Abstract
Adipose tissue is the largest endocrine organ, producing various adipokines and many other substances. Almost all blood vessels are surrounded by perivascular adipose tissue (PVAT), which has not received research attention until recently. This review will discuss the paracrine actions of PVAT on the growth of underlying vascular smooth muscle cells (VSMCs). PVAT can release growth factors and inhibitors. Visfatin is the first identified growth factor derived from PVAT. Decreased adiponectin and increased tumour necrosis factor-α in PVAT play a pathological role for neointimal hyperplasia after endovascular injury. PVAT-derived angiotensin II, angiotensin 1–7, reactive oxygen species, complement component 3, NO and H2S have a paracrine action on VSMC contraction, endothelial or fibroblast function; however, their paracrine actions on VSMC growth remain to be directly verified. Factors such as monocyte chemoattractant protein-1, interleukin-6, interleukin-8, leptin, resistin, plasminogen activator inhibitor type-1, adrenomedullin, free fatty acids, glucocorticoids and sex hormones can be released from adipose tissue and can regulate VSMC growth. Most of them have been verified for their secretion by PVAT; however, their paracrine functions are unknown. Obesity, vascular injury, aging and infection may affect PVAT, causing adipocyte abnormality and inflammatory cell infiltration, inducing imbalance of PVAT-derived growth factors and inhibitors, leading to VSMC growth and finally resulting in development of proliferative vascular disease, including atherosclerosis, restenosis and hypertension. In the future, using cell-specific gene interventions and local treatments may provide definitive evidence for identification of key factor(s) involved in PVAT dysfunction-induced vascular disease and thus may help to develop new therapies.
LINKED ARTICLES
This article is part of a themed section on Fat and Vascular Responsiveness. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-3
Keywords: perivascular adipose tissue, paracrine, vascular smooth muscle cell, adipokine, nitric oxide, hydrogen sulphide, reactive oxygen species, obesity, inflammation, vascular disease
Introduction
Obesity, defined as excess body fat, is epidemic (Ogden et al., 2006) and harmful to health because it increases the risk of cardiovascular disease, type 2 diabetes and some cancers (Wisse et al., 2007). Adipose tissue is far from being considered simply a passive reservoir for energy storage. Instead, adipose tissue is the largest endocrine organ (Halvorsen et al., 2000; Kahn, 2008) that can produce and secrete many substances, such as various proteins/peptides termed adipokines, free fatty acids (FFA) and steroid hormones, which have essential roles in energy homeostasis, glucose and lipid metabolism, cell viability, control of feeding, thermogenesis, neuroendocrine function, reproduction, immunity and cardiovascular function (Flier, 2004; Kershaw and Flier, 2004). Most research so far has focused on the endocrine roles of those substances in the development of vascular disease without considering the paracrine role of the adipose tissue surrounding the blood vessels. In fact, blood vessels are composed of three layers: intima, media and adventitia (Figure 1). The adventitia harbouring nerve endings can be divided into two sub-layers: adventitial compacta containing as main cell-type fibroblasts and adventitial fat containing as main cell-type adipocytes. Adventitial fat is also called perivascular fat or perivascular adipose tissue (PVAT); it surrounds almost all blood vessels except the cerebral vasculature (Gao, 2007). Traditionally, the study of vascular function regulation has been based on layer-specific mechanisms. The intimal endothelial cells can produce and release a variety of vasoactive substances to modulate medial vascular smooth muscle cell (VSMC) function (Busse et al., 2002; Miao et al., 2005; Feletou et al., 2008). The nerves play an important role in the regulation of medial function (Gutterman, 1999). And the adventitial compacta may also regulate medial function (Gonzalez et al., 2001; Sartore et al., 2001; Somoza et al., 2005). However, PVAT had long been thought to provide only structural support for the blood vessel and has been routinely removed in the traditional isolated blood vessel studies.
Figure 1.
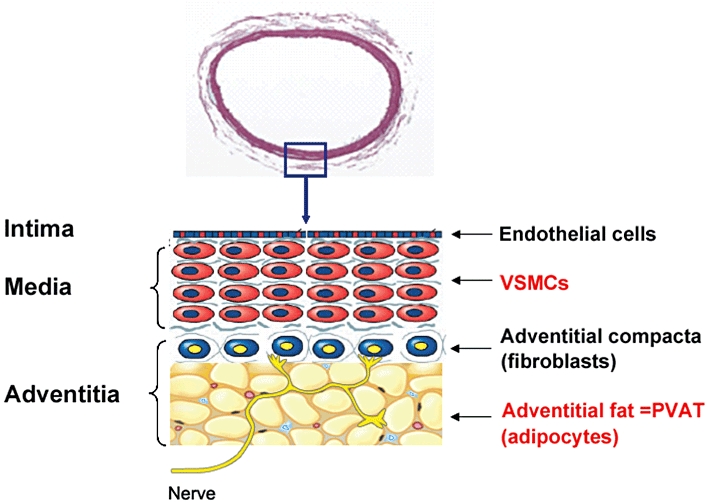
Vascular layer structure. In the PVAT, the yellow, red and blue, respectively, indicate adipocytes, vasa vasorum and other cells (macrophages, adipocyte stem/progenitor cells, lymphocytes, fibroblasts, etc.).
Given that adipocytes can secrete numerous active substances, and since perivascular adipocytes encroach into the adventitial compacta without an anatomical barrier, mediators secreted by PVAT may readily gain access into the blood vessel wall, and PVAT may function as a paracrine organ that transduces metabolic signals to blood vessels (Rajsheker et al., 2010). However, little attention had been paid to this possibility until Soltis and Cassis demonstrated that PVAT attenuates the contractile response to norepinephrine in the rat aorta (Soltis and Cassis, 1991), and Lohn et al. demonstrated that PVAT releases an unknown relaxing factor (Lohn et al., 2002). In 2005, Barandier et al. demonstrated that PVAT secreted growth factors, which can stimulate the proliferation of VSMCs, providing a new notion that PVAT plays roles in not only vascular tone but also other biological properties of VSMCs and thus opening new perspectives in vascular biology. Thereafter, we identified visfatin, an adipokine, as a PVAT-derived growth factor for VSMCs (Wang et al., 2009). Most recently, in vivo experiments demonstrated that implanting adipose tissue to surround the blood vessel may affect neointimal formation following endovascular injury, indicating the role of PVAT in VSMC growth and migration (Takaoka et al., 2009). The vascular surrounding tissue (PVAT mainly) was also pointed out being important for saphenous vein graft patency in pioneer human studies (Souza et al., 2001; Souza et al., 2006). Here, we will review the influence of PVAT on VSMC growth and migration and discuss its possible role and potential therapy in proliferative vascular diseases.
PVAT structure and heterogeneity
Two types of adipose tissue exist in the body: brown adipose tissue as non-shivering thermogenerator and white adipose tissue as depository for excessive energy. They are endowed with distinct vascular and nervous supplies: the brown adipose tissue has a greater density of capillaries and noradrenergic fibres than white adipose tissue. PVAT, like other adipose depots, has a mixed composition of both types of adipose tissue, with different white/brown adipose tissue ratio in different organs (Cinti, 2011). In Sv129 mice, the mediastinal depot (coronary artery and thoracic aorta) consists mainly of brown adipose tissue, the abdomino-pelvic depot (iliofemoral vessels) is equally composed of white and brown adipose tissue, while the retroperitoneal and mesenteric depots (abdominal aorta and mesenteric artery) contain mainly white adipose tissue (Frontini and Cinti, 2010; Cinti, 2011). Our results showed that in Sprague–Dawley rats, PVAT surrounding thoracic aorta is mainly brown adipose tissue (Wang et al., 2009). Moreover, it is obviously different from the PVAT surrounding abdominal aorta (Figure 2A) and mesenteric artery (Figure 2B) and the inguinal subcutaneous adipose tissue (Figure 2C), which are mainly white adipose tissue. In humans, brown adipose tissue is mainly located in PVAT around the aorta and its main branches including carotid, subclavian, intercostal and renal arteries in relation to the need to distribute the heat produced by brown adipose tissue to the body via the circulation (Frontini and Cinti, 2010). The data for human coronary PVAT are conflicting: one study indicates that epicardial adipose tissue harvested from the origin of human right coronary arteries expresses higher levels of brown adipocyte-related genes compared with other regional adipose depots (Sacks et al., 2009), while another study indicates that the gene expression profile in human perivascular adipocytes surrounding coronary arteries reflects white, rather than brown, adipocytes (Chatterjee et al., 2009). This difference may reflect different methods, individual variations or be due to harvesting epicardial adipose tissues from different parts of the coronary arteries. It should be noted that epicardial adipose tissue is a mixture of that surrounding the large coronary vessels but is also present in significant quantities in humans on the surface of the ventricles, especially the right, and the apex of the heart (Sacks and Fain, 2007). There are no obvious anatomical boundaries between coronary PVAT and epicardial adipose tissue in humans. Adipocyte transdifferentiation may occur; in conditions of chronic cold exposure, white-to-brown conversion meets the need for thermogenesis, whereas an obesogenic diet induces brown-to-white conversion to meet the need for storing energy (Cinti, 2011). Species differences may exist; in contrast to humans, little or no epicardial adipose tissue is seen in rats and mice (Marchington et al., 1989).
Figure 2.
