Abstract
BACKGROUND AND PURPOSE
Microparticles (MPs), small membrane-bound particles originating from different cell types during activation or apoptosis, mediate intercellular communication, exert pro-coagulant activity and affect inflammation and other pathophysiological conditions. Monocyte-derived MPs have undergone little investigation and, to our knowledge, have never been evaluated for their possible autocrine effects. Therefore, we assessed the ability of monocyte-derived MPs to stimulate human monocytes and monocyte-derived macrophages (MDM).
EXPERIMENTAL APPROACH
MPs were generated from supernatants of human monocytes stimulated by the calcium ionophore A23187 (12 µM), and then characterized. Human monocytes and MDM of healthy donors were isolated by standard procedures. Cells were challenged by MPs or phorbol 12-myristate 13-acetate (PMA, used as standard stimulus), in the absence or presence of PPARγ agonists and antagonists. Superoxide anion production (measured spectrophotometrically), cytokine release (elisa), PPARγ protein expression (immunoblotting) and NF-κB activation (EMSA assay) were evaluated.
KEY RESULTS
Monocyte-derived MPs induced, in a concentration-dependent manner, oxygen radical production, cytokine release and NF-κB activation in human monocytes and macrophages, with lower effects than PMA. In both cell types, the PPARγ agonists rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) inhibited MPs-induced stimulation and this inhibition was reversed by a PPARγ antagonist. In human monocyte/macrophages, MPs as well as rosiglitazone and 15d-PGJ2 induced PPARγ protein expression.
CONCLUSION AND IMPLICATIONS
In human monocyte/macrophages, monocyte-derived MPs exert an autocrine activation that was modulated by PPARγ ligands, inducing both pro-inflammatory (superoxide anion production, cytokine release and NF-κB activation) and anti-inflammatory (PPARγ expression) effects.
Keywords: Monocyte-derived microparticles, human monocytes, monocyte-derived macrophages, oxy-radical production, inflammatory cytokines, NF-κB, PPARγ, rosiglitazone
Introduction
Microparticles (MPs), also referred to as microvesicles, are small (diameter 0.1–1 µm) membrane-bound particles that originate from the cell surface of most (if not all) cell types during activation, shear stress or apoptosis. MPs are heterogeneous in nature, varying in both size and content, and present cell surface markers and cytoplasmic components of the parent cells from which they originate. Therefore, surface markers are largely used to identify the derivation of MPs. As an example, CD14 is the major marker for monocyte-derived MPs, whereas CD3, CD41a and CD146 are markers for lymphocyte-, platelet- and endothelial cell-derived MPs respectively (Dalli et al., 2008).
In addition to cell-specific markers, MPs carry a broad variety of proteins, nucleic acids and lipids, that enable them to mediate intercellular communication (thereby acting as novel signalling elements), to transfer genetic information (due to their content of nucleic acids and proteins), to exert pro-coagulant activity and to affect inflammation, as well as other pathophysiological conditions (Mallat et al., 1999; Hugel et al., 2004; Distler et al., 2005b; Ratajczak et al., 2006; Dalli et al., 2008; Beyer and Pisetsky, 2010). For these reasons, MPs have been the focus of intense experimental research in the last decade and are now recognized to play differing roles, depending on the cell of origin.
Although present at low levels (5–50 µg·mL−1; Ratajczak et al., 2006) in the blood from healthy donors, markedly increased numbers of circulating MPs have been detected under different pathological conditions, such as atherosclerosis, diabetes, sepsis, arthritis and cancer (Mallat et al., 2000; Nieuwland et al., 2000; Distler et al., 2005b; Ratajczak et al., 2006; Anderson et al., 2010; Beyer and Pisetsky, 2010). The majority (about 80%) of in vivo circulating MPs derive from platelets (Ratajczak et al., 2006) and most experimental work so far performed deals with platelet-derived MPs. As an example, platelet-derived MPs enhance neutrophil aggregation and adhesion (even when L-selectin is blocked), deliver arachidonic acid to other cells, and increase monocyte chemotaxis and/or adhesion to the endothelium (Barry et al., 1998; Distler et al., 2005a). Moreover, neutrophil-derived MPs induce the expression of IL-6 and the chemokine CCL2 (MCP-1) in endothelial cells (Mesri and Altieri, 1999), but, under certain circumstances, can also exert anti-inflammatory effects (Gasser and Schifferli, 2004; Hugel et al., 2004; Dalli et al., 2008).
Although less thoroughly investigated, monocyte-derived MPs also play a key role in haemostasis and thrombosis. In fact, they express tissue factor (TF) at high levels, in addition to P-selectin glycoprotein ligand-1 (PSGL-1). Therefore, these MPs not only directly activate platelets (via PSGL-1), but also fuse with them and transfer TF to platelet membranes, thereby promoting optimal coagulation by activated platelets (Del Conde et al., 2005). Recently, Li et al. (2010) demonstrated that exposure of THP-1 cells or human monocyte-derived macrophages (MDM) to tobacco smoke extract induces the generation of highly pro-coagulant MPs that contribute to the pathological hypercoagulability of smokers. Moreover, atherosclerotic plaques have been shown to contain pro-coagulant MPs (Mallat et al., 1999) that are mainly derived from monocyte/macrophages, as demonstrated by co-labelling for CD14 and IgG (>90% of IgG containing MPs is CD14+; Mayr et al., 2009). In addition, plaque-derived MPs have been shown to transfer the adhesion molecule ICAM-1 to endothelial cells, thus promoting monocyte adhesion and transendothelial migration (Rautou et al., 2011).
Evidence is also emerging that monocyte-derived MPs affect endothelial cells (Essayagh et al., 2007; Aharon et al., 2008) as well as human bronchial epithelial and alveolar cell lines (Cerri et al., 2006; Neri et al., 2011). In human airway cells, monocyte-derived MPs have been shown to up-regulate the synthesis of IL-8, CCL2 and ICAM-1 (Cerri et al., 2006). Moreover, MPs-induced pro-inflammatory effects are mediated by NF-κB activation and modulated by PPARγ agonists (Neri et al., 2011). This is an interesting finding, as activation of PPARγ (expressed in a large variety of cells, including human airway cells and monocyte/macrophages) by selective agonists results in consistent anti-inflammatory effects (Jiang et al., 1998; Ricote et al., 1998; Amoruso et al., 2007, 2008, 2010; Neri et al., 2011).
To our knowledge, there is no information concerning a possible autocrine effect of monocyte-derived MPs. Therefore, we evaluated the ability of monocyte-derived MPs to stimulate human monocytes and monocyte-derived macrophages (MDM).
In this study we demonstrate that monocyte-derived MPs induce, in a concentration-dependent manner, oxygen radical production, cytokine release, NF-κB activation and PPARγ protein expression in human monocytes and MDM. Moreover, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) and rosiglitazone, two PPARγ agonists which exert relevant anti-inflammatory activity in human monocyte/macrophages (Jiang et al., 1998; Amoruso et al., 2007, 2008, 2010), modulate MPs-induced effects.
