Abstract
Fat cells or adipocytes are distributed ubiquitously throughout the body and are often regarded purely as energy stores. However, recently it has become clear that these adipocytes are engine rooms producing large numbers of metabolically active substances with both endocrine and paracrine actions. White adipocytes surround almost every blood vessel in the human body and are collectively termed perivascular adipose tissue (PVAT). It is now well recognized that PVAT not only provides mechanical support for any blood vessels it invests, but also secretes vasoactive and metabolically essential cytokines known as adipokines, which regulate vascular function. The emergence of obesity as a major challenge to our healthcare systems has contributed to the growing interest in adipocyte dysfunction with a view to discovering new pharmacotherapeutic agents to help rescue compromised PVAT function. Very few PVAT studies have been carried out on human tissue. This review will discuss these and the hypotheses generated from such research, as well as highlight the most significant and clinically relevant animal studies showing the most pharmacological promise.
LINKED ARTICLES
This article is part of a themed section on Fat and Vascular Responsiveness. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-3
Keywords: perivascular adipose tissue, PVAT, adipocytes, adipokines, obesity, metabolic syndrome, adiponectin, leptin, epicardial adipose tissue, coronary vessels
Introduction: the clinical problem
Obesity, defined as a body mass index (BMI) of greater or equal to 30 kg·m−2 is a major problem in acculturated and developing societies. It often coexists with a number of other diseases including hypertension, dyslipidaemia and insulin resistance. Such a constellation has been labelled the metabolic syndrome (MetS). The international obesity taskforce (IOTF) estimates that approximately 1 billion adults are currently overweight (BMI 25–29.9 kg·m−2), and of these 475 million are obese (≥30 kg·m−2) (IOTF, 2010).
The enormity of this epidemic highlights the need for novel approaches to obesity management and a furthering of our knowledge of the mechanisms responsible for the consequences of being overweight.
A number of reports has indicated that the distribution of fat around the body determines not only the obese phenotype but its consequences. For example, intra-abdominal and visceral fat depots have been linked with an increased cardiometabolic risk and the mortality associated with obesity (Fox et al., 2007; Gesta et al., 2007). The total amount of internal fat rises with increasing subcutaneous adipocity, but even individuals classed as thin may have more visceral fat than some obese individuals. In addition, increased gluteofemoral fat mass has been negatively linked to levels of inflammatory cytokines and positively linked to raised concentrations of adipokines resulting in decreased metabolic and cardiovascular risk (Manolopoulos et al., 2010).
Our current understanding of this problem focuses on our ancestors and the fact that fat was the energy store developed in times of plenty, which could then be burned during famine. Therefore genes which predisposed to obesity would confer survival benefits and such individuals would live long enough to reproduce. Several reviews have suggested that it is the breakdown of this system that is responsible for the contemporary problems associated with obesity, where susceptible individuals no longer have periods of famine and are instead constantly overeating readily available high energy foods (Neel, 1962; Diamond, 2003). Whilst in hibernating mammals, short-term obesity and insulin resistance have the beneficial effect of directing glucose to the brain, only man has developed chronic obesity with its associated cardiovascular morbidity and mortality (Scott and Grant, 2006).
Yudkin et al. (2005) originally postulated that perivascular adipose tissue (PVAT) might hold the key to linking obesity with the development of MetS and diabetes as a result of an adverse influence upon the vasculature (Yudkin et al., 2005). In health, PVAT could produce adipokines that profoundly influence metabolism and the control of local vascular tone via vasocrine actions. It was suggested that the loss of such substances would result in a change in vascular function and development of insulin resistance. These authors suggested that the effect of circulating insulin on NO-mediated vasodilatation, which is of paramount importance in modulating the postprandial increase in nutritive flow, could be challenged by the paracrine action of adipokines released from local fat stores in obesity. They further highlighted the role inflammation may play and suggested elevated levels of TNF-α in obesity could disrupt the crosstalk between fat and blood vessels.
In this review we intend to focus on the vasoactive properties of PVAT as mediated by adipokines.
What are the principle adipokines released from adipocytes?
The recent interest in adipose tissue as a dynamic endocrine organ has resulted in a number of studies examining the shared, and in cases distinct, properties of different fat depots. PVAT surrounds subcutaneous small arteries, coronary vessels (peri-coronary and epicardial fat), aorta and systemic vessels and secretes a number of important adipokines (Figure 1).
Figure 1.
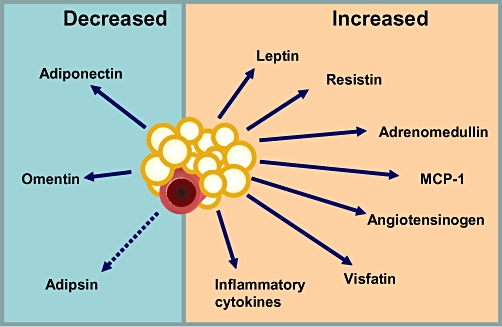
PVAT is the source of a number of vasoactive and metabolically significant adipokines.
Amongst the adipokines, leptin and adiponectin have been subject of recent reviews (Ren, 2004; Kadowaki and Yamauchi, 2005; Kadowaki et al., 2006; Sweeney, 2010). Here, we review briefly the most significant findings on leptin, adiponectin and adrenomedullin. A more comprehensive list of adipokines and their roles with regards to the MetS has been published recently (Deng and Scherer, 2011).
Leptin
In rats leptin has a direct vasodilator effect acting via an endothelium- and NO-dependent mechanism (Lembo et al., 2000). However, it has an endothelium-independent vasodilator effect on segments of human subclavian vein and internal mammary arteries harvested during surgery, thus highlighting the need for careful selection of vascular tissue in order to design clinically relevant studies (Momin et al., 2006).
The role of leptin as an indirect vasoconstrictor has also been studied. Fruhbeck et al. have shown that leptin has a sympathoexcitatory effect leading to a rise in BP in Wistar rats (Fruhbeck, 1999). They demonstrated a significant rise in both systolic and diastolic BP when rats were given an intravenous bolus of the NO inhibitor NG-nitro-L-arginine methyl ester (L-NAME) followed by leptin, demonstrating the role NO plays in facilitating the vasorelaxant effects of leptin. In the same experiment they showed that leptin administration, following postganglionic blockade using chlorisondamine, resulted in a reduction in BP, which was abolished by NO inhibition.
Weight gain is often associated with increased insulin resistance and resultant hyperinsulinaemia. Interestingly, Vecchione et al. have demonstrated that insulin potentiates leptin-induced NO release and even at physiological levels, insulin enhances the vasodilator effects of leptin (Vecchione et al., 2003).
That leptin, a pro-inflammatory adipokine, possesses vasodilator properties, and that obesity is associated with leptin resistance, implies its role in obesity is not understood fully. In MetS leptin levels are increased within the epicardial fat adjacent to the coronary vessels (Payne et al., 2010). In dogs, hyperleptinaemia has been associated with endothelial dysfunction (Knudson et al., 2005), and work on a swine model of MetS has shown that leptin exacerbates endothelial dysfunction via a protein kinase C-β dependent pathway (Payne et al., 2009).
It is unclear whether elevated levels of leptin in epicardial fat are of benefit to the adjacent coronary vessels, or whether they may contribute to endothelial dysfunction in human arteries.