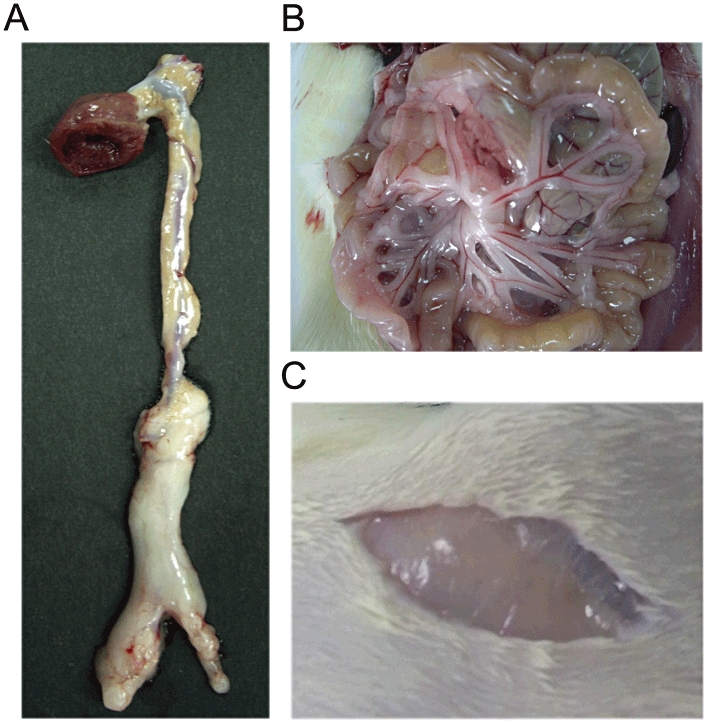
Photographs of periaortic (A), mesenteric (B) and inguinal subcutaneous (C) adipose tissue in Sprague–Dawley rats.
PVAT is a kind of connective tissue. Besides cell components (mainly adipocytes), it contains collagen and elastic fibres, nerve bundles and vasa vasorum (Lebona, 1993). Adventitial vasa vasorum neovascularization occurs during vascular injury and inflammation, providing a direct way to transmit substances released by PVAT to the inner vasculature (Kwon et al., 1998; Gossl et al., 2009). Luminal terminations of the vasa vasorum in the saphenous vein have been described, and this microvascular network may be a system transporting vasoactive factors within the vascular wall (Dashwood et al., 2007), providing a structural basis for the cell crosstalk between different layers of blood vessel. The cell types in PVAT include adipocytes, endothelial cells, macrophages, adipocyte stem/progenitor cells, lymphocytes, fibroblasts, etc. The percentage of these cells can change with age, nutritional status and environmental conditions. In obese individuals, the periaortic PVAT has more inflammatory cells infiltrated and is expanding in response to metabolic stimuli (Skilton et al., 2009). Perivascular adipocytes surrounding human coronary arteries are more heterogeneous in shape and smaller in size and exhibit a reduced state of adipogenic differentiation than subcutaneous adipocytes (Chatterjee et al., 2009). Throacic periaortic adipocytes are also much smaller in size as compared with perimesenteric adipocytes in the rat (Galvez-Prieto et al., 2008). The mean adipocyte surface and differentiating preadipocyte density in epicardial adipose tissue near the proximal tract of the right coronary artery obtained from coronary artery disease (CAD) patients are larger and increased, respectively, compared with those of non-CAD patients (Silaghi et al., 2007).
PVAT influences VSMC growth and migration
Extensive plasticity is an inherent property exhibited by fully mature VSMCs, which are ordinarily in a non-proliferative, contractile state, but switch to a proliferative, synthetic phenotype during response to injury (Carmeliet, 2000). VSMC growth (proliferation and/or hypertrophy) and migration play a major role in proliferative vascular diseases, such as atherosclerosis, restenosis, graft vasculopathy and hypertension. Atherosclerosis is the most widely acknowledged example in which VSMC phenotypic switching to proliferation and migration plays a key role. Restenosis at sites of vascular injury following angioplasty is due to intimal hyperplasia, in which VSMC proliferation and VSMC migration from media to intima are involved. The local microenvironment, such as growth factors/inhibitors and mechanical influences, is very important for the phenotypic conversion (Owens et al., 2004). Factors released from perivascular fat may be regulators for VSMC proliferation and migration, to judge from the available direct and indirect evidence.
Direct evidence
PVAT-derived growth factors
Adipocytes promote proliferation in some types of cells (Manabe et al., 2003; Amemori et al., 2007). However, the effect of adipocytes on VSMC growth was unknown until Yang et al. (Barandier et al., 2005) cultured VSMCs in conditioned medium from 3T3-L1-derived differentiated adipocytes or from 3T3-L1 preadipocytes. They found that VSMC proliferation can be induced by the conditioned medium from differentiated adipocytes rather than that of preadipocytes. The growth factor(s) present in the conditioned medium from mature adipocytes may be a protein(s), since this proliferation is abolished by proteinase K treatment and not sensitive to phospholipase B (to hydrolyze lysophosphatidic acid). In addition, the protein(s) is (are) resistant to trypsin. Further experiments demonstrated that the adipocyte-derived growth factor(s) is (are) hydrosoluble and present in a fraction of molecular mass larger than 100 kDa. Another study also showed that adipocyte-conditioned media generated from human adipocytes can induce a prominent proliferation and migration of human VSMCs. This effect is enhanced in a synergistic way by the combination with oleic acid, a FFA (Lamers et al., 2011).
Moreover, Yang et al. (Barandier et al., 2005) showed that the conditioned medium from rat periaortic adipose tissue can stimulate VSMC proliferation, which is partly reduced by heat and proteinase K, suggesting that a protein component is present in conditioned medium from PVAT and is involved in the proliferation-promoting effect. The activity of PVAT-conditioned medium is also resistant to trypsin. But 40% of the biological activity of PVAT-conditioned medium is retained after treatment with heat plus proteinase K, implying that conditioned medium from PVAT has a more complex feature than that from cultured adipocytes. The difference between cultured 3T3-L1-derived adipocytes and adipocytes in PVAT and heterogeneous cell types in periaortic adipose tissue may contribute to this diversity. It seems that PVAT-derived growth factors are composed of both protein and non-protein components. Of interest, the growth-promoting effect of PVAT is significantly enhanced in aged rats and in high-fat diet-induced obese rats, suggesting that PVAT may be involved in vascular disease associated with aging and obesity (Barandier et al., 2005).
Visfatin
Visfatin is an adipokine discovered in 2005 (Fukuhara et al., 2005). This protein is also known as pre-B cell colony-enhancing factor and identified as nicotinamide phosphoribosyltransferase that biosynthesizes nicotinamide mononucleotide (NMN) from nicotinamide (Wang et al., 2011). As a new adipokine, it is mainly expressed in and secreted from visceral fat as opposed to subcutaneous fat. The circulating visfatin level is increased in obesity (Fukuhara et al., 2005; Sandeep et al., 2007; Filippatos et al., 2008; Wang et al., 2010a).
We demonstrated the expression and secretion of visfatin in aortic PVAT (Wang et al., 2009). Visfatin protein expression in PVAT of the rat thoracic aorta was the highest, 3.7-fold and 1.8-fold higher than that in subcutaneous and visceral adipose tissue respectively. Similar results were obtained in monkey adipose tissues. Moreover, visfatin was detected in PVAT-conditioned medium, indicating that visfatin can be released from PVAT.
To characterize the paracrine function of PVAT-derived visfatin, we tested its role in VSMC contraction and proliferation (Wang et al., 2009). The anti-contractile effect of PVAT was not affected by visfatin-specific antibodies and by the visfatin chemical inhibitor FK866. Exogenous visfatin (up to 50 nM) caused neither relaxation nor contraction in aortae with and without endothelium. These observations imply that PVAT-derived visfatin is not involved in the regulation of vascular tone. However, PVAT-derived visfatin was found to be a VSMC growth factor. PVAT-conditioned medium stimulated VSMC proliferation, which was significantly attenuated by visfatin specific antibodies. Exogenous visfatin stimulated VSMC proliferation, which also could be totally blocked by the visfatin antibody. In addition, visfatin did not show any proliferative effect on VSMCs during co-incubation with FK866. These results indicate that the proliferative effect of PVAT is partly mediated by visfatin. This was the first report that an adipokine secreted by PVAT could be identified as a growth factor for VSMCs (Wang et al., 2009).
Our further experiments demonstrated that exogenous visfatin at physiological concentrations stimulated VSMC proliferation in a dose- and time-dependent manner via extracellular signal-regulated kinase (ERK) 1/2 and p38 rather than c-Jun N-terminal kinase and phosphatidylinositol 3-kinase/Akt signalling pathways. Visfatin exerted anti-apoptotic effects when VSMC apoptosis was induced by H2O2, but not under normal conditions. Insulin receptor knockdown did not affect the response to visfatin. Visfatin acted as a nicotinamide phosphoribosyltransferase to biosynthesize NMN, which mediated proliferative signalling pathways and cell proliferation similar to the visfatin effect (Wang et al., 2009).
Another report focused on the pro-inflammatory effects of exogenous visfatin in VSMCs (Romacho et al., 2009) and showed that visfatin induced inducible nitric oxide synthase (iNOS) in a concentration-dependent manner and elicited a sustained activation of ERK1/2 signalling with induction of NF-κB. Intriguingly, consistent with our observations with exogenous NMN (Wang et al., 2009), the authors found that NMN mimicked NF-κB activation and iNOS induction by visfatin, and that this could be prevented by FK866 (Romacho et al., 2009).
The above two studies may open new areas of research related to the role of visfatin in pathology, as proliferation of and inflammation in VSMCs are closely related to the genesis, development and rupture of atherosclerotic plaques (Hansson, 2005). Regarding the relationship between circulating visfatin and atherosclerosis, the available clinical studies yield different conclusions, reporting presence (Zhong et al., 2008; Kadoglou et al., 2010; Mu et al., 2011) and absence (Kato et al., 2009; Rho et al., 2010) of correlation. Local PVAT visfatin was also studied in atherosclerotic patients. Epicardial adipose tissue thickness was correlated with plasma visfatin levels and local visfatin expression in epicardial adipose tissue adjacent to the proximal right coronary artery was increased in CAD patients (Cheng et al., 2008). Moreover, aortic and coronary atherosclerosis was positively correlated with visfatin expression in the corresponding PVAT (Spiroglou et al., 2010). In obesity, visfatin may be increased in systemic circulation and local PVAT. This elevated visfatin may play a role in certain types of atherosclerosis, for example, obesity-related atherosclerosis.