Methods
Generation of monocyte-derived MPs and MPs characterization
MPs were generated according to Neri et al. (2011). Briefly, human monocytes (about 2 × 106 cells per well; see below for monocyte isolation) were stimulated by the calcium ionophore A23187 (12 µM), in a final volume of 0.5 mL, for 20 min at 37°C. The supernatant was recovered and cleared by centrifugation (14 000×g, 5 min, room temperature) to remove cell debris.
Such supernatants from six individual non-smoker volunteers (mean age 34.6 + 2.9 yr, range 27-45 yr, 3 males, 3 females; no present medication) were used in these experiments and the MPs characterized by assessing CD14+ elements, phosphatidylserine (PS) expression and TF activity.
PS-positive MPs in each sample were detected using the Zymuphen MP-activity kit (Hyphen BioMed, Neuville-sur-Oise, France), according to the manufacturer's instructions and expressed as PS equivalents; the PS concentration in supernatants was about 2 nM (see also Results). TF activity was measured by a one-stage clotting time as described by Celi et al. (1994). Briefly, 100 µL MPs were mixed with 100 µL normal human plasma at 37°C for 5 min; 100 µL of 25 mM CaCl2 was added to the mixture and the time to clot formation was recorded. The test tube was kept in a transparent water bath at 37°C throughout the test. The values were converted to arbitrary units (A.U.) of pro-coagulant activity by comparison with a standard curve obtained using a human brain thromboplastin standard; this preparation was assigned a value of 1000 A.U. for a clotting time of 20 s (Celi et al., 1994).
To characterize the expression of CD14 in MPs generated from monocytes, samples were analysed by flow cytometry using a FACS Calibur (Becton Dickinson, Oxford, UK). Data acquisition and analysis were performed using WinMDi 2.9 software (Joseph Trotter, The Scripps Institute). To determinate the percentage of CD14+ MPs, 100 µL of resuspended human monocytes and 100 µL of MPs were added for 30 min on ice to the fluorescent dye-labelled antibody against surface marker CD14+ PE (Phycoerythrin)-conjugated (Dako Cytomation, Denmark). Incubation was performed in the dark and the expression of the surface marker was analysed. CD14 expression positivity was defined on FL2 (PE fluorescence) versus FSC dot plot representation. Regions (R1) corresponding to monocytes and MPs were defined on forward versus side angle light scatter intensity dot plot representation. The forward light scatter setting was E-01 and a total of 10 000 events was analysed.
Following procedures outlined by Scanu et al. (2008) and Carpintero et al. (2010), we also measured the total protein content in the supernatant samples; this allows a quantitative measure, as well as a better evaluation of possible concentration-dependent effects of MPs. The determination of protein content was measured using a bicinchoninic acid (BCA) assay: 100 µL of supernatant from A23187-challenged monocytes usually contained about 10 µg proteins (see also Results).
In order to ensure that the effects observed (see below) with our MPs preparations were not attributable to soluble mediators that may be present within supernatants, supernatants were ultracentrifuged at 100 000×g for 2 h. In this latter case, both supernatant (from which MPs have been cleared) and pellet (that contains MPs and is resuspended in the same volume as the starting material) were evaluated.
Preparation of monocytes and MDM
This study and the research protocol were approved by the Ethical Committee of the Azienda Ospedaliera Maggiore della Carità, Novara (Italy) and informed written consent was obtained from all participants.
Human monocytes were isolated either from fresh buffy coats, obtained from the local blood bank, or from heparinized venous blood (30–40 mL) of healthy non-smoker volunteers by the standard techniques of dextran sedimentation, Histopaque (density = 1.077 g·cm−3)-gradient centrifugation (400×g, 30 min, room temperature) and finally recovered by thin suction at the interface, as described (Amoruso et al., 2007, 2008, 2009a). Cells were resuspended in RPMI 1640 medium, supplemented with 5% heat-inactivated foetal bovine serum (FBS), 2 mM glutamine, 10 mM HEPES, 50 µg·mL−1 streptomycin and 5 U·mL−1 penicillin. Purified monocyte populations were obtained by adhesion (2 h, 37°C, 5% CO2), non-adherent cells (mainly lymphocytes) being gently removed with sterile PBS. Cell viability (Trypan blue dye exclusion) was usually >98%. MDM were prepared from monocytes, by culture (8–10 days) in RPMI 1640 medium containing 20% FBS, glutamine and antibiotics; MDM were defined as macrophage-like cells by evaluating surface markers CD14, MHCII, CD1a and CD68, as described (Amoruso et al., 2008, 2009b). The effects of different treatments (see below) on cell viability were determined by the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. In these experiments, monocytes (1 × 105) were challenged with the compounds under study for different times (up to 24 h). Thereafter, the medium was replaced by the MTT solution (1 mg·mL−1) and cells were incubated for 2 h at 37°C in the dark. The supernatant was removed and DMSO was added in order to dissolve the purple formazan; the absorbance of the samples was read at 580 and 675 nm, as described (Amoruso et al., 2010).
Different numbers of cells were used according to the type of experiments – 2 × 106 cells for Western blot experiments, 5 × 106 cells for EMSA assays, 0.5–1 × 106 cells for superoxide anion production and 1 × 106 cells for cytokine release.
Superoxide anion (O2−) production in monocytes and MDM
Adherent cells (0.5–1 × 106 cells per well) were washed twice with PBS, incubated in RPMI 1640 medium (without phenol red, no antibiotics and no FBS) and challenged with different aliquots (1–300 µL, corresponding to 0.1 and 30 µg protein concentration respectively) of MPs-containing supernatants from A23187-stimulated monocytes for 30 min, in order to evaluate concentration-dependent effects. MPs effects were compared with those evoked by maximally effective concentrations of the PKC activator, phorbol 12-myristate 13-acetate (PMA; 1 µM). In some experiments, cells were pretreated with 15d-PGJ2 (10 µM), an endogenous PPARγ agonist, for 30 min and then challenged with MPs or PMA. O2− production, evaluated by the superoxide dismutase (SOD)-inhibitable cytochrome C reduction, was expressed as nmol cytochrome C reduced per 106 cells per 30 min, using an extinction coefficient of 21.1 mM (Bardelli et al., 2005). To avoid interference with spectrophotometric recordings of O2− production, human monocytes and MDM were incubated with RPMI 1640 without phenol red. Experiments were performed in duplicate; control values (e.g. basal O2− production in the absence of stimuli) were subtracted from all determinations.
Cytokine release in monocyte and MDM
Human monocytes and MDM were challenged with increasing concentrations of MPs or PMA (0.1 µM) for 24 h. This time period has been previously shown to ensure maximal cytokine release (Bardelli et al., 2005; Amoruso et al., 2010). Supernatants were collected and stored at −80°C. Cytokine content in the samples was measured using enzyme-linked immunoassay kit (R&D Systems, Minneapolis, USA).
TNF-α and IL-6 were evaluated as the most relevant pro-inflammatory cytokines; the amount of each cytokine was expressed in pg·mL−1, as indicated by the manufacturer's instructions. The minimum detectable concentrations of human TNF-α and IL-6 were 1.4 pg·mL−1 and 0.5 pg·mL−1 respectively. No cross-reactivity was observed with any other known cytokine. Control values (e.g. cytokine release from untreated, un-stimulated cells) were subtracted from all determinations. In some experiments, cells were pretreated for 1 h with 15d-PGJ2 (10 µM) or rosiglitazone (1 µM), in the absence or presence of the PPARγ antagonist 2-chloro-5-nitro-N-phenylbenzamide (GW9662; 1 µM), and then stimulated by MPs or PMA for 24 h. At the concentrations reported above, all the compounds had no relevant cytotoxicity (MTT assay; data not shown).
