Abstract
BACKGROUND AND PURPOSE
Transient receptor potential ion channel vanilloid 3 (TRPV3) is expressed in skin keratinocytes and plays an important role in thermal and chemical nociceptions in the periphery. The presence of TRPV3 inhibitors would improve our understanding of TRPV3 function and help to develop receptor-specific analgesics. However, little is known about physiological substances that specifically inhibit TRPV3 activity. Here, we investigated whether 17(R)-resolvin D1 (17R-RvD1), a naturally occurring pro-resolving lipid specifically affects TRPV3 activity.
EXPERIMENTAL APPROACH
We examined the effect of 17R-RvD1 on sensory TRP channels using Ca2+ imaging and whole cell electrophysiology experiments in a HEK cell heterologous expression system, cultured sensory neurons and keratinocytes. We also examined changes in sensory TRP agonist-specific acute licking/flicking or flinching behaviours and mechanical and thermal pain behaviours using Hargreaves, Randall-Selitto and von Frey assay systems in the absence and presence of inflammation.
KEY RESULTS
We showed that 17R-RvD1 specifically suppresses TRPV3-mediated activity at nanomolar and micromolar concentrations. The voltage-dependence of TRPV3 activation by camphor was shifted rightwards by 17R-RvD1, which indicates its inhibitory mechanism is as a result of a shift in voltage-dependence. Consistently, TRPV3-specific acute pain behaviours were attenuated by locally injected 17R-RvD1. Moreover, the administration of 17R-RvD1 significantly reversed the thermal hypersensitivity that occurs during an inflammatory response. Knockdown of epidermal TRPV3 blunted these antinociceptive effects of 17R-RvD1.
CONCLUSIONS AND IMPLICATIONS
17R-RvD1 is a novel natural inhibitory substance specific for TRPV3. The results of our behavioural studies suggest that 17R-RvD1 has acute analgesic potential via TRPV3-specific mechanisms.
Keywords: 17(R)-resolvin D1, TRPV3, specific inhibitor, pain, keratinocytes, nociceptive behaviours, inflammation
Introduction
Epidermal keratinocytes and sensory neurons innervating the skin act as primary peripheral detectors of environmental changes. Sensory transient receptor potential (TRP) ion channels are expressed in these cells and transduce the changes to electrical signals. TRPV3 expressed in keratinocytes is a sensory TRP channel that detects heat (Moqrich et al., 2005) and chemicals (Vriens et al., 2009; Bang et al., 2010a). The peripheral signals evoked by TRPV3 activation appear to be relayed to the brain leading to pain perception according to studies using genetically modified animals (Moqrich et al., 2005; Huang et al., 2008; Bang et al., 2010a). Indeed, a TRPV3-specific pain phenotype was shown to be suppressed by broad-spectrum TRP inhibitors (Bang et al., 2010b; 2011). Hence, regulating TRPV3 activity may be important for pain modulation. However, the limited availability of specific TRPV3 inhibitors has restricted studies on the physiological outcomes of the pharmacological inhibition of TRPV3.
While many natural compounds that activate or inhibit TRPs have been reported, few endogenous or naturally produced substances that inhibit TRPV3 have been described. Resolvins are anti-inflammatory and pro-resolving lipidergic molecules generated endogenously by ω-3 lipid metabolism (Ariel and Serhan, 2007). A member of docosahexaenoic acid-derived series of resolvins, 17(R)-resolvin D1 (17R-RvD1) was discovered in mammalian tissues (Serhan et al., 2002). Recently, 17R-RvD1 and its precursor molecule were demonstrated to display potent antinociceptive activities (Lima-Garcia et al., 2011). Furthermore, related substances 17S-RvD1 and RvE1 have been shown to suppress multiple TRP channel activities (Xu et al., 2010; Bang et al., 2010b). In the present study, we demonstrated that 17R-RvD1 is a specific inhibitor of TRPV3. We also showed that 17R-RvD1 attenuates TRPV3-mediated nociceptive behaviours in animals.
Methods
Cell cultures
HEK293T cells and human keratinocyte cell lines (HaCaT) were maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin (Bang et al., 2007a,b). The HEK293T cells were transfected transiently with 3 µg of individual TRP channel plasmid DNA (mTRPA1, rTRPV1, rTRPV2 or mTRPV4 in pcDNA3.1; hTRPV3 or mTRPM8 in pcDNA5/FRT) using Fugene HD (Roche Diagnostics Corp., Indianapolis, IN). Primary cultures of mouse dorsal root ganglion (DRG) neurons were prepared as described previously (Bang et al., 2007a). All cells were grown at 37°C and 5% CO2.
Ca2+ imaging experiments
Ca2+ imaging experiments were performed as described previously (Kim et al., 2008). The cells were loaded with 5 µM Fura-2AM and 0.02% pluronic F127 for 30 min, and the cells were resuspended in (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, titrated to pH 7.4 with NaOH). Images of Fura-2 loaded cells were captured with a cooled CCD camera (Retiga-SRV, Q-imaging Corp., Burnaby, BC, Canada). The ratio of fluorescence intensity at 340/380 nm wavelengths in each experiment was analysed using MetaFluor (Molecular Devices, Sunnyvale, CA). The threshold of intracellular Ca2+ increase was defined as 20% increase from the basal Ca2+ level (Story et al., 2003; Ryu et al., 2010).
Patch-clamp electrophysiology
Whole-cell voltage clamp recordings were performed using the same bath solution as in the Ca2+ imaging experiments. The pipette solution contained (in mM) 140 CsCl, 5 EGTA, 10 HEPES, 2.0 MgATP, 0.2 NaGTP titrated to pH 7.2 with CsOH. The holding potential was −60 mV. For the current–voltage analysis, 800 ms voltage-ramp pulses from −80 to +80 mV were used. For whole cell voltage-dependence analysis, a step protocol composed of 32 episodes of 800 ms step pulses with a 10 mV increment starting from −120 mV was used. Each step pulse was followed by a 400 ms invariant step to −120 mV. The peak inward tail currents at −120 mV were collected for each step, normalized to the maximum tail current and fitted using the Boltzmann equation.