When considering the potential role of visfatin in atherosclerosis, it should be taken into account that it is a multifunctional protein. Visfatin has potent pro-survival effects on the three most important cell types (macrophages, VSMCs and endothelial cells) involved in atherosclerosis, implying a protective role of the adipokine in these cells (van der Veer et al., 2007; Li et al., 2008; Borradaile and Pickering, 2009; Ho et al., 2009; Wang et al., 2009). Together with the fact that dysfunction or death of macrophages, VSMCs and endothelial cells are believed to be critical processes in the genesis and development of atherosclerosis, it remains uncertain whether visfatin is friend or foe.
Adiponectin
Adiponectin is an adipocyte-specific adipokine, independently characterized in 1995 and 1996 by four groups using different methods, hence its alternative names of apM1, Acrp30, adipoQ and GBP28 (Scherer et al., 1995; Hu et al., 1996; Maeda et al., 1996; Nakano et al., 1996). Adiponectin expression is higher in human subcutaneous than visceral adipose tissue (Fain et al., 2004). Circulating adiponectin levels decline in obesity, suggesting that hypoadiponectinemia contributes to the pathological conditions associated with overweight. Most of the available information indicates that adiponectin is a beneficial adipokine having anti-diabetic, anti-inflammatory and anti-atherogenic effects (Matsuzawa, 2010).
Adiponectin is a vasodilator substance but is not involved in the modulation of vascular tone by PVAT (Fesus et al., 2007). However, it has been identified as responsible for the protection by PVAT against neointimal formation after vascular injury (Takaoka et al., 2009). This study provided in vivo data supporting the paracrine effect of PVAT on VSMC growth and migration. The authors developed a novel mouse model replacing PVAT with exogenous fat after endovascular wire injury. In the femoral artery, PVAT removal enhanced neointimal hyperplasia following endovascular injury. Transplantation of subcutaneous fat from a normal mouse to surround the injured artery markedly attenuated neointimal formation. These observations suggest that PVAT may have a protective role in neointimal hyperplasia. Supporting this viewpoint, the atheroprotective effect of exogenous fat was not observed when subcutaneous fat was from obese mice. The explanation may be phenotypic changes in adipose tissue with the production of anti-inflammatory adiponectin decreasing and that of pro-inflammatory adipokines [interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), tumour necrosis factor-α (TNF-α) and plasminogen activator inhibitor type-1 (PAI-1)] increasing in subcutaneous fat-conditioned medium from obese mice compared with that from normal mice. The conditioned medium from the subcutaneous fat of normal mice attenuated VSMC proliferation produced by platelet-derived growth factor (PDGF)-BB. By contrast, the conditioned medium from the subcutaneous fat of obese mice increased VSMC proliferation, which was prevented by pretreatment with anti-TNF-α antibodies. Also, the conditioned medium of adiponectin-deficient subcutaneous fat stimulated VSMC proliferation. These findings reveal that TNF-α secreted from fat increased VSMC growth, and that adiponectin secreted from fat suppressed VSMC growth in response to PDGF-BB stimulation. Further experiments demonstrated that exogenous adiponectin suppressed PDGF-BB-induced VSMC proliferation via AMP-activated kinase pathway.
To analyse which molecule(s) contributes to the protection exerted by PVAT, Takaoka et al. focused on adiponectin. Adiponectin-deficient mice exhibited increased neointimal smooth muscle proliferation in response to endovascular injury, which was reversed by local perivascular delivery, but not systemic administration, of recombinant adiponectin. This indicates that adiponectin secreted from PVAT has a protective role in lesion formation in response to endovascular injury. Supporting this notion, the adiponectin expression was down-regulated in the PVAT of obese mice, and endovascular injury-induced neointimal formation was enhanced in the artery of these mice.
In contrast to subcutaneous fat, transplantation of visceral fat to surround the injured artery exerted no protection (Takaoka et al., 2009). The explanation for this difference may be the lower expression of adiponectin in visceral than subcutaneous fat in mice, similar to humans (Fain et al., 2004). When considering the paracrine function of PVAT, it should be noted that PVAT heterogeneity exists in different vascular regions, different species and under different physiological and pathological conditions. In addition, the evidence for the protection by PVAT in the mouse femoral artery was mainly obtained using implanting subcutaneous fat rather than real PVAT, as the authors pointed out that it was impossible to obtain enough amount of PVAT for the transplantation to coat the artery of another mouse (Takaoka et al., 2009).
In humans, plasma adiponectin levels in the cardiac vein were higher than those in the coronary artery, suggesting that adiponectin is produced locally in the coronary circulation (Date et al., 2006). Adiponectin was expressed in both periaortic and pericoronary adipose tissue and negatively correlated with age and atherosclerosis (Spiroglou et al., 2010). Adiponectin expression in epicardial adipose tissue surrounding the right coronary artery was decreased in metabolic syndrome (Teijeira-Fernandez et al., 2011) and CAD patients (Iacobellis et al., 2005; Karastergiou et al., 2010). These studies suggest that low adiponectin level in local PVAT contributes to human atherosclerosis, as does systemic hypoadiponectinaemia.
TNF-α
Since it was reported in 1993 that adipose tissue expressed both the substantial TNF-α gene and one of candidate molecules inducing insulin resistance (Hotamisligil et al., 1993), TNF-α has been recognized as an important adipokine. Adipose TNF-α expression and plasma TNF-α level are increased in most animal models and human subjects with obesity and insulin resistance. In addition, this cytokine has a variety of functions in inflammation and atherosclerosis (Matsuzawa, 2005).
After identifying adiponectin for the protection of PVAT against neointimal formation of the injured mouse femoral artery (Takaoka et al., 2009), the same group proposed that TNF-α is involved in rapid phenotypic changes in PVAT following endovascular injury (Takaoka et al., 2010). In addition to the model of wire injury in the mouse femoral artery, they demonstrated in another model (rat iliac artery balloon injury) that endovascular injury significantly up-regulated pro-inflammatory adipokines (MCP-1, TNF-α, IL-6 and PAI-1) and down-regulated the anti-inflammatory adipokine adiponectin within PVAT. TNF-α knockout attenuated up-regulation of pro-inflammatory adipokine expression in PVAT, with reduced neointimal hyperplasia after vascular injury. Local delivery of TNF-α to the perivascular area enhanced pro-inflammatory adipokine expression, and this was associated with augmented neointimal hyperplasia in TNF-α-deficient mice. Conditioned medium from a coculture of 3T3-L1 adipocytes and RAW264 macrophages stimulated VSMC proliferation. An anti-TNF-α neutralizing antibody in the coculture abrogated the stimulating effect of the conditioned medium. These findings suggest that endovascular injury-induced phenotypic changes in PVAT may play a role in the pathogenesis of neointimal hyperplasia after angioplasty, in which TNF-α is a major mediator. One possible mechanism for PVAT phenotypic alterations is that endothelial injury brings about infiltration of inflammatory cells (such as macrophages) into the vascular wall from the luminal side. Inflammatory cell-derived TNF-α may trigger adipocyte dysfunction and then lead to a vicious cycle between adipocytes and macrophages, which may aggravate inflammatory changes in the PVAT, demonstrated as up-regulation of the pro-inflammatory adipokines (MCP-1, TNF-α, IL-6 and PAI-1) and down-regulation of the anti-inflammatory adipokine (adiponectin) (Takaoka et al., 2010).
However, the pathological effect of PVAT in this study (Takaoka et al., 2010) is in contradiction with their previous finding that removal of PVAT enhanced neointimal hyperplasia after endovascular injury, indicating that PVAT may protect against neointimal formation after angioplasty (Takaoka et al., 2009). Thus, the in vivo effect of PVAT needs to be studied further in different vascular regions, different species and under different physiological and pathological conditions.
In addition, TNF-α was found to be highly expressed in the balloon-injured rat aorta, but not in normal blood vessels (Jovinge et al., 1997). Blockade of TNF-α activity by soluble TNF-α receptor suppressed coronary artery neointimal formation after cardiac transplantation in rabbits (Clausell et al., 1994). TNF-α stimulated VSMC proliferation and migration in vitro (Wang et al., 2001a; Kim et al., 2007). The secretion of TNF-α in epicardial fat adjacent to the proximal coronary artery and abdominal fat was higher in CAD patients (Cheng et al., 2008). These observations suggest PVAT-derived TNF-α may be involved in the VSMC growth and migration during vascular injury. To exactly identify the major molecule(s) for the role of PVAT in the proliferative vascular disease, the function of local PVAT from normal and diseased arteries should be compared using PVAT-conditioned medium and various specific blockers. For these studies, adipose-specific over- and under-expression would be more useful than systemic over- and under-expression.
Adipose-derived factors potentially involved in regulating VSMC growth by PVAT
Renin–angiotensin system (RAS)
RAS is very important in cardiovascular physiopathology and pharmacology (Fyhrquist and Saijonmaa, 2008; Abassi et al., 2009). Adipose RAS and its functional importance have attracted closer attention (Kershaw and Flier, 2004; Thatcher et al., 2009). Angiotensinogen was the first RAS component demonstrated in adipocytes and is abundantly expressed in adipose tissue. Adipose tissue, rather than the liver, may serve as a primary source of angiotensinogen in the foetus (Gomez et al., 1988). Adipocyte-specific overexpression of angiotensinogen elevated both circulating angiotensinogen and blood pressure, indicating that adipocyte-derived angiotensinogen contributes to the systemic RAS and to the regulation of arterial blood pressure (Massiera et al., 2001). In our study of rat gene microarrays, we analysed the expression of all RAS genes in adipose tissue (Table 1). The results showed that almost all RAS genes were expressed, except renin, AT2 and insulin-regulated aminopeptidase (IRAP) receptor. The gene expression of the AT1A receptor was high, whereas that of other genes encoding angiotensin receptors (AT1B, AT2, Mas and IRAP) was low in adipose tissue, indicating that effects of Ang II on adipose tissue are mediated mainly by AT1A under physiological conditions. The two types of renin/prorenin receptors were both highly expressed in adipose tissue, indicating that adipose has the ability for renin/prorenin uptake and clearance, and that circulating renin/prorenin may affect adipose through the specific renin/prorenin receptor-mediated intracellular signalling and renin/prorenin activation (Nguyen, 2007).
Table 1.
Gene microarrays of renin-angiotensin system in rat perivascular and subcutaneous adipose tissue
| Mean expression value | Differential expression score | |||||
|---|---|---|---|---|---|---|
| Gene | PVAT | MAT | SAT | PVAT versus MAT | PVAT versus SAT | MAT versus SAT |
| Renin | 1 | −1 | 3 | 0.0 | 0.0 | 0.0 |
| Atp6ap2 (renin/prorenin receptor) | 2046 | 4024 | 5339 | −39.6* | −55.6* | −7.0 |
| Igf2r (renin/prorenin clearance receptor) | 2332 | 1829 | 2004 | 5.9 | 0.2 | −2.1 |
| Angiotensinogen | 2217 | 3967 | 3122 | −25.1* | −6.9 | 4.9 |
| ACE | 114 | 68 | 351 | 3.9 | −55.5* | −101.5* |
| ACE2 | 89 | 37 | 52 | 8.1 | 5.3 | −0.6 |
| Chymase | 1260 | 1016 | 4880 | 1.9 | −82.5* | −99.1* |
| AT1A | 1224 | 1556 | 4259 | −3.1 | −79.8* | −62.7* |
| AT1B | 11 | 6 | 7 | 0.0 | 0.0 | 0.0 |
| AT2 | −4 | −4 | −2 | 0.0 | 0.0 | 0.0 |
| Mas (previous AT3) | 31 | 13 | 67 | 0.0 | −1.4 | −14.9* |
| IRAP (previous AT4) | 0 | −6 | 1 | 0.0 | 0.0 | −0.1 |
| Aminopeptidase A | 20 | 8 | 8 | 0.0 | 0.0 | 0.0 |
| Aminopeptidase N | 454 | 328 | 1967 | 6.4 | −71.2* | −90.8* |
Sprague–Dawley rats are used at the age of 27–28 weeks. n = 8.
P < 0.05, 13 < differential expression score < −13; **P < 0.01, 20 < differential expression score < −20.
MAT, mesenteric adipose tissue; SAT, subcutaneous adipose tissue of inguinal region.
In the rat aorta, the majority of angiotensinogen mRNA expression was localized in PVAT, and this protein was released into the medium from incubated adipose tissue with levels increasing over a 2 h period (Cassis et al., 1988a; Cassis et al., 1988b). Expression of angiotensinogen mRNA has been detected in human epicardial adipose tissue close to the course of the right coronary artery and significantly increased by acute surgery stress (Roubicek et al., 2008). In addition to angiotensinogen mRNA, angiotensin-converting enzyme (ACE), ACE2 and chymase mRNAs were expressed in PVAT surrounding rat aortae and mesenteric arteries (Galvez-Prieto et al., 2008) (Table 1). Renin/prorenin uptake was present, as indicated by high expression of two renin/prorenin receptors in perivascular fat (Table 1). Thus, PVAT contains the components necessary for the synthesis of angiotensin (Ang) II and Ang 1–7, two major effectors in the RAS. Ang II and Ang 1–7 were detected in rat periaortic and/or perimesenteric adipose tissue (Galvez-Prieto et al., 2008; Lee et al., 2009; Lu et al., 2010). The expression of the specific renin/prorenin receptor and angiotensinogen was higher in mesenteric than periaortic adipose tissue (Table 1), supporting the finding that Ang II content was higher in mesenteric fat (Galvez-Prieto et al., 2008). PVAT-derived Ang II is critically involved in the potentiation of vasoconstriction to perivascular neuronal stimulation in rat mesenteric arteries (Lu et al., 2010), whereas PVAT-derived Ang 1–7 acted as a relaxing factor in rat aortas (Lee et al., 2009; Lu et al., 2011), indicating that the local RAS in perivascular fat plays a paracrine role in the regulation of vascular tone.
Although there has been no direct evidence regarding the effect of perivascular adipose RAS on VSMC growth and migration, it is reasonable to deduce that this local RAS may have this effect under physiological and/or pathological conditions. Indeed, Ang II is a powerful growth factor, stimulating VSMC growth (hypertrophy and hyperplasia) and migration in vitro and in vivo following activation of AT1 (Kim and Iwao, 2000). ACE inhibitors and AT1 antagonists reverse or reduce vascular remodelling in response to hypertension and vascular injury (Heeneman et al., 2007). Of note, AT2, expressed in the developing vascular system and re-expressed in adult VSMCs in response to vascular injury, has opposite functions, inhibiting proliferation (Nakajima et al., 1995) and migration (Chassagne et al., 2002). In addition, exogenous Ang 1–7 inhibits VSMC proliferation in a balloon-catheter injury model (Strawn et al., 1999). It inhibits Ang II-induced VSMC proliferation and migration partly through negative modulation of Ang II-induced ERK1/2 activity (Zhang et al., 2010) and down-regulation of AT1 receptors in VSMCs (Clark et al., 2001). The systemic infusion of Ang II induces local PVAT inflammation which may participate in hypertensive vascular remodelling (Guzik et al., 2007) and abdominal aortic aneurysm formation (Police et al., 2009). Nevertheless, further studies are needed to directly test whether local RAS in PVAT is involved in vascular diseases, as well as to identify which components play a role. Using adipose-specific over- or under-expression of individual RAS components would provide definitive evidence in this regard.
Reactive oxygen species (ROS)
The production of ROS increased selectively in adipose tissue of obese mice, and this was accompanied by an augmented expression of NADPH oxidase and a decreased expression of antioxidative enzymes (Furukawa et al., 2004). PVAT-derived ROS was involved in the modulation of vascular contraction (Gao et al., 2006; Gao et al., 2007) and promoted endothelial dysfunction in diet-induced obese mice (Ketonen et al., 2010). In New Zealand obese mice, a model for metabolic syndrome, PVAT of mesenteric resistance arteries showed increased superoxide anion production and NADPH oxidase activity, accompanied by hypertrophic vascular remodelling and loss of PVAT anti-contractile properties (Marchesi et al., 2009). Epicardial adipose tissue suffered greater oxidative stress than subcutaneous adipose tissue in patients with cardiovascular disease (Salgado-Somoza et al., 2010). ROS are important signalling molecules or second messengers that regulate proliferation, hypertrophy and migration of VSMCs (Taniyama and Griendling, 2003). It is generally believed that low concentrations of ROS induce cell growth, whereas high concentrations result in cell death (Taniyama and Griendling, 2003). Several factors have been shown to induce ROS-dependent migration of VSMCs, including PDGF (Weber et al., 2004), thrombin (Wang et al., 2004), vascular endothelial growth factor (Wang et al., 2001b), MCP-1 (Lo et al., 2005) and insulin-like growth factor 1 (Meng et al., 2008). Actually, H2O2 treatment is sufficient to induce VSMC migration (Kim et al., 2004).
Complement component 3 (C3)
PVAT-derived C3 stimulated adventitial fibroblast migration and differentiation. Indeed, C3 was increased in PVAT and associated with adventitial thickening and myofibroblast clustering around PVAT in deoxycorticosterone acetate-salt-hypertensive rats (Ruan et al., 2010). C3 is involved in the synthetic phenotype and exaggerated growth of VSMCs from spontaneously hypertensive rats (Lin et al., 2004), is essential for C-reactive protein-mediated exaggeration of neointimal formation in injured mouse carotid arteries (Hage et al., 2010), is increased in atherosclerotic lesions and contributes to the development of the latter by stimulation of several cytokines in vivo (Buono et al., 2002).
NO
NO can be released by adipocytes and endothelial cells of vasa vasorum in adipose tissue (Dashwood et al., 2007; Malinowski et al., 2008). NO production was increased in mesenteric PVAT, improving relaxation to acetylcholine and sodium nitroprusside, during early diet-induced obesity (Gil-Ortega et al., 2010). Of the three NOS isoenzymes, eNOS immunostaining was the most abundant in PVAT of the saphenous vein, and eNOS activity was comparable in PVAT and the underlying vein, suggesting that PVAT-derived NO plays a role in the improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery (Dashwood et al., 2007). NO induces opposite effects on cell migration and proliferation depending on the different phenotypes of VSMCs. It inhibited the proliferation and migration of relatively dedifferentiated VSMCs, including subcultured cells or cells isolated from newborn rats (Garg and Hassid, 1989; Tanner et al., 2000). For differentiated VSMCs isolated from the adult rat aorta, NO potentiated the effect of growth factors on cell proliferation and induced migration (Brown et al., 1999; Chang et al., 2002). In addition, different NOS isoforms produce converse effects on VSMC growth and migration. Gene konockout studies indicated that eNOS suppresses vascular injury-induced neointima formation (Moroi et al., 1998), whereas iNOS promotes this neointima formation (Chyu et al., 1999). iNOS expression in adipose tissue is up-regulated in obesity, a low-grade chronic inflammation (Perreault and Marette, 2001). It remains to be answered whether this iNOS up-regulation occurs in the inflammatory PVAT and thus contributes to the obesity-associated vascular disease.
