Abstract
Visceral fat has been linked to metabolic disturbances and increased risk for cardiovascular disease and type 2 diabetes. Recent studies propose a paracrine role for periadventitial adipose tissue in the control of arterial vascular tone. This regulation depends on the anatomical integrity of the vessels and involves a transferable mediator(s) (adipokine) released from either periadventitial adipocytes or perivascular adipose tissue. Although a number of adipokines with vasoactive properties have been identified, a still unidentified adipocyte-derived relaxing factor (ADRF) plays a major role in the periadventitial vasoregulation of visceral arteries, such as the aorta and mesenteric arteries. ADRF is released by visceral periadventitial adipocytes and primarily produces endothelium-independent vasorelaxation by opening voltage-dependent (Kv) K+ channels in the plasma membrane of smooth muscle cells. At least in part, KCNQ (Kv7) channels could represent the subtype of Kv channels involved. Glibenclamide-sensitive KATP channels are not involved or play a minor role. The ‘third gas’, namely H2S, could represent ADRF. Alterations in the paracrine control of arterial tone by visceral periadventitial adipose tissue have been found in animal models of hypertension and metabolic disease. ADRF, or perhaps its putative targets, might represent exciting new targets for the development of drugs for treatment of cardiovascular and metabolic disorders.
LINKED ARTICLES
This article is part of a themed section on Fat and Vascular Responsiveness. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-3
Keywords: adipocyte-derived relaxing factor, perivascular fat, ADRF, KCNQ channels, KV channels, hydrogen sulphide, obesity, periadventitial dysfunction
Obesity is one of today's most blatantly visible public health problems. There is major concern that medical progress in reducing coronary heart disease, high cholesterol and hypertension could be reversed by the escalating global epidemic of overweight and obesity –‘globesity’ (World Health Organization, 1998). Obesity is a risk factor for hypertension, type 2 diabetes, congestive heart failure, stroke, renal dysfunction, gallbladder disease, obstructive sleep apnea, cancer, osteoarthritis, and impaired quality of life (Sharma, 2003; Flier, 2004). Up to 50% of obese individuals have concomitant hypertension (Sharma, 2003). However, the mechanisms underlying these observations have yet to be fully understood. Increased sympathetic nerve activity, insulin resistance and hyperinsulinaemia, sodium and volume retention, renal abnormalities and, more recently, hyperleptinaemia have all been implicated in obesity-related hypertension (Hall et al., 2001). The haemodynamic profile of obese hypertensive subjects is characterized by high intravascular volume, high cardiac output and increased peripheral vascular resistance (Messerli et al., 1983; Fortuno et al., 2003). Increased vascular resistance in obesity is known to be accompanied by structural changes in peripheral resistance vessels (Rocchini et al., 1992) and blunted Ca2+ regulation of vascular smooth muscle tone by insulin (Zemel, 1998). However, the role of periadventitial adipocytes in vascular reactivity has received little attention. This is surprising since adipose tissue secretes a number of bioactive substances.
Vascular effects of adipokines
Recent in vitro and in vivo evidence identifies adipose tissue as a critical endocrine organ that secretes a variety of bioactive signalling molecules into the circulation. These molecules include vascular endothelial growth factor (VEGF), interleukin 6, interleukin-1, tumour necrosis factor-α (TNF-α), leptin, adiponectin (ACRP30), resistin, omentin, acylation stimulation protein (ASP), apelin, adipsin, agouti, insulin-like growth factor (IGF-1), angiotensinogen, plasminogen activator protein (PAI-1), reactive oxygen species and sex steroids (Fortuno et al., 2003; Havel, 2004; Thalmann and Meier, 2007; Yamawaki et al., 2010). These substances can play an autocrine role in the regulation of adipocyte metabolism. Upon secretion into the bloodstream, they can also play an endocrine role in the regulation of other cellular processes, including vascular function and peripheral resistance. Although a number of adipocyte-derived substances can influence vascular tone (Ohkawa et al., 1994; Loughrey et al., 2003; Salcedo et al., 2007; Yamawaki et al., 2009; Yamawaki et al., 2010), the vasoactive effects of leptin and TNF-α deserve highlighting. Leptin regulates blood pressure by at least two opposing mechanisms, that is release of nitric oxide (NO) and excitation of the sympathetic nervous system (Fruhbeck, 1999; Lembo et al., 2000). Leptin induces direct vasodilation by stimulation of NO and endothelium-derived hyperpolarizing factor (EDHF) (Lembo et al., 2000) but not involving glibenclamide-sensitive (KATP) and TEA-sensitive (BK) K+ channels (Kimura et al., 2000; Sahin and Bariskaner, 2007). On the other hand, leptin induces indirect vasoconstriction via central activation of the sympathetic nervous system (Fruhbeck, 1999). TNF-α is also both a vasoconstrictor (Wagner, 2000; Zhang et al., 2002) and a vasodilator (Baudry and Vicaut, 1993; Shibata et al., 1996; Brian and Faraci, 1998; Johns and Webb, 1998). TNF-α-mediated vasorelaxation can involve NO and prostaglandin production (Shibata et al., 1996; Brian and Faraci, 1998) or NAD(P)H oxidase-dependent Ca2+ spark activation (Cheranov and Jaggar, 2006). Moreover, TNF-α plays a role in endothelial dysfunction (Park et al., 2011; Zhang et al., 2006; Yang et al., 2009). Notably, a complete renin–angiotensin system (RAS) has been identified in adipose tissue (Engeli et al., 2003; Galvez-Prieto et al., 2008a). Thus, adipose cells play a more dynamic role in regulating cellular processes than previously recognized. However, most of these studies have been focused on the identification of substances released from adipose tissue and their possible humoral vasoactive effects but not on a possible paracrine function for adipose tissue in the regulation of arterial tone and peripheral vascular resistance.