Evaluation of NF-κB activation
The activation of NF-κB induced by MPs or PMA was evaluated by measuring its nuclear migration (by EMSA), as previously described (Bardelli et al., 2005). In EMSA assays, nuclear extracts (5 µg) from monocytes or MDM were incubated with 2 µg poly (dI-dC) and [32P] ATP-labelled oligonucleotide probe (100 000–150 000 cpm; Promega) in binding buffer for 30 min at room temperature. The NF-κB consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC–3′) was from Promega. The nucleotide–protein complex was separated on a polyacrylamide gel, the gel was then dried and radioactive bands were detected by autoradiography (Bardelli et al., 2005; Neri et al., 2011). Densitometric analysis of NF-κB nuclear translocation was also performed, with values for un-stimulated cells being set to 1. In some experiments, cells were pretreated with 15d-PGJ2 (10 µM) or rosiglitazone (5 µM) for 1 h and then challenged with MPs or PMA. At the concentrations used, neither 15d-PGJ2 nor rosiglitazone exerted cytotoxic effects (evaluated by MTT assay; not shown).
PPARγ protein expression and semi-quantitative analysis
The constitutive expression of PPARγ protein was evaluated in monocytes and MDM, as described (Amoruso et al., 2007, 2008). Cells (2 × 106), seeded in six-well plates, were washed twice with ice-cold PBS, scraped off in radioimmunoprecipitation assay buffer and lysed by sonication; when necessary, cell lysates were stored at −80°C. Protein concentration was measured by the BCA assay. Protein samples (20 µg) were analysed by SDS-PAGE (10% acrylamide) and electro-blotted on nitrocellulose membrane (Protran, Perkin Elmer Life Sciences, Boston, MA, USA). Immunoblots were performed using polyclonal rabbit anti-human PPARγ (Abcam, UK), that detects both PPARγ 1 and 2 isoforms, and monoclonal mouse anti-human β-actin (Sigma, Milan, Italy) antibodies; anti-mouse and anti-rabbit secondary antibodies were coupled to horseradish peroxidase. Chemiluminescence signals were analysed under non-saturating conditions with an image densitometer (Versadoc, Bio-Rad, Hercules, USA). Semi-quantitative evaluation of PPARγ protein was performed by calculating the ratio between its total expression (i.e. the sum of the two isoforms) and the expression of the reference housekeeping protein, β-actin (Amoruso et al., 2007, 2008).
Statistical analysis
All statistical analyses were performed using spss statistical software (version 15.0, SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± SEM of ‘n’ independent experiments on cells isolated from different healthy donors; cytokine and oxy-radical determinations were performed in duplicate. Concentration–response curves for MPs were constructed and EC50 values were interpolated from curves of best-fit. Statistical evaluation was performed by anovaand Bonferroni correction or, when required, by unpaired, two-tailed Student's t-test. A non-parametric test (Mann–Whitney test) was used to evaluate the data transformed to percentage, as in the case of cytokine and O2− inhibition, and EMSA quantification. Differences were considered statistically significant when P < 0.05.
Materials
FBS was from Gibco (Paisley, UK). Rosiglitazone, GW9662 and 15d-PGJ2 were from Cayman Chemicals (Milan, Italy). Histopaque, PBS, RPMI 1640 medium (with or without phenol red), glutamine, HEPES, streptomycin, penicillin, PMA, SOD, cytochrome C and monoclonal mouse anti-human β-actin antibody were obtained from Sigma (Milan, Italy). All the reagents for EMSA assays were purchased from Promega Corporation (St. Louis, USA). The polyclonal rabbit anti-human PPARγ antibody was from Abcam (UK). Tissue-culture plates were from Nunc Ltd (Denmark); all cell culture reagents, with the exception of FBS, were endotoxin-free according to details provided by the manufacturer. TNF-α and IL-6 immunoassay kits were obtained from R&D Systems (Minneapolis, USA). The Zymuphen MP-activity kit was purchased from Hyphen BioMed (Neuville-sur-Oise, France).
Results
Characterization of monocyte-derived MPs
The MPs used in this study are generated from A23187-challenged human monocytes (isolated from six healthy donors). In order to characterize these MPs, supernatants (100 µL) from un-stimulated or A23187-challenged monocytes were evaluated for CD14 expression, TF activity and PS equivalents (Figures 1 and 2). Figure 1 shows a forward versus side angle light scatter dot plot and CD14+ expression of un-stimulated monocytes (A) and MPs (B). As expected, monocyte-derived MPs are smaller than monocytes, as demonstrated by physical parameters analysed by FACS. Since about 95% of our MPs preparation is CD14+, we can conclude that they have a monocytic origin. Moreover, as shown in Figure 2, only supernatants from A23187-stimulated monocytes demonstrated significant TF activity and PS concentration, thus confirming MPs formation. In an attempt to quantify MPs and in view of their possible concentration-dependent effects, we have evaluated the total protein content of the supernatants from A23187-stimulated monocytes (n = 6), using different aliquots (1, 5, 10, 100 or 300 µL) containing 0.1, 0.5, 1, 10 and 30 µg protein respectively (data not shown). Since a final volume of 1 mL is used in all the experimental assays performed (see below), MPs were used in the range 0.1–30 µg protein mL−1.
Figure 1.
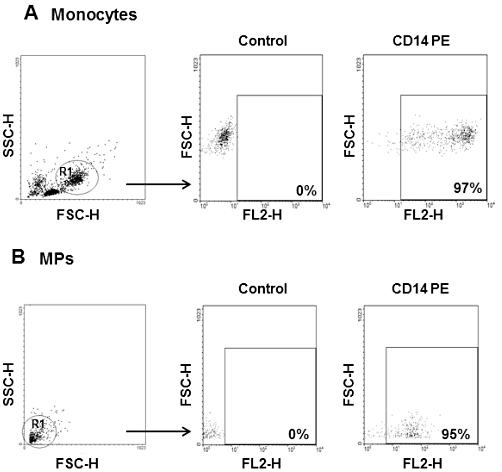
Flow cytometry analysis of monocyte-derived microparticles (MPs). Human monocytes (1 × 106; resuspended in 100 µL PBS) and 100 µL of MPs were analysed. Regions (R1) corresponding to monocytes (A) and MPs (B) were defined on forward (FSC) versus side angle light scatter (SSC) intensity dot plot representation and CD14 expression positivity was defined on FL2 (PE fluorescence) versus FSC dot plot representation. MPs have smaller physical parameters and show similar percentage of CD14 positivity compared to monocyte population.
Figure 2.