Hydrogen sulphide (H2S)
Rat aortic PVAT expressed cystathionase, a key enzyme for H2S generation, and released H2S. The H2S production decreased in an age-dependent manner (Fang et al., 2009). PVAT-derived H2S has been identified as a relaxing factor (Fang et al., 2009). Atorvastatin increases net H2S production in PVAT, but not media, by inhibiting its mitochondrial oxidation and results in the augmentation of anti-contractile effect of PVAT (Wojcicka et al., 2011). H2S inhibited VSMC proliferation and induced VSMC apoptosis in vitro (Du et al., 2004). Endogenous H2S may protect against atherosclerosis (Qiao et al., 2010) and neointima hyperplasia following endovascular injury (Meng et al., 2007). Enhanced cell proliferation has been detected in the aortic media from cystathionase knockout mice (Yang et al., 2010).
Other factors
PVAT expresses some other known adipokines, most of which have been verified for their secretion from PVAT. Given their roles in the regulation of VSMC growth, they may be potentially PVAT-derived growth factors or inhibitors. These factors include MCP-1 (Egashira et al., 2002; Chatterjee et al., 2009; Takaoka et al., 2010), IL-6 (Yao et al., 2007; Langheim et al., 2010; Takaoka et al., 2010), IL-8 (Yue et al., 1994; Henrichot et al., 2005; Chatterjee et al., 2009), leptin (Li et al., 2005; Zeidan et al., 2005; Cheng et al., 2008; Ketonen et al., 2010), resistin (Calabro et al., 2004; Langheim et al., 2010), PAI-1 (Chen et al., 2006; Takaoka et al., 2009; Karastergiou et al., 2010), adrenomedullin (Kano et al., 1996; Iwasaki et al., 1998; Shibasaki et al., 2010), heparin-binding epidermal growth factor-like growth factor (HB-EGF) (Igura et al., 1996; Matsumoto et al., 2002), IL-10 (Selzman et al., 1998; Eiras et al., 2010), IL-1β (Dinarello, 1996; Lohmann et al., 2009) and macrophage migration inhibitory factor (MIF) (Pan et al., 2004; Karastergiou et al., 2010).
Epicardial adipose tissue has a greater capacity for FFA release than the perirenal and popliteal adipose tissue (Marchington et al., 1989). Saturated fatty acids are higher and unsaturated fatty acids are lower in epicardial adipose tissue near the proximal right coronary artery than subcutaneous adipose tissue (Pezeshkian et al., 2009). Actually, the nature of FFAs released by different regional perivascular fats is still unclear, and the compositions of PVAT-derived FFAs should be defined when verifying their local effect, since different FFAs exert distinct effects on VSMC growth (Rao et al., 1995; Pakala and Benedict, 1999; Schauer and Reusch, 2009; Lamers et al., 2011).
Glucocorticoids inhibit VSMC proliferation and migration in vitro (Longenecker et al., 1984; Pross et al., 2002). They prevent restenosis after angioplasty in patients (Liu et al., 2004). Adipocytes highly express 11-β-hydroxysteroid dehydrogenase 1 (11β-HSD1) that converts inactive glucocorticoid precursors (cortisone) to active glucocorticoids (cortisol) in humans (Seckl and Walker, 2001). 11β-HSD1 mRNA is increased in epicardial adipose tissue near the proximal tract of the right coronary artery in CAD patients (Silaghi et al., 2007). However, there is no information concerning the function of PVAT-derived glucocorticoids.
Adipose tissue produces sex hormones from precursors. It is speculated that 10–20% of the body production of sex steroids indeed are formed in adipose tissue (Frayn et al., 2003). Oestrogen suppresses (Sullivan et al., 1995; Jiang et al., 2010) or enhances (Keyes et al., 1996; Ling et al., 2004) VSMC growth, depending on cellular phenotype (Song et al., 1998). Androgen reduces neointimal formation in rabbit carotid balloon injury model (Ii et al., 2009) and exerts an anti-proliferative, pro-apoptotic and anti-migrative effect on cultured VSMCs (Furutama et al., 1998; Bowles et al., 2007).
Overall, among the abovementioned factors, mainly visfatin, adiponectin and TNF-α appear to participate in the paracrine regulation by PVAT of VSMC growth and migration (Figure 3). PVAT-derived Ang II, Ang 1–7, ROS, C3, NO and H2S have a paracrine action on VSMC contraction, endothelial function or fibroblast function; however, their paracrine action on VSMC growth and migration remains to be directly verified. More work is needed to conclude as to the importance of the remaining factors (MCP-1, IL-6, IL-8, leptin, resistin, PAI-1, adrenomedullin, HB-EGF, IL-10, IL-1β, MIF, FFAs, glucocorticoids and sex hormones) as regards a contribution to the paracrine role of PVAT in the regulation of VSMC growth and migration.
Figure 3.
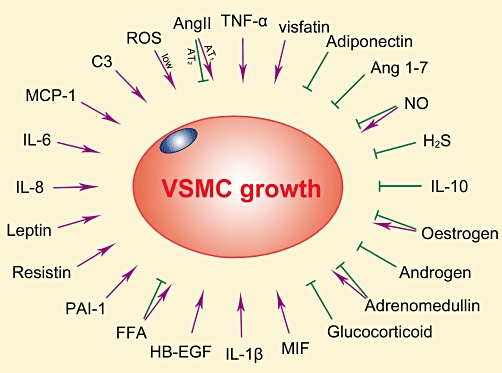
Effects of adipose-derived factors on VSMC growth.
PVAT and vascular disease
The epicardial fat volume is well correlated with cardiovascular risk factors (Rosito et al., 2008). Epicardial fat, but not intrathoracic fat, is associated with coronary artery calcification, whereas intrathoracic fat, but not epicardial fat, is associated with abdominal aortic calcification (Rosito et al., 2008). CAD is negatively related to coronary flow hyperaemia, and among the fat depots, epicardial fat is the only independent predictor of hyperaemic myocardial perfusion (Bucci et al., 2011). These studies support the hypothesis that PVAT exerts a paracrine effect in vascular disease. A correlation also exists between coronary PVAT and atherosclerosis, as the amount of local pericoronary fat appears to be related to the plaque burden in the underlying coronary artery segment (Mahabadi et al., 2010; Wang et al., 2010b). The relationship between PVAT and atherosclerosis has been reviewed elsewhere (Verhagen and Visseren, 2011).
PVAT may participate in restenosis after angioplasty. Indeed, inflammatory cells increased in PVAT of the porcine coronary artery after angioplasty (Okamoto et al., 2001). Similar results were observed in PVAT of mouse femoral artery and rat iliac artery after endovascular injury, with up-regulation of pro-inflammatory and down-regulation of anti-inflammatory adipokines (Takaoka et al., 2010). These phenotypic changes in PVAT were enhanced by diet-induced obesity in mice and were involved in neointimal formation following endovascular injury (Takaoka et al., 2009; Takaoka et al., 2010).
PVAT surrounding saphenous vein may reduce vein graft stenosis, improving early (1.5 years) and long-term (8.5 years) graft patency in patients with coronary artery bypass grafting (Souza et al., 2001; Souza et al., 2006). These suggest that PVAT prevents intimal hyperplasia and atherosclerosis after saphenous vein grafting (Souza et al., 2006). Several mechanisms may be considered for the beneficial effects of PVAT-intact saphenous vein grafts, such as anti-spasm, anti-platelet, anti-proliferative and mechanical actions as well as the minimal injury on the vascular wall (Souza et al., 2001; Souza et al., 2006). PVAT-derived NO may be one of the potential molecules for the protection of PVAT in saphenous vein grafts (Dashwood et al., 2007).
Besides local adipocyte dysfunction, the increase of inflammatory cells in PVAT is involved in vascular disease. Macrophages and T cells in PVAT are increased in obesity (Police et al., 2009; Takaoka et al., 2009), vascular injury (Takaoka et al., 2010), aging (Fei et al., 2010), acute systemic infection (Madjid et al., 2007) and atherosclerosis (Henrichot et al., 2005; Lohmann et al., 2009). T cells play a role in the genesis of Ang II-induced hypertension and vascular dysfunction (Guzik et al., 2007). Systemic infusion of Ang II induces local PVAT inflammation and obvious T-cell infiltration, which may participate in hypertensive vascular remodelling (Guzik et al., 2007). Adipose tissue secretes chemokines (Henrichot et al., 2005) and has a loose structure to recruit and accommodate inflammatory cells, which can further alter adipose tissue function (Weisberg et al., 2003). The resulting adipocyte abnormality and inflammatory cell infiltration may induce imbalance of PVAT-derived factors, for example, secreting more growth factors and less inhibitors, leading to VSMC growth and migration, and finally resulting in development of proliferative vascular disease (Figure 4). One of the key features of cell migration is the capacity of the cells to breakdown extracellular matrix components by matrix metalloproteinases (MMPs). In VSMCs, MMPs can be activated by TNF-α, Ang II, ROS, leptin, resistin, IL-6 and MIF (Moon et al., 2004; Kong et al., 2005; Li et al., 2005; Wang et al., 2005; Chen et al., 2007; Hu et al., 2009; Ding et al., 2011), which can be released by PVAT, especially during inflammation. These further support the proposed mechanisms for involvement of PVAT dysfunction in vascular disease (Figure 4).
Figure 4.
