savage et al., 2001). Macrophage scavenger receptors (SRs), such as class A SR (SR-AI, SR-AII, SR-AIII) and class B SR (SR-BI, SR-BII, CD36), are responsible for the internalization of oxidized LDL (oxLDL) (Kunjathoor et al., 2002). Macrophage-derived foam cells play a critical role in the initiation and progression of atherosclerosis (Glass and Witztum, 2001; Li and Glass, 2002). These cells infiltrate arteries and initiate or promote atherogenesis by secreting various pro-inflammatory cytokines (Ross, 1999).
As mentioned before, resistin levels are elevated in ACS, which has been hypothesized to be due to release of resistin from atherosclerotic plaques during plaque rupture (Chu et al., 2008). Meanwhile, macrophages infiltrating atherosclerotic aneurysms have been found to secrete resistin, which, in turn, affects endothelial function and VSMC migration, thus contributing to atherogenesis (Jung et al., 2006). Reciprocally, resistin has been shown to increase the uptake of oxLDL by macrophages, thereby promoting foam cell formation (Xu et al., 2006; Lee et al., 2009); this appears to be mediated by a resistin-induced increase in SR-A and CD36 and reduction in the cholesterol efflux regulatory protein ATP-binding cassette transporter 1 (Lee et al., 2009). A resistin-induced phenotypic change into foam cells has been demonstrated in a variety of contexts, and it has been shown that resistin directly affects the metabolism of fatty acids by increasing cholesterol esterification into lipid, increasing the intracellular availability of non-esterified fatty acids in human macrophages (Rae et al., 2007). In light of the different sources of resistin in mice and humans (adipose tissue vs. macrophages), a novel transgenic mouse model which expresses macrophage-specific human resistin but lacks mouse resistin has been generated (Qatanani et al., 2009). It was found that these mice, when fed a high-fat diet, demonstrated exacerbated diet-induced insulin resistance when compared with controls. This was associated with marked white adipose tissue inflammation, increased lipolysis and elevated serum free fatty acids.
Resistin and cytokines
Cytokines are small cell-signalling molecules mediate inflammation. Cytokines bind their matching cell-surface receptors and trigger intracellular signalling pathways, which in turn alter cellular functions. This may lead to up-regulation and/or down-regulation of several genes and their transcription factors, resulting in the production of other cytokines, an increase in the number of cell surface receptors for other molecules, or the suppression of their own effect by feedback inhibition. It has been demonstrated that resistin promotes endothelial cell activation through the release of ET-1 and up-regulation of vascular cell adhesion molecule and intercellular adhesion molecule-1; meanwhile, resistin leads to down-regulation of the expression of tumour necrosis factor (TNF) receptor-associated factor-3 (TRAF-3), an inhibitor of TNF receptor superfamily member 5 (CD40) ligand signalling (Verma et al., 2003). In addition, resistin has been shown to induce pentraxin 3, an inflammatory mediator involved in atherosclerosis, in human endothelial cells (Kawanami et al., 2004).
Resistin itself is an adipokine and has been found to induce the expression of cytokines and chemokines in human articular chondrocytes (Zhang et al., 2010). Resistin has been shown to induce the mRNA expression of 20 tested cytokines and chemokines in normal human chondrocytes as well as chondrocytes from the preserved area of osteoarthritic cartilage; these included TNF-α, IL-1α, IL-1β, CCL2, CCL3, CCL3L1, CCL4, CCL5, CCL8, CXCL1, CXCL2 and CXCL3. In order to explore the potential mechanisms whereby resistin induces up-regulation of inflammatory chemokines and cytokines in chondrocytes, computational analysis was performed on the differentially expressed genes; it was found that the genes that were most highly up-regulated have a nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) binding motif, which was confirmed using an NFκB luciferase reporter construct in human chondrocytes. Meanwhile, the transcription factor C/EBPβ was also found to have a high binding score, and cotransfection of the C/EBPβ expression vector enhanced the promoter activity of CCL3 and CCL4. Taken together, these data suggest that NFκB and C/EBPβ might play key roles in the high level of cytokine and chemokine expression in human chondrocytes following resistin treatment.
So far there has been only one published report where the authors show that resistin competes with lipopolysaccharide for binding to TLR4 receptor in human myeloid and epithelial cells (Tarkowski et al., 2010). TLR activation initiates a cascade of intracellular events leading to alterations in transcription and signalling pathways, including NFκB signalling; as such, antibody blocking of TLR4 was used to demonstrate that the binding of resistin to human leucocytes and cytokine production by PBMCs in response to resistin stimulation were abolished. Similarly, resistin binding was observed in TLR4-transfected human epithelial kidney cell line HEK293, but not with myeloid differentiation factor 2/CD14-transfected, TLR2-transfected or HEK null cells. As TLR4 binds to exogenous bacterial and viral structures and mediates the protective inflammatory reactions of the host, the authors evaluated the role of intracellular signalling pathways in resistin-mediated pro-inflammatory effects in PBMCs. Cells were pretreated with inhibitors specific for NFκB (parthenolide), mitogen-activated protein kinases (MAPKs) (PD98059 for p44/p42 and SB203580 for p38) and phosphatidylinositol 3-kinase (PI3K) (LY294002) and then stimulated with resistin. Inhibition of NFκB and MAPKs led to blockage of resistin-induced expression of IL-6, TNF-α and IL-1β in a dose-dependent manner at both the mRNA and protein levels. In contrast, the inhibition of PI3K increased the effect of resistin leading to increased expression of the cytokine IL-6 and IL-1β, because PI3K acts a negative regulator of inflammatory effects triggered through TLR4 and TLR2 (Williams et al., 2006). These results indicate that the pro-inflammatory intracellular signals elicited by resistin are mediated through NFκB and MAPK signalling mechanisms and are likely initiated by resistin binding to the TLR4 receptor.