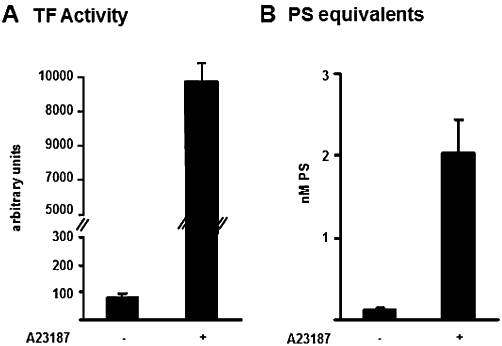
Characterization of monocyte-derived microparticles (MPs). The TF activity in supernatant (100 µL) from un-stimulated or stimulated (A23187, 12 µM) monocytes is shown in A and the corresponding concentrations of PS equivalents in B. Data are expressed as mean ± SEM of six separate MPs preparations. See text for further details.
Monocyte-derived MPs induce oxygen radical production in human monocytes and MDM
Monocyte-derived MPs induce, in a concentration-dependent manner (0.1–30 µg protein mL−1), O2− production in human monocytes and MDM (Figure 3A), with a maximal effect at 10–30 µg·mL−1 and with EC50 values of 1.55 µg·mL−1 (monocytes) and 2 µg·mL−1 (MDM). As shown in Figure 3B, MPs-induced oxygen radical production is significantly lower than that evoked by PMA (1 µM). Moreover, spontaneous O2− production is minimal in both cell types, and ultra-centrifuged supernatants (from which MPs have been removed) or supernatants from un-stimulated human monocytes do not affect the respiratory burst. Conversely, the pellet from ultra-centrifuged samples induced O2− production, although less effectively that the original MPs. Interestingly, 4 µM A23187 (the concentration present in the 300 µL aliquot of supernatant) has minor effects (Figure 3B), and even at 12 µM evokes only a low respiratory burst (not shown). As expected, the endogenous PPARγ agonist, 15d-PGJ2, used at 10 µM, potently inhibited PMA-induced O2− production: about 90–95% in both monocytes and MDM (Figure 3C). In human monocytes, the endogenous PPARγ agonist is less potent (P < 0.01) in inhibiting MPs-induced O2− production than PMA-evoked O2− production (Figure 3C). In keeping with previous observations (Amoruso et al., 2008, 2009b), the PPARγ antagonist GW9662 completely abolishes the inhibitory effect of 15d-PGJ2 on oxygen radical production (not shown).
Figure 3.
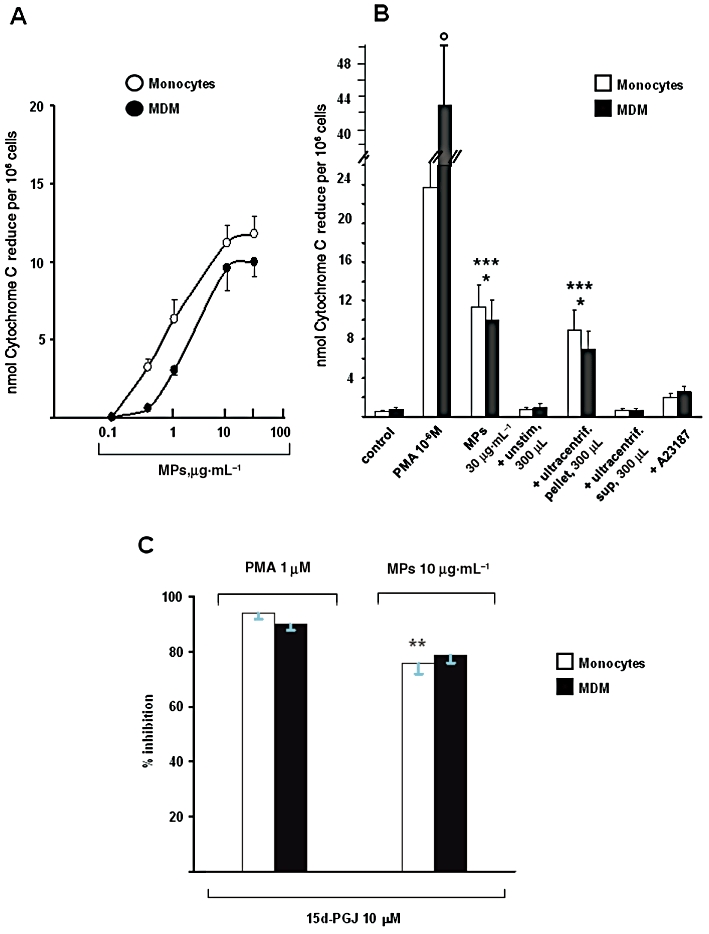
Monocyte-derived MPs induce superoxide anion (O2−) production in human monocytes and monocyte-derived macrophages (MDM). Human monocytes and MDM were challenged with the different stimuli for 30 min. In A: concentration-dependent (0.1–30 µg·mL−1) effects of MPs. Values are means ± SEM; n = 14 (monocytes), 8 (MDM). In B: superoxide anion production induced by MPs, PMA, A23187, ultra-centrifuged supernatants and pellets. Cells were challenged with PMA (1 µM), MPs (30 µg·mL−1), A23187 (4 µM), supernatant (300 µL) from un-stimulated monocytes, ultra-centrifuged supernatant (300 µL) from A23187-stimulated monocytes or ultra-centrifuged pellet (300 µL) from A23187-stimulated monocytes. Please, note that, in these experiments, spontaneous (control) O2− production was not subtracted. +unstim, 300 µL = supernatant (300 µL) from un-stimulated monocytes added to cells; +ultracentrif, sup 300 µL = ultra-centrifuged supernatant (300 µL) from A23187-challenged monocytes added to cells; +ultracentrif, pellet 300 µL = ultra-centrifuged pellet (300 µL) from A23187-challenged monocytes added to cells. Mean ± SEM (n = 8–14 for control, PMA and MPs; n = 3 for the others). °P < 0.05 versus monocytes; *P < 0.05 versus PMA; ***P < 0.001 versus control. In C: % inhibition of PMA- or MPs-evoked O2− production by 15d-PGJ2 (15d-PGJ; 10 µM) in both cell types. Cells were treated with the endogenous PPARγ agonist for 30 min and then challenged with MPs (10 µg·mL−1) or PMA (1 µM). Data are expressed as mean ± SEM of five separate experiments in each cell type. **P < 0.01 versus PMA.
Monocyte-derived MPs induce cytokine release in human monocytes and MDM
As shown in Figure 4, PMA and MPs induced TNF-α and IL-6 release in human monocytes and MDM. In both cell types, the phorbol ester evokes a significantly higher cytokine production than MPs. Furthermore, MPs, evaluated in the range 1–30 µg protein mL-1, exerted concentration-dependent effects with maximal effects at 10 µg·mL−1 (protein concentration; Figure 4).
Figure 4.