Adiponectin
Adiponectin is a 30 kDa protein made up of 244 amino acids. It is the most abundant adipokine (Dridi and Taouis, 2009; Liu et al., 2010) and exists in two forms, full length, or a smaller globular fragment. (Kadowaki and Yamauchi, 2005; Kadowaki et al., 2006; Dridi and Taouis, 2009). The full length form acts via the R2 receptor and the globular form via R1 (Yamauchi et al., 2003a). In human adipocytes, the expression of Adipo R1 is ∼15-fold higher than that of Adipo R2 (Rasmussen et al., 2006). Circulating adiponectin levels are reduced in obesity (Sowers, 2008), diabetes (Kadowaki et al., 2006), and there is a down-regulation of adiponectin receptors in the adipose tissue of obese individuals (Rasmussen et al., 2006). Given its anti-diabetic (Berg et al., 2001; Yamauchi et al., 2001; 2003b; Maeda et al., 2002; Pajvani and Scherer, 2003; Davis and Scherer, 2008), anti-atherosclerotic (Yamauchi et al., 2003b; Han et al., 2009) and vasodilator (Fesus et al., 2007; Greenstein et al., 2009) properties, adiponectin is believed to be the link between obesity and MetS. Its mechanism of action has yet to be determined fully.
In the liver, full-length adiponectin activates adenosine monophosphate-activated protein kinase (AMPK) and the trimeric form is known to activate AMPK in adipose tissue and muscle. Activation of AMPK leads to phosphorylation of Acetyl-Coenzyme-A Carboxylase (ACC), which results in fatty acid β-oxidation and inhibition of triacylglycerol and fatty acid synthesis (Kadowaki and Yamauchi, 2005; Liu et al., 2010).
Deng et al. have demonstrated that adiponectin improves endothelial dysfunction by increasing NO production via phosphorylation of endothelial NO in the aorta of high-fat-fed obese Sprague-Dawley rats (Deng et al., 2010). Moreover, in adiponectin receptor-knockout mice, there is a significant attenuation of endothelium-dependent vasodilatation in response to ACh (Ouchi et al., 2003). Of relevance, adiponectin suppresses both basal and oxidized-LDL induced superoxide generation in bovine endothelial cells (Motoshima et al., 2004), and also suppresses excess reactive oxygen species (ROS) production under high-glucose conditions via a cAMP/PKA-dependent pathway (Ouedraogo et al., 2006). Given the elevated levels of oxidized-LDL (Holvoet et al., 2008) and plasma glucose in patients with MetS, the concomitant reduction in adiponectin levels may explain partly the endothelial dysfunction observed by our group (Greenstein et al., 2009) in the cohort of patients with MetS.
Incubation of healthy human vessels with a blocking peptide for the adiponectin R1 receptor almost abolished completely the anticontractile effect of PVAT in response to cumulative noradrenaline doses (Greenstein et al., 2009). Our unpublished proteomic analysis of adipose tissue from obese patients shows a significant reduction in adiponectin levels as compared with lean individuals. Therefore, we suspect that the absence of the anticontractile function of PVAT in obesity is accounted for by a reduction in adiponectin levels in PVAT at least in part. It has been suggested that the high levels of adiponectin in some disease states may be a compensatory response to the development of ‘adiponectin resistance’ (i.e. a dysfunction in the adiponectin signalling pathway) (Sam and Walsh, 2010). Clearly, further research is required to describe the exact mechanisms of action of adiponectin in health and to explain how these pathways become affected in disease.
Adrenomedullin
Amongst the adipokines, adrenomedullin (AM) has received the least attention in recently published literature. It is a 52 amino acid peptide first isolated from a sample of human phaeochromocytoma, but later shown to be synthesized by adrenal, heart, and vascular endothelial and smooth muscle cells as well as white adipocytes of both rodents and humans (Fukai et al., 2005; Silaghi et al., 2007). Its dose-dependent vasodilator effect on the rat mesenteric vessels was first reported in 1993 (Nuki et al., 1993). Its direct and potent vasodilatory action on blood vessels would suggest that it plays an important role in the control of vessel tone. Human studies have shown that intravenous infusion of the peptide leads to significant vasodilatation of pulmonary vessels providing a potential therapeutic strategy for pulmonary hypertension (Nagaya et al., 2000; 2003). Also there is evidence of AM-induced human coronary (Terata et al., 2000) and skeletal artery (Nakamura et al., 1997) vasodilatation via an NO-dependent pathway.
The exact role of AM in obesity needs further evaluation, but we know that AM reduces levels of ROS in vascular smooth muscle cells (Yoshimoto et al., 2005), and AM knockout mice express higher levels of ROS (Shimosawa et al., 2003).
Catecholamine stimulation of β3 adrenoceptors on adipocytes results in lipolysis and release of stored adipokines (Robidoux et al., 2006). AM inhibits lipolysis via an NO-dependent mechanism (Harmancey et al., 2005) which in theory, would affect vessel tone indirectly by blocking the release of vasoactive adipokines such as adiponectin (Figure 2). Gettys et al. have reported that stimulation of the β3-adrenergic receptor on white rat adipocytes by the selective agonist CL316,243 leads to the inhibition of the release of leptin (Gettys et al., 1996). The balance between the potential lipolysis-induced release of adiponectin and the inhibition of leptin release and their relevance to vascular tone needs further clarification.
Figure 2.
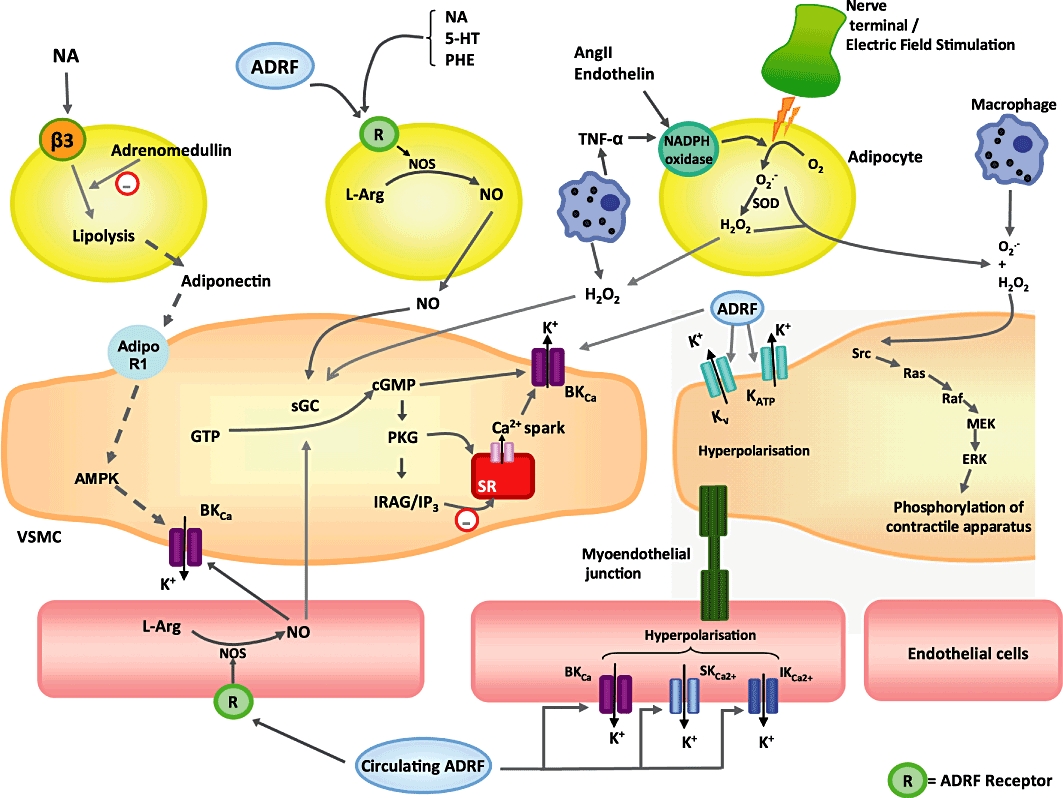
Potential mechanisms via which perivascular adipocytes, vascular smooth muscle cells and endothelial cells interact. Dotted lines represent unproven pathways. ADRF, adventitium-derived relaxing factor; AMPK, AMP-activated protein kinase; Ang II, angiotensin II; BKca, large conductance calcium-activated potassium channel; H2O2, hydrogen peroxide; ERK, extracellular signal-regulated kinases; 5-HT, 5-hydroxytryptamine (serotonin); GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate; IP3, inositol triphosphate; IRAG, IP3 receptor-associated cGMP kinase substrate; IKca, intermediate conductance calcium-activated potassium channel; Kv, voltage-gated potassium channel; KATP, ATP-sensitive potassium channel; L-Arg, L-Argine; NA, noradrenaline; NO, nitric oxide; NOS, nitric oxide synthase; O2.-, superoxide anion; ONOO, peroxynitrite; PCS, prostacyclin pathway; PHE, phenylephrine; PGH2,prostaglandin H2; PGI2, prostaglandin I2 (prostacyclin); PKG, protein kinase G; R, receptor; sGC, soluble guanylate cyclise; SKca, small conductance calcium-activated potassium channel; SOD, superoxide dismutase; SR, sarcoplasmic reticulum; TNF, tumour necrosis factor; VSMC, vascular smooth muscle cell.
Epicardial adipose tissue from patients with coronary artery disease (CAD) displays higher levels of AM than those without CAD. Given its vasorelaxant properties, one would assume this may serve as a protective mechanism for the diseased coronary vessels (Shibasaki et al., 2010). Plasma AM concentrations are also elevated in disease states, which may provide further protection against oxidative stress and vasoconstrictors. Despite its antioxidant and vasorelaxant properties, it is possible that elevated AM levels in disease states may actually contribute to the vascular dysfunction.
PVAT and control of local vascular tone
PVAT function has been assessed in canine, swine and rodent models and demonstrated different functional and structural properties of PVAT, which vary both between species and anatomical site. Examples of structural differences include the fact that PVAT from rat aorta comprises smaller adipocytes compared with mesenteric vessels (Galvez-Prieto et al., 2008). Whilst the murine thoracic aorta is surrounded by brown adipocytes, peri-abdominal fat is comprised of white adipocytes (Police et al., 2009). From a functional perspective, coronary artery PVAT in healthy dogs attenuates acetylcholine-induced relaxation (marker of endothelial function) (Payne et al., 2008; 2009), but does not affect bradykinin-mediated dilatation in healthy pig arteries (Payne et al., 2010).
Three studies have shown that healthy human PVAT exerts an anticontractile effect on adjacent vessels. Rodent (both mouse and rat) mesenteric and aortic vascular beds have been the most frequently studied models of PVAT function. Data from these models have matched closely the data obtained from limited human studies.
In 1991, Soltis and Cassis were the first to report that vessels with intact PVAT were less responsive to noradrenaline than naked vessels (Soltis and Cassis, 1991). Later studies used solution transfer protocols to demonstrate the existence of a transferrable adventitium-derived relaxing factor (ADRF) (Lohn et al., 2002; Gao et al., 2005b; Malinowski et al., 2008; Greenstein et al., 2009). The solution transfer experiments involved using a small volume of solution from a tissue bath with PVAT, adding it to a preconstricted vessel and measuring the vascular response. These suggest that the observed anticontractile property of PVAT is not merely a consequence of it acting as an obstacle to diffusion for vasoconstrictors, but as a dynamic tissue which secretes adipokines with anticontractile properties.
There are likely to be a number of substances which account for the anticontractile effects of PVAT. Indeed, both endothelium-dependent and endothelium-independent mechanisms have been demonstrated. Protocols using rat mesenteric vessels have shown that both PVAT and exogenous adiponectin exert anticontractile effects on preconstricted small arteries (Greenstein et al., 2009); the effects of PVAT have been corroborated by experiments on rat aorta and its surrounding PVAT, which mostly consists of brown adipocytes (Lohn et al., 2002; Gao et al., 2007). Gao has shown that the endothelium-independent mechanism involves hydrogen peroxide (H2O2) and subsequent activation of soluble guanylyl cyclase. At low concentrations (10–100 µM) of H2O2, mesenteric vessels preconstricted with phenylephrine undergo further constriction, but higher concentrations of H2O2 (0.3–1 mM) result in a biphasic response, with an early constriction followed by relaxation. Hydrogen peroxide released from both adipocytes and macrophages can act via the raf/MEK/ERK pathway and influence the phosphorylation of contractile apparatus in vascular smooth myocytes (Figure 2).
Interestingly, exposure of rat mesenteric arteries to electric field stimulation (EFS) leads to a contractile response via stimulation of α1-adrenoceptors by the perivascular sympathetic nerves. PVAT enhances this contractile response by stimulating superoxide generation and activation of tyrosine kinase and MAPK/ERK pathway (Gao et al., 2006) (Figure 2). More recently it has been shown that Angiotensin II derived from adipocytes potentiates the contractile response to EFS (Lu et al., 2010), thereby highlighting the role of the renin-angiotensin system (RAS) within PVAT with regards to local vascular tone modulation.
Physiological release and action of ADRF: possible role of the potassium channel
The exact mechanisms by which adipocytes exert their effects on adjacent arteries are not well understood. Figure 2 shows a number of the potential mechanisms involved. Downstream in the PVAT anticontractile pathway are vascular potassium (K) channels, which occupy a central position in the maintenance and regulation of vascular tone. Early experiments on potassium channels have identified roles for a number of K channels. In rat mesentery the anticontractile effect is attenuated by blockade of the delayed rectifier K channel (KV) (Verlohren et al., 2004). Studies of rat aorta suggest roles for adenosine triphosphate (ATP)-sensitive K channels (KATP) (Lohn et al., 2002) as well as small and intermediate conductance calcium-sensitive potassium channels (SKCa and IKCa) (Gao et al., 2007). In human internal mammary artery, the relaxing factor appears to work via large conductance calcium-sensitive K channels (BKCa) (Gao et al., 2005b).
More recent experiments have highlighted the pivotal role of the BKCa channel in mediating the PVAT effect. Work from our group supports the role of the BKCa channel in the vasodilator function of PVAT. Pharmacological inhibition of the channel using paxilline or using mesenteric arteries from BKCa knockout mice (Slo-/-, BKβ1-/-) results in a significant impairment of the response (Lynch et al., 2010b). This highlights the role of BKCa channels in facilitating the ADRF effect, but further work is needed to clarify whether BKCa channels are also present on adipocytes as well as the vascular myocytes. Additionally, the removal of endothelium or inhibition of NOS can significantly attenuate the response in these mouse models (Lynch et al., 2010a).
Elegant micro-electrode studies of de-endothelialized rat mesenteric arteries have shown that in non-constricted vessels the hyperpolarization to exogenous adiponectin is inhibited by selective blockade of BKCa (Egner et al., 2010). This group has reported also that stimulation of β3 adrenoceptors releases a factor which indirectly activates myocyte BKCa channels. They have suggested that this factor is adiponectin working via myocyte adipoR1 receptors to activate AMPK. These protocols were performed in the absence of endothelium. However, there are BKCa channels present on the endothelium (Hughes et al., 2010) and stimulation of these channels by circulating ADRF could result in hyperpolarization of endothelial cells and subsequent hyperpolarization of vascular myocytes via the myoendothelial junction, thus leading to their relaxation (Figure 2).
Obesity is a state of adrenergic overdrive with increased circulating noradrenaline levels (Prezio et al., 1964) and an overactive sympathetic nervous system (Lambert et al., 2010) releasing noradrenaline at nerve terminals which are present in PVAT. Noradrenaline can bind to β3 adrenoceptors, although with lower affinity than to β1 and β2 adrenoceptors. In obesity one would expect that overstimulation of the β3 adrenoceptor by noradrenaline would result in an increase in adiponectin release from adipocytes thus lowering vessel tone and enhancing metabolic homeostasis. However, in obesity, there are reduced blood and adipocyte adiponectin levels (Asayama et al., 2003; Kern et al., 2003) as well as down-regulation of adiponectin receptors (Kadowaki and Yamauchi, 2005). Further research is required to explain the reduced levels of adiponectin and its receptors. There may be an increase in breakdown of adiponectin secondary to oxidative stress and inflammation or a reduction in its production due to obesity-induced damage to the intracellular mechanisms involved in production of the protein. We shall discuss further evidence for inflammation-induced PVAT damage later in this review.