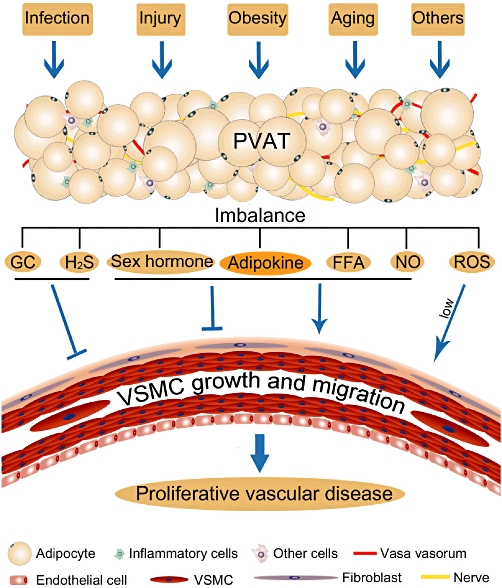
Schematic diagram for perivascular fat dysfunction and vascular disease. Obesity, aging, vascular injury, infection and other factors may affect PVAT, causing adipocyte abnormality and inflammatory cell infiltration, leading to imbalance of PVAT-secreted growth factors and inhibitors, tending towards VSMC growth and migration and finally resulting in development of proliferative vascular disease. GC, glucocorticoid.
Perspective
Current research on perivascular fat opens a new field in vascular biology. Both the intimal endothelium and the adventitial fat regulate contraction and growth of medial VSMCs. The crosstalk between cells in the blood vessel wall is vital for normal vascular function. Imbalance of PVAT-derived factors may play a role in proliferative vascular diseases such as atherosclerosis, restenosis and hypertension. To identify key factors altered in distinct sites of PVAT and involved in stimulation of proliferation may help to develop new therapies for the prevention and treatment of vascular disease. Using in vivo models of cell-specific gene over- and under-expression, systemic gene over- and under-expression, and local pharmacological interventions may provide definitive evidence for drug target identification. Antibodies, recombinant proteins and chemicals may be used locally, for example during angioplasty or vein grafting. Preservation of PVAT may be as important as preservation of endothelium for a successful vascular intervention.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China for Distinguished Young Scholars (30525045 to C-YM), the National Basic Research Program of China (2009CB521902 to C-YM), the National Science and Technology Major Project (2009ZX09303-002 to C-YM), the Program of Shanghai Subject Chief Scientist (10XD1405300 to C-YM) and the Shanghai ‘Shu Guang’ Project (10GG19 to C-YM).
Glossary
- CAD
coronary artery disease
- PVAT
perivascular adipose tissue
- VSMC
vascular smooth muscle cell
Conflict of interest
None.
References
- Abassi Z, Winaver J, Feuerstein GZ. The biochemical pharmacology of renin inhibitors: implications for translational medicine in hypertension, diabetic nephropathy and heart failure: expectations and reality. Biochem Pharmacol. 2009;78:933–940. doi: 10.1016/j.bcp.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G923–G929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–H1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell. 2009;8:100–112. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- Bowles DK, Maddali KK, Dhulipala VC, Korzick DH. PKCdelta mediates anti-proliferative, pro-apoptic effects of testosterone on coronary smooth muscle. Am J Physiol Cell Physiol. 2007;293:C805–C813. doi: 10.1152/ajpcell.00127.2007. [DOI] [PubMed] [Google Scholar]
- Brown C, Pan X, Hassid A. Nitric oxide and C-type atrial natriuretic peptide stimulate primary aortic smooth muscle cell migration via a cGMP-dependent mechanism: relationship to microfilament dissociation and altered cell morphology. Circ Res. 1999;84:655–667. doi: 10.1161/01.res.84.6.655. [DOI] [PubMed] [Google Scholar]
- Bucci M, Joutsiniemi E, Saraste A, Kajander S, Ukkonen H, Saraste M, et al. Intrapericardial, but not extrapericardial, fat is an independent predictor of impaired hyperemic coronary perfusion in coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:211–218. doi: 10.1161/ATVBAHA.110.213827. [DOI] [PubMed] [Google Scholar]
- Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, et al. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–3031. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Lynch KR, Peach MJ. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988a;62:1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger RNA. Hypertension. 1988b;11((6 Pt 2)):591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- Chang Y, Ceacareanu B, Dixit M, Sreejayan N, Hassid A. Nitric oxide-induced motility in aortic smooth muscle cells: role of protein tyrosine phosphatase SHP-2 and GTP-binding protein Rho. Circ Res. 2002;91:390–397. doi: 10.1161/01.res.0000033524.92083.64. [DOI] [PubMed] [Google Scholar]
- Chassagne C, Adamy C, Ratajczak P, Gingras B, Teiger E, Planus E, et al. Angiotensin II AT(2) receptor inhibits smooth muscle cell migration via fibronectin cell production and binding. Am J Physiol Cell Physiol. 2002;282:C654–C664. doi: 10.1152/ajpcell.00318.2001. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- Chen H, Xia T, Zhou L, Chen X, Gan L, Yao W, et al. Gene organization, alternate splicing and expression pattern of porcine visfatin gene. Domest Anim Endocrinol. 2007;32:235–245. doi: 10.1016/j.domaniend.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- Chyu KY, Dimayuga P, Zhu J, Nilsson J, Kaul S, Shah PK, et al. Decreased neointimal thickening after arterial wall injury in inducible nitric oxide synthase knockout mice. Circ Res. 1999;85:1192–1198. doi: 10.1161/01.res.85.12.1192. [DOI] [PubMed] [Google Scholar]
- Cinti S. Between brown and white: novel aspects of adipocyte differentiation. Ann Med. 2011;43:104–115. doi: 10.3109/07853890.2010.535557. [DOI] [PubMed] [Google Scholar]
- Clark MA, Diz DI, Tallant EA. Angiotensin-(1-7) downregulates the angiotensin II type 1 receptor in vascular smooth muscle cells. Hypertension. 2001;37:1141–1146. doi: 10.1161/01.hyp.37.4.1141. [DOI] [PubMed] [Google Scholar]
- Clausell N, Molossi S, Sett S, Rabinovitch M. In vivo blockade of tumor necrosis factor-alpha in cholesterol-fed rabbits after cardiac transplant inhibits acute coronary artery neointimal formation. Circulation. 1994;89:2768–2779. doi: 10.1161/01.cir.89.6.2768. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Does periadventitial fat-derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery? J Vasc Res. 2007;44:175–181. doi: 10.1159/000099833. [DOI] [PubMed] [Google Scholar]
- Date H, Imamura T, Ideguchi T, Kawagoe J, Sumi T, Masuyama H, et al. Adiponectin produced in coronary circulation regulates coronary flow reserve in nondiabetic patients with angiographically normal coronary arteries. Clin Cardiol. 2006;29:211–214. doi: 10.1002/clc.4960290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Ding Q, Chai H, Mahmood N, Tsao J, Mochly-Rosen D, Zhou W. Matrix metalloproteinases modulated by protein kinase Cepsilon mediate resistin-induced migration of human coronary artery smooth muscle cells. J Vasc Surg. 2011;53:1044–1051. doi: 10.1016/j.jvs.2010.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, et al. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels. 2004;19:75–80. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- Egashira K, Zhao Q, Kataoka C, Ohtani K, Usui M, Charo IF, et al. Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 2002;90:1167–1172. doi: 10.1161/01.res.0000020561.03244.7e. [DOI] [PubMed] [Google Scholar]
- Eiras S, Teijeira-Fernandez E, Salgado-Somoza A, Couso E, Garcia-Caballero T, Sierra J, et al. Relationship between epicardial adipose tissue adipocyte size and MCP-1 expression. Cytokine. 2010;51:207–212. doi: 10.1016/j.cyto.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- Fang L, Zhao J, Chen Y, Ma T, Xu G, Tang C, et al. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J Hypertens. 2009;27:2174–2185. doi: 10.1097/HJH.0b013e328330a900. [DOI] [PubMed] [Google Scholar]
- Fei J, Cook C, Blough E, Santanam N. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis. 2010;212:488–494. doi: 10.1016/j.atherosclerosis.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Tang EH, Vanhoutte PM. Nitric oxide the gatekeeper of endothelial vasomotor control. Front Biosci. 2008;13:4198–4217. doi: 10.2741/3000. [DOI] [PubMed] [Google Scholar]
- Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD, et al. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur J Clin Invest. 2008;38:71–72. doi: 10.1111/j.1365-2362.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27:875–888. doi: 10.1038/sj.ijo.0802326. [DOI] [PubMed] [Google Scholar]
- Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutama D, Fukui R, Amakawa M, Ohsawa N. Inhibition of migration and proliferation of vascular smooth muscle cells by dehydroepiandrosterone sulfate. Biochim Biophys Acta. 1998;1406:107–114. doi: 10.1016/s0925-4439(97)00085-9. [DOI] [PubMed] [Google Scholar]
- Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264:224–236. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Prieto B, Bolbrinker J, Stucchi P, Heras AI, Merino B, Arribas S, et al. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des. 2007;13:2185–2192. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, et al. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151:3299–3306. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- Gomez RA, Cassis L, Lynch KR, Chevalier RL, Wilfong N, Carey RM, et al. Fetal expression of the angiotensinogen gene. Endocrinology. 1988;123:2298–2302. doi: 10.1210/endo-123-5-2298. [DOI] [PubMed] [Google Scholar]
- Gonzalez MC, Arribas SM, Molero F, Fernandez-Alfonso MS. Effect of removal of adventitia on vascular smooth muscle contraction and relaxation. Am J Physiol Heart Circ Physiol. 2001;280:H2876–H2881. doi: 10.1152/ajpheart.2001.280.6.H2876. [DOI] [PubMed] [Google Scholar]
- Gossl M, Herrmann J, Tang H, Versari D, Galili O, Mannheim D, et al. Prevention of vasa vasorum neovascularization attenuates early neointima formation in experimental hypercholesterolemia. Basic Res Cardiol. 2009;104:695–706. doi: 10.1007/s00395-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman DD. Adventitia-dependent influences on vascular function. Am J Physiol. 1999;277((4 Pt 2)):H1265–H1272. doi: 10.1152/ajpheart.1999.277.4.H1265. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage FG, Oparil S, Xing D, Chen YF, McCrory MA, Szalai AJ. C-reactive protein-mediated vascular injury requires complement. Arterioscler Thromb Vasc Biol. 2010;30:1189–1195. doi: 10.1161/ATVBAHA.110.205377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen YD, Wilkison WO, Briggs MR. Human adipocyte proteomics – a complementary way of looking at fat. Pharmacogenomics. 2000;1:179–185. doi: 10.1517/14622416.1.2.179. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Heeneman S, Sluimer JC, Daemen MJ. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- Ho C, van der Veer E, Akawi O, Pickering JG. SIRT1 markedly extends replicative lifespan if the NAD+ salvage pathway is enhanced. FEBS Lett. 2009;583:3081–3085. doi: 10.1016/j.febslet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Hu T, Luan R, Zhang H, Lau WB, Wang Q, Zhang Y, et al. Hydrogen peroxide enhances osteopontin expression and matrix metalloproteinase activity in aortic vascular smooth muscle cells. Clin Exp Pharmacol Physiol. 2009;36:626–630. doi: 10.1111/j.1440-1681.2008.05124.x. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Igura T, Kawata S, Miyagawa J, Inui Y, Tamura S, Fukuda K, et al. Expression of heparin-binding epidermal growth factor-like growth factor in neointimal cells induced by balloon injury in rat carotid arteries. Arterioscler Thromb Vasc Biol. 1996;16:1524–1531. doi: 10.1161/01.atv.16.12.1524. [DOI] [PubMed] [Google Scholar]
- Ii M, Hoshiga M, Negoro N, Fukui R, Nakakoji T, Kohbayashi E, et al. Adrenal androgen dehydroepiandrosterone sulfate inhibits vascular remodeling following arterial injury. Atherosclerosis. 2009;206:77–85. doi: 10.1016/j.atherosclerosis.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Eguchi S, Shichiri M, Marumo F, Hirata Y. Adrenomedullin as a novel growth-promoting factor for cultured vascular smooth muscle cells: role of tyrosine kinase-mediated mitogen-activated protein kinase activation. Endocrinology. 1998;139:3432–3441. doi: 10.1210/endo.139.8.6144. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhang Y, Hou D, Zhu L, Xu W, Ding L, et al. 17beta-estradiol inhibits oleic acid-induced rat VSMC proliferation and migration by restoring PGC-1alpha expression. Mol Cell Endocrinol. 2010;315:74–80. doi: 10.1016/j.mce.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Jovinge S, Hultgardh-Nilsson A, Regnstrom J, Nilsson J. Tumor necrosis factor-alpha activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:490–497. doi: 10.1161/01.atv.17.3.490. [DOI] [PubMed] [Google Scholar]
- Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou A, Tsanikidis H, Vitta I, et al. Visfatin (nampt) and ghrelin as novel markers of carotid atherosclerosis in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2010;118:75–80. doi: 10.1055/s-0029-1237360. [DOI] [PubMed] [Google Scholar]
- Kahn CR. Medicine. Can we nip obesity in its vascular bud? Science. 2008;322:542–543. doi: 10.1126/science.1165667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano H, Kohno M, Yasunari K, Yokokawa K, Horio T, Ikeda M, et al. Adrenomedullin as a novel antiproliferative factor of vascular smooth muscle cells. J Hypertens. 1996;14:209–213. doi: 10.1097/00004872-199602000-00009. [DOI] [PubMed] [Google Scholar]
- Karastergiou K, Evans I, Ogston N, Miheisi N, Nair D, Kaski JC, et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- Kato A, Odamaki M, Ishida J, Hishida A. Relationship between serum pre-B cell colony-enhancing factor/visfatin and atherosclerotic parameters in chronic hemodialysis patients. Am J Nephrol. 2009;29:31–35. doi: 10.1159/000148648. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010;74:1479–1487. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- Keyes LE, Moore LG, Walchak SJ, Dempsey EC. Pregnancy-stimulated growth of vascular smooth muscle cells: importance of protein kinase C-dependent synergy between estrogen and platelet-derived growth factor. J Cell Physiol. 1996;166:22–32. doi: 10.1002/(SICI)1097-4652(199601)166:1<22::AID-JCP3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- Kim J, Min G, Bae YS, Min DS. Phospholipase D is involved in oxidative stress-induced migration of vascular smooth muscle cells via tyrosine phosphorylation and protein kinase C. Exp Mol Med. 2004;36:103–109. doi: 10.1038/emm.2004.15. [DOI] [PubMed] [Google Scholar]
- Kim HM, Bae SJ, Kim DW, Kim BK, Lee SB, Lee US, et al. Inhibitory role of magnolol on proliferative capacity and matrix metalloproteinase-9 expression in TNF-alpha-induced vascular smooth muscle cells. Int Immunopharmacol. 2007;7:1083–1091. doi: 10.1016/j.intimp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Kong YZ, Huang XR, Ouyang X, Tan JJ, Fingerle-Rowson G, Bacher M, et al. Evidence for vascular macrophage migration inhibitory factor in destabilization of human atherosclerotic plaques. Cardiovasc Res. 2005;65:272–282. doi: 10.1016/j.cardiores.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, et al. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2072–2079. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- Lamers D, Schlich R, Greulich S, Sasson S, Sell H, Eckel J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signaling in human vascular smooth muscle cells. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2010.01099.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–H753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- Lebona GT. The presence of paraganglia in the human ascending aortic fold: histological and ultrastructural studies. J Anat. 1993;183((Pt 1)):35–41. [PMC free article] [PubMed] [Google Scholar]
- Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- Li L, Mamputu JC, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 2005;54:2227–2234. doi: 10.2337/diabetes.54.7.2227. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, et al. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008;283:34833–34843. doi: 10.1074/jbc.M805866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZH, Fukuda N, Jin XQ, Yao EH, Ueno T, Endo M, et al. Complement 3 is involved in the synthetic phenotype and exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2004;44:42–47. doi: 10.1161/01.HYP.0000129540.83284.ca. [DOI] [PubMed] [Google Scholar]
- Ling S, Dai A, Dilley RJ, Jones M, Simpson E, Komesaroff PA, et al. Endogenous estrogen deficiency reduces proliferation and enhances apoptosis-related death in vascular smooth muscle cells: insights from the aromatase-knockout mouse. Circulation. 2004;109:537–543. doi: 10.1161/01.CIR.0000109699.45186.30. [DOI] [PubMed] [Google Scholar]
- Liu X, De Scheerder I, Desmet W. Dexamethasone-eluting stent: an anti-inflammatory approach to inhibit coronary restenosis. Expert Rev Cardiovasc Ther. 2004;2:653–660. doi: 10.1586/14779072.2.5.653. [DOI] [PubMed] [Google Scholar]
- Lo IC, Shih JM, Jiang MJ. Reactive oxygen species and ERK 1/2 mediate monocyte chemotactic protein-1-stimulated smooth muscle cell migration. J Biomed Sci. 2005;12:377–388. doi: 10.1007/s11373-005-1703-2. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Schafer N, von Lukowicz T, Sokrates Stein MA, Boren J, Rutti S, et al. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets. Atherosclerosis. 2009;207:360–367. doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- Longenecker JP, Kilty LA, Johnson LK. Glucocorticoid inhibition of vascular smooth muscle cell proliferation: influence of homologous extracellular matrix and serum mitogens. J Cell Biol. 1984;98:534–540. doi: 10.1083/jcb.98.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM, Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II. Eur J Pharmacol. 2010;634:107–112. doi: 10.1016/j.ejphar.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lu C, Zhao AX, Gao YJ, Lee RM. Modulation of vein function by perivascular adipose tissue. Eur J Pharmacol. 2011;657:111–116. doi: 10.1016/j.ejphar.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Madjid M, Vela D, Khalili-Tabrizi H, Casscells SW, Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34:11–18. [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothorac Surg. 2008;33:225–231. doi: 10.1016/j.ejcts.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol. 2003;201:221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989;94:225–232. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Kishida K, Shimomura I, Maeda N, Nagaretani H, Matsuda M, et al. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem Biophys Res Commun. 2002;292:781–786. doi: 10.1006/bbrc.2002.6720. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Adipocytokines and metabolic syndrome. Semin Vasc Med. 2005;5:34–39. doi: 10.1055/s-2005-871744. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896–1901. doi: 10.2174/138161210791208893. [DOI] [PubMed] [Google Scholar]
- Meng QH, Yang G, Yang W, Jiang B, Wu L, Wang R. Protective effect of hydrogen sulfide on balloon injury-induced neointima hyperplasia in rat carotid arteries. Am J Pathol. 2007;170:1406–1414. doi: 10.2353/ajpath.2007.060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- Miao CY, Villeneuve N, Brunel-Jacquemin C, Petit C, Guillaumin JP, Gransagne D, et al. Chronic mild hyperhomocysteinemia induces aortic endothelial dysfunction but does not elevate arterial pressure in rats. J Vasc Res. 2005;42:148–156. doi: 10.1159/000083972. [DOI] [PubMed] [Google Scholar]
- Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–427. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- Moroi M, Zhang L, Yasuda T, Virmani R, Gold HK, Fishman MC, et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Feng B, Ye Z, Yuan F, Zeng W, Luo Z, et al. Visfatin is related to lipid dysregulation, endothelial dysfunction and atherosclerosis in patients with chronic kidney disease. J Nephrol. 2011;24:177–184. doi: 10.5301/jn.2010.3488. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, et al. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci U S A. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- Nguyen G. The (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Curr Opin Nephrol Hypertens. 2007;16:129–133. doi: 10.1097/MNH.0b013e328040bfab. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, et al. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- Owens G, Kumar M, Wamhoff B. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pakala R, Benedict C. Eicosapentaenoic acid and docosahexaenoic acid selectively attenuate U46619-induced smooth muscle cell proliferation. Lipids. 1999;34:915–920. doi: 10.1007/s11745-999-0440-2. [DOI] [PubMed] [Google Scholar]
- Pan JH, Sukhova GK, Yang JT, Wang B, Xie T, Fu H, et al. Macrophage migration inhibitory factor deficiency impairs atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:3149–3153. doi: 10.1161/01.CIR.0000134704.84454.D2. [DOI] [PubMed] [Google Scholar]
- Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- Pezeshkian M, Noori M, Najjarpour-Jabbari H, Abolfathi A, Darabi M, Shaaker M, et al. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab Syndr Relat Disord. 2009;7:125–131. doi: 10.1089/met.2008.0056. [DOI] [PubMed] [Google Scholar]
- Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pross C, Farooq MM, Angle N, Lane JS, Cerveira JJ, Xavier AE, et al. Dexamethasone inhibits vascular smooth muscle cell migration via modulation of matrix metalloproteinase activity. J Surg Res. 2002;102:57–62. doi: 10.1006/jsre.2001.6220. [DOI] [PubMed] [Google Scholar]
- Qiao W, Chaoshu T, Hongfang J, Junbao D. Endogenous hydrogen sulfide is involved in the pathogenesis of atherosclerosis. Biochem Biophys Res Commun. 2010;396:182–186. doi: 10.1016/j.bbrc.2010.04.061. [DOI] [PubMed] [Google Scholar]
- Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GN, Alexander RW, Runge MS. Linoleic acid and its metabolites, hydroperoxyoctadecadienoic acids, stimulate c-Fos, c-Jun, and c-Myc mRNA expression, mitogen-activated protein kinase activation, and growth in rat aortic smooth muscle cells. J Clin Invest. 1995;96:842–847. doi: 10.1172/JCI118130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho YH, Chung CP, Solus JF, Raggi P, Oeser A, Gebretsadik T, et al. Adipocytokines, insulin resistance, and coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2010;62:1259–1264. doi: 10.1002/art.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romacho T, Azcutia V, Vazquez-Bella M, Matesanz N, Cercas E, Nevado J, et al. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia. 2009;52:2455–2463. doi: 10.1007/s00125-009-1509-2. [DOI] [PubMed] [Google Scholar]
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- Roubicek T, Dolinkova M, Blaha J, Haluzikova D, Bosanska L, Mraz M, et al. Increased angiotensinogen production in epicardial adipose tissue during cardiac surgery: possible role in a postoperative insulin resistance. Physiol Res. 2008;57:911–917. doi: 10.33549/physiolres.931315. [DOI] [PubMed] [Google Scholar]
- Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, et al. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Arterioscler Thromb Vasc Biol. 2010;30:2568–2574. doi: 10.1161/ATVBAHA.110.215525. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol. 2010;299:H202–H209. doi: 10.1152/ajpheart.00120.2010. [DOI] [PubMed] [Google Scholar]
- Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:565–570. doi: 10.1016/j.metabol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- Schauer IE, Reusch JE. Nonesterified fatty acid exposure activates protective and mitogenic pathways in vascular smooth muscle cells by alternate signaling pathways. Metabolism. 2009;58:319–327. doi: 10.1016/j.metabol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1 – a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- Selzman CH, McIntyre RC, Jr, Shames BD, Whitehill TA, Banerjee A, Harken AH. Interleukin-10 inhibits human vascular smooth muscle proliferation. J Mol Cell Cardiol. 1998;30:889–896. doi: 10.1006/jmcc.1998.0642. [DOI] [PubMed] [Google Scholar]
- Shibasaki I, Nishikimi T, Mochizuki Y, Yamada Y, Yoshitatsu M, Inoue Y, et al. Greater expression of inflammatory cytokines, adrenomedullin, and natriuretic peptide receptor-C in epicardial adipose tissue in coronary artery disease. Regul Pept. 2010;165:210–217. doi: 10.1016/j.regpep.2010.07.169. [DOI] [PubMed] [Google Scholar]
- Silaghi A, Achard V, Paulmyer-Lacroix O, Scridon T, Tassistro V, Duncea I, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293:E1443–E1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- Skilton MR, Serusclat A, Sethu AH, Brun S, Bernard S, Balkau B, et al. Noninvasive measurement of carotid extra-media thickness: associations with cardiovascular risk factors and intima-media thickness. JACC Cardiovasc Imaging. 2009;2:176–182. doi: 10.1016/j.jcmg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- Somoza B, Gonzalez MC, Gonzalez JM, Abderrahim F, Arribas SM, Fernandez-Alfonso MS. Modulatory role of the adventitia on noradrenaline and angiotensin II responses role of endothelium and AT2 receptors. Cardiovasc Res. 2005;65:478–486. doi: 10.1016/j.cardiores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Song J, Wan Y, Rolfe BE, Campbell JH, Campbell GR. Effect of estrogen on vascular smooth muscle cells is dependent upon cellular phenotype. Atherosclerosis. 1998;140:97–104. doi: 10.1016/s0021-9150(98)00122-1. [DOI] [PubMed] [Google Scholar]
- Souza DS, Bomfim V, Skoglund H, Dashwood MR, Borowiec JW, Bodin L, et al. High early patency of saphenous vein graft for coronary artery bypass harvested with surrounding tissue. Ann Thorac Surg. 2001;71:797–800. doi: 10.1016/s0003-4975(00)02508-x. [DOI] [PubMed] [Google Scholar]
- Souza DS, Johansson B, Bojo L, Karlsson R, Geijer H, Filbey D, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg. 2006;132:373–378. doi: 10.1016/j.jtcvs.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- Strawn WB, Ferrario CM, Tallant EA. Angiotensin-(1-7) reduces smooth muscle growth after vascular injury. Hypertension. 1999;33((1 Pt 2)):207–211. doi: 10.1161/01.hyp.33.1.207. [DOI] [PubMed] [Google Scholar]
- Sullivan TR, Jr, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, et al. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96:2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, et al. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- Tanner FC, Meier P, Greutert H, Champion C, Nabel EG, Luscher TF. Nitric oxide modulates expression of cell cycle regulatory proteins: a cytostatic strategy for inhibition of human vascular smooth muscle cell proliferation. Circulation. 2000;101:1982–1989. doi: 10.1161/01.cir.101.16.1982. [DOI] [PubMed] [Google Scholar]
- Teijeira-Fernandez E, Eiras S, Grigorian Shamagian L, Salgado Somoza A, Delgado C, Gonzalez-Juanatey JR. Lower epicardial adipose tissue adiponectin in patients with metabolic syndrome. Cytokine. 2011;54:185–190. doi: 10.1016/j.cyto.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111–117. doi: 10.1016/j.mce.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Wang Z, Castresana MR, Newman WH. NF-kappaB is required for TNF-alpha-directed smooth muscle cell migration. FEBS Lett. 2001a;508:360–364. doi: 10.1016/s0014-5793(01)03109-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Castresana MR, Newman WH. Reactive oxygen and NF-kappaB in VEGF-induced migration of human vascular smooth muscle cells. Biochem Biophys Res Commun. 2001b;285:669–674. doi: 10.1006/bbrc.2001.5232. [DOI] [PubMed] [Google Scholar]
- Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009;81:370–380. doi: 10.1093/cvr/cvn288. [DOI] [PubMed] [Google Scholar]
- Wang P, Bai C, Xu QY, Xu TY, Su DF, Sassard J, et al. Visfatin is associated with lipid metabolic abnormalities in Lyon hypertensive rats. Clin Exp Pharmacol Physiol. 2010a;37:894–899. doi: 10.1111/j.1440-1681.2010.05402.x. [DOI] [PubMed] [Google Scholar]
- Wang TD, Lee WJ, Shih FY, Huang CH, Chen WJ, Lee YT, et al. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis. 2010b;213:279–287. doi: 10.1016/j.atherosclerosis.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Tian WW, Viollet B, Rui YC, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, et al. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE, Kim F, Schwartz MW. Physiology. An integrative view of obesity. Science. 2007;318:928–929. doi: 10.1126/science.1148032. [DOI] [PubMed] [Google Scholar]
- Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Bryan S, Khaper N, Mani S, Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res. 2010;86:487–495. doi: 10.1093/cvr/cvp420. [DOI] [PubMed] [Google Scholar]
- Yao JS, Zhai W, Fan Y, Lawton MT, Barbaro NM, Young WL, et al. Interleukin-6 upregulates expression of KDR and stimulates proliferation of human cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab. 2007;27:510–520. doi: 10.1038/sj.jcbfm.9600365. [DOI] [PubMed] [Google Scholar]
- Yue TL, Wang X, Sung CP, Olson B, McKenna PJ, Gu JL, et al. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994;75:1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]
- Zeidan A, Purdham DM, Rajapurohitam V, Javadov S, Chakrabarti S, Karmazyn M. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J Pharmacol Exp Ther. 2005;315:1075–1084. doi: 10.1124/jpet.105.091561. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hu Y, Xu Q, Ye S. Different effects of angiotensin II and angiotensin-(1-7) on vascular smooth muscle cell proliferation and migration. PloS One. 2010;5:e12323. doi: 10.1371/journal.pone.0012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Tan HW, Gong HP, Wang SF, Zhang Y, Zhang W. Increased serum visfatin in patients with metabolic syndrome and carotid atherosclerosis. Clin Endocrinol (Oxf) 2008;69:878–884. doi: 10.1111/j.1365-2265.2008.03248.x. [DOI] [PubMed] [Google Scholar]