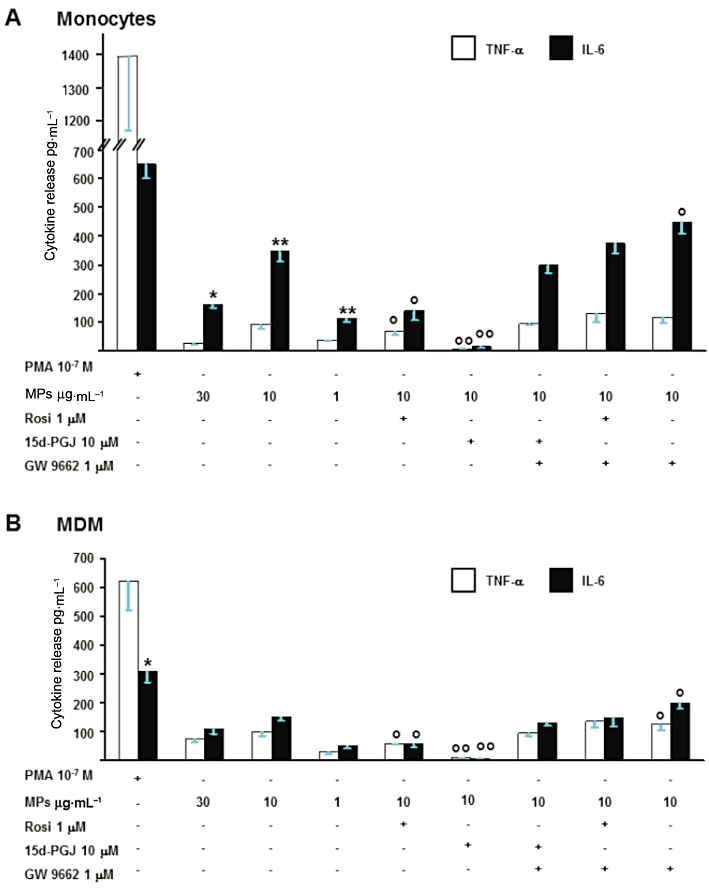
Monocyte-derived MPs induce cytokine production in monocytes (A) and MDM (B). Concentration-dependent (1–30 µg·mL−1) effects of MPs and modulation by PPARγ agonists (15d-PGJ2 (15d-PGJ; 10 µM), rosiglitazone (Rosi, 1 µM) and antagonist (GW9662, 1 µM). TNF-α and IL-6 release by PMA 10−7 M are shown for comparison. Values are mean ± SEM; n = 5. *P < 0.05 versus TNF-α; **P < 0.01 versus TNF-α; °°P < 0.01 versus MPs 10 µg·mL−1; °P < 0.05 versus MPs 10 µg·mL−1.
In human monocytes (Figure 4A), MPs released higher amounts of IL-6 than of TNF-α, whereas PMA-evoked release was not significantly different. Basal cytokine release from un-stimulated human monocytes (TNF-α = 10 ± 4 pg·mL−1 and IL-6 = 38 ± 14 pg·mL−1; n = 5) was subtracted from all determinations. Maximal release by MPs of TNF-α or of IL-6 was observed at 10 µg·mL−1 protein concentration (Figure 4A) and was enhanced by the PPARγ antagonist, GW9662 (1 µM). Rosiglitazone (1 µM) and 15d-PGJ2 (10 µM), previously shown to exert maximal effects with no evident sign of cytotoxicity (Amoruso et al., 2008, 2009b, 2010), inhibited MPs-induced cytokine release (see also Table 1) and this inhibition was completely reversed by GW9662 (Figure 4A).
Table 1.
Rosiglitazone (1 µM) and 15d-PGJ2 (10 µM) inhibit PMA- and MPs-evoked cytokine release in all cell types
| % inhibition of MPs 10 µg·mL−1 | % inhibition of PMA 10−7 M | |||
|---|---|---|---|---|
| TNF-α | IL-6 | TNF-α | IL-6 | |
| Monocytes (n = 5) | ||||
| Rosi | 27 ± 1 | 60 ± 6** | 62 ± 2§§ | 58 ± 3 |
| 15d-PGJ2 | 92 ± 1°° | 96 ± 1°° | 95 ± 2°° | 82 ± 4°° |
| MDM (n = 5) | ||||
| Rosi | 28 ± 5 | 30 ± 3 | 40 ± 8 | 42 ± 7 |
| 15d-PGJ2 | 40 ± 6 | 54 ± 5°° | 52 ± 3 | 58 ± 5 |
Cells were pretreated with (or without) PPARγ agonists for 1 h and then stimulated by PMA or MPs for 24 h; the percentage of cytokine release inhibition produced by each agonist was calculated. Rosi: rosiglitazone. Data are expressed as mean + SEM of five experiments.
P < 0.01 versus TNF-α
P < 0.01 versus rosiglitazone
P < 0.01 versus MPs.
In human MDM (Figure 4B), PMA and MPs induced TNF-α and IL-6 release (in lower amounts than in human monocytes) that was potently inhibited by the two PPARγ ligands (15d-PGJ2 exerting greater effects than rosiglitazone). This inhibition was reversed by GW9662. As described previously, basal cytokine release (TNF-α = 20 ± 5 pg·mL−1, IL-6 = 25 ± 10 pg·mL−1; n = 5) is subtracted from all determinations.
As shown in Table 1, some differences are observed concerning the inhibitory potency of the two PPARγ agonists on cytokine release, which appears to depend on both the cell type and the stimulus. The inhibition afforded by PPARγ ligands is more evident in monocytes than in MDM (Table 1). In these experiments, the inhibition by rosiglitazone on MPs-induced cytokine release was usually less (although not always reaching statistical significance) than PMA-induced cytokine release. This effect was particularly evident in human monocytes, in which rosiglitazone inhibited by more than 60% the PMA-induced TNF-α release, while it reduced MPs-evoked release by only 27% (Table 1).
Monocyte-derived MPs induce NF-κB activation in human monocytes and MDM
In unstimulated monocytes, DNA binding of NF-κB is minimal, although detectable, whereas it is greatly increased following challenge with PMA or MPs (Figure 5A). The specificity of the NF-κB DNA binding is confirmed by the reversal of the binding by a 100-fold molar excess of unlabelled probe (data not shown). Nuclear translocation of the transcription factor is maximal when monocytes are stimulated by PMA (1 µM) for 1 h (>threefold increase vs. control; Figure 5A), or by TNF-α 10 ng·mL−1 (data not shown). MPs, evaluated at different concentrations (1, 5, 10 and 30 µg protein mL-1), also enhance NF-κB nuclear migration, with maximal effect at 10 µg·mL−1 (Figure 5A). Previous reports (Tesse et al., 2008; Amoruso et al., 2009b, 2010; Neri et al., 2011) documented the ability of PPARγ agonists to inhibit NF-κB activation. When used at a near maximal concentration (5 µM), rosiglitazone potently inhibited MP-triggered NF-κB translocation, yielding values similar to unstimulated monocytes in densitometric evaluations, while it was only modestly effective in reducing PMA-induced translocation (Figure 5A).
Figure 5.
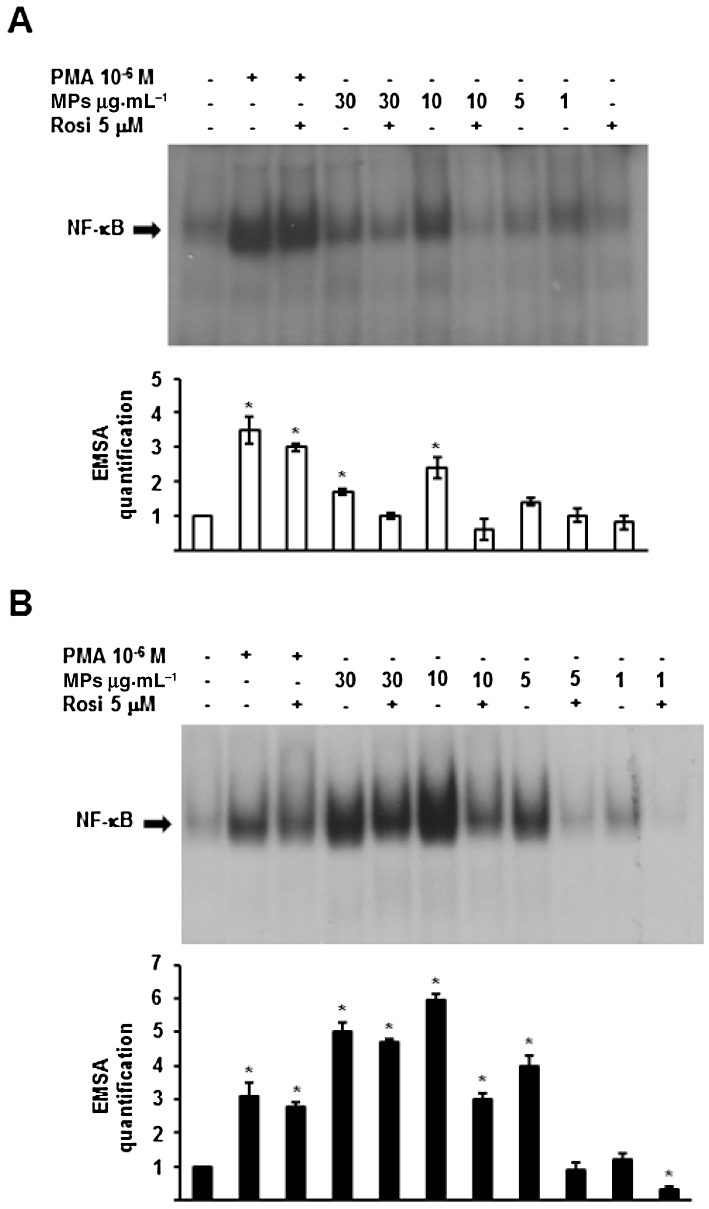
Monocyte-derived MPs induce NF-κB activation in human monocytes (A) and MDM (B). Concentration-dependent (1–30 µg·mL−1) effects of MPs and modulation by rosiglitazone (Rosi, 5 µM) and a PPARγ antagonist (GW9662, 1 µM). The effects produced by PMA 1 µM are shown for comparison. Histograms below each blot are densitometric analysis for EMSA quantification, control, unstimulated cells being = 1. Mean ± SEM; n = 4. *P < 0.05 versus control.
In human MDM, MPs exert a concentration (1–30 µg protein mL-1)-dependent effect and, at the highest 10 and 30 µg·mL−1 concentrations, were even more potent than PMA in evoking NF-κB nuclear migration (Figure 5B). EMSA quantification of NF-κB translocation (setting unstimulated MDM = 1) is illustrated in the lower half of Figure 5B; the effect of 10 µg·mL−1 MPs was almost double that of PMA (Figure 5B). In human MDM, rosiglitazone potently reduced NF-κB activation evoked by MPs, while it was less effective against PMA-induced activation (Figure 5B).
In order to confirm that MPs, rather than soluble molecules present within supernatant, were responsible for NF-κB nuclear translocation, we performed some experiments with ultra-centrifuged (100 000×g) materials, as well as direct stimulation with the calcium ionophore. As shown in Figure 6, in human monocytes, NF-κB translocation induced by MPs (10 or 30 µg protein mL−1) is significantly higher than that evoked by A23187 (used at 1.2 and 4 µM, corresponding to 100 and 300 µL aliquots of supernatant). As expected, supernatants from both un-stimulated monocytes and ultra-centrifuged materials were devoid of activity. The pellet from the ultra-centrifuged samples still induced some NF-κB translocation, but it was less effective (Figure 6), as already observed for superoxide anion production (see Figure 3B). To explain this apparent reduced activity, we suggest that a loss of bioactive material occurs during the experimental procedures or, alternatively, there is a higher ‘concentrated’ MPs content. By evaluating total protein concentration, we have observed that 300 µL of ultra-centrifuged pellet contain about 40 µg protein (instead of 30 µg protein, as usually observed in MPs). As the activity of MPs follows a bell-shaped dose-response curve, the 30 µg·mL−1 concentration displaying a lesser effect than the 10 µg·mL−1 concentration, it is conceivable that a more elevated concentration (as in the case of ultra-centrifuged pellet) could exert a lower activity.
Figure 6.
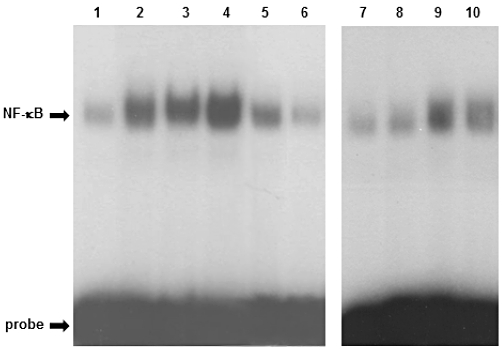
Monocyte-derived MPs, but not ultra-centrifuged supernatants, induce NF-κB activation in human monocytes. Lane 1: un-stimulated monocytes; lane 2: +PMA 10−6 M; lane 3: +MPs 30 µg·mL−1; lane 4: +MPs 10 µg·mL−1; lane 5: +ultra-centrifuged pellet (300 µL) from A 23187-stimulated monocytes; lane 6: +ultra-centrifuged supernatant (300 µL) from A23187-stimulated monocytes; lane 7: unstimulated monocytes; lane 8: +supernatant (300 µL) from un-stimulated monocytes; lane 9: +A23187 1.2 µM; lane 10: +A23187 4 µM. This blot is representative of another one.
Monocyte-derived MPs induce PPARγ protein expression in human monocytes and MDM
As shown in Table 2, human monocytes constitutively express low, although detectable, levels of PPARγ protein, that are up-regulated during differentiation to MDM, as previously reported (Amoruso et al., 2007, 2009a). The two PPARγ agonists enhance PPARγ protein expression about twofold in both cell types, which is in good accord with previous reports (Amoruso et al., 2007, 2009a). Interestingly, when evaluated at 10 µg protein mL-1, MPs induce PPARγ protein expression in both monocytes and MDM, slightly less than that after the selective ligands (Table 2). A representative Western blot is also provided (Figure 7).
Table 2.
PPARγ protein expression in human monocytes and macrophages
| Treatment | Monocytes (n = 5) | MDM (n = 5) |
|---|---|---|
| Control | 0.4 ± 0.03 | 1.5 ± 0.1°° |
| +15d-PGJ2 (10 µM) | 0.9 ± 0.05* | 3 ± 0.2* |
| +Rosi (1 µM) | 1.1 ± 0.1* | 3.5 ± 0.2* |
| +MPs (10 µg·mL−1) | 0.8 ± 0.1* | 2.9 ± 0.2* |
| +MPs (1 µg·mL−1) | 0.45 ± 0.1 | 1.7 ± 0.1 |
PPARγ protein expression was semi-quantified by measuring the ratio between PPARγ expression and β-actin expression. Rosi: rosiglitazone. Data are expressed as mean ± SEM of five experiments.