PVAT and human vessels
Harvesting human arteries for PVAT studies is fraught with difficulty, which may explain the limited number of published studies. The most accessible human blood vessel is the internal thoracic artery, obtained during coronary artery bypass graft operations (Gao et al., 2005b; Malinowski et al., 2008) and small arteries from gluteal fat biopsies (Greenstein et al., 2009). Both are clinically relevant vascular beds. However, the atraumatic harvest of saphenous vein grafts, via the ‘no-touch’ technique has been shown to result in vessels with intact PVAT and immunohistochemical evidence of the potent vasodilator NO (Dashwood et al., 2007; 2009). Venous PVAT has been shown to exert an anticontractile effect on adjacent tissue by releasing Ang-(1–7) which activates Kv channels and relaxes vascular myocytes through eNOS release (Lu et al., 2011b).
Gao et al. first studied the anticontractile properties of human PVAT using segments of human internal thoracic artery. It was shown that human PVAT exerted an anticontractile effect on its adjacent vessel upon exposure to contractile agents including U46619, which is a thromboxane A2/prostaglandin H2 receptor antagonist (Gao et al., 2005b). This is a significant finding because thromboxane A2 and its stable metabolite thromboxane B2 (TxB2) are known to be potent vasoconstrictors and the levels of TxB2 are known to be increased during cardiopulmonary bypass (Davies et al., 1980). Perioperative spasm of the internal thoracic artery is a commonly encountered issue (He et al., 1994), therefore leaving the perivascular fat intact may help counteract the vasoconstricting effects of the increased plasma thromboxane levels. In this study, the anticontractile effect of PVAT was shown to be due to a transferrable relaxing factor (ADRF) that activated BKCa channels.
Malinowski et al. also used segments of human internal thoracic artery and investigated whether it is PVAT per se, or adipose tissue in general, that is capable of exerting an anticontractile effect (Malinowski et al., 2008). Following a coronary artery bypass operation, the in situ graft (taken from the internal thoracic artery) heals in close proximity to pleural tissue. Dose-responses to serotonin were constructed for skeletonized vessel segments with and without incubation with pleural fat. The study showed that, despite the presence of white adipocytes in pleural fat, there was no anticontractile effect. The study also demonstrated clearly that adipokines released by PVAT are able to affect the blood vessel even if the fat tissue has been loosely added to the tissue bath and is not in direct contact with the artery.
In order to assess whether ADRF acts via the endothelium, Malinowski et al. incubated their vessels with inhibitors of NO synthase and cyclooxygenase (COX). It is well known that NO and prostacyclin produced in the endothelium have vasodilatory effects. This study showed that inhibition of NO and COX did not abolish the anticontractile effect of PVAT. This does not mean that PVAT function is endothelium-independent; rather that it can exert its effect independent of the NO and COX pathways.
The most recent study of human PVAT has been carried out on small arteries harvested from adipose tissue samples obtained by gluteal biopsies in patients with MetS. Figure 3A demonstrates the anticontractile function of PVAT in healthy individuals whilst Figure 3B shows that PVAT did not exhibit an anticontractile function in the group with MetS (Greenstein et al., 2009). Incubating healthy PVAT intact human vessels with a fragment of the human type 1 adiponectin receptor completely abolished PVAT anticontractile function, thus identifying adiponectin as an ADRF in human PVAT. Also presence of NO in human PVAT was demonstrated by incubating PVAT intact healthy vessels with L-NMMA which resulted in attenuation of the anticontractile property of PVAT. This study will be discussed further in the next section.
Figure 3.
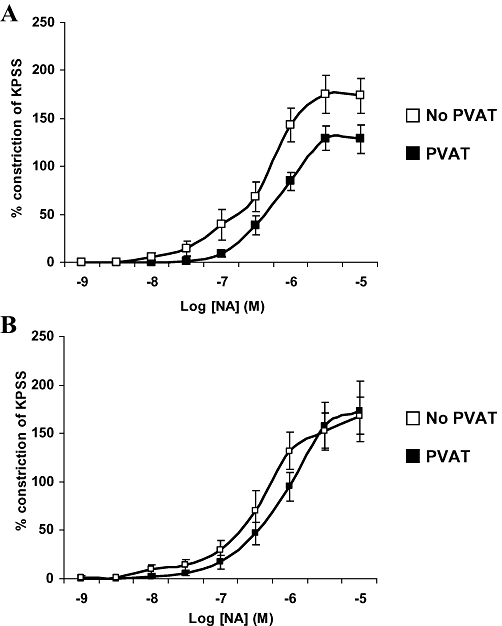
Effect of obesity and the metabolic syndrome on anticontractile capacity of PVAT on small arteries from subcutaneous gluteal fat, *P < 0.01 (Greenstein et al., 2009). (A) In healthy control participants PVAT exerts a significant anticontractile effect (P = 0.009, multiple anova) when compared with contractility of arteries without PVAT (n = 10). (B) In patients with obesity and metabolic syndrome, presence of PVAT has no effect on contractility (n = 10). KPSS, high potassium physiological saline solution; NA, noradrenaline.
PVAT, obesity and inflammation
The effect of obesity on PVAT function was first reported in 2005. It was shown that, prenatal exposure of rats to nicotine caused postnatal obesity and an increased amount of PVAT. However, the rise in PVAT volume which was associated with weight gain resulted in a reduction in the anticontractile function of PVAT surrounding the aorta. It was thought that this may be due to a change in the nature of PVAT or a reduction in secretion of relaxation factor(s) from PVAT (Gao et al., 2005a).
Often obesity is associated with hyperglycaemia and hyperinsulinaemia in the context of the MetS. The role of circulating insulin on vascular tone has been well documented (Eringa et al., 2004), but its effect on PVAT-mediated vascular responses has not been established. However, both acute and chronic hyperglycaemia enhance the PVAT anticontractile effect (Lee et al., 2009b). This highlights the complex nature of the MetS and the difficulty of controlling for variables in ex vivo experiments.
Adipocytes undergo significant hypertrophy in obesity. The cross-sectional area of adipocytes in obese individuals with MetS is up to 1000 µm2 larger than that of healthy individuals (Greenstein et al., 2009). Given that the diffusion limit of oxygen is thought to be around 100 µm (Hosogai et al., 2007), the hypertrophied adipocytes are likely to be subjected to a decreased oxygen tension. In obese individuals, there is no increase in blood supply to match the increase in adipocyte size, and the postprandial increase of blood flow that occurs in lean subjects is also absent in obesity (Bulow et al., 1987; Coppack et al., 1992). This implies that hypertrophied adipocytes exist in a state of relative hypoxia. This has been confirmed by pimonidazole staining (a 2-nitroimidazole which is activated at low oxygen concentrations) of adipocytes from obese mice (Hosogai et al., 2007), as well as measurements of partial pressures of oxygen in abdominal subcutaneous adipose tissue of obese humans (Pasarica et al., 2009). In the context of hypoxia, vasa vasorum in the adipose tissue surrounding vessels plays an important role in providing oxygen to the hypoxic PVAT. Recent work has shown that PVAT induces the formation of vasa vasorum (Manka et al., 2010), which would be beneficial in obesity.
Hypoxia reduces PVAT anticontractile effects in rat mesenteric arteries (Greenstein et al., 2009); however, in aorta the opposite occurs, namely an enhancement of PVAT function (Maenhaut et al., 2010). Clearly, these regional differences merit further evaluation.
From a therapeutic perspective, experimental hypoxia-induced damage to PVAT function can be rescued using free radical scavengers (Greenstein et al., 2009) and Eplerenone (Withers et al., 2011). Future clinical trials can explore the possibility of using such drugs in the treatment of patients with obesity.