ADRF and the paracrine regulation of arterial tone by periadventitial adipose tissue
Virtually all blood vessels are surrounded by variable amounts of adventitial adipose tissue (Cinti, 2002). Soltis and Cassis (1991) found that periadventitial fat significantly attenuates vascular responsiveness of Sprague–Dawley aortic ring preparations to noradrenaline in vitro. The study was aimed to clarify whether vasoconstriction is due to release of noradrenaline from nerves stimulating the vascular smooth muscle directly or from the innervation of the adipose tissue in rat aorta. Using three methods that cause the release of noradrenaline from sympathetic nerve terminals, the authors demonstrated that the innervation to the adipose tissue influences responsiveness of the aortic smooth muscle. The authors suggested that periaortic adipose tissue contains sufficient sympathetic nervous system innervation to influence in vitro aortic vascular responsiveness. They suggested that the anti-contractile effect on the response to noradrenaline was due to uptake of noradrenaline by the surrounding adipose tissue. However, according to this theory, vessels with perivascular fat are expected to exhibit greater contractile responses to depolarizing external K+ (60–100 mM) solutions than vessels without fat. However, this appears not to be the case. Therefore, the authors concluded that the reason for the lack of difference in KCl responsiveness between intact vessels and vessels without fat is not known. They appreciated limitations of their adipose tissue innervation theory to explain the reduced vascular responsiveness of intact aortic ring preparations with fat in response to noradrenaline. Lohn et al. (2002) reexamined the idea of periadventitial vasoregulation. Their studies on Sprague–Dawley rat aorta confirmed the inhibitory effect of periadventitial adipose tissue on vasoconstriction induced by serotonin, angiotensin II and phenylephrine. In contradistinction to noradrenaline, these substances are not subject to reuptake by adrenergic nerves. The anti-contractile effects did not involve neuronal pre-synaptic N-type Ca2+ and Na+ channels or vanilloid, cannabinoid, and calcitonin gene-related peptide receptors (Dubrovska et al., 2004). Rather, this regulation depends on the anatomical integrity of the vessels and is mediated by a transferable ‘adipocyte-derived relaxing factor’ (ADRF) (Lohn et al., 2002; Gollasch and Dubrovska, 2004; Verlohren et al., 2004c). Blocking NO synthase by L-NAME, cyclo-oxygenase by indomethacin or cytochrome P450 by miconazole did not affect the effects of ADRF (Lohn et al., 2002). Blocking adenosine receptors or removal of the endothelium did affect the anti-contractile effects of perivascular fat (Lohn et al., 2002). Lack of functional leptin receptors in the Zucker fa/fa rat did not modify the anticontractile effect of periadventitial fat, indicating that the ADRF effects are independent of functional leptin receptors (Lohn et al., 2002). Studies on adiponectin gene knockout mice demonstrated that ADRF is not adiponectin (Fesus et al., 2007).
ADRF and smooth muscle Kv channels
Though the chemical nature of ADRF(s) is unknown and there might be more than one ADRF, there is evidence that the substance produces relaxation by opening smooth muscle K+ channels. The disappearance of the anti-contractile effect of periadventitial adipose tissue in high (60 mM) external K+ solutions suggested that K+ channels are critically involved in this effect (Lohn et al., 2002). By donor-acceptor transfer bioassay experiments, we and others showed that the effects are mediated by a transferable factor (Dubrovska et al., 2004; Gao et al., 2005), which opens Kv channels (ADRF-Kv pathway) to produce vasorelaxation (Verlohren et al., 2004c; Fesus et al., 2007). Periadventitial adipose tissue was also reported to inhibit arterial contraction of human mesenteric arteries (Verlohren et al., 2004a). The Kv-subfamily involved in the effects of ADRF was recently explored using a pharmacological approach with XE991 (10,10-bis(4-Pyridinylmethyl)-9(10H)-anthracenone dihydrochloride) (Schleifenbaum et al., 2010). XE991 inhibited the anti-contractile effects of perivascular fat in mesenteric arteries and in aortas. We also re-examined the effects of glibenclamide (3 µM) in the rat aorta (Lohn et al., 2002) and found that this drug antagonizes the anti-contractile effect of fat by a non-specific, ADRF-independent mechanism (Schleifenbaum et al., 2010). In particular, we found that glibenclamide (3 µM) increased contractile responses of (+) fat aortas to serotonin. However, dose-response curves of (-) fat aortas for serotonin were similarly shifted to the left by glibenclamide. These results indicate that KATP channels play no or only a minor role in vasorelaxation induced by ADRF. Instead, the pharmacological data suggest that, at least in part, KCNQ channels represent the Kv channel family involved in the effects of ADRF (Figure 1).
Figure 1.