Resistin and endothelial function
Endothelial cells form the main physical barrier between blood and the arterial wall and control the movement of solutes and fluid from the vascular space to the surrounding tissues. Endothelial cells release vasoactive and trophic substances such as prostacyclin, endothelium-derived relaxing factor/nitric oxide (NO), angiotensin II and ET-1 (Caldwell et al., 1976; Moncada et al., 1976; Furchgott and Zawadzki, 1980; Hickey et al., 1985; Ignarro et al., 1987; Yanagisawa et al., 1988). These substances are essential for controlling vascular growth, vasomotor reactivity, platelet function, coagulation, and immunologic and inflammatory responses (Libby, 2001). Endothelial cells are connected through specialized structures called endothelial cell junctions, which provide the primary endothelial barrier function. Endothelial dysfunction due to breakdown of the endothelial cell–cell barrier promotes atherogenesis as a result of enhanced permeability through the endothelial layer, increased adherence of leukocytes, monocytes and macrophages, and subendothelial accumulation of cholesterol-bearing lipoproteins (Widlansky et al., 2003; Endemann and Schiffrin, 2004). It has been reported that high concentrations of resistin generated in conditional media from epicardial adipose tissue (EAT) of patients with ACS profoundly influence in vitro endothelial function by significantly increasing endothelial cell permeability (Langheim et al., 2010). These findings suggest that EAT-secreted resistin is a major inducer of endothelial damage through the induction of hyper-permeability in human umbilical vein endothelial cells (HUVECs). Apart from resistin, other members of the family of RLM have been shown to be involved in the maintenance of epithelial cell barrier function. For example, RLM-β is expressed predominantly by goblet cells and epithelial cells within the colonic epithelium and plays a critical role in the maintenance of colonic epithelial cell barrier function (Hogan et al., 2006).
Recently, we have investigated whether resistin impairs endothelial functions by affecting the endothelial nitric oxide synthase (eNOS) system in human coronary artery endothelial cells (HCAECs) (Chen et al., 2010). eNOS was selected for investigation because of its important roles in controlling vascular tone and neovascularization (Palmer et al., 1987; Murohara et al., 1998; Chen et al., 2010). In our study, clinically relevant concentrations of resistin significantly reduced eNOS mRNA, protein and activity levels, and eNOS mRNA stability and cellular NO levels were diminished. Cellular levels of reactive oxygen species (ROS) including superoxide anion were significantly increased in resistin-treated HCAECs, whereas mitochondrial membrane potential and the activities of catalase and superoxide dismutase were reduced in comparison with untreated cells. Antioxidants effectively blocked resistin-induced eNOS down-regulation.
Furthermore, resistin immunoreactivity is increased in atherosclerotic regions of human aorta and carotid arteries (Chen et al., 2010). In an investigation of the mechanisms underlying resistin's action, we determined that resistin activated the MAPK p38 and c-Jun NH2-terminal kinase (JNK); administration of a specific p38 inhibitor effectively blocked resistin-induced ROS production and eNOS down-regulation. These results indicate that resistin directly induces eNOS down-regulation through overproduction of ROS and activation of p38 and JNK MAPK. We also investigated the effects of resistin treatment on cultured porcine coronary artery endothelial cells (PCAECs) (Kougias et al., 2005). As observed in HCAECs, the eNOS mRNA levels in PCAECs treated with resistin were decreased in a dose-dependent manner. Immunoreactivity for eNOS in resistin-treated pulmonary artery rings was also substantially reduced. Meanwhile, superoxide anion levels were increased by 88% in the vessel rings treated with 40 ng·mL−1 resistin when compared with controls (P < 0.05). These results indicate that resistin reduces eNOS expression in PCAECs and acts as a pro-oxidant mediator of coronary endothelial dysfunction.
Impaired vasorelaxation in response to pharmacological agents is a useful indicator of vascular dysfunction. We determined that resistin can affect vasomotor function in porcine coronary arteries (Kougias et al., 2005). Endothelium-dependent relaxation in response to bradykinin was significantly reduced in a dose-dependent manner in artery rings treated with resistin. Endothelium-independent relaxation in response to sodium nitroprusside was also reduced by 11% after treatment with 40 ng·mL−1 of resistin (P < 0.05). This represents compelling evidence that resistin reduces both endothelium-dependent and endothelium-independent vasorelaxation, and this is likely mediated by increased superoxide production in porcine coronary artery rings.
The effects of resistin on coronary vasomotor function have also been studied by other investigators both in vitro and in vivo (Dick et al., 2006). Experiments were conducted to determine the effects of resistin on superoxide anion production in coronary arteries and vasomotor functions in response to endothelium-dependent relaxants in anesthetized dogs and isolated coronary artery rings. These investigations demonstrated that administration of resistin into the coronary artery did not change coronary blood flow, mean arterial pressure, heart rate or acetylcholine-induced relaxation of artery rings; however, resistin did impair bradykinin-induced relaxation in isolated coronary rings in vitro and also attenuated bradykinin-induced vasodilation in vivo. In order to determine whether resistin-mediated attenuation of bradykinin-induced vasodilation is due the generation of superoxide anion as was previously shown (Kougias et al., 2005) or impaired production of vasoactive substances such as NO or prostaglandin I2 (pGI2), the effects of adding specific inhibitors were assessed. Tempol was used as a superoxide dismutase mimetic, NG-nitro-L-arginine methyl ester (L-NAME) as an eNOS inhibitor and indomethacin as a pGI2 inhibitor. The resistin-mediated attenuation of bradykinin-induced canine coronary vasodilation persisted in the presence of each treatment: Tempol, L-NAME and indomethacin. Therefore, it was concluded that the inhibitory effect of resistin is likely mediated through the bradykinin receptor or signal transduction pathways upstream of the NO synthase and cyclooxygenase signalling pathways (Dick et al., 2006). The lack of involvement of superoxide production in this study is in contrast to the data described above for porcine coronary arteries (Kougias et al., 2005), as resistin treatment at 10 ng·mL−1 and 40 ng·mL−1 had no effect on superoxide anion production in isolated canine coronary arteries (Dick et al., 2006). Inter-species variation (dog vs. pig), the size of the coronary arteries used, and differences in experimental conditions may be the reason for the discrepancy in the data between dog and pig arteries. And again, the exact mechanisms whereby resistin affects endothelial functions in humans are not known.
A recent study of the effects of resistin on vascular function and insulin-evoked vasorelaxation found that administration of resistin in young and old C57BL/6 mice and to cultured endothelial cells significantly impaired dose-dependent insulin-evoked vasodilation by reducing eNOS enzymatic activity both in vivo and in vitro (Gentile et al., 2008). Insulin has been previously shown to induce vasodilation by eNOS-mediated NO release (Zeng and Quon, 1996). Resistin's effects in this study were specific for insulin on vascular action, as vasodilation induced by increasing doses of acetylcholine or nitroglycerin was not influenced by resistin (Gentile et al., 2008). In addition, it was determined that resistin impaired insulin-evoked AKT and eNOS phosphorylation in endothelial cells and insulin receptor substrate-1 tyrosine/serine phosphorylation, subsequently altering its interaction with PI3K and thereby interrupting the pro-vasorelaxant pathway. These collective studies demonstrate that resistin alters coronary vasomotor functions both in vivo and in vitro. More studies are needed in order to more clearly define the roles and pathways whereby resistin influences coronary physiology and vascular disease formation.
Resistin and thrombosis
Thrombosis occurs when clot forms inside a blood vessel, obstructing the flow of blood through the circulatory system. This can occur when a blood vessel is injured, resulting in recruitment of platelets and fibrin to the injured area which form a blood clot to prevent loss of blood. Increasing evidence suggests a central role of thrombosis in the progression and complications of atherosclerosis, and thrombosis has been linked to the clinical occurrence of ACS (Libby and Aikawa, 2002; Libby et al., 2002). A number of experimental and clinical studies indicate that tissue factor (TF) plays a pivotal role in the pathophysiology of ACS by triggering the formation of intracoronary thrombi following endothelial injury (Wilcox et al., 1989; Pawashe et al., 1994; Annex et al., 1995; Ragni et al., 1996). Several studies have shown that resistin may enhance thrombus formation during atherosclerotic plaque formation. For example, treatment of HCAECs with resistin has been shown to cause up-regulation of TF expression, which appears to be mediated by oxygen-free radicals and the activation of the transcription factor NFκB, thereby promoting a pro-thrombotic state (Calabro et al., 2011).
In a recent clinical study, the serum resistin level of 90 patients with the MetS was determined and compared with serum levels of mediators of thrombosis (Fang et al., 2011). It was determined that the average level of resistin in MetS patients with or without acute myocardial or cerebral infarction was significantly higher than that of the control patients. And, in the patients with MetS and infarction, resistin levels correlated significantly with TF and plasminogen activator inhibitor-1. In vitro effects of resistin on gene expression were also determined using microarray analysis, and it was found that treatment of HUVECs with resistin led to a dramatic increase in the expression levels of apolipoprotein C-I, angiotensin-converting enzyme, TNF receptor superfamily member 1A (TNFRSF1A) and CD40 (Fang et al., 2011). These findings suggest that resistin may induce thrombotic complications via mediating the lipoprotein metabolism and stimulating inflammation in a hypercoagulable and hyperfibrinolytic environment. Resistin has long been associated with insulin resistance and obesity, and increased TF activity has been described in vitro in monocytes from obese young adults when compared with matched lean adults, although no relationship between resistin treatment and TF activity could be established (Ayer et al., 2010).
Although there is presently no direct evidence for a role of resistin in thrombus formation, resistin may be involved in thrombosis through its regulation of the eNOS enzyme. eNOS constitutively generates NO in the endothelium through its conversion of L-arginine to form L-citrulline and NO. The signalling molecule NO relaxes VSMC, thereby controlling vascular tone. NO has also been implicated in platelet aggregation and adhesion in vitro; meanwhile, the roles of NO and the three other NOS enzymes (eNOS, iNOS and nNOS) are still under investigation in vivo. Moore et al. (2011, 2010) investigated the role of eNOS in mice and found that eNOS-derived endogenous NO plays a critical role in regulating platelet function in vivo. The authors found that eNOS plays a significant role in platelet aggregation, whereas iNOS and nNOS appear to have minimal roles in this process. Up and downstream regulators of eNOS such as resistin could therefore represent important targets for anti-thrombotic effects; this needs to be further evaluated through appropriate experimental models. As increasing evidence indicates that resistin participates in the pathogenesis of atherosclerosis, the role of resistin in thrombosis merits further investigation.
Resistin and angiogenesis
Angiogenesis refers to the formation of new blood vessels from existing blood vessels. Angiogenesis is a complex process involving increases in vascular permeability, matrix degradation, and migration and proliferation of endothelial cells. It is an important physiological process, in both normal development and pathologies such as ischemic CVD and cancers. We have shown that human recombinant resistin stimulates proliferation, migration and capillary-like tube formation by HCAECs on matrigel (Mu et al., 2006). Resistin also up-regulates the mRNA and protein expression of several angiogenesis-promoting molecules, including vascular endothelial growth factor receptors (VEGFR-1 and VEGFR-2) and matrix metalloproteinases (MMP-1 and MMP-2). These findings suggest resistin may enhance angiogenesis. Potential signalling mechanisms were also investigated, and it was found that resistin treatment transiently increased phosphorylated ERK1/2 and p38 in HCAECs; when specific inhibitors were used to block ERK1/2 and p38, the resistin-induced cell proliferation and migration previously observed were completely blocked (Mu et al., 2006). Similarly, treatment of HUVECs with resistin induced VEGF production and stimulated endothelial cell tube formation in a separate in vitro model (Di Simone et al., 2006). Like human resistin, mouse resistin has been observed to induce endothelial cell migration and sprouting of cellular networks by mouse aortic arch explants, primary aortic endothelial cells and in a ‘wound healing’ model utilizing mouse b.End5 endothelioma cells (Robertson et al., 2009a).
The effects of murine resistin on angiogenesis have been investigated as well; the effects of resistin on angiogenesis may be mediated by TNF-α-like weak inducer of apoptosis (TWEAK), the levels of which have been found to be significantly increased in mouse b.End5 endothelioma cells following resistin treatment. Neutralization of TWEAK, meanwhile, has been found to block resistin-mediated cell proliferation and migration (Robertson et al., 2009b). Soluble TWEAK protein exerts proinflammatory and angiogenic responses and has been found to promote blood vessel formation in a rat cornea angiogenesis assay; also, expression of TWEAK and its specific receptor, Fn14 (FGF-inducible molecule 14 receptors), have been found to be up-regulated in chronic tissue injury and disease, including rheumatoid arthritis (RA) and cerebral ischaemia (Burkly et al., 2007). Accordingly, Robertson et al. concluded that up-regulated expression of TWEAK may contribute to the enhanced inflammation and angiogenesis during atheroma formation (2009b).
The mechanisms behind murine resistin-induced increases in migration and sprouting of endothelial cells have been determined to involve PI3K/AKT phosphorylation and NKκB based on abolishment of the angiogenic properties of resistin-treated cells after specific inhibition of these mediators. The Akt/I-κβ-kinase pathway has been found to promote angiogenic and metastatic gene expression in colorectal cancer through activation of NFκB and β-catenin (Agarwal et al., 2005). Meanwhile, PI3K/Akt induces NFκB activation and production of VEGF in murine epithelial cells (Li et al., 2005). Thus, murine resistin may influence angiogenesis and contribute to the development of cancerous metastasis. Meanwhile, enhancing angiogenesis has been intensely investigated as a possible therapeutic tool in treating ischemic heart disease, even as angiogenesis and intimal neovascularization have been proposed as pro-atherosclerotic events (Carmeliet, 2005; Khurana et al., 2005); so, it is important to further elucidate the role of resistin on angiogenesis in these settings. While mounting evidence suggests that resistin plays a role in promoting CVD formation, it is possible that investigation of its role in angiogenesis may provide useful clinical information for treatment of ischemic heart disease.
Resistin and vascular smooth muscle cell function
Vascular smooth muscle cells form layers within the vessel wall and control blood flow by contracting or relaxing in response to external stimuli. VSMCs do not proliferate under normal physiological conditions. However, in response to injury or inflammatory stimuli, VSMCs begin to grow and divide. Aberrant proliferation of VSMCs can lead to pathological changes in the vessel walls (Boettger et al., 2009). Indeed, resistin has been found to induce human aortic smooth muscle cell proliferation in a dose-dependent manner, and this appears to be mediated by ERK1/2 and Akt signalling pathways (Calabro et al., 2004).
Resistin also promotes VSMC migration (Jung et al., 2006; Jiang et al., 2009). Homocysteine has been shown to accumulate in adipose tissue and induce resistin expression (Li et al., 2008). Recently, it has been demonstrated that homocysteine-induced resistin expression stimulates VSMC migration in an adipocyte–VSMC coculture; small interfering RNA (siRNA) against resistin significantly attenuated VSMC migration in the system. The VSMC migration appeared to be mediated by resistin-induced cytoskeletal changes and α5β1-integrin activation through a α5β1-integrin-focal adhesion kinase/paxillin-Ras-related C3 botulinum toxin substrate 1 pathway (Jiang et al., 2009). The enhancement in VSMC proliferation and migration exerted by resistin provides further evidence of interaction between this adipokine and vascular cells, and this may represent an important factor in pathological vessel changes.
Therapeutic considerations
Several cholesterol lowering drugs have been used to investigate the possibility of lowering resistin levels in human cells and in the serum of patients with DM2. HMG-CoA reductase inhibitors (‘statins’) work to inhibit a critical enzyme in cholesterol production in the liver while also having profound anti-inflammatory effects; this class of drugs includes atorvastatin and simvastatin. Treatment with atorvastatin (10 mg·day−1 for 6 months) reduced resistin levels in patients with DM2, although the results did not reach statistical significance; meanwhile, in vitro it was found that treatment with atorvastatin reduced resistin mRNA levels in 3T3-L1 adipocytes and human monocytes/macrophages according to qPCR analysis (Ichida et al., 2006). In another in vitro study, CRP was found to induce resistin mRNA expression in human PBMC; co-incubation with simvastatin significantly inhibited this CRP-induced up-regulation of mRNA and protein expression of resistin (Hu et al., 2007b). As such, interplay between CRP and resistin might be involved in the pathogenesis of atherosclerosis, and therapy with statins may abrogate these effects. In fact, Shyu et al. (2009) found that atorvastatin was able to inhibit TNF-α-induced resistin expression in human macrophages; this inhibitory effect of atorvastatin was mediated through the inhibition of Rac phosphorylation and AP1 transcription factor binding to the resistin promoter. Statin therapy therefore could be another therapeutic strategy for controlling resistin associated cardiovascular dysfunction in humans.
Resistin is a proinflammatory cytokine and its effect is mediated by TNF-α (Silswal et al., 2005). Therefore, the effect of anti-TNF-α treatment was investigated in patients with RA, a chronic inflammatory disease. Administration of infliximab, an anti-TNF-α monoclonal antibody, resulted in a significant reduction of serum resistin levels in patients with RA (Gonzalez-Gay et al., 2008).
Folic acid-fortified foods have been used to reduce plasma homocysteine levels, and hyperhomocysteinaemia is a well-known risk factor for CVD. The effects of folic acid consumption on serum levels of resistin and endothelial health were thus studied in a mouse model. High-dose folic acid consumption (71 µg·kg−1) caused a significantly reduction in resistin levels in obese diabetic mice (Seto et al., 2010). Meanwhile, oleic acid, the predominant monounsaturated fatty acid of olive oil, has also been shown to reduce resistin gene expression in isolated adipocytes (Rea and Donnelly, 2006).
Therapy targeting the reduction of serum resistin levels is a promising strategy for clinical translation of our developing knowledge of the role of resistin in disease formation. Specifically, if resistin is confirmed to play key roles in insulin sensitivity, diabetes, MetS, various forms of CVD, thrombosis, and dysfunction of endothelial cells and macrophages, resistin could be a useful therapeutic target for CVD. In addition to anti-inflammatories and statins, new drugs specifically targeting resistin may include antisense oligonucleotides, antibodies and small molecular inhibitors. Furthermore, if resistin-induced signalling pathways are clearly mapped out, additional downstream targets of resistin could be evaluated for inhibition through drug development. Clearly, this is an exciting field for future study and the translation of basic science discoveries to clinical application.
Summary
Since the discovery of resistin in 2001 as a ‘link’ between obesity and diabetes, researchers have increasingly focused on the pleiotropic role of resistin and its biological functions. Although it is classified as an adipokine, resistin is importantly expressed in macrophages and plays important roles in inflammation throughout the body. Also, resistin has been implicated in a variety of disease processes besides obesity and diabetes; with respect to CVD and atherosclerosis, resistin has been found to have possible roles in the development of endothelial dysfunction, thrombosis, angiogenesis, inflammation and smooth muscle cell dysfunction (Figure 1). Ongoing work in this area should continue to provide insight into the mechanisms by which resistin can affect multiple organs and tissues. The detailed molecular pathways whereby resistin interacts with cells and specific molecules, such as receptors, proteins, transcription factors and target genes, as well as individual genomic variability within these mediators, represent the most critical areas of research. Ultimately, new agents and targets for pharmacological intervention to reduce the serum level of resistin will be identified, thereby preventing its adverse effects on the cardiovascular system and providing a novel therapeutic strategy in the treatment of a range of CVDs.
Figure 1.
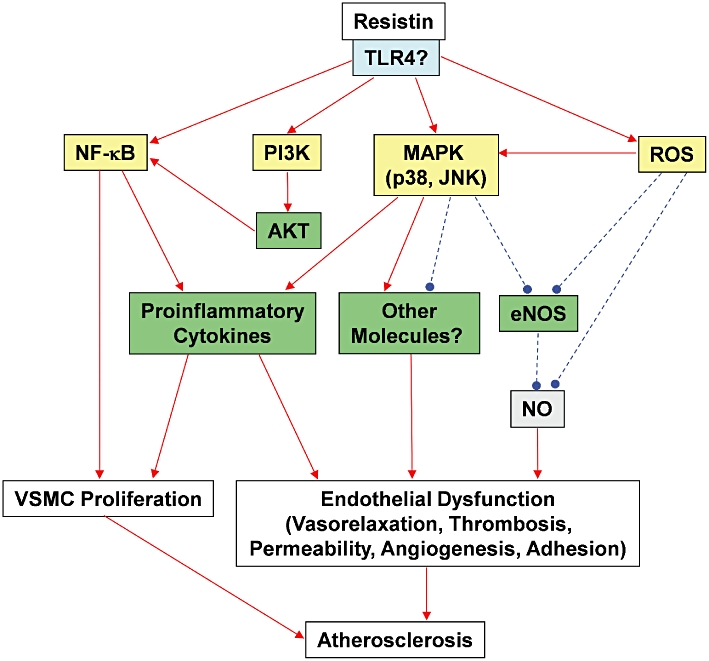
A schematic representation of potential mechanisms by which resistin may mediate cardiovascular dysfunction. Resistin possibly binds to the membrane bound TLR4 receptor, which then activates the intracellular signalling pathway. Resistin can activate the translocation of NFκB into the nucleus, which in turn, activates the transcription of pro-inflammatory cytokine genes, contributing to the proliferation of VSMCs and endothelial dysfunction. Activation of NFκB can also be mediated by the resistin-induced activation of PI3K/AKT pathway. Resistin can also stimulate the production of pro-inflammatory cytokines through MAPK p38 and JNK. Resistin can cause oxidative stress, which is another factor for MAPK activation and eNOS inhibition. Resistin increases the production of superoxide anions, which inhibit eNOS gene expression and reduce bioavailability of NO. VSMC proliferation and endothelial dysfunction including impaired vasorelaxation, enhanced thrombosis, hyper-permeability, angiogenesis and increased cell adhesion collectively contribute to the formation of atherosclerosis. Solid red arrows indicates activation, while broken blue lines indicate inhibition. eNOS, endothelial nitric oxide synthase; JNK, c-Jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor kappa B; NO, nitric oxide; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; TLR4, toll-like receptor 4; VSMC, vascular smooth muscle cell.