The chronic low-grade inflammatory state which develops as a consequence of hypoxia in obesity is marked by an increase in blood levels of ROS and pro-inflammatory cytokines such as CRP, IL-6 and TNF-α (Trayhurn et al., 2008). Incubation with TNF reduces the anticontractile effect of PVAT in vessels harvested from healthy individuals, suggesting that the higher levels of the inflammatory cytokine in obesity may partly account for the reduced anticontractile function of PVAT. Similar results were shown in healthy rat tissue where incubation with TNF and IL-6 resulted in a reduction of the anticontractile function of PVAT (Greenstein et al., 2009).
Further human studies have shown a link between high levels of IL-6 and increased risk of CAD (Pai et al., 2004). Epicardial adipose tissue IL-6 mRNA levels have been shown to be higher in CAD than non-CAD patients, with higher levels correlating with greater degrees of angiographically defined vascular injury (Eiras et al., 2008). The up-regulation of inflammatory cytokines in the adipose tissue suggest a role for oxidative stress as a mechanism for damage to PVAT function. Proteomic analysis of epicardial adipose tissue and subcutaneous adipose tissue obtained from CAD patients has demonstrated higher levels of ROS in epicardial adipose tissue, and mRNA analysis has revealed lower levels of the antioxidant enzyme catalase (Salgado-Somoza et al., 2010).
The role of the macrophage
Increased macrophage numbers in adipose tissue of obese animals (Rausch et al., 2008) and humans (Weisberg et al., 2003) also support the hypoxia theory, as hypoxic cells secrete chemokines to attract macrophages (Pasarica et al., 2009). Further data from our group suggest that when PVAT from mouse vessels is rendered hypoxic, the presence and activation of macrophages is the key modulator of increased vascular contractility. Moreover, following the conditional ablation of macrophages, hypoxic insult has no effect on contractility of the vessels (Figure 4), further implicating a role for macrophages in mediating the response to inflammatory stimuli (Withers et al., 2011).
Figure 4.
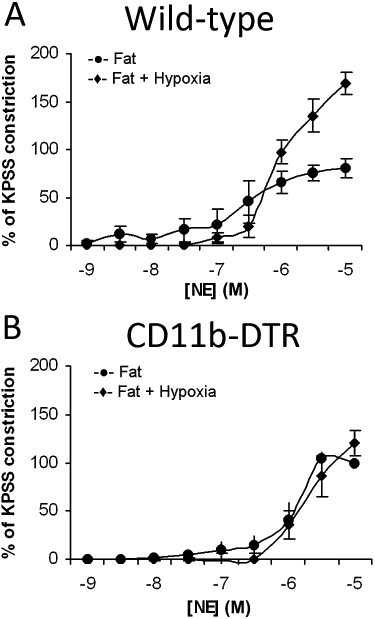
The presence of macrophages is the key modulator of increased vascular contractility in vessels with hypoxic PVAT (Withers et al., 2011). (A) When PVAT from mouse vessels is rendered hypoxic, there is increased sensitivity of the vessel to cumulative doses of noradrenaline. (B) In CD11b–diphtheria toxin (DT) receptor (DTR) transgenic mice (DT administration selectively kills monocytes/macrophages), hypoxia has no effect on vascular contractility. KPSS, high potassium physiological saline solution; NE, norepinephrine.
The levels of monocyte chemotactic protein-1 (MCP-1) are raised in plasma and adipose tissue of both genetically obese and diet-induced obese mice. In MCP-1 knockout diet-induced obese mice, there is a significant reduction in numbers of macrophages in the adipose tissue as well as improved insulin sensitivity (Kanda et al., 2006). Paradoxically, however, while it is likely that activated macrophages in obese patients contribute to PVAT damage, in health, perivascular macrophages enable structural remodelling of the small artery wall in response to flow. Bakker et al. have shown that in mouse mesenteric arteries, inactivation of macrophages by clodronate, reduces flow-induced remodelling (Bakker et al., 2006) which is observed in arteries from diabetic animals (Belin de Chantemele et al., 2009).
We propose that reduced levels of adiponectin in obesity enhances macrophage effects on PVAT function as adiponectin suppresses macrophage phagocytosis and TNF-α production (Yokota et al., 2000). Moreover, it has been shown that hypoxia and ROS decrease adiponectin production from 3T3-L1 adipocytes (Chen et al., 2006).
A major process in the development of atherosclerosis is the recruitment of leucocytes to the endothelium and their subsequent migration into the subendothelial space. Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) are two of the adhesion molecules expressed on vascular endothelial cells and are responsible for facilitating the recruitment of the white blood cells. In patients with coronary heart disease and carotid artery atherosclerosis, there are increased levels of ICAM-1 (Hwang et al., 1997). Resistin is produced by adipocytes and is also expressed in macrophages which are present in larger numbers in the PVAT of obese animals and humans. It has been shown that resistin induces the expression of ICAM-1 and VCAM-1 in human endothelial cells and that adiponectin can block this effect of resistin (Kawanami et al., 2004). PPARγ agonists have been shown to reduce the expression of resistin mRNA in macrophages (Patel et al., 2003) whilst increasing plasma levels of adiponectin (Long et al., 2010). Ouchi et al. have also reported that adiponectin can dose-dependently inhibit the expression of VCAM-1 and ICAM-1 in human aortic endothelial cells (Ouchi et al., 1999).
The RAS within PVAT
The RAS is an important regulator of blood pressure and vascular function. Circulating angiotensinogen is cleaved by renin to produce angiotensin 1 (Ang I). Angiotensin-converting enzyme (ACE) converts this to Ang II (Engeli et al., 1999) which binds to two receptors, AT1 and AT2. Adipocytes secrete products of the RAS and the functional significance of this has been reviewed in depth (Gorzelniak et al., 2002; Engeli et al., 2003). RAS products such as ACE, angiotensinogen and AT1 and AT2 receptors have been identified on human and rodent adipocytes (Cassis et al., 1988; Harp and DiGirolamo, 1995; Crandall et al., 1999; Schling et al., 1999; Cassis, 2000). However, it is becoming apparent that the adipocyte RAS system influences tissues beyond its local environment and that it plays a role in the development of vascular diseases such as obesity and hypertension. Much is known about adipose RAS per se; however, this section will focus on the RAS component of PVAT.
There appears to be differential distribution of components of the RAS system expressed in both white and brown PVAT (Cassis et al., 1988; Campbell et al., 1993; Campbell et al., 1995; Engeli et al., 1999; Galvez et al., 2006). Angiotensinogen and Ang I expression is similar between white and brown tissue (Galvez et al., 2006); however, Ang II expression appears to vary between brown and white perivascular adipocytes (Jonsson et al., 1994; Engeli et al., 1999; Schling et al., 1999; Giacchetti et al., 2002). There is conflicting evidence of renin expression in perivascular brown or white adipose tissue with some groups reporting no detectable levels of the protein and others reporting its presence (Engeli et al., 1999; Giacchetti et al., 2002; Galvez-Prieto et al., 2008).
There is a growing body of evidence to suggest that elements of adipocyte-derived RAS play vital roles in the normal and pathogenic responses of blood vessels. Ang 1–7 has recently been identified as an important PVAT diffusible RAS product (Lee et al., 2009a). It is released by PVAT and acts on the endothelium to stimulate the release of NO, which in turn hyperpolarizes smooth muscle cells through KCa channels. Ang 1–7 is thought to counterbalance the contractile influence of Ang II (Yagil and Yagil, 2003; Ferrario et al., 2005; Reudelhuber, 2006). Increased expression of Ang II and decreased expression of Ang 1–7 has been reported in hypertensive models. Indeed treatment of hypertensive models with ACE inhibitors lowers Ang II and increases Ang 1–7 (Yagil and Yagil, 2003). Ang I antagonism using Losartan restored PVAT-induced anticontractility in fructose fed rats (Huang et al., 2010). Ang II type 1 receptor antagonism using olmesartan can reduce blood pressure and increase adiponectin levels in a rat model of MetS (Mizukawa et al., 2009). Lu et al. have recently shown that PVAT-induced anticontractility is impaired in spontaneously hypertensive rats and that the anticontractile response could be inhibited with an Ang 1–7 antagonist in normotensive rats (Lu et al., 2011a). Interestingly Ang 1–7 is also thought to be vital for the PVAT-induced anticontractile response observed in venous rings (Lu et al., 2011b).
Epicardial adipose tissue and its clinical correlates
Given its proximity to the coronary vessels, epicardial and pericoronary fat present an attractive therapeutic target for CAD and the treatment of its consequent symptoms. Epicardial adipose tissue thickness correlates well with waist circumference, visceral adipose tissue mass, fasting insulin and diastolic blood pressure (Iacobellis et al., 2003a,b) and has been shown to be significantly greater in patients with MetS than those without (Iacobellis et al., 2008).
In man, Chatterjee et al. have shown that PVAT surrounding coronary vessels is made up of smaller, more irregularly shaped adipocytes which exhibit a reduced differentiation state as compared with visceral and subcutaneous fat depots. They also report that PVAT from the coronary vessels secretes lower levels of the anti-inflammatory cytokine adiponectin and higher levels of pro-inflammatory cytokines IL-8 and IL-6 as compared with subcutaneous and visceral adipocytes (Chatterjee et al., 2009). Moreover, exposure to IL-6 has been linked with a reduction in adiponectin production by human adipocytes (Simons et al., 2007). There is a high level of macrophage infiltration in the epicardial fat tissue of CAD patients (Baker et al., 2006); indeed there is a greater pro-inflammatory profile of the epicardial adipose tissue as compared with the subcutaneous adipose tissue of individuals with CAD (Mazurek et al., 2003). Also it has been shown that peri-coronary adipose tissue contains higher levels of MCP-1 as compared with visceral and subcutaneous tissue (Chatterjee et al., 2009).
There are lower adiponectin mRNA levels in the epicardial adipose tissue of patients with CAD as compared with samples from those undergoing thoracic operations for non-CAD-related indications (Iacobellis et al., 2005; 2009; Eiras et al., 2008). Lower levels of adiponectin in epicardial tissue have also been associated with hypertension (Ohashi et al., 2006; Teijeira-Fernandez et al., 2008) and increased risk of myocardial infarction (Pischon et al., 2004).
Greif et al. (Greif et al., 2009) have studied the relationship between epicardial adipose tissue and coronary atherosclerosis in 286 consecutive patients with an intermediate pretest likelihood for CAD using dual-source multi-slice CT coronary angiography. Interestingly, they found no correlation between BMI and coronary atherosclerosis, but those with atherosclerotic lesions were found to have higher volumes of pericardial adipose tissue (226 ± 92 cm3) than those without lesions (134 ± 56 cm3; P < 0.001). Those with larger volumes of pericardial adipose tissue had lower levels of plasma adiponectin and HDL, but higher levels of the pro-inflammatory cytokine TNF and highly sensitive CRP.
A recent comprehensive post-mortem study on 41 human cadavers carried out by Spiroglou et al. has shown that adiponectin is present in both peri-aortic and peri-coronary human fat depots and its levels inversely correlate with the degree of atherosclerosis (Spiroglou et al., 2010). In animal models of vascular injury, adiponectin knockout mice exhibit more severe neointimal thickening and increased proliferation of vascular smooth muscle cells than wild-type mice (Matsuda et al., 2002).
Conclusions and perspectives
In this review we have discussed clinically significant PVAT studies and highlighted a number of potential mechanisms via which PVAT may exert its anticontractile effect in health and how these can become disordered in obesity (Figure 2).
Our current understanding of the intricate nature of interactions between adipocytes, vascular myocytes and endothelial cells is not sufficient to explain precisely the role adipokines play and the receptors and signalling pathways involved in facilitating their actions. We can conclude that in response to vasoconstrictor challenges, adipocytes release adipokines with anticontractile effects on the smooth myocytes of adjacent vessels. The mechanism of action of the ADRF(s) may involve direct action on potassium channels of vascular myocytes. Circulating ADRF(s) can also work via potassium channels on the endothelium and there is also the possibility that the potassium channels present on white adipocytes may be involved in facilitating the ADRF action. ADRF action on its receptors can also lead to NO release from adipocytes and endothelial cells. We have also discussed the contribution of macrophages and ROS in compromising the anticontractile effect of PVAT in hypoxia.
It is clear that animal studies have contributed significantly to our current understanding of the mechanism of action of PVAT. However, the studies have been conducted using different vascular beds in different animal models using different pharmacological approaches. Also it has become apparent that PVAT property varies amongst species and vascular beds, and not all animal studies are clinically relevant thus highlighting the paramount importance of research using human tissue.
Obesity and its associated co-morbidities pose a huge threat to public health and to our increasingly fragile economies. Clearly, prevention of obesity by effective educational programmes needs to be coupled with treatments for those currently suffering from the consequences of the disorder. Currently, the most radical and arguably the only effective intervention in treatment of obesity is bariatric surgery which is available only to a limited number of patients worldwide. The recent appreciation of the contribution of PVAT to metabolic homeostasis and control of local vessel tone, as well as its involvement in disease states such as obesity, hypertension and atherosclerosis has generated great interest in the possibility of reversing the PVAT damage and rescuing its pre-morbid properties. This field of research will no doubt continue to provide new challenges and opportunities in the years to come.
Acknowledgments
Our group is supported by the Manchester Wellcome Trust Clinical Research Facility and Dr Aghamohammadzadeh is a British Heart Foundation and NIHR Manchester Biomedical Research Centre Clinical Research Fellow.
Glossary
- ADRF
adventitium-derived relaxing factor
- BMI
body mass index
- CAD
coronary artery disease
- ICAM-1
intercellular adhesion molecule-1
- L-NAME
NG-nitro-L-arginine methyl ester
- MCP-1
monocyte chemotactic protein-1
- MetS
metabolic syndrome
- PVAT
perivascular adipose tissue
- ROS
reactive oxygen species
- VCAM-1
vascular cell adhesion molecule-1
Conflict of interest
The authors declare no conflict of interest.
References
- Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, et al. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obesity. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker EN, Pistea A, Spaan JA, Rolf T, de Vries CJ, van Rooijen N, et al. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res. 2006;99:86–92. doi: 10.1161/01.RES.0000229657.83816.a7. [DOI] [PubMed] [Google Scholar]
- Belin de Chantemele EJ, Vessieres E, Guihot AL, Toutain B, Maquignau M, Loufrani L, et al. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc Res. 2009;81:788–796. doi: 10.1093/cvr/cvn334. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bulow J, Astrup A, Christensen NJ, Kastrup J. Blood flow in skin, subcutaneous adipose tissue and skeletal muscle in the forearm of normal man during an oral glucose load. Acta Physiol Scand. 1987;130:657–661. doi: 10.1111/j.1748-1716.1987.tb08189.x. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Kladis A, Duncan AM. Nephrectomy, converting enzyme inhibition, and angiotensin peptides. Hypertension. 1993;22:513–522. doi: 10.1161/01.hyp.22.4.513. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Duncan AM, Kladis A, Harrap SB. Converting enzyme inhibition and its withdrawal in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1995;26:426–436. doi: 10.1097/00005344-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Cassis LA. Fat cell metabolism: insulin, fatty acids, and renin. Curr Hypertens Rep. 2000;2:132–138. doi: 10.1007/s11906-000-0072-5. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Lynch KR, Peach MJ. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988;62:1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, et al. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549–556. doi: 10.1016/j.bbrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism. 1992;41:264–272. doi: 10.1016/0026-0495(92)90269-g. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Armellino DC, Busler DE, McHendry-Rinde B, Kral JG. Angiotensin II receptors in human preadipocytes: role in cell cycle regulation. Endocrinology. 1999;140:154–158. doi: 10.1210/endo.140.1.6430. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Does periadventitial fat-derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery? J Vasc Res. 2007;44:175–181. doi: 10.1159/000099833. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Savage K, Tsui JC, Dooley A, Shaw SG, Fernandez Alfonso MS, et al. Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension-induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J Thorac Cardiovasc Surg. 2009;138:334–340. doi: 10.1016/j.jtcvs.2008.11.060. [DOI] [PubMed] [Google Scholar]
- Davies GC, Sobel M, Salzman EW. Elevated plasma fibrinopeptide A and thromboxane B2 levels during cardiopulmonary bypass. Circulation. 1980;61:808–814. doi: 10.1161/01.cir.61.4.808. [DOI] [PubMed] [Google Scholar]
- Davis KE, Scherer PE. Adiponectin: no longer the lone soul in the fight against insulin resistance? Biochem J. 2008;416:e7–e9. doi: 10.1042/BJ20082033. [DOI] [PubMed] [Google Scholar]
- Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann NY Acad Sci. 2011;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes (Lond) 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- Diamond J. The double puzzle of diabetes. Nature. 2003;423:599–602. doi: 10.1038/423599a. [DOI] [PubMed] [Google Scholar]
- Dridi S, Taouis M. Adiponectin and energy homeostasis: consensus and controversy. J Nutr Biochem. 2009;20:831–839. doi: 10.1016/j.jnutbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Egner I, Weston A, Porter E, Edwards G. Hyperpolarising effect of perivascular adipose tissue in rat mesenteric artery myocyte: stimulation by beta-3 adrenoceptor activation. Proceedings of the British Pharmacological Society. 2010 http://www.pA2online.org/abstracts/vol8Issue1abst141P.pdf. (accessed 11 June 2011) [Google Scholar]
- Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine. 2008;43:174–180. doi: 10.1016/j.cyto.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Engeli S, Gorzelniak K, Kreutz R, Runkel N, Distler A, Sharma AM. Co-expression of renin-angiotensin system genes in human adipose tissue. J Hypertens. 1999;17:555–560. doi: 10.1097/00004872-199917040-00014. [DOI] [PubMed] [Google Scholar]
- Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, et al. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- Eringa EC, Stehouwer CD, van Nieuw Amerongen GP, Ouwehand L, Westerhof N, Sipkema P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am J Physiol Heart Circ Physiol. 2004;287:H2043–H2048. doi: 10.1152/ajpheart.00067.2004. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes. 1999;48:903–908. doi: 10.2337/diabetes.48.4.903. [DOI] [PubMed] [Google Scholar]
- Fukai N, Yoshimoto T, Sugiyama T, Ozawa N, Sato R, Shichiri M, et al. Concomitant expression of adrenomedullin and its receptor components in rat adipose tissues. Am J Physiol Endocrinol Metab. 2005;288:E56–E62. doi: 10.1152/ajpendo.00586.2003. [DOI] [PubMed] [Google Scholar]
- Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- Galvez-Prieto B, Bolbrinker J, Stucchi P, Heras AI, Merino B, Arribas S, et al. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Zeng ZH, Lim GE, Petrik JJ, Foster WG, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005a;13:687–692. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. 2005b;130:1130–1136. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- Giacchetti G, Faloia E, Mariniello B, Sardu C, Gatti C, Camilloni MA, et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens. 2002;15:381–388. doi: 10.1016/s0895-7061(02)02257-4. [DOI] [PubMed] [Google Scholar]
- Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. J Hypertens. 2002;20:965–973. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- Han SH, Sakuma I, Shin EK, Koh KK. Antiatherosclerotic and anti-insulin resistance effects of adiponectin: basic and clinical studies. Prog Cardiovasc Dis. 2009;52:126–140. doi: 10.1016/j.pcad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Harmancey R, Senard JM, Pathak A, Desmoulin F, Claparols C, Rouet P, et al. The vasoactive peptide adrenomedullin is secreted by adipocytes and inhibits lipolysis through NO-mediated beta-adrenergic agonist oxidation. FASEB J. 2005;19:1045–1047. doi: 10.1096/fj.04-2868fje. [DOI] [PubMed] [Google Scholar]
- Harp JB, DiGirolamo M. Components of the renin-angiotensin system in adipose tissue: changes with maturation and adipose mass enlargement. J Gerontol A Biol Sci Med Sci. 1995;50:B270–B276. doi: 10.1093/gerona/50a.5.b270. [DOI] [PubMed] [Google Scholar]
- He GW, Buxton BF, Rosenfeldt FL, Angus JA, Tatoulis J. Pharmacologic dilatation of the internal mammary artery during coronary bypass grafting. J Thorac Cardiovasc Surg. 1994;107:1440–1444. [PubMed] [Google Scholar]
- Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- Huang F, Lezama MAR, Ontiveros JAPr, Bravo G, Villafaña S, del-Rio-Navarro BE, et al. Effect of losartan on vascular function in fructose-fed rats: the role of perivascular adipose tissue. Clin Exp Hypertens. 2010;32:98–104. doi: 10.3109/10641960902993129. [DOI] [PubMed] [Google Scholar]
- Hughes JM, Riddle MA, Paffett ML, Gonzalez Bosc LV, Walker BR. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am J Physiol Heart Circ Physiol. 2010;299:H1439–H1450. doi: 10.1152/ajpheart.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003a;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003b;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 2008;16:887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, di Gioia CR, Cotesta D, Petramala L, Travaglini C, De Santis V, et al. Epicardial adipose tissue adiponectin expression is related to intracoronary adiponectin levels. Horm Metab Res. 2009;41:227–231. doi: 10.1055/s-0028-1100412. [DOI] [PubMed] [Google Scholar]
- IOTF. 2010. Obesity the global epidemic Vol. 2010.
- Jonsson JR, Game PA, Head RJ, Frewin DB. The expression and localisation of the angiotensin-converting enzyme mRNA in human adipose tissue. Blood Press. 1994;3:72–75. doi: 10.3109/08037059409101524. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56:351–358. doi: 10.1161/HYPERTENSIONAHA.110.155663. [DOI] [PubMed] [Google Scholar]
- Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009a;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- Lee RM, Lu C, Su LY, Werstuck G, Gao YJ. Effects of hyperglycemia on the modulation of vascular function by perivascular adipose tissue. J Hypertens. 2009b;27:118–131. doi: 10.1097/HJH.0b013e3283163cc9. [DOI] [PubMed] [Google Scholar]
- Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, Amati G, et al. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- Liu Q, Gauthier M-S, Sun L, Ruderman N, Lodish H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. FASEB J. 2010;24:4229–4239. doi: 10.1096/fj.10-159723. fj.10-159723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- Long Q, Lei T, Feng B, Yin C, Jin D, Wu Y, et al. Peroxisome proliferator-activated receptor-gamma increases adiponectin secretion via transcriptional repression of endoplasmic reticulum chaperone protein ERp44. Endocrinology. 2010;151:3195–3203. doi: 10.1210/en.2009-1501. [DOI] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM, Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II. Eur J Pharmacol. 2010;634:107–112. doi: 10.1016/j.ejphar.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011a;656:68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Lu C, Zhao AX, Gao YJ, Lee RM. Modulation of vein function by perivascular adipose tissue. Eur J Pharmacol. 2011b;657:111–116. doi: 10.1016/j.ejphar.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Lynch F, Withers S, Werner M, Heagerty AM. Attenuation of anticontractile properties of perivascular adipose tissue in isolated mesenteric arteries from Slo-/- mice: the role of Bkca channels: Pp.11.423. J Hypertens. 2010a;28:e17. 10.1097/1001.hjh.0000378748.0000311779.d0000378741. [Google Scholar]
- Lynch F, Withers SB, Werner M, Heagerty AM. Abstract 17063: the {beta}-subunit of the BkCa channel plays a vital role in mediating perivascular adipose tissue induced contractility in isolated small arteries. Circulation. 2010b;122:A17063. (21_MeetingAbstracts) [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Maenhaut N, Boydens C, Van de Voorde J. Hypoxia enhances the relaxing influence of perivascular adipose tissue in isolated mice aorta. Eur J Pharmacol. 2010;641:207–212. doi: 10.1016/j.ejphar.2010.05.058. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothorac Surg. 2008;33:225–231. doi: 10.1016/j.ejcts.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Manka D, Chatterjee TK, Moseley N, LaSance K, Lemen L, Hui DY, et al. Abstract 15733: perivascular adipose tissue triggers vasa vasorum formation and injury-induced neointimal hyperplasia in mice. Circulation. 2010;122:A15733. (21_MeetingAbstracts) [Google Scholar]
- Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- Mizukawa M, Ohmori K, Obayashi A, Ishihara Y, Yoshida J, Noma T, et al. Effects of combined olmesartan and pravastatin on glucose intolerance and cardiovascular remodeling in a metabolic-syndrome model. Hypertens Res. 2009;32:617–624. doi: 10.1038/hr.2009.63. [DOI] [PubMed] [Google Scholar]
- Momin AU, Melikian N, Shah AM, Grieve DJ, Wheatcroft SB, John L, et al. Leptin is an endothelial-independent vasodilator in humans with coronary artery disease: evidence for tissue specificity of leptin resistance. Eur Heart J. 2006;27:2294–2299. doi: 10.1093/eurheartj/ehi831. [DOI] [PubMed] [Google Scholar]
- Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Nishikimi T, Uematsu M, Satoh T, Oya H, Kyotani S, et al. Haemodynamic and hormonal effects of adrenomedullin in patients with pulmonary hypertension. Heart. 2000;84:653–658. doi: 10.1136/heart.84.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Miyatake K, Kyotani S, Nishikimi T, Nakanishi N, Kangawa K. Pulmonary vasodilator response to adrenomedullin in patients with pulmonary hypertension. Hypertens Res. 2003;26(Suppl):S141–S146. doi: 10.1291/hypres.26.s141. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yoshida H, Makita S, Arakawa N, Niinuma H, Hiramori K. Potent and long-lasting vasodilatory effects of adrenomedullin in humans. Comparisons between normal subjects and patients with chronic heart failure. Circulation. 1997;95:1214–1221. doi: 10.1161/01.cir.95.5.1214. [DOI] [PubMed] [Google Scholar]
- Neel JV. Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- Nuki C, Kawasaki H, Kitamura K, Takenaga M, Kangawa K, Eto T, et al. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun. 1993;196:245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–213. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Bratz IN, Roell WC, Bohlen HG, Dick GM, et al. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation. 2008;15:417–426. doi: 10.1080/10739680701858447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H460–H465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezio JA, Carreon G, Clerkin E, Meloni CR, Kyle LH, Canary JJ. Influence of body composition on adrenal function in obesity. J Clin Endocrinol Metab. 1964;24:481–485. doi: 10.1210/jcem-24-6-481. [DOI] [PubMed] [Google Scholar]
- Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B. Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 2006;14:28–35. doi: 10.1038/oby.2006.5. [DOI] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- Ren J. Leptin and hyperleptinemia – from friend to foe for cardiovascular function. J Endocrinol. 2004;181:1–10. doi: 10.1677/joe.0.1810001. [DOI] [PubMed] [Google Scholar]
- Reudelhuber TL. A place in our hearts for the lowly angiotensin 1-7 peptide? Hypertension. 2006;47:811–815. doi: 10.1161/01.HYP.0000209020.69734.73. [DOI] [PubMed] [Google Scholar]
- Robidoux J, Kumar N, Daniel KW, Moukdar F, Cyr M, Medvedev AV, et al. Maximal beta3-adrenergic regulation of lipolysis involves Src and epidermal growth factor receptor-dependent ERK1/2 activation. J Biol Chem. 2006;281:37794–37802. doi: 10.1074/jbc.M605572200. [DOI] [PubMed] [Google Scholar]
- Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol. 2010;299:H202–H209. doi: 10.1152/ajpheart.00120.2010. [DOI] [PubMed] [Google Scholar]
- Sam F, Walsh K. What Can Adiponectin Say About Left Ventricular Function? Heart. 2010;96:331–332. doi: 10.1136/hrt.2009.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schling P, Mallow H, Trindl A, Loffler G. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. Int J Obes Relat Metab Disord. 1999;23:336–341. doi: 10.1038/sj.ijo.0800821. [DOI] [PubMed] [Google Scholar]
- Scott EM, Grant PJ. Neel revisited: the adipocyte, seasonality and type 2 diabetes. Diabetologia. 2006;49:1462–1466. doi: 10.1007/s00125-006-0280-x. [DOI] [PubMed] [Google Scholar]
- Shibasaki I, Nishikimi T, Mochizuki Y, Yamada Y, Yoshitatsu M, Inoue Y, et al. Greater expression of inflammatory cytokines, adrenomedullin, and natriuretic peptide receptor-C in epicardial adipose tissue in coronary artery disease. Regul Pept. 2010;10:210–217. doi: 10.1016/j.regpep.2010.07.169. [DOI] [PubMed] [Google Scholar]
- Shimosawa T, Ogihara T, Matsui H, Asano T, Ando K, Fujita T. Deficiency of adrenomedullin induces insulin resistance by increasing oxidative stress. Hypertension. 2003;41:1080–1085. doi: 10.1161/01.HYP.0000066846.46422.2C. [DOI] [PubMed] [Google Scholar]
- Silaghi A, Achard V, Paulmyer-Lacroix O, Scridon T, Tassistro V, Duncea I, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293:E1443–E1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- Simons PJ, van den Pangaart PS, Aerts JM, Boon L. Pro-inflammatory delipidizing cytokines reduce adiponectin secretion from human adipocytes without affecting adiponectin oligomerization. J Endocrinol. 2007;192:289–299. doi: 10.1677/JOE-06-0047. [DOI] [PubMed] [Google Scholar]
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Endocrine functions of adipose tissue: focus on adiponectin. Clin Cornerstone. 2008;9:32–38. doi: 10.1016/s1098-3597(08)60026-5. discussion 39–40. [DOI] [PubMed] [Google Scholar]
- Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7:22–29. doi: 10.1038/nrcardio.2009.224. [DOI] [PubMed] [Google Scholar]
- Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Fernandez A, Adrio B, Gonzalez-Juanatey JR. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens. 2008;22:856–863. doi: 10.1038/jhh.2008.75. [DOI] [PubMed] [Google Scholar]
- Terata K, Miura H, Liu Y, Loberiza F, Gutterman DD. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K(+) channels. Am J Physiol Heart Circ Physiol. 2000;279:H2620–H2626. doi: 10.1152/ajpheart.2000.279.6.H2620. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100:227–235. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- Vecchione C, Aretini A, Maffei A, Marino G, Selvetella G, Poulet R, et al. Cooperation between insulin and leptin in the modulation of vascular tone. Hypertension. 2003;42:166–170. doi: 10.1161/01.HYP.0000082806.73530.68. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers BS, Agabiti-Rosei C, Linvingstone DM, Little MC, Aslam R, Malik RA, et al. Macrophage activation is responsible for loss f anticontractile function in inflamed perivascular fat. Arterioscler Thromb Vasc Biol. 2011;31:908–913. doi: 10.1161/ATVBAHA.110.221705. [DOI] [PubMed] [Google Scholar]
- Yagil Y, Yagil C. Hypothesis: ACE2 modulates blood pressure in the mammalian organism. Hypertension. 2003;41:871–873. doi: 10.1161/01.HYP.0000063886.71596.C8. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003a;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003b;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- Yoshimoto T, Gochou N, Fukai N, Sugiyama T, Shichiri M, Hirata Y. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertens Res. 2005;28:165–172. doi: 10.1291/hypres.28.165. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehouwer CD. ‘Vasocrine’ signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]