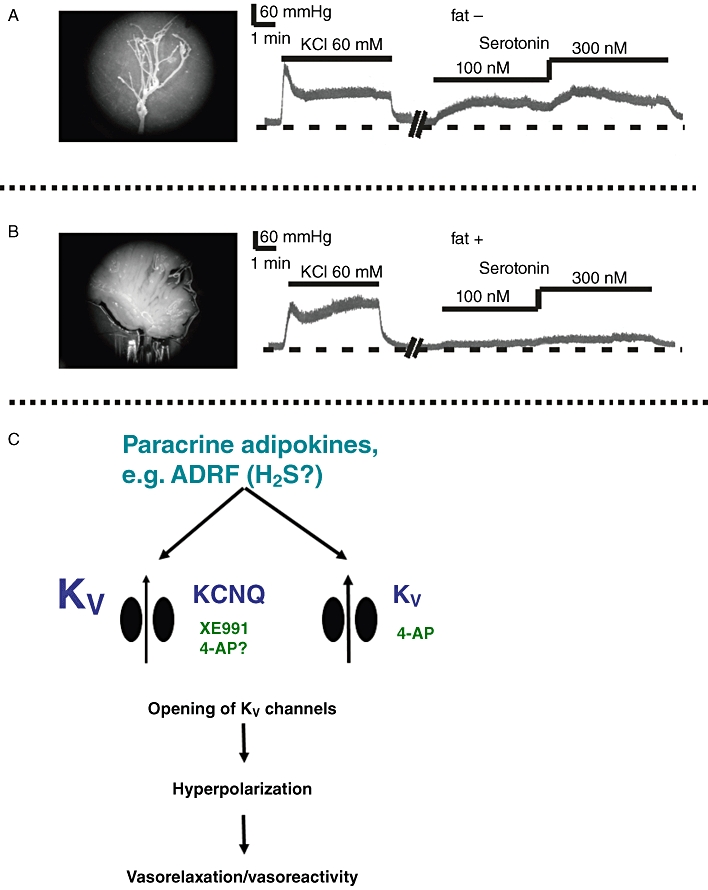
Perivascular fat limits vascular reactivity to serotonin in mouse mesenteric vascular beds. Shown are original recordings of perfusion pressure for perfused isolated mesenteric vascular beds in the absence (fat –) and presence (fat +) of perivascular fat (modified from Fesus et al., 2007). Dashed lines represent 30 mmHg. Horizontal bars show the presence of the drugs. The perivascular fat was carefully removed in fat – beds (A) or left intact (B). C. Periadventitial vasoregulation of visceral arteries by ADRF. ADRF is released by periadventitial adipocytes in response to vasoconstrictors (such as serotonin) and primarily produces endothelium-independent vasorelaxation by opening voltage-dependent (Kv) K+ channels in the plasma membrane of smooth muscle cells. At least in part, KCNQ (Kv7) channels could represent the subtype of Kv channels involved. KCNQ channels are blocked by XE991. It is possible that additional subtypes of Kv channels play a role in the effects of ADRF, which are sensitive to 4-AP. H2S could represent ADRF.
KCNQ channels were previously described as targets for vasopressin in aortic smooth A7r5 cells (Brueggemann et al., 2007). Yeung et al. (2007) detected mRNA of KCNQ1, 4 and 5 mesenteric arteries, in aorta, carotid arteries and femoral arteries of mice, with KCNQ4 being the most abundant (Yeung et al., 2007). Notably, intracellular tetraethylammonium ions (TEA) are known to block KCNQ current by a mechanism that is distinct from the inhibition by polyvalent cations (Hadley et al., 2000; 2003; Shah et al., 2002; Koyama and Appel, 2006; Schwarz et al., 2006; Suh and Hille, 2007). These effects may explain why some authors have observed that the anti-contractile effects of perivascular fat are inhibited by TEA (Gao et al., 2007; Takemori et al., 2007; Zeng et al., 2009). The role of BKCa channels has been ruled out using a genetic approach. Accordingly, mice with genetically inactivated large Ca-activated K+ channels (BKCa) in smooth muscle (Pluger et al., 2000; Sausbier et al., 2005) showed normal anti-contractile effects of ADRF (Fesus et al., 2007). Nevertheless, the KCNQ channel subtype involved in the ADRF effects is unknown. Furthermore, direct evidence for membrane hyperpolarizing effects of ADRF in arterial smooth muscle is shown only for rat mesenteric arteries (Verlohren et al., 2004c). Direct evidence for activation of KCNQ channels is still missing. Further studies with gene-silencing or knock-out technologies might help to confirm these observations and to determine the KCNQ channel subtypes and possibly other Kv channels activated by ADRF (Figure 1). Nonetheless, the data support the novel concept that periadventitial adipose tissue represents a paracrine tissue to produce membrane hyperpolarization and vasorelaxation by opening Kv channels in arterial smooth muscle cells. The effects appear to be mediated largely by opening KCNQ-type Kv channels. These data suggest that a promising candidate for ADRF should fulfill the requirement to mimic ADRF characteristic effects on K+ channels, i.e. being a potent KCNQ channel opener.
Notably, KCNQ channels in arterial smooth muscle cells can contribute to the resting membrane and carry remarkably large outward currents in response to membrane depolarisations (Yeung and Greenwood, 2005; Mackie et al., 2008; Greenwood and Ohya, 2009; Gurney et al., 2010). Thus, KCNQ channels exhibit a large hyperpolarisation reserve and are enabled to fulfil physiological vasodilatory functions in the presence of perivascular fat, i.e. in response to ADRF. Moreover, since ADRF is released from perivascular adipocytes, KCNQ channels seem to play a unique role for vasodilatory signals from outside the vessel (‘outside control’). KCNQ channels are not targeted by endothelium-derived relaxing factors (EDRF) (‘inside control’), including NO, prostaglandin I2 (prostacyclin) and endothelium-derived hyperpolarizing factor (EDHF). Although exogenous H2O2 as candidate EDHF has been reported to open 4-aminopyridine (4-AP)-sensitive Kv channels in certain vascular beds (Rogers et al., 2007), endogenously produced H2O2 induces EDHF-like responses independent of Kv (KCNQ) channels (Hercule et al., 2009). Moreover, superoxide produced by perivascular fat in response to electrical field nerve stimulation does not produce vasorelaxation, but rather vasoconstriction (Gao et al., 2006). Furthermore, H2O2 generated by perivascular adipose tissue has been reported to produce vasodilation via smooth muscle soluble guanylyl cyclase (sGC) without activation of large-conductance, Ca2+ activated K+ (BKCa) channels (Gao et al., 2007), which is in contrast to previous findings demonstrating that BKCa channels are strongly activated by the sGC/cGMP/PKG pathway [for example (Robertson et al., 1993)]. Ang-(1–7) released by perivascular adipose tissue has been reported to cause endothelial release of NO, which leads to vasorelaxation by opening large-conductance, Ca2+ activated K+ (BKCa) channels (Lee et al., 2009). Together, it can be concluded that KCNQ channels in visceral arteries are not targeted by physiological vasodilators released from the luminal side of arteries, i.e. from endothelial cells (‘inside control’), and from perivascular nerve endings. KCNQ channels are also not targeted by other perivascular candidates, such as H2O2, superoxide and Ang (1–7). The data support the novel concept that ADRF (hydrogen sulphide, H2S) is the physiological activator of KCNQ channels, at least in part, in visceral arteries (Figure 1). Should our hypothesis be confirmed, we will have identified a novel role of KCNQ channels in arterial smooth muscle function.
ADRF and small vessels
The aforementioned studies addressed ADRF effects in relatively large vessels, vessels that do not contribute greatly to peripheral vascular resistance. In contrast, smooth muscle tone in small arteries and arterioles of the microcirculation is an important determinant of peripheral vascular resistance and hence blood pressure. In more recent studies, periadventitial vasoregulation was studied in smaller visceral arteries of rats and mice (Verlohren et al., 2004b; Fesus et al., 2007). In these studies, periadventitial adipose tissue significantly attenuated vascular responsiveness of small mesenteric arteries to several hormonal agonists, including serotonin, phenylephrine and endothelin-1. Inhibition of the contractile response to serotonin by fat depended on the amount of fat on each ring but did not depend on the presence of endothelium. The anti-contractile effect of periadventitial fat was abolished by inhibition of Kv channels using 4-AP or 3,4-diaminopyridine. It was largely inhibited by XE991 (Schleifenbaum et al., 2010). However, it was not abolished by pharmacological inhibition of BKCa channels, KATP channels, small-conductance calcium-activated K+ channels and inward rectifying K+ channels (Verlohren et al., 2004b; Fesus et al., 2007). Experiments on knockout mice lacking the β1 subunit of BKCa channels confirmed no involvement of BKCa channels in ADRF effects (Fesus et al., 2007). The resting membrane potential of smooth muscle cells in intact mesenteric artery rings surrounded by fat was more hyperpolarized than rings without periadventitial fat; this difference was abolished by Kv channel inhibition with 4-AP (Verlohren et al., 2004b). Collectively, these data provide strong evidence that the anti-contractile effects of adipose tissue in small visceral arteries are mediated by activation of smooth muscle Kv channels, leading to membrane hyperpolarization, reduced Ca2+ influx into smooth muscle cells and vasorelaxation. KCNQ-type Kv channels seem to play a major role in these effects.
Interestingly, ADRF effects occur also in mesenteric resistance vessels exhibiting myogenic tone. Fesus et al. (2007) perfused the isolated mesenteric vascular bed at a constant flow in the absence and presence of perivascular fat. KCl (60 mM) induced similar increases in perfusion pressure in the presence and absence of perivascular fat. However, serotonin induced stronger increases in perfusion pressure in isolated mesenteric vascular beds without fat, compared with mesenteric vascular beds with fat of wild-type mice. Kv channel inhibition with 4-AP abrogated the anti-contractile effects of perivascular fat. The experiments with fat-removed mesenteric vascular beds showed that these effects were solely dependent on the presence of perivascular fat (Figure 1). Adiponectin was not involved. This report was the first to show a direct vasodilating action of perivascular fat on resistance vessels exhibiting myogenic tone in a perfused organ and under flow (Fesus et al., 2007). These results did also demonstrate that the anti-contractile effects of perivascular fat occur through access of ADRF from outside the vessel (‘outside control’). This consideration is important because most studies on periadventitial vasoregulation rely on open-ended ring preparations, which are commonly used in wire myography. In wire myography, substances released from the adipose tissue could have had their effects simply as a result of their diffusion to the intraluminal side of the vessel through the open ends (‘inside control’). In a normal in vivo situation, this could not occur. Thus, the results of Fesus et al. indicate that periadventitial vasoregulation is not only a phenomenon of open-ended isolated arteries in wire myography but is also present in intact visceral mesenteric beds in vivo.
Candidates for ADRF
Protein bands with relative masses of 74.0, 59.8, 54.4, 28.7 and 13.8 kDa have been identified as candidates for ADRF in the rat aorta (Yang et al., 2005). ADRF is released by periadventitial adipose tissue in a Ca2+-dependent manner. Although half-maximal release of ADRF occurred at relatively high Ca2+ concentrations (4.7 mM), the Hill coefficient of ∼1 indicates that the release can be modulated by changes of the plasma Ca2+ concentrations in vivo (Dubrovska et al., 2004). This release is also inhibited by blockers of tyrosine kinase and protein kinase A (Dubrovska et al., 2004). Moreover, bioassay experiments showed that ADRF is inactivated or caused to disappear by heating (65°C) and is not adsorbed by essentially fatty acid-free serum albumin (Lohn et al., 2002). Interestingly, hypoxia aggravates ADRF effects in the aorta (Maenhaut et al., 2010). We explored the possibility that ADRF might be a volatile, gaseous mediator that disappears into the atmosphere during purification (Schleifenbaum et al., 2010). Previously, we demonstrated that NO is not ADRF (Lohn et al., 2002; Verlohren et al., 2004c). We tested the possibility that ADRF might be another biological active gaseous mediator, such as H2S or carbon monoxide (CO). H2S is a newly discovered physiologic vasorelaxant generated by cystathionine gamma lyase (CSE) (Yang et al., 2008). This enzyme is expressed in perivascular adipose tissue and endogenously generates H2S (Feng et al., 2009). Lack of CSE induces systemic hypertension in mice (Yang et al., 2008) (but see Ishii et al., 2010). We found that inhibitors of CSE inhibited the anti-contractile effects of perivascular fat (Gollasch and Dubrovska, 2009; Schleifenbaum et al., 2010). Moreover, we found that the effects of ADRF can be mimicked by synthetic KCNQ channel openers in conditions of reduced H2S release from perivascular adipose tissue. Furthermore, the H2S donor NaHS produced dose-dependent vasorelaxation, which was blocked by the KCNQ channel blocker XE991 (Gollasch and Dubrovska, 2009; Schleifenbaum et al., 2010). In contrast, the heme oxygenase inhibitors tin mesoporphyrin and zinc deuteroporphyrin IX had no effects on the anti-contractile effects of perivascular fat. Hemin, a substrate of heme oxygenase, induced vasorelaxation of tibial and mesenteric arteries. The effects were not inhibited by XE991 (Schleifenbaum et al., 2010). The effects were also neither inhibited by the Kv channel blocker 4-AP nor by the Ca2+-activated large-conductance BKCa channel blocker iberiotoxin or KATP channel blocker glibenclamide (Essin et al., 2008). Notably, CO generated by heme oxygenase in astrocytes can induce cerebral vasodilation by activating smooth muscle BKCa channels (Li et al., 2008). Nonetheless, the data indicate that H2S represents a candidate or modulator of ADRF (Figure 1).
Notably, Fang et al. (2009) obtained similar results with CSE inhibitors in the rat aorta. In an elegant series of experiments, they measured endogenous H2S production in the aorta and found that serotonin, phenylephrine and angiotensin II increase H2S release from periadventitial adipose tissue. Release of H2S is decreased with aging of rats and can be blocked by CSE inhibitors (Fang et al., 2009). We are aware that the H2S data summarized here are preliminary, and that several weaknesses remain in the methodological approaches (Tangerman, 2009). There are no data on genetically engineered mice available that might define the importance of H2S that might open KCNQ channels. More studies are needed to demonstrate KCNQ channel activation by H2S and to determine the molecular mechanisms of channel activation.
Perivadventitial regulation in human arteries
The first demonstration that perivascular adipose tissue mediates an anti-contractile effect in human arteries compared to K+-induced contraction comes from studies on isolated small mesenteric arteries (Verlohren et al., 2004a). Although Kv channel blockade was not tested in this study, the aforementioned data indicate critical roles of Kv channels and H2S in the periadventitial vasoregulation of this type of arteries. Subsequently, periadventitial adipose tissue was reported to inhibit arterial contraction of human internal thoracic arteries (Gao et al., 2004). Significantly, blocking the BKCa channel by TEA or iberiotoxin attenuated the anti-contractile effects of periadventitial fat in human internal thoracic arteries, suggesting activation of large-conductance, calcium-activated potassium (BKCa) channels is functionally relevant, and that there are regional vascular or species differences (Gao et al., 2004). However, there is no direct evidence that membrane hyperpolarization and BKCa channel opening occurred by the transferable factor released from perivascular fat in internal thoracic arteries. Greenstein et al. (2009) were the first to demonstrate a complete loss of the anti-contractile effect of perivascular fat in patients with obesity and metabolic syndrome. In their study, periadventitial vasoregulation has been studied in small arteries taken from subcutaneous gluteal fat biopsy samples (Greenstein et al., 2009). This study showed that healthy adipose tissue around small arteries secretes factors that influence vasodilation by increasing NO bioavailability. Adiponectin was suggested to play a major role in this effect (Greenstein et al., 2009). Notably, in subcutaneous arteries from obese subjects with metabolic syndrome, the dilator effect was lost, suggesting malfunction of adiponectin. Epicardial perivascular fat-derived leptin has been found to exacerbate coronary endothelial dysfunction in a swine model of metabolic syndrome (Payne et al., 2010). These latter findings have been implicated to play a role in ‘outside to inside’ signalling for perivascular adipose tissue-derived factors in the regulation of coronary tone and pathogenesis of coronary artery disease. Of note, previous studies on adiponectin gene-deficient mice and Zucker fa/fa rats (that lack functional leptin receptors) ruled out that adiponectin and leptin are ADRFs in visceral arteries of rats and mice (Fesus et al., 2007; Lohn et al., 2002), despite the fact the adiponectin exhibits humoral vasodilatory properties possibly via activation of 4-AP-sensitive Kv channels (Fesus et al., 2007). Interestingly, no correlation could be detected between perivascular adipose tissue and local endothelial function in conduit skeletal arteries (i.e. the brachial artery) of humans (Rittig et al., 2008), arguing against ‘outside to inside’ signalling as universal paradigm of perivascular vasoregulation. Together, these data indicate that there are regional vascular and species differences in periadventitial vasoregulation.
Animal models of hypertension and metabolic diseases
Aging and obesity are independent risk factors for hypertension. Spontaneously hypertensive (SHR) rats and New Zealand obese mice are models of lean and obese hypertension respectively. Fang et al. (2009) recently showed that in aging normotensive rats, endogenous H2S production in aortic tissues and isolated perivascular adipose tissue were decreased, while CSE protein expression levels increased. In SHR rats, gene expression of CSE and activity of CSE were suppressed in thoracic aorta, whereas plasma levels of H2S were decreased (Yan et al., 2004). ADRF also malfunctions in visceral arteries of SHR rats and New Zealand obese mice with increased age (Galvez et al., 2006; Fesus et al., 2007; Galvez-Prieto et al., 2008b). This malfunction has been confirmed by others (Marchesi et al., 2009; Zeng et al., 2009). It does not involve adiponectin (Fesus et al., 2007) but may be improved by atorvastatin, which inhibits the mevalonate pathway or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway (Zeng et al., 2009). Although CSE expression and endogenous H2S production have not been studied in perivascular adipose tissue of those animal models, the findings raise the notion that a malfunctioning CSE/H2S pathway is likely involved in the pathogenesis of hypertension. With the advent of NO, a precedent for novel gas molecules as pivotal signalling pathway regulators is now routinely accepted. We predict the same for H2S. ADRF, or perhaps its putative targets, might represent exciting new targets for the development of drugs for treatment of cardiovascular and metabolic disorders.
Targeting the amount of perivascular fat
There is evidence that changes in lifestyle, including diet, exercise and nicotine, can affect the paracrine regulation of arterial tone by perivascular fat. Porcine coronary perivascular adipose tissue exhibits blunting of vasoconstrictor responses to endothelin-1 similar to those reported previously in rat aorta and visceral arteries (Reifenberger et al., 2007). This effect is not influenced by diet. However, exercise training abolished the effect of perivascular adipose tissue on endothelin-1-induced vasoconstriction in pigs on normal fat diet but not in the pigs on high fat diet (Reifenberger et al., 2007). Moreover, periadventitial vasoregulation was also studied in Wistar rats on a high-fat diet for 6 months. The results show that rats on high-fat diet had higher periaortic fat mass compared with rats on a chow diet. Interestingly, obesity induced impairment of endothelium-dependent relaxation of the aorta, which was markedly attenuated by temporary periaortic fat removal. (Ma et al., 2010). Furthermore, periadventitial fat can also promote endothelial dysfunction in diet-induced obese C57Bl/6 mice via mechanisms that are linked to increased NADPH oxidase-derived oxidative stress and increased production of pro-inflammatory cytokines (Ketonen et al., 2010). Finally, fetal and neonatal exposure of Wistar–Kyoto rats to nicotine increased the number of adipocytes in aortic perivascular adipose tissue. This increase was associated with blood pressure elevation and reduced periadventitial vasodilation in response to vasoconstrictors (Gao et al., 2008). These data may indicate that there is a reciprocal relationship between the amount of perivascular fat and periadventitial vasorelaxation by perivascular adipose tissue. However, this appears not to be the case. Takemori et al. studied using lipoatrophic A-ZIP/F1 transgenic mice. Only a small amount of brown fat was found around the aorta but not around mesenteric arteries. Blood pressure was measured by the tail-cuff method. Blood pressure of A-ZIP/F1 mice became higher than wild-type mice from 10 weeks of age. The presence of perivascular fat reduced the contraction of wild-type aorta to phenylephrine and serotonin, whereas this effect was either absent or less prominent in A-ZIP/F1 aorta. These results suggest that the absence of perivascular fat tissue, which enhances the contractile response of the blood vessels to agonists, and an up-regulation of vascular Ang II type 1 receptors in A-ZIP/F1 mice are some of the mechanisms underlying the blood pressure elevation in these lipoatrophic mice (Takemori et al., 2007). Together, the data indicate that a certain amount of perivascular adipose tissue is required for optimum periadventitial vasoregulation and blood pressure regulation. Notably, targeting existing vessels in white adipose tissue by a pro-apoptotic peptide to prohibitin caused ablation of white fat in obese mice and rapid obesity reversal and metabolic normalization (Kolonin et al., 2004). Targeted ablation of the vasculature that feeds adipose tissue might not only cause weight loss but also represent a novel therapeutic approach to treat periadventitial vasodysregulation and blood pressure.
Concluding remarks
The focus on vascular research has changed over the past years. Most early functional studies characterized the effects of various agents on arterial smooth muscle and the involved receptor subtypes. The identification of NO as EDRF in the 1980s reoriented vascular research. The endothelium is now considered a paracrine tissue that controls the homeostasis of the underlying smooth muscle cell layer by production of a number of substances. Endothelial dysfunction plays an important role in atherosclerosis. Recent studies proposed the concept of paracrine role for periadventitial adipose tissue in the regulation of vascular tone (Gollasch and Dubrovska, 2004). This regulation involves ADRF, which is released by periadventitial adipocytes and produces vasorelaxation by opening Kv channels in smooth muscle cells. The H2S findings as candidate ADRF warrant diligent pursuit. We believe that there is more to ADRF than H2S; further research is necessary. Adipocyte-derived factors and their targets, including KCNQ channels, could represent novel drug targets for regulating vascular tone and for the treatment of obesity-associated cardiovascular disorders.
Acknowledgments
The experiments cited here obviously represent work of a large number of scientists, and I would like to thank previous and current members of our laboratory in Berlin. In particular, I wish to express my special gratitude to Galyna Dubrovska. Without her exemplary persistence and experimental skills, we would not have been able to make the progress presented here. I wish to thank Johanna Schleifenbaum, Yoland-Marie Anistan, Carolin Köhn, Mario Kaßmann, Wolf-Hagen Schunck, Michael Rothe, Friedrich C. Luft and Rudolf Schubert for collaborative work on this emerging area of interest over the past several years. I would also like to express my thanks to the Deutsche Forschungsgemeinschaft for continuous support, which enabled us to pursue this exciting research direction.
Glossary
- 4-AP
4-aminoyporidine
- ADRF
adipocyte-derived relaxing factor
- ASP
acylation stimulation protein
- BKCa, large-conductance
Ca2+-activated K+ channel
- CSE
cystathionine gamma lyase
- SHR
spontaneously hypertensive rats
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- HMG-CoA
hydroxy-3-methylglutaryl-coenzyme A
- IGF-1
insulin-like growth factor
- PAI-1
plasminogen activator protein
- KATP
glibenclamide-sensitive K+ channel
- Kv
voltage-dependent K+ channel
- RAS
renin–angiotensin system
- sGC
soluble guanylyl cyclase
- ACRP30
adiponectin (ACRP30)
- XE991
10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride
Conflicts of interest
There is no conflict of interest.
References
- Baudry N, Vicaut E. Role of nitric oxide in effects of tumor necrosis factor-alpha on microcirculation in rat. J Appl Physiol. 1993;75:2392–2399. doi: 10.1152/jappl.1993.75.6.2392. [DOI] [PubMed] [Google Scholar]
- Brian JE, Jr, Faraci FM. Tumor necrosis factor-alpha-induced dilatation of cerebral arterioles. Stroke. 1998;29:509–515. doi: 10.1161/01.str.29.2.509. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Moran CJ, Barakat JA, Yeh JZ, Cribbs LL, Byron KL. Vasopressin stimulates action potential firing by protein kinase C-dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H1352–H1363. doi: 10.1152/ajpheart.00065.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. TNF-alpha dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation. Am J Physiol Cell Physiol. 2006;290:C964–C971. doi: 10.1152/ajpcell.00499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest. 2002;25:823–835. doi: 10.1007/BF03344046. [DOI] [PubMed] [Google Scholar]
- Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1107–H1113. doi: 10.1152/ajpheart.00656.2003. [DOI] [PubMed] [Google Scholar]
- Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, et al. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- Essin K, Schleifenbaum J, Luft FC, Gollasch M. Carbon monoxide targets the pore-forming BK alpha subunit in vascular smooth muscle Ca2+-activated large-conductance K+ channels. FASEB J. 2008;22:1206.5–1206. [Google Scholar]
- Fang L, Zhao J, Chen Y, Ma T, Xu G, Tang C, et al. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens. 2009;27:1034–1049. doi: 10.1097/HJH.0b013e328330a900. [DOI] [PubMed] [Google Scholar]
- Feng X, Chen Y, Zhao J, Tang C, Jiang Z, Geng B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem Biophys Res Commun. 2009;380:153–159. doi: 10.1016/j.bbrc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- Fortuno A, Rodriguez A, Gomez-Ambrosi J, Fruhbeck G, Diez J. Adipose tissue as an endocrine organ: role of leptin and adiponectin in the pathogenesis of cardiovascular diseases. J Physiol Biochem. 2003;59:51–60. doi: 10.1007/BF03179868. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes. 1999;48:903–908. doi: 10.2337/diabetes.48.4.903. [DOI] [PubMed] [Google Scholar]
- Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, et al. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008a;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- Galvez-Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, Gonzalez MC, et al. A reduction in the amount and anti-contractile effect of periadventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res. 2008b;31:1415–1423. doi: 10.1291/hypres.31.1415. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zeng ZH, Teoh K, Abouzahr L, Cybulsky I, Lamy A, et al. Perivascular adipose tissue releases a vasodilator in human internal thoracic artery. J Vasc Res. 2004;41(Suppl. 1):20. [Google Scholar]
- Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. 2005;130:1130–1136. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee RM. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol. 2008;590:264–268. doi: 10.1016/j.ejphar.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25:647–653. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gollasch M, Dubrovska G. Adventitia derived relaxing factor. MOVD. 2009;1 SSY-5, 96. [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Ohya S. New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol. 2009;156:1196–1203. doi: 10.1111/j.1476-5381.2009.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney AM, Joshi S, Manoury B. KCNQ potassium channels: new targets for pulmonary vasodilator drugs? Adv Exp Med Biol. 2010;661:405–417. doi: 10.1007/978-1-60761-500-2_26. [DOI] [PubMed] [Google Scholar]
- Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels. Br J Pharmacol. 2000;129:413–415. doi: 10.1038/sj.bjp.0703086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, et al. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J Neurosci. 2003;23:5012–5019. doi: 10.1523/JNEUROSCI.23-12-05012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14((Pt 2)):103S–115S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl. 1):S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, et al. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, Suematsu M. Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem. 2010;285:26358–26368. doi: 10.1074/jbc.M110.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Webb RC. TNF-alpha-induced endothelium-independent vasodilation: a role for phospholipase A2-dependent ceramide signaling. Am J Physiol. 1998;275:H1592–H1598. doi: 10.1152/ajpheart.1998.275.5.H1592. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010;74:1479–1487. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, et al. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun. 2000;273:745–749. doi: 10.1006/bbrc.2000.3005. [DOI] [PubMed] [Google Scholar]
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- Koyama S, Appel SB. Characterization of M-current in ventral tegmental area dopamine neurons. J Neurophysiol. 2006;96:535–543. doi: 10.1152/jn.00574.2005. [DOI] [PubMed] [Google Scholar]
- Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, et al. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, et al. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. Faseb J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- Loughrey JP, Laffey JG, Moore BJ, Lynch F, Boylan JF, McLoughlin P. Interleukin-1 beta rapidly inhibits aortic endothelium-dependent relaxation by a DNA transcription-dependent mechanism. Crit Care Med. 2003;31:910–915. doi: 10.1097/01.CCM.0000053516.15727.E5. [DOI] [PubMed] [Google Scholar]
- Ma L, Ma S, He H, Yang D, Chen X, Luo Z, et al. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res. 2010;33:446–453. doi: 10.1038/hr.2010.11. [DOI] [PubMed] [Google Scholar]
- Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, et al. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2008;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenhaut N, Boydens C, Van de Voorde J. Hypoxia enhances the relaxing influence of perivascular adipose tissue in isolated mice aorta. Eur J Pharmacol. 2010;641:207–212. doi: 10.1016/j.ejphar.2010.05.058. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- Messerli FH, Sundgaard-Riise K, Reisin E, Dreslinski G, Dunn FG, Frohlich E. Disparate cardiovascular effects of obesity and arterial hypertension. Am J Med. 1983;74:808–812. doi: 10.1016/0002-9343(83)91071-9. [DOI] [PubMed] [Google Scholar]
- Ohkawa F, Ikeda U, Kawasaki K, Kusano E, Igarashi M, Shimada K. Inhibitory effect of interleukin-6 on vascular smooth muscle contraction. Am J Physiol. 1994;266((Pt 2)):H898–H902. doi: 10.1152/ajpheart.1994.266.3.H898. [DOI] [PubMed] [Google Scholar]
- Park Y, Yang J, Zhang H, Chen X, Zhang C. Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106:111–123. doi: 10.1007/s00395-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, et al. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- Reifenberger MS, Turk JR, Newcomer SC, Booth FW, Laughlin MH. Perivascular fat alters reactivity of coronary artery: effects of diet and exercise. Med Sci Sports Exerc. 2007;39:2125–2134. doi: 10.1249/mss.0b013e318156e9df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig K, Staib K, Machann J, Bottcher M, Peter A, Schick F, et al. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia. 2008;51:2093–2099. doi: 10.1007/s00125-008-1128-3. [DOI] [PubMed] [Google Scholar]
- Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265((Pt 1)):C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Rocchini AP, Moorehead C, Katch V, Key J, Finta KM. Forearm resistance vessel abnormalities and insulin resistance in obese adolescents. Hypertension. 1992;19:615–620. doi: 10.1161/01.hyp.19.6.615. [DOI] [PubMed] [Google Scholar]
- Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- Sahin AS, Bariskaner H. The mechanisms of vasorelaxant effect of leptin on isolated rabbit aorta. Fundam Clin Pharmacol. 2007;21:595–600. doi: 10.1111/j.1472-8206.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- Salcedo A, Garijo J, Monge L, Fernandez N, Luis Garcia-Villalon A, Sanchez Turrion V, et al. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul Pept. 2007;144:50–55. doi: 10.1016/j.regpep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, et al. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, et al. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, et al. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol. 2002;544:29–37. doi: 10.1113/jphysiol.2002.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AM. Obesity and cardiovascular risk. Growth Horm IGF Res. 2003;13(Suppl. A):S10–S17. doi: 10.1016/s1096-6374(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Shibata M, Parfenova H, Zuckerman SL, Leffler CW. Tumor necrosis factor-alpha induces pial arteriolar dilation in newborn pigs. Brain Res Bull. 1996;39:241–247. doi: 10.1016/0361-9230(95)02142-6. [DOI] [PubMed] [Google Scholar]
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Electrostatic interaction of internal Mg2+ with membrane PIP2 Seen with KCNQ K+ channels. J Gen Physiol. 2007;130:241–256. doi: 10.1085/jgp.200709821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori K, Gao YJ, Ding L, Lu C, Su LY, An WS, et al. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007;49:365–372. doi: 10.1161/01.HYP.0000255576.16089.b9. [DOI] [PubMed] [Google Scholar]
- Tangerman A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Dubrovska G, Luft FC, Gollasch M. Control of arterial tone by perivascular adipose tissue. Recent Res Devel Physiol. 2004a;2:259–264. [Google Scholar]
- Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004b;44:1–6. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004c;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- Wagner EM. TNF-alpha induced bronchial vasoconstriction. Am J Physiol Heart Circ Physiol. 2000;279:H946–H951. doi: 10.1152/ajpheart.2000.279.3.H946. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The World Health Report 1998:Life in the 21st Century. Geneva: World Health Organization; 1998. [Google Scholar]
- Yamawaki H, Hara N, Okada M, Hara Y. Visfatin causes endothelium-dependent relaxation in isolated blood vessels. Biochem Biophys Res Commun. 2009;383:503–508. doi: 10.1016/j.bbrc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–672. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- Yang L, Hu BR, Xiang JZ, Wang JL. Adventitium-derived relaxing factor may be a protein factor secreted by adipocytes with non-species-specificity and not limited to periadventitial fat. Chin J Pharmacol Toxicol. 2005;19:401–406. [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, et al. Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1850–H1858. doi: 10.1152/ajpheart.01199.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SY, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SY, Pucovsky V, Moffatt JD, Saldanha L, Schwake M, Ohya S, et al. Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol. 2007;151:758–770. doi: 10.1038/sj.bjp.0707284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel MB. Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem. 1998;188:129–136. [PubMed] [Google Scholar]
- Zeng ZH, Zhang ZH, Luo BH, He WK, Liang LY, He CC, et al. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin Exp Hypertens. 2009;31:355–363. doi: 10.1080/10641960902977916. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Yi FX, Zou AP, Li PL. Role of ceramide in TNF-alpha-induced impairment of endothelium-dependent vasorelaxation in coronary arteries. Am J Physiol Heart Circ Physiol. 2002;283:H1785–H1794. doi: 10.1152/ajpheart.00318.2002. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006;40:247–257. doi: 10.1016/j.yjmcc.2005.11.010. [DOI] [PubMed] [Google Scholar]