P < 0.05 versus Control (unstimulated cells of the same type)
P < 0.01 versus control monocytes.
Figure 7.
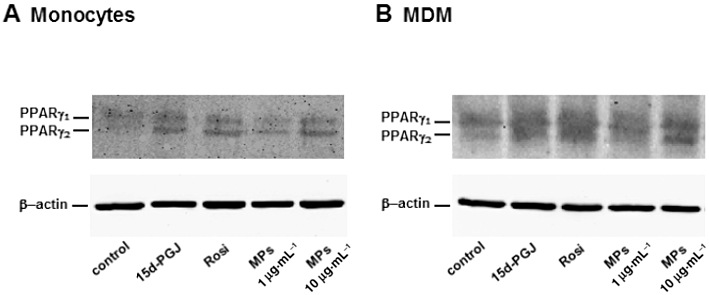
Monocyte-derived MPs induce PPARγ expression in human monocytes (A) and MDM (B). Cells were incubated with 15d-PGJ2 (15d-PGJ; 10 µM), rosiglitazone (Rosi, 1 µM) or MPs (1 or 10 µg·mL−1) for 6 h. This representative blot shows PPARγ (both one and two isoforms) and β-actin expression. The corresponding quantification is presented in Table 2.
Discussion and conclusions
The results presented in this paper show, for the first time, that MPs generated from A23187-stimulated human monocytes significantly affect human cells of the same lineage. We showed that monocyte-derived MPs induce oxygen radical production, cytokine release, NF-κB activation and PPARγ protein expression in both isolated human circulating monocytes and human macrophages (MDM).
Several studies have demonstrated that MPs from different cell types exert pro-inflammatory activities in monocyte/macrophages (Barry et al., 1998; Distler et al., 2005a; Baj-Krzyworzeka et al., 2007; Scanu et al., 2008; Carpintero et al., 2010), but our novel finding is that these effects are produced even by monocyte-derived MPs. These results suggest that MPs may not only mediate cell-to-cell communication but may also represent a key component for autocrine stimulation.
Berckmans et al., (2005) have demonstrated that synovial fluid MPs induced an enhanced release of CCL2, IL-8 and IL-6 in fibroblast-like synoviocytes. However, as stated by the authors (Berckmans et al., 2005), synovial fluid MPs are mainly derived from monocytes and neutrophils, and therefore may not represent an autocrine effect.
There are two well-known processes which lead to MPs formation: (i) chemical and physical cell activation (e.g. activated complement components, PMA, bacterial LPS, calcium ionophores, inflammatory cytokines, shear stress) and (ii) apoptosis, induced by growth factor deprivation or pro-apoptotic agents. As far as human monocytes are concerned, Essayagh et al. (2007) produced MPs from apoptotic monocytes, challenged with the Fas ligand (0.2 µg·mL−1 for 6 h), whereas Gauley and Pisetsky (2010) used LPS (0.05–50 µg·mL−1) for 24 h to induce MPs formation in RAW 264.7 cells, and stated that, under these conditions, MPs production resulted either from cells undergoing apoptosis or from activated cells. Moreover, Aharon et al. (2008) used starvation (20 h) or challenge with LPS (1 µg·mL−1) for 5 h + A23187 (10 µM) for 15 min to induce MPs generation in THP-1 cells. We prepared MPs as previously described (Neri et al., 2011), by treating monocytes with A23187 (12 µM). We are aware that calcium ionophores are not physiological activators, but A23187 induces a rapid and complete MPs release, and is regularly used experimentally. For example, Satta et al. (1994), although demonstrating MPs formation from human monocytes challenged with LPS (5 µg·mL−1 for 5 h), used A23187 (3 µM) to characterize monocyte and shed MP populations. These authors also reported that, using LPS, the degree of MPs formation ‘was strongly donor-dependent’. In addition, Cerri et al. (2006) demonstrated that A23187 (1–24 µM) evokes a concentration-dependent MPs release from human monocytes, 12 µM representing the EC50 concentration. According to these authors, histamine, at 30 µM, induces MPs formation from human monocytes, but it requires a longer stimulation time (1–2 h) instead of 10 min (Cerri et al., 2006).
Characterization of MPs is neither a standardized nor unequivocal procedure: we have addressed this problem by evaluating the percentage of CD14+ elements, TF activity, PS equivalent concentration, as well as protein concentration in the samples. In our MPs preparations, 100 µL of supernatants from A23187-challenged monocytes corresponds to 10 µg protein (that, in our experiments, usually ensures maximal effects) and was 2 nM in PS equivalents, in good agreement with other reports (Scanu et al., 2008; Tesse et al., 2008; Carpintero et al., 2010). In fact, Scanu et al. (2008) and Carpintero et al. (2010) report maximal effects with 30 and 6 µg·mL−1 protein concentrations for T cell-derived MPs respectively, whilst Tesse et al. (2008), in experiments on vascular reactivity, use 30 nM PS equivalents of T cell-derived MPs.
We can also speculate that monocyte-derived MPs directly interact with monocyte/macrophages, leading to further cell activation, which may represent an amplification loop that perpetuates inflammation. Such a direct interaction is plausible since our MPs preparations are PS-positive and macrophages express PS receptors (Fadok et al., 2000).
Another interesting finding of our study is that monocyte-derived MPs exert concentration-dependent effects, with a somewhat varied sensitivity according to the cell type and/or the type of test. As previously documented (Essayagh et al., 2007), MPs generated from apoptotic monocytes induce the production of reactive oxygen species (ROS), mainly superoxide anion, by human endothelial cells, as well as transient platelet recruitment and TF expression. Our results substantiate and further extend this observation, since we demonstrate that in human monocytes and MDM (which are major phagocytes and present a functionally active NADPH oxidase), monocyte-derived MPs induce a concentration-dependent O2− production, that peaks at 10–30 µg·mL−1 protein concentration.
Furthermore, in keeping with previous studies in other cell types (Mesri and Altieri, 1999; Tesse et al., 2008; Neri et al., 2011), this study provides evidence that monocyte-derived MPs induced, in a concentration-dependent manner, TNF-α and IL-6 release from human monocyte/macrophages, and also demonstrated that rosiglitazone and 15d-PGJ2 reduced these effects. Amoruso et al. (2008, 2009b, 2010) have shown that, at maximally effective concentrations, 15d-PGJ2 often exerts a significantly higher inhibition than rosiglitazone, which is independent of the stimulus (except in the case of TNF-α release in human monocytes). Tesse et al. (2008) previously documented that rosiglitazone, used at 5 µM, prevented the increase of IL-6, IL-1β and IL-8 mRNA expression induced by T cell-derived MPs in human endothelial cells. In human lung epithelial cells, Neri et al. (2011) demonstrated that monocyte-derived MPs increased IL-8 and CCL2 synthesis, which was inhibited by rosiglitazone and 15d-PGJ2. This inhibitory effect is likely to be mediated via interaction with PPARγ as rosiglitazone and 15d-PGJ2 are PPARγ agonists. However, since both rosiglitazone and 15d-PGJ2 have the potential to exert biological effects independent of PPARγ activation, we used the specific PPARγ inhibitor GW9662 to evaluate the role of this receptor in the modulation of MPs-induced cytokine release. As the antagonist completely reversed the effects of the two PPARγ agonists, we confirmed the direct involvement of this receptor. Moreover, another PPARγ agonist, pioglitazone, has been shown to reduce the circulating levels of endothelial MPs in the metabolic syndrome (Esposito et al., 2006), with an effect independent of its ability to ameliorate insulin sensitivity.