Behavioural studies
The study was performed in accordance with protocols approved by the University Committee on Laboratory Animals. Six-week-old male ICR mice (Hanlim Animal Co., Korea), TRPV1-knockout mice (C57BL/6J strain, a generous gift from Dr Uhtaek Oh (Seoul National University), or 200–300 g male Sprague–Dawley rats (Hanlim Animal Co.) were used. The animals were acclimated for 1 h to the test environment before assays. Time engaged in drug (capsaicin, cinnamaldehyde or KCl)-induced hind paw licking, flicking or flinching was measured for 10 min (Bang et al., 2007a). Hargreaves (Plantar Analgesia meter, for heat hyperalgesia), von Frey (Dynamic Plantar Aesthesiometer, for mechanical allodynia) and Randall-Selitto apparatus (Analgesy-meter, for blunt pressure-evoked nociceptive flexion reflex) were from UGO Basile (Italy). Assays for changes in thermal or mechanical behaviours during inflammation were also conducted (Bang et al., 2010a). For complete Freund's adjuvant (CFA)-induced inflammation, 50 µL CFA was injected into a hind paw 24 h prior to the 17R-RvD1 injection. Baseline responses were measured 5 min before the 17R-RvD1 injection. Farnesyl pyrophosphate (FPP)-induced licking and lifting test was conducted during carrageenan-induced inflammation (Bang et al., 2010a). For carrageenan-induced inflammation, 2% λ-carrageenan in 10 µL was injected into a hind paw 3 h before the FPP injection. Drugs were injected in 10 µL vehicle (PBS containing 0.5% Tween 80) into mice hind paws i.d. at the doses detailed in the results. Data are shown as means ± SEM. The results were analysed by use of Student's t-test unless stated otherwise (*P < 0.05, **P < 0.01, ***P < 0.001). In vivo TRPV3 knockdown was performed as described previously (Bang et al., 2010a). Briefly, 10 µg of shRNA in 20 µL saline with 20 µL EzWay™ transfection reagent (Komabiotech, Korea) were injected into a hind paw 48 h before the FPP or 17R-RvD1 injection.
Compounds
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. 17R-RvD1 was purchased from Cayman Chemical Co. (Ann Arbor, MI). Cinnamaldehyde was purchased from MP Biomedicals (Solon, OH). mTRPV3-shRNA (5′-CCGGGCTGGAAATCATCGTCTACAACTCGAGTTGTAGACGATGATTTCCAGCTTTTTG-3′) was purchased from Open Biosystems (Huntsville, AL). A non-target scrambled shRNA (5′- CCTAAGGTTAAGTCGCCCTCGCTCTAGCGAGGGCGACTTAACCTTAG G-3′) was purchased from Sigma-Aldrich. Stock solutions were made using water or ethanol and were diluted with test solutions before use. 17R-RvD1 was kept at −80°C between uses. Immediately before use, RvD1 was directly diluted to test concentrations with the bath solution and briefly sonicated. All drug/molecular target nomenclature used here conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2011).
Results
17R-RvD1 inhibits TRPV3 in heterologous expression systems
In the Fura-2 Ca2+ imaging, 17R-RvD1 alone did not cause Ca2+ influx in the non-transfected or TRPV3-transfected HEK cells (data not shown and Figure 1A). The Ca2+ influxes elicited by the three TRPV3 agonists, 4 mM camphor, 30 µM 2-aminoethoxydiphenyl borate (2-APB) and 1 µM FPP, in the TRPV3-transfected cells were blocked by the addition of 17R-RvD1 (Figure 1A,B). 17R-RvD1 also suppressed heat (37°C)-induced TRPV3-mediated Ca2+ influx (Figure 1A). Immediately after washout of 17R-RvD1, the agonists or heat stimulation were able to evoke intracellular Ca2+ increases in the same cells, indicating that the 17R-RvD1 effects are reversible (Figure 1A). In the whole cell voltage clamp mode, all the current responses to any of the three TRPV3 agonists (4 mM camphor, 30 µM 2-APB or 1 µM FPP) were blocked by the addition of 17R-RvD1 (Figure 1B,C). 17R-RvD1 alone did not evoke a current response indicating no partial agonistic activity on TRPV3 (n = 5, data not shown). Collectively, both the Ca2+ imaging and electrophysiology data consistently demonstrated that TRPV3 activity is inhibited by 17R-RvD1.
Figure 1.
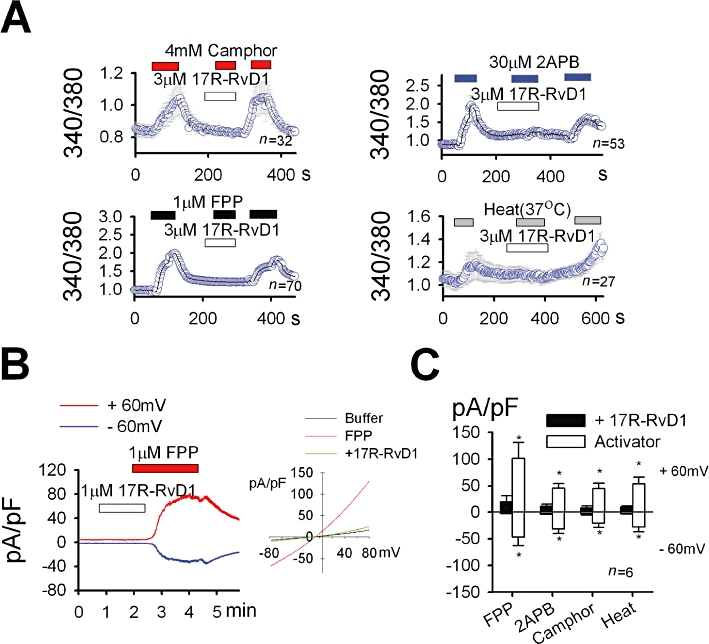
17R-RvD1 inhibits TRPV3 activity in the heterologous expression system. (A) 17R-RvD1 attenuated intracellular Ca2+ increases in response to TRPV3 agonists and 37°C heat stimulation. The test concentrations of all three agonists were between their EC50 values and ones that elicit maximal efficacy (Hu et al., 2009; Bang et al., 2010a). (B-C) 17R-RvD1 attenuated current responses to TRPV3 agonists in the whole cell voltage clamp experiments using TRPV3-expressing HEK cells. (B) FPP evoked an outwardly rectifying current increase (n = 7). The current was inhibited by co-application of 1 µM 17R-RvD1. Inset: current–voltage curves for the responses to FPP alone and FPP plus 17R-RvD1 are superimposed. (C) Average current densities induced by TRPV3 activation at ±60 mV are displayed.
We next examined the specificity of the inhibitory effects of 17R-RvD1 on sensory TRP channels. To prevent unnecessary competition with an excessive amount of an agonist, the concentrations used were: 0.2 µM capsaicin for TRPV1; 300 µM probenecid for TRPV2; 4 mM camphor for TRPV3; 10 µM 4α-PDD for TRPV4; 300 µM menthol for TRPM8; 300 µM cinnamaldehyde for TRPA1 (Tominaga et al., 1998; McKemy et al., 2002; Bandell et al., 2004; Bang et al., 2007a; Vogt-Eisele et al., 2007; Thorneloe et al., 2008; Hu et al., 2009; Vincent et al., 2009). The Ca2+ influxes through the other five TRP channels were not affected by 17R-RvD1 and no Ca2+ influx via any of TRPs during 17R-RvD1 application was observed (Figure 2A), confirming that 17R-RvD1 is a full antagonist specific for TRPV3.
Figure 2.
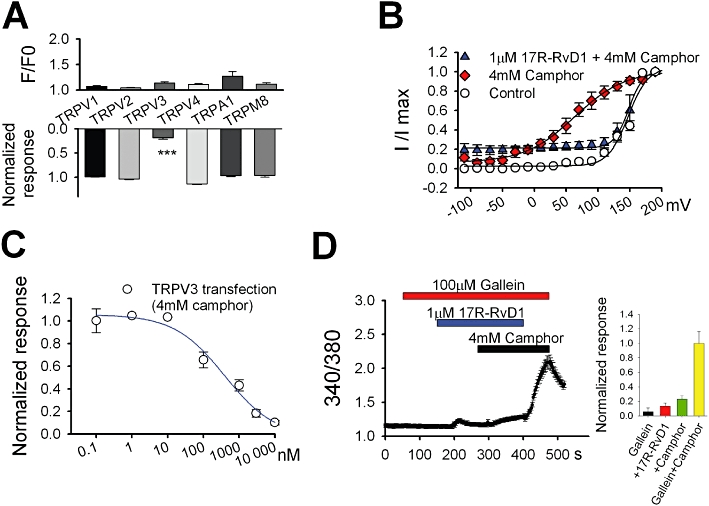
Specificity and potency of 17R-RvD1. (A) Changes in intracellular Ca2+ level in cells expressing each TRP were measured during application with 3 µM 17R-RvD1 alone (upper; n = 31–81 for each TRP). The 17R-RvD1 (3 µM)-induced reduction in intracellular Ca2+ increases in response to the agonists was also examined (lower). For each test, the cells were incubated with 17R-RvD1 for 1–3 min. Data for the reduction test were normalized to the averaged responses from each TRP with agonist alone (n = 31–81 for each TRP). (B) Voltage-dependence of TRPV3 activation. The peak inward tail currents at −120 mV from the step protocol were plotted by a function of test voltage steps. Control indicates the data obtained with no drug application. (C) The dose–response curve for 17R-RvD1 on TRPV3 inhibition obtained by Fura-2 Ca2+ imaging. The curve was fitted by the Hill equation. Symbols represent mean values of responses of Ca2+ influx via TRPV3 activation by 4 mM camphor at each 17R-RvD1 dose (n = 17–41). (D) Gallein (100 µM) had no effect on TRPV3 inhibition by 17R-RvD1 in the Fura-2 Ca2+ imaging. Inset: summary of intracellular Ca2+ levels under each drug application normalized to that under 4 mM camphor alone.
To investigate the mechanisms of the inhibition induced by 17R-RvD1, we next tested the voltage- dependence of TRPV3 activation. 17R-RvD1 shifted the voltage-dependence of TRPV3 activation by camphor rightwards (Figure 2B), increasing the V1/2 from 64 to 148 mV. Thus, TRPV3 inhibition by 17R-RvD1 is caused by a shift in the voltage-dependence. Analysis of the dose–response curve for TRPV3 inhibition (IC50 = 398 nM; Hill coefficient = 0.8) indicated that 17R-RvD1 has an inhibitory effect at nanomolar and micromolar concentrations (Figure 2C). 17S-RvD1 is known to activate GPCRs (Krishnamoorthy et al., 2010), therefore, we determined whether the TRPV3 inhibition by 17R-RvD1 involves GPCR signalling. Gallein, a small molecule inhibitor of Gβγ, did not alter TRPV3 activation by camphor (data not shown), and also failed to affect the TRPV3 inhibition by 17R-RvD1, indicating that GPCR signalling does not mediate this inhibition (Figure 2D). Collectively, the data suggest that 17R-RvD1 specifically and reversibly inhibits TRPV3 activity.
17R-RvD1 inhibits TRPV3 expressed in native cells
Next we determined whether 17R-RvD1 inhibits endogenous TRPV3 using HaCaT keratinocytes that are known to natively express TRPV3. As observed in the above heterologous systems, 17R-RvD1 could block the agonist-induced or heat-induced intracellular Ca2+ increases, which indicates that TRPV3 inhibition by 17R-RvD1 is reproducible in the native TRPV3-expressing cells (Figure 3A). Whole cell voltage clamp experiments using keratinocytes confirmed the inhibitory effect of 17R-RvD1 on TRPV3 (Figure 3B,C). The dose–response analysis of the effects of 17R-RvD1 in the keratinocytes showed that it had a similar inhibitory potency in these cells as in the HEK cells (IC50 = 155 nM; Hill coefficient = 0.9; Figure 3D). Sensitization upon recurrent stimuli is a particular feature of TRPV3 activation (Moqrich et al., 2005). Using electrophysiological experiments, we confirmed that sensitization to repeated heat pulses occurred in the keratinocytes; 17R-RvD1 application also significantly blunted this sensitization (Figure 3E).
Figure 3.
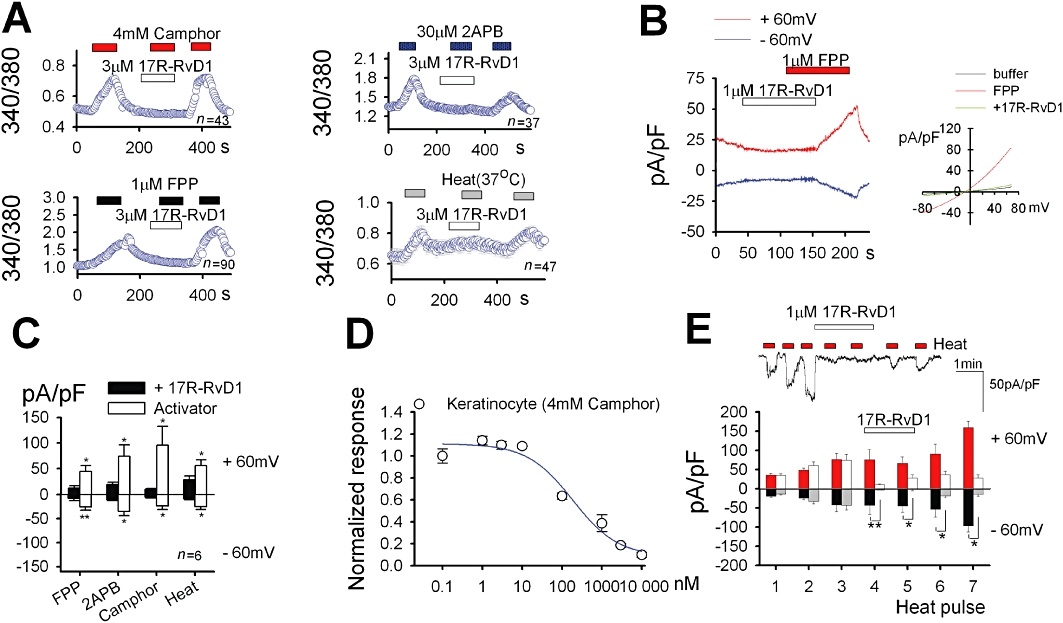
17R-RvD1 inhibits TRPV3 activity in keratinocytes. (A) In HaCaT keratinocytes, 17R-RvD1 attenuated intracellular Ca2+ increases in response to TRPV3 agonists and 37°C heat stimulation. (B-C) 17R-RvD1 attenuated current responses to TRPV3 agonists in the whole cell voltage clamp experiments. (B) FPP evoked an outwardly rectifying current increase (n = 7). The current was inhibited by the co-application of 1 µM 17R-RvD1. Inset: current–voltage curves for the responses to FPP alone and FPP plus 17R-RvD1 are superimposed. (C) Average current densities in keratinocyte induced by TRPV3 activation at ±60 mV. (D) The dose–response curve for the inhibitory effect of 17R-RvD1 on 4 mM camphor-induced Ca2+ influx obtained by Fura-2 Ca2+ imaging (n = 17–41). (E) 17R-RvD1 inhibits the TRPV3-mediated response to repeated heat stimulation in the whole cell voltage clamp experiments. Upper: representative current trace for 17R-RvD inhibition at −60 mV. Lower: average current densities in keratinocytes induced by TRPV3 activation at ±60 mV (n = 4–5). During the application of 17R-RvD1 (open and grey columns), the heat responses were decreased by 92.2 ± 9.2% and by 96.8 ± 4.2% at fourth and fifth heat pulses, respectively, compared to the heat responses without 17R-RvD1 (red and black columns) at −60 mV. The final heat responses were increased by 27.8. ± 16.5% and 461.3 ± 138.5% compared to the initial heat responses at −60 mV, with and without 17R-RvD1 addition, respectively.
Nociceptive signals are relayed from keratinocytes to sensory neurons (Huang et al., 2008; Mandadi et al., 2009; Bang et al., 2010a). The mouse sensory neurons are known to lack functional TRPV3 (Peier et al., 2002; Moqrich et al., 2005; Huang et al., 2008; Mandadi et al., 2009; Bang et al., 2010a), and we examined other pleiotropic effects of 17R-RvD1 on DRG neuronal excitation. KCl-induced depolarization causes Ca2+ influxes in the sensory neurons via voltage-gated cation channel activation (Bang et al., 2010a,b; 2011; Ryu et al., 2010). 17R-RvD1 did not affect mouse DRG neuronal Ca2+ influxes evoked by 60 mM KCl-induced depolarization (Figure 4A), suggesting that 17R-RvD1 does not regulate the functions of voltage-gated cation channels that confer general neuronal excitability. TRPV1 is the major nociceptive sensor ion channel in the DRG neurons. The TRP specificity experiments described above had already shown that TRPV1 activity is not modified by 17R-RvD1 (Figure 2A). Similarly, DRG neuronal capsaicin responses, which are mediated via neuronal TRPV1 activation, were unaffected by 17R-RvD1 (Figure 4B). An acute inflammatory mediator bradykinin evokes DRG neuronal excitation leading to acute pain. The neuronal excitation by bradykinin is known to result from intracellular signal transductions, which use extensive cellular machineries such as the B2 GPCR, PLC, PLA2, lipoxygenase, TRPV1, M-type K+ channels and anoctamin Cl– channels, etc. (Chuang et al., 2001; Shin et al., 2002; Liu et al., 2010). 17R-RvD1 application had no significant effect on the responses (Figure 4C), which suggests that DRG neuronal functions that involve such intracellular signalling components are largely unaffected by 17R-RvD1.
Figure 4.
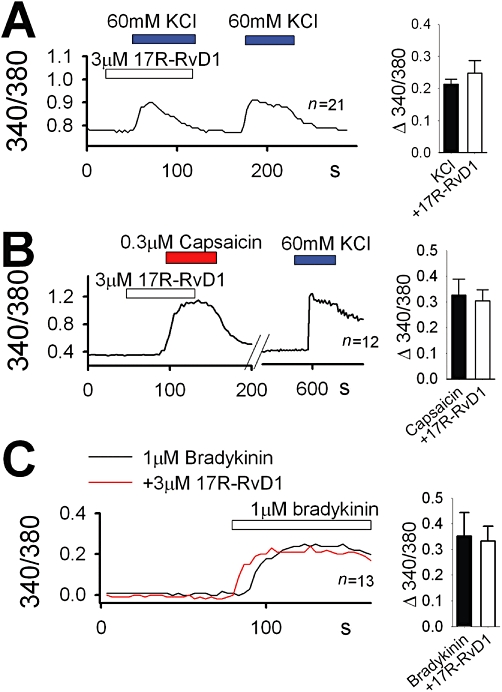
17R-RvD1 does not affect DRG neuronal responses. In cultured mouse DRG neurons, 17R-RvD1 failed to affect intracellular Ca2+ increases in response to 60 mM KCl-evoked depolarization (A), capsaicin (B) or bradykinin (C). For the bradykinin experiments, different neurons were tested for the 17R-RvD1 effects due to strong desensitization of bradykinin responses. Insets: summary of each stimulation-evoked neuronal Ca2+ influxes with or without 3 µM 17R-RvD1 (n = 21 for KCl, n = 12 for capsaicin and n = 13 for bradykinin).
17R-RvD1 attenuates TRPV3-mediated nociceptive behaviours
Activation of peripheral TRPV3 contributes to thermal and chemical pain sensation (Moqrich et al., 2005; Huang et al., 2008; Bang et al., 2010a). Firstly, we determined whether 17R-RvD1 has an antinociceptive effect on the thermal and mechanical hypersensitivities that occur during an inflammatory response. Resolvins have anti-inflammatory and pro-resolving actions that may alleviate nociception under long-term incubation, irrespective of their effect on TRPV3. We therefore administered 17R-RvD1 locally and immediately before the test measurements to avoid these effects. To assess the 17R-RvD1 effect on heat nociception, we performed Hargreaves assays using an infrared heat-radiating Plantar Analgesia meter. Intraplantar 17R-RvD1 treatment (30 µM in 20 µL) did not change the heat threshold in hind paws of normal animals (n = 5, data not shown). On the other hand, the same dose of 17R-RvD1 did reduce the heat threshold in animals with a CFA-inflamed hind paw (Figure 5A). Although not as robustly as normal animals, TRPV1-decifient mice also showed significantly decreased heat thresholds during inflammation, suggesting that TRPV1-independent components also contribute to this thermal hypersensitivity. 17R-RvD1 could still reduce this hypersensitivity, suggesting that the thermal anti-nociceptive action of 17R-RvD1 does not involve TRPV1. No such suppression effect was detected in mechanical nociception tests using von Frey (mechanical allodynia) and Randall-Selitto (for mechanical hyperalgesia) apparatuses with the inflamed animals (Figure 5B,D), indicating that 17R-RvD1 does not affect the functions of peripheral machinery that enables mechanical hypersensitivity such as noxious mechanosensitive channels like TRPV4 or TRPA1.
Figure 5.
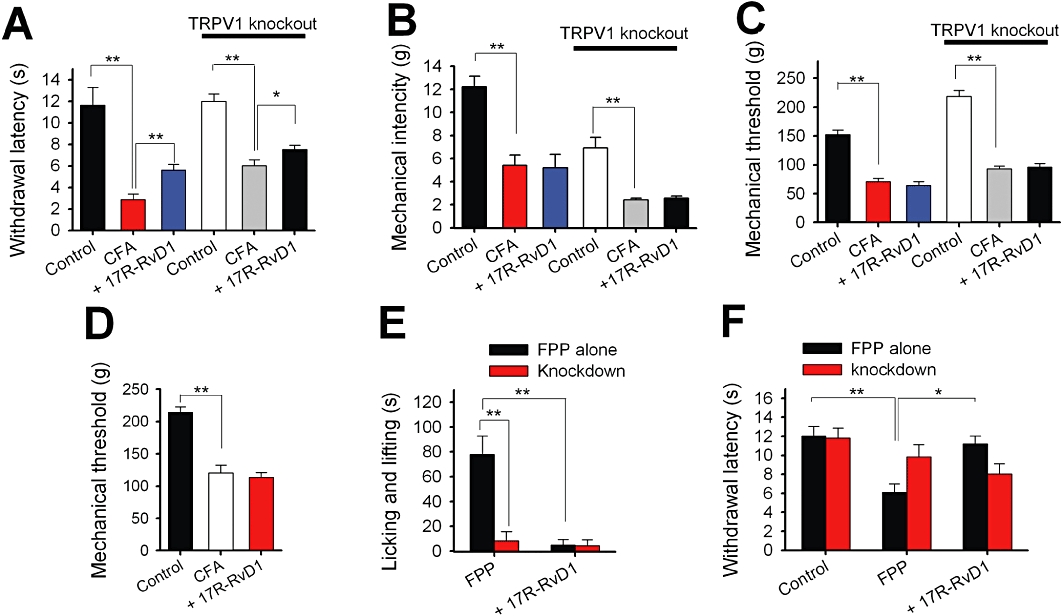
17R-RvD1 attenuates TRPV3-mediated thermal and chemical nociception. (A) Summary of the changes in hind paw withdrawal latencies upon 17R-RvD1 treatment obtained in Hargreaves assays. The average decrease ratios of the Hargreaves latencies induced by CFA inflammation were 75.3 ± 4.2% (control for wild-type mice, n = 5) and 49.8 ± 5.6% (control for TRPV1-null mice, n = 5). Administration of 17R-RvD1 (30 µM in 20 µL, i.d.) reversed the paw withdrawal latency decreases by 23.5 ± 4.4% (wild type, n = 5) and by 12.2 ± 5.8% (TRPV1-null mice, n = 5) compared with the control withdrawal latencies during inflammation. (B) Summary of the mechanical intensities eliciting hind paw withdrawal in von Frey assays. The average decrease ratios of the von Frey intensities during CFA inflammation were 55.9 ± 7.2% (wild-type mice, n = 6) and 63.5 ± 4.3% (control for TRPV1-null mice, n = 5). Administration of 17R-RvD1 i.d. into the hind paw failed to reverse these decreases in the von Frey intensities (a −1.6 ± 9.5% change compared with the control intensity during inflammation and n = 5 for wild-type mice; a 7.1 ± 9.3% change and n = 5 for TRPV1-null mice). (C–D) Summary of the changes in the mechanical threshold induced by inflammation in Randall-Selitto assays. (C) The average decrease ratios of the mechanical threshold during CFA-induced inflammation were 53.7 ± 3.9% (wild-type mice, n = 6) and 57.1 ± 1.4% (control for TRPV1-null mice, n = 5). Administration of 17R-RvD1 i.d. into the hind paw failed to reverse the decreases in mechanical threshold (a −9.0 ± 8.5% change compared with the control threshold under inflammation; n = 6; a 3.5 ± 8% change and n = 5 for TRPV1-null mice). (D) The average decreased ratios of the mechanical threshold during CFA-induced inflammation were 43 ± 7.4% (rats, n = 5) and i.d. administration of 17R-RvD1 into the hind paw failed to reverse this decrease in mechanical threshold (a −9.4 ± 17% change compared with the control threshold during inflammation; n = 5). (E) A summary of the time spent in licking/lifting behaviours of mice during carrageenan-induced inflammation for 10 min immediately after FPP injection (1 mM in 10 µL). 17R-RvD1 i.d. suppressed FPP-evoked behaviours by 86.6 ± 5.4%. mTRPV3 knockdown with shRNA suppressed FPP-evoked behaviours by 83.4 ± 5.3%, and no further suppression was detected upon the treatment with 17R-RvD1. (F) 17R-RvD1 reversed FPP-induced heat hypersensitivity. In Hargreaves assays, the i.d. FPP treatment significantly reduced Hargreaves latencies by 45.4 ± 5.9% (n = 6). Immediate pretreatment with 17R-RvD1 reversed this reduction (n = 6). mTRPV3 knockdown with shRNA suppressed FPP-induced heat-hypersensitivity (n = 6). No further change in the Hargreaves latencies of the knockdown animals occurred upon the pretreatment with 17R-RvD1 (n = 5). Statistical comparisons were done by anova followed by Bonferroni's post hoc test.
Previously, we reported that TRPV3 mediates chemical nociception by FPP via a direct interaction with this endogenous substance (Bang et al., 2010a). Thus, we tested whether 17R-RvD1 affects this chemical nociception. The acute licking/lifting responses to i.d. FPP injection during carrageenan inflammation were successfully reproduced here (Figure 5E), but these response were not evoked by 17R-RvD1 injection (Figure 6A,B). However, 17R-RvD1 pretreatment robustly attenuated the FPP-evoked responses (Figure 5E). Local TRPV3 knockdown also abolished the FPP-evoked acute behavioural responses as previously reported (Bang et al., 2010a), and no further suppression occurred with 17R-RvD1 treatment (Figure 5E). A mismatched control shRNA did not affect FPP-evoked nociception or 17R-RvD1's antinociceptive activity (data not shown). Hence, our knockdown strategy confirmed the specific actions of FPP and 17R-RvD1 on TRPV3.
Figure 6.
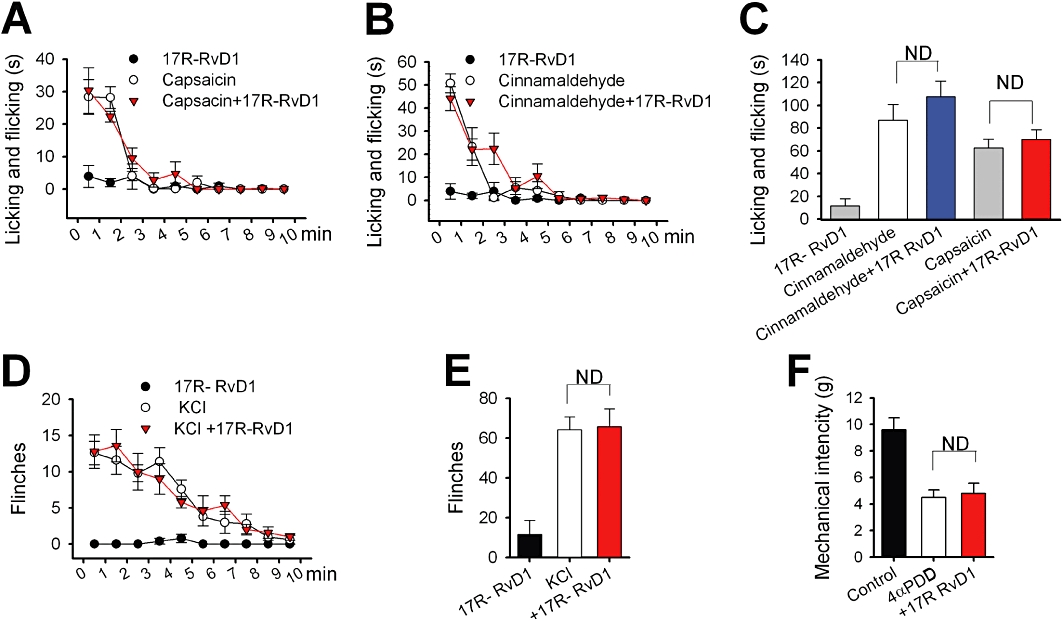
17R-RvD1 failed to affect other related channel-mediated nociceptions. Summaries of the time courses of licking/flicking behaviours in mice treated i.d. with a TRPV1 agonist capsaicin (300 µM in 10 µL) (A), or with a TRPA1 agonist cinnamaldehyde (10 mM in 10 µL) (B), immediately following the injections (n = 5 respectively). Mice were pretreated with 17R-RvD1 (30 µM in 20 µL) immediately before the agonist administrations (n = 5). (C) Summary of the accumulating licking/flicking time of panels (A) and (B). The mean values of the sum of the licking/flicking time during the recording period (10 min) are displayed. (D) Summary of the time course of the flinching behaviours of mice injected intraplantarly with 10 µL KCl solution (140 mM, which evokes sensory neuronal excitation via voltage-gated channel activation) immediately following the injection (n = 5). 17R-RvD1 was administered immediately before the KCl (n = 5). (E) Summary of the total flinching numbers of (D). The mean values of the accumulated flinching numbers during the recording period (10 min) are displayed. (F) 17R-RvD1 failed to reverse a TRPV4 agonist 4-α-phorbol 12,13-didecanoate (4α-PDD)-induced mechanical hypersensitivity. Intraplantar injection with 4α−PDD elicits a decreased von Frey threshold via a TRPV4-specific mechanism (Grant et al., 2007). In our von Frey assays, the i.d. 4α-PDD treatment (1 mM in 10 µL) also significantly reduced von Frey mechanical intensities by 52.2 ± 6.7% (n = 5). Immediate pretreatment with 17R-RvD1 did not significantly affect this reduction (n = 5). Statistical comparisons were done by anova followed by Bonferroni's post hoc test. ND, not significantly different.
FPP not only directly activates TRPV3 but also sensitizes TRPV3 responses to heat, thereby reducing the in vivo heat threshold (Bang et al., 2010a). 17R-RvD1 also reversed this reduction in the heat threshold (Figure 5F). TRPV3 knockdown again eliminated these effects of FPP and 17R-RvD1.
17R-RvD1 administration (30 µM in 20 µL) did not affect the acute nociception mediated by TRPV1, TRPA1, TRPV4 or voltage-gated channels (Figure 6), indicating that the behavioural outcomes from the i.d. 17R-RvD1 treatment are consistently limited to TRPV3-mediated phenotypes. Overall these data suggest that 17R-RvD1 inhibits TRPV3 activity, and that this effect attenuates TRPV3-specific nociception.
Discussion
In the present study, we showed that 17R-RvD1 is a TRPV3-specific inhibitor. We have previously shown that 17S-RvD1, a stereoisomer of 17R-RvD1, is also an inhibitory ligand with a relatively high potency (IC50 = ∼80 nM for 17S-RvD1 vs. hundreds of nM for 17R-RvD1), but that the 17S isomer has a broad specificity for other TRPs (Bang et al., 2010b). Thus, 17R-RvD1 is the first naturally occurring lipid found to be a specific blocker of TRPV3. Earlier, epimeric configurations of 5-hydroxyl residue of hydroxytetraenoic acids were shown to be important for TRPV1 activation potencies (Hwang et al., 2000). Likewise, configurational differences in the 17-hydroxyl residue of RvD1 may determine receptor-specific recognition and the potency of the molecule. This might be caused by a different three-dimensional twist of the carbon chains, and future studies on fatty acid molecular architectures and their structural interaction with TRPV3 protein binding pockets will shed light on this hypothesis.
In this study, we found a novel physiological role for 17R-RvD1 and also suggest its molecular mechanism. 17R-RvD1 is known to suppress cytokine production, neutrophil migration and infiltration, which contributes to its resolution of inflammation (Sun et al., 2007). The molecular identity of the 17R-RvD1 receptor expressed in the relevant cell types is unclear. The potency of 17R-RvD1 (picomolar to nanomolar) for these effects in leucocytes is much lower than its potency for TRPV3 (hundreds of nanomolar), indicating that the leucocyte effects do not involve an inhibitory action on TRPV3. The pro-resolving actions of 17R-RvD1 are comparable with those of 17S-RvD1 in terms of spectrum of effects and potencies. Recently, GPR32 and formyl peptide receptor 2 were suggested as the 17S-RvD1 receptors (Krishnamoorthy et al., 2010). Possibly, those receptors may be bound without stereoselectivity, which may cause the similar in vitro and in vivo outcomes. Again, the discrepancy between the potencies of the leucocyte actions and of the present TRPV3 inhibition and the results from our gallein experiment also reduces the possibility that the two GPCRs are involved in the peripheral anti-nociceptive actions. Therefore, TRPV3 is probably a newly found molecular target for 17R-RvD1 that is independent of its known pro-resolving action. Nonetheless, the pro-resolving effect of resolvins is also likely to be beneficial for alleviating inflammatory pain as it would biochemically alter the injury conditions. Activation of the GPCRs in the central sensory pathway may also negatively modify nociceptive transmission (Xu et al., 2010; Bang et al., 2010b). Thus, though the present study tried to isolate the effect on TRPV3 in the periphery, the parallel processes in all these independent mechanisms may be practically important for anti-nociception (Lima-Garcia et al., 2011).
To exclude GPCR-mediated or other possible systemic effects, we used restricted time scale and limited injection areas for our in vivo assays with 17R-RvD1 and TRPV3 shRNA. The analgesic effects of peripheral 17R-RvD1 only occurred on heat nociception and FPP-induced chemical nociception, both of which are mediated by TRPV3. Interestingly, recently developed synthetic TRPV3 blockers have a broad spectrum of analgesic effects including mechanical phenotypes, when administered centrally or systemically (Moran et al., 2007; Gullapalli et al., 2008). The drugs may potentially affect TRPV3 expressed in unknown brain regions as well as in the periphery, leading to a broad analgesic effect. Thus, the limited effects of peripherally injected 17R-RvD1 or shRNA on thermal and FPP phenotypes in the present study confirms that peripheral TRPV3 does not directly alter mechanical signals but only heat- and agonist-induced signals. Those modality-specific results also teleologically suggest that our peripherally administered 17R-RvD1 does not diffuse out to a central region.
Interactions between TRP channels and lipidergic molecules have been often reported (see the review: Bang et al., 2010c). Interestingly, TRP sensitizers or activators were found in ω-6 fatty acid-derivatives. Arachidonic acid potentiates TRPV3 activation (Hu et al., 2006), epoxyeicosatrienoic acids activate TRPV4 (Watanabe et al., 2003), hydroperoxytetraenoic acids and hydroxyoctadecadienoic acids activate TRPV1 (Hwang et al., 2000; Shin et al., 2002; Patwardhan et al., 2009). Cyclopentenone prostaglandins with αβ-unsaturated aldehydes are covalent TRPA1 activators (Andersson et al., 2008; Taylor-Clark et al., 2008). Consequently, ω-6 derivatives are likely to elicit or exacerbate pain. On the other hand, the ω-3 derived 17R-RvD1 was found to inhibit TRPV3. 17S-RvD1 is also an inhibitory ligand for TRPV3-4 and TRPA1 (Huang et al., 2008; Bang et al., 2010b). These D series ω-3 resolvins seem to alleviate pain via TRP inhibition in our hands. This difference prompted the mediator switching principle during inflammation. That is, the pro-inflammatory mediators generated in the early stage of inflammation are mostly composed of ω-6-derived mediators, and by contrast, ω-3-derived mediators contribute to the resolution of inflammation in the late stage (Levy et al., 2001). This interesting coincidence of differential actions observed in nociception and inflammation will give further insights about lipid connection with the pain transducer TRPs in progress and termination of nociception.
Sensory TRP inhibitors are regarded as having analgesic potential. Structural information on natural ligands may provide important insights. For example, the structure of capsaicin has been frequently applied to the syntheses of novel TRPV1 antagonists (Patapoutian et al., 2009). A synthetic TRPA1 antagonist AP18 shares the backbone with an agonist cinnamaldehyde (Petrus et al., 2007). 17R-RvD1 will be similarly useful in this context. Moreover, endogenously-produced, specific antagonistic molecules are extremely rare in ion channel studies (Stone, 1993; Hilmas et al., 2001; Puntambekar et al., 2004). Such unusual molecules including 17R-RvD1 may be helpful in defining antagonist-specific binding domains as well as in developing biocompatible therapeutic agents.
Taken together, our results show that 17R-RvD1 is a novel natural inhibitory substance specific for TRPV3. Inhibition of peripherally expressed TRPV3 by 17R-RvD1 may lead to modality-specific antinociception, suggesting that 17R-RvD1 may be a useful analgesic substance. Stimulating the endogenous production of 17R-RvD1 or the extraneous administration of it or its possible synthetic analogues might help reverse TRPV3-mediated pain states.
Acknowledgments
This work was supported by the Korea Research Foundation Grant (code KRF-2008–331-E00457 and 2009–0076543), the Republic of Korea.
Glossary
- 17R-RvD1
17(R)-resolvin D1 (7S,8R,17R–trihydroxy-4Z,9E,11E,13Z,15E,19Z–docosahexaenoic acid)
- 2-APB
2-aminoethoxydiphenyl borate
- 4α−PDD
4-α-phorbol 12,13-didecanoate
- BK
bradykinin
- CFA
complete Freund's adjuvant
- DRG
dorsal root ganglion
- FPP
farnesyl pyrophosphate
- RvD1
resolvin D1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid)
- shRNA
small hairpin RNA
- TRP
transient receptor potential
- TRPV3
TRP ion channel vanilloid 3
Conflict of interest
SB and SWH applied for Korean patents regarding the uses of 17R-RvD1 for TRPV3 inhibition (patent application No. 10–2011-0000309)
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;153(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci. 2007a;26:2516–2523. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007b;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem. 2010a;285:19362–19371. doi: 10.1074/jbc.M109.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. 2010b;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Yoo S, Oh U, Hwang SW. Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch Pharm Res. 2010c;33:1509–1520. doi: 10.1007/s12272-010-1004-9. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain. 2011;152:1156–1164. doi: 10.1016/j.pain.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullapalli STA, Lingam PR, Kattige V, Gudi GS, Khairatkar-Joshi N. GRC 15133 – A Novel, Selective TRPV3 Antagonist with Anti-Hyperalgesic Effects in Inflammatory and Neuropathic Pain. Philadelphia, PA: CHI-World Pharmaceutical Congress; 2008. [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, et al. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208:201–212. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Grandl J, Bandell M, Petrus M, Patapoutian A. Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc Natl Acad Sci USA. 2009;106:1626–1631. doi: 10.1073/pnas.0812209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Bang S, Han S, Nguyen YH, Kang TM, Kang KW, et al. TRP-independent inhibition of the phospholipase C pathway by natural sensory ligands. Biochem Biophys Res Commun. 2008;370:295–300. doi: 10.1016/j.bbrc.2008.03.077. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Lima-Garcia J, Dutra R, da Silva K, Motta E, Campos M, Calixto J. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Moran MM, Wei DS, Zhen X, Mandel-Brehm J, Witek J, Yaksh T, et al. 2007. Potent and selective antagonists validate TRPV3 as a target for analgesic therapeutics. In: Society of Neuroscience Annual Meeting pp 2007 2143.2005/J2006. San Diego.
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci USA. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS, Ramkumar V. Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci. 2004;24:3663–3671. doi: 10.1523/JNEUROSCI.4773-03.2004. http://www.molecularpain.com/content/3/1/40/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JJ, Yoo S, Kim KY, Park JS, Bang S, Lee SH, et al. Laser-modulation of heat and capsaicin receptor TRPV1 leads to thermal antinociception. J Dent Res. 2010;89:1455–1460. doi: 10.1177/0022034510381394. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, et al. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, et al. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]