Acknowledgments
This work was partially supported by a NIH grant (R01HL083471 to Chen C.) from the National Institutes of Health and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX, USA. Weakley S.M. was supported from a NIH training grant (T32HL083774).
Glossary
- aa
amino acid
- ACS
acute coronary syndrome
- CD40
TNF receptor superfamily member 5
- CVD
cardiovascular disease
- DM2
type 2 diabetes mellitus
- EAT
epicardial adipose tissue
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- GDM
gestational diabetes
- HCAEC
human coronary artery endothelial cell
- hs-CRP
high-sensitive C-reactive protein
- HUVEC
human umbilical vein endothelial cell
- JNK
c-Jun NH2-terminal kinase
- L-NAME
NG-nitro-L-arginine methyl ester
- MAPK
mitogen-activated protein kinase
- MetS
metabolic syndrome
- MMP
matrix metalloproteinase
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- oxLDL
oxidized LDL
- PBMC
peripheral blood mononuclear cell
- PI3K
phosphatidylinositol 3-kinase
- RA
rheumatoid arthritis
- RLM
resistin-like molecules
- ROS
reactive oxygen species
- TF
tissue factor
- TNF
tumour necrosis factor receptor
- TNFRSF1A
tumour necrosis factor receptor superfamily member 1A
- TRAF-3
tumour necrosis factor receptor-associated factor-3
- VEGFR
vascular endothelial growth factor receptor
- VSMC
vascular smooth muscle cell
Conflict of interest
None.
References
- Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- Annex BH, Denning SM, Channon KM, Sketch MH, Jr, Stack RS, Morrissey JH, et al. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation. 1995;91:619–622. doi: 10.1161/01.cir.91.3.619. [DOI] [PubMed] [Google Scholar]
- Ayer JG, Song C, Steinbeck K, Celermajer DS, Ben Freedman S. Increased tissue factor activity in monocytes from obese young adults. Clin Exp Pharmacol Physiol. 2010;37:1049–1054. doi: 10.1111/j.1440-1681.2010.05430.x. [DOI] [PubMed] [Google Scholar]
- Banerjee RR, Lazar MA. Dimerization of resistin and resistin-like molecules is determined by a single cysteine. J Biol Chem. 2001;276:25970–25973. doi: 10.1074/jbc.M103109200. [DOI] [PubMed] [Google Scholar]
- Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16. doi: 10.1016/j.cyto.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Calabro P, Cirillo P, Limongelli G, Maddaloni V, Riegler L, Palmieri R, et al. Tissue factor is induced by resistin in human coronary artery endothelial cells by the NF-kB-dependent pathway. J Vasc Res. 2011;48:59–66. doi: 10.1159/000318775. [DOI] [PubMed] [Google Scholar]
- Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- Caldwell PR, Seegal BC, Hsu KC, Das M, Soffer RL. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976;191:1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang J, Lu JM, Chai H, Wang X, Lin PH, et al. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2010;299:H193–H201. doi: 10.1152/ajpheart.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang L, Boeg YS, Xia B, Wang J. Differential dimerization and association among resistin family proteins with implications for functional specificity. J Endocrinol. 2002;175:499–504. doi: 10.1677/joe.0.1750499. [DOI] [PubMed] [Google Scholar]
- Chu S, Ding W, Li K, Pang Y, Tang C. Plasma resistin associated with myocardium injury in patients with acute coronary syndrome. Circ J. 2008;72:1249–1253. doi: 10.1253/circj.72.1249. [DOI] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, et al. Serum resistin (FIZZ3) protein is increased in obese humans. Clin Endocrinol Metab. 2003;88:5452–5455. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Peralta S, Carrascosa JM, Ros M, Andrés A, Arribas C. Alternative splicing generates a novel non-secretable resistin isoform in Wistar rats. FEBS Lett. 2003;555:243–249. doi: 10.1016/s0014-5793(03)01241-9. [DOI] [PubMed] [Google Scholar]
- Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D'Asta M, Caforio L, et al. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol. 2006;189:691–699. doi: 10.1677/joe.1.06610. [DOI] [PubMed] [Google Scholar]
- Dick GM, Katz PS, Farias M, 3rd, Morris M, James J, Knudson JD, et al. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. Am J Physiol Heart Circ Physiol. 2006;291:H2997–H3002. doi: 10.1152/ajpheart.01035.2005. [DOI] [PubMed] [Google Scholar]
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- Fain JN, Cheema PS, Bahouth SW, Lloyd Hiler M. Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun. 2003;300:674–678. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- Fang W, Zhang Q, Peng Y, Chen M, Lin X, Wu J, et al. Resistin level is positively correlated with thrombotic complications in southern Chinese metabolic syndrome patients. J Endocrinol Invest. 2011;34:e36–e42. doi: 10.1007/BF03347059. [DOI] [PubMed] [Google Scholar]
- Filippidis G, Liakopoulos V, Mertens PR, Kiropoulos T, Stakias N, Verikouki C, et al. Resistin serum levels are increased but not correlated with insulin resistance in chronic hemodialysis patients. Blood Purif. 2005;23:421–428. doi: 10.1159/000088017. [DOI] [PubMed] [Google Scholar]
- Filkova M, Haluzik M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gentile MT, Vecchione C, Marino G, Aretini A, Di Pardo A, Antenucci G, et al. Resistin impairs insulin-evoked vasodilation. Diabetes. 2008;57:577–583. doi: 10.2337/db07-0557. [DOI] [PubMed] [Google Scholar]
- Gerber M, Boettner A, Seidel B, Lammert A, Bar J, Schuster E, et al. Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab. 2005;90:4503–4509. doi: 10.1210/jc.2005-0437. [DOI] [PubMed] [Google Scholar]
- Gharibeh MY, Al Tawallbeh GM, Abboud MM, Radaideh A, Alhader AA, Khabour OF. Correlation of plasma resistin with obesity and insulin resistance in type 2 diabetic patients. Diabetes Metab. 2010;36:443–449. doi: 10.1016/j.diabet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003;305:27–34. doi: 10.1016/s0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Gnacinska M, Malgorzewicz S, Stojek M, Lysiak-Szydlowska W, Sworczak K. Role of adipokines in complications related to obesity: a review. Adv Med Sci. 2009;54:150–157. doi: 10.2478/v10039-009-0035-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, Garcia-Unzueta MT, Gonzalez-Juanatey C, Miranda-Filloy JA, Vazquez-Rodriguez TR, De Matias JM, et al. Anti-TNF-alpha therapy modulates resistin in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:311–316. [PubMed] [Google Scholar]
- Hasegawa G, Ohta M, Ichida Y, Obayashi H, Shigeta M, Yamasaki M, et al. Increased serum resistin levels in patients with type 2 diabetes are not linked with markers of insulin resistance and adiposity. Acta Diabetol. 2005;42:104–109. doi: 10.1007/s00592-005-0187-x. [DOI] [PubMed] [Google Scholar]
- Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248:C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WL, Qiao SB, Hou Q, Yuan JS. Plasma resistin is increased in patients with unstable angina. Chin Med J (Engl) 2007a;120:871–875. [PubMed] [Google Scholar]
- Hu WL, Qiao SB, Li JJ. Decreased C-reactive protein-induced resistin production in human monocytes by simvastatin. Cytokine. 2007b;40:201–206. doi: 10.1016/j.cyto.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Ichida Y, Hasegawa G, Fukui M, Obayashi H, Ohta M, Fujinami A, et al. Effect of atorvastatin on in vitro expression of resistin in adipocytes and monocytes/macrophages and effect of atorvastatin treatment on serum resistin levels in patients with type 2 diabetes. Pharmacology. 2006;76:34–39. doi: 10.1159/000088948. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Seshadri P, Stern L, Loh J, Kundu S, Jafar T, et al. Serum resistin is not associated with obesity or insulin resistance in humans. Eur Rev Med Pharmacol Sci. 2005;9:161–165. [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes Res. 2002;10:1–5. doi: 10.1038/oby.2002.1. [DOI] [PubMed] [Google Scholar]
- Jiang C, Zhang H, Zhang W, Kong W, Zhu Y, Zhang H, et al. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am J Physiol Cell Physiol. 2009;297:C1466–C1476. doi: 10.1152/ajpcell.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan CC, Kan LS, Huang CC, Chen SS, Ho LT, Au LC. Production and characterization of bioactive recombinant resistin in Escherichia coli. J Biotechnol. 2003;103:113–117. doi: 10.1016/s0168-1656(03)00099-3. [DOI] [PubMed] [Google Scholar]
- Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, et al. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Adipocyte-derived cytokine resistin causes endothelial dysfunction of porcine coronary arteries. J Vasc Surg. 2005;41:691–698. doi: 10.1016/j.jvs.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, et al. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol. 2009;25:258–263. doi: 10.1080/09513590802653825. [DOI] [PubMed] [Google Scholar]
- Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–H753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- Laudes M, Oberhauser F, Schulte DM, Freude S, Bilkovski R, Mauer J, et al. Visfatin/PBEF/Nampt and resistin expressions in circulating blood monocytes are differentially related to obesity and type 2 diabetes in humans. Horm Metab Res. 2010;42:268–273. doi: 10.1055/s-0029-1243638. [DOI] [PubMed] [Google Scholar]
- Le Lay S, Boucher J, Rey A, Castan-Laurell I, Krief S, Ferre P, et al. Decreased resistin expression in mice with different sensitivities to a high-fat diet. Biochem Biophys Res Commun. 2001;289:564–567. doi: 10.1006/bbrc.2001.6015. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bullen JW, Jr, Stoyneva VL, Mantzoros CS. Circulating resistin in lean, obese, and insulin-resistant mouse models: lack of association with insulinemia and glycemia. Am J Physiol Endocrinol Metab. 2005;288:E625–E632. doi: 10.1152/ajpendo.00184.2004. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- Lee TS, Lin CY, Tsai JY, Wu YL, Su KH, Lu KY, et al. Resistin increases lipid accumulation by affecting class A scavenger receptor, CD36 and ATP-binding cassette transporter-A1 in macrophages. Life Sci. 2009;84:97–104. doi: 10.1016/j.lfs.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang C, Xu G, Wang N, Zhu Y, Tang C, et al. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes. 2008;57:817–827. doi: 10.2337/db07-0617. [DOI] [PubMed] [Google Scholar]
- Li YM, Zhou BP, Deng J, Pan Y, Hay N, Hung MC. A hypoxia-independent hypoxia-inducible factor-1 activation pathway induced by phosphatidylinositol-3 kinase/Akt in HER2 overexpressing cells. Cancer Res. 2005;65:3257–3263. doi: 10.1158/0008-5472.CAN-04-1284. [DOI] [PubMed] [Google Scholar]
- Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- McTernan PG, Fisher FM, Valsamakis G, Chetty R, Harte A, McTernan CL, et al. Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J Clin Endocrinol Metab. 2003;88:6098–6106. doi: 10.1210/jc.2003-030898. [DOI] [PubMed] [Google Scholar]
- Milan G, Granzotto M, Scarda A, Calcagno A, Pagano C, Federspil G, et al. Resistin and adiponectin expression in visceral fat of obese rats: effect of weight loss. Obes Res. 2002;10:1095–1103. doi: 10.1038/oby.2002.149. [DOI] [PubMed] [Google Scholar]
- Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moore C, Sanz-Rosa D, Emerson M. Distinct role and location of the endothelial isoform of nitric oxide synthase in regulating platelet aggregation in males and females in vivo. Eur J Pharmacol. 2011;651:152–158. doi: 10.1016/j.ejphar.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Moore C, Tymvios C, Emerson M. Functional regulation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb Haemost. 2010;104:342–349. doi: 10.1160/TH09-11-0764. [DOI] [PubMed] [Google Scholar]
- Morash BA, Willkinson D, Ur E, Wilkinson M. Resistin expression and regulation in mouse pituitary. FEBS Lett. 2002;526:26–30. doi: 10.1016/s0014-5793(02)03108-3. [DOI] [PubMed] [Google Scholar]
- Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–157. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Gallego R, Gualillo O, Caminos JE, Garcia-Caballero T, Casanueva FF, et al. Resistin is expressed in different rat tissues and is regulated in a tissue- and gender-specific manner. FEBS Lett. 2003;548:21–27. doi: 10.1016/s0014-5793(03)00708-7. [DOI] [PubMed] [Google Scholar]
- Ohmori R, Momiyama Y, Kato R, Taniguchi H, Ogura M, Ayaori M, et al. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46:379–380. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- Pawashe AB, Golino P, Ambrosio G, Migliaccio F, Ragni M, Pascucci I, et al. A monoclonal antibody against rabbit tissue factor inhibits thrombus formation in stenotic injured rabbit carotid arteries. Circ Res. 1994;74:56–63. doi: 10.1161/01.res.74.1.56. [DOI] [PubMed] [Google Scholar]
- Pravenec M, Kazdová L, Landa V, Zidek V, Mlejnek P, Jansa P, et al. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem. 2003;278:45209–45215. doi: 10.1074/jbc.M304869200. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009;119:531–539. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Robertson SA, Taylor JM, Graham A. Resistin induces lipolysis and re-esterification of triacylglycerol stores, and increases cholesteryl ester deposition, in human macrophages. FEBS Lett. 2007;581:4877–4883. doi: 10.1016/j.febslet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Ragni M, Cirillo P, Pascucci I, Scognamiglio A, D'Andrea D, Eramo N, et al. Monoclonal antibody against tissue factor shortens tissue plasminogen activator lysis time and prevents reocclusion in a rabbit model of carotid artery thrombosis. Circulation. 1996;93:1913–1918. doi: 10.1161/01.cir.93.10.1913. [DOI] [PubMed] [Google Scholar]
- Rajala MW, Lin Y, Ranalletta M, Yang XM, Qian H, Gingerich R, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol. 2002;16:1920–1930. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- Rea R, Donnelly R. Effects of metformin and oleic acid on adipocyte expression of resistin. Diabetes Obes Metab. 2006;8:105–109. doi: 10.1111/j.1463-1326.2005.00477.x. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Rae CJ, Graham A. Induction of angiogenesis by murine resistin: putative role of PI3-kinase and NO-dependent pathways. Regul Pept. 2009a;152:41–47. doi: 10.1016/j.regpep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Rae CJ, Graham A. Resistin: TWEAKing angiogenesis. Atherosclerosis. 2009b;203:34–37. doi: 10.1016/j.atherosclerosis.2008.05.040. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- Schinke T, Haberland M, Jamshidi A, Nollau P, Rueger JM, Amling M. Cloning and functional characterization of resistin-like molecule gamma. Biochem Biophys Res Commun. 2004;314:356–362. doi: 10.1016/j.bbrc.2003.12.100. [DOI] [PubMed] [Google Scholar]
- Seto SW, Lam TY, Or PM, Lee WY, Au AL, Poon CC, et al. Folic acid consumption reduces resistin level and restores blunted acetylcholine-induced aortic relaxation in obese/diabetic mice. J Nutr Biochem. 2010;21:872–880. doi: 10.1016/j.jnutbio.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Sheng CH, Di J, Jin Y, Zhang YC, Wu M, Sun Y, et al. Resistin is expressed in human hepatocytes and induces insulin resistance. Endocrine. 2008;33:135–143. doi: 10.1007/s12020-008-9065-y. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Chua SK, Wang BW, Kuan P. Mechanism of inhibitory effect of atorvastatin on resistin expression induced by tumor necrosis factor-alpha in macrophages. J Biomed Sci. 2009;16:50. doi: 10.1186/1423-0127-16-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001a;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001b;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiotra PC, Tsigos C, Anastasiou E, Yfanti E, Boutati E, Souvatzoglou E, et al. Peripheral mononuclear cell resistin mRNA expression is increased in type 2 diabetic women. Mediators Inflamm. 2008 doi: 10.1155/2008/892864. DOI: 10.1155/2008/892864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttolomondo A, La Placa S, Di Raimondo D, Bellia C, Caruso A, Lo Sasso B, et al. Adiponectin, resistin and IL-6 plasma levels in subjects with diabetic foot and possible correlations with clinical variables and cardiovascular co-morbidity. Cardiovasc Diabetol. 2010;9:50. doi: 10.1186/1475-2840-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen DY, Cao J, He ZY, Zhu BP, Long M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin Med Sci J. 2009;24:161–166. doi: 10.1016/s1001-9294(09)60082-1. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- Xu W, Yu L, Zhou W, Luo M. Resistin increases lipid accumulation and CD36 expression in human macrophages. Biochem Biophys Res Commun. 2006;351:376–382. doi: 10.1016/j.bbrc.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996;98:894–898. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xing X, Hensley G, Chang LW, Liao W, Abu-Amer Y, et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum. 2010;62:1993–2003. doi: 10.1002/art.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]