Some of the anti-inflammatory properties of PPARγ are attributed, at least partially, to its trans-repression ability. PPARγ can physically interact with the p65 subunit of NF-κB, so preventing its nuclear translocation (Chen et al., 2003; Tesse et al., 2008), or it can be sumoylated and indirectly inhibit NF-κB binding (Pascual et al., 2005). In our hands, monocyte-derived MPs induced NF-κB nuclear translocation, which was significantly reduced by PPARγ agonists, in good agreement with previous reports (Tesse et al., 2008; Neri et al., 2011). Moreover, MPs-induced NF-κB nuclear migration was similar to that evoked by PMA and significantly higher than that induced by direct A23187 stimulation.
Another novel finding of our research is that monocyte-derived MPs stimulated (about twofold) PPARγ protein expression in human monocytes and MDM, with maximal effects that were slightly lower than those induced by the selective ligands rosiglitazone and 15d-PGJ2. Therefore, these results support and further extend previous data describing the anti-inflammatory properties of MPs (Gasser and Schifferli, 2004; Koeppler et al., 2006; Dalli et al., 2008). For example, the transfer of Kato cell-derived MPs to human monocytes results in a decreased release of the pro-inflammatory cytokines GM-CSF and TNF-α and an enhanced release of the anti-inflammatory IL-10 (Koeppler et al., 2006). The release of PPARγ, in association with platelet MPs, is another intriguing finding in the literature (Ray et al., 2008). Platelet MPs can be internalized by the monocytic THP-1 cells and, in their presence, rosiglitazone significantly attenuates THP-1 activation, suggesting a novel transcellular mechanism of regulation (Ray et al., 2008).
We now demonstrate that monocyte-derived MPs not only induce pro-inflammatory effects (ROS production, cytokine release, NF-κB activation) in human monocyte/macrophages, but also enhance PPARγ protein expression in the same cells, so providing a possible counter-regulatory, anti-inflammatory mechanism. PPARγ is highly expressed in human MDM and alveolar macrophages (from healthy individuals or animal models), whereas the amount of PPARγ protein in monocytes is markedly lower (Asada et al., 2004; Reddy et al., 2004; Amoruso et al., 2007, 2008).
In conclusion, these results demonstrate that monocyte-derived MPs have relevant pro-inflammatory effects, that are reduced by PPARγ agonists, in human monocytes and MDM. This observation suggests that monocyte-derived MPs are relevant to autocrine stimulation in human cells. Moreover, our results also indicate a novel activity, enhancement of PPARγ protein expression, for monocyte-derived MPs, that might support a possible anti-inflammatory loop.
Since MPs represent a disseminated storage pool of bioactive effectors, their properties need to be carefully evaluated, according to the nature of MPs and particular recipient cells, given the possibility of dual effects of MPs.
Acknowledgments
This research was supported by PRIN grant no. 2007 4S9KXF_001. We also wish to thank Dr Simone Merlin for help with FACS experiments.
Glossary
- GW9662
2-chloro-5-nitro-N-phenylbenzamide
- ICAM
intercellular adhesion molecule
- MDM
monocyte-derived macrophages
- MPs
microparticles
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- PMA
phorbol 12-myristate 13-acetate
- PS
phosphatidylserine
- PSGL
P-selectin glycoprotein ligand-1
- ROS
reactive oxygen species
- TF
tissue factor
Conflict of interest
None.
References
- Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100:878–885. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- Amoruso A, Bardelli C, Gunella G, Fresu LG, Ferrero V, Brunelleschi S. Quantification of PPAR-γ protein in monocyte/macrophages from healthy smokers and non-smokers: a possible direct effect of nicotine. Life Sci. 2007;81:906–915. doi: 10.1016/j.lfs.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Amoruso A, Bardelli C, Gunella G, Ribichini F, Brunelleschi S. A novel activity for substance P: stimulation of peroxisome proliferator-activated receptor-γ protein expression in human monocytes and macrophages. Br J Pharmacol. 2008;54:144–152. doi: 10.1038/bjp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoruso A, Bardelli C, Fresu LG, Palma A, Vidali M, Ferrero V, et al. Enhanced Peroxisome Proliferator-Activated Receptor-γ expression in monocyte/macrophages from coronary artery disease patients and possible gender differences. J Pharmacol Exp Ther. 2009a;331:531–539. doi: 10.1124/jpet.109.154419. [DOI] [PubMed] [Google Scholar]
- Amoruso A, Gunella G, Rondano E, Bardelli C, Fresu LG, Ferrero V, et al. Tobacco smoke affects expression of peroxisome proliferator-activated receptor-gamma in monocyte/ macrophages of patients with coronary heart disease. Br J Pharmacol. 2009b;58:1272–1284. doi: 10.1111/j.1476-5381.2009.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoruso A, Bardelli C, Fresu LG, Poletti E, Palma A, Federici Canova D, et al. The nitric oxide donating pravastatin, NCX 6550, inhibits cytokine release and NF-κB activation while enhancing PPARγ expression in human monocyte/macrophages. Pharmacol Res. 2010;62:391–399. doi: 10.1016/j.phrs.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90:1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- Asada K, Sasaki S, Suda T, Chida K, Nakamura H. Antiinflammatory roles of Peroxisome Proliferator-activated Receptor γ in human alveolar macrophages. Am J Respir Crit Care Med. 2004;169:195–200. doi: 10.1164/rccm.200207-740OC. [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Zmbala M. Tumour-derived microvesicles modulate activity of human monocytes. Immun Lett. 2007;113:76–82. doi: 10.1016/j.imlet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Bardelli C, Gunella G, Varsaldi F, Balbo P, Del Boca E, Bernardone IS, et al. Expression of functional NK1 receptors in human alveolar macrophages: superoxide anion production, cytokine release and involvement of NF-κB pathway. Br J Pharmacol. 2005;145:385–396. doi: 10.1038/sj.bjp.0706198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;99:2118–2127. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berckmans RJ, Nieuwland R, Kraan MC, Schaap MCL, Pots D, Smeets TJM, et al. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res Ther. 2005;7:R536–R544. doi: 10.1186/ar1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–29. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- Carpintero R, Gruaz L, Brandt KJ, Scanu A, Faille D, Combes V, et al. HDL interfere with the binding of T cell microparticles to human monocytes to inhibit pro-inflammatory cytokine production. PLoS ONE. 2010;5:e11869. doi: 10.1371/journal.pone.0011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, et al. P-selectin induces the expression of tissue factor in monocytes. Proc Natl Acad Sci USA. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri C, Chimenti D, Conti I, Neri T, Paggiaro P, Celi A. Monocyte/macrophage-derived microparticles up-regulate inflammatory mediator synthesis by human airway epithelial cells. J Immun. 2006;177:1975–1980. doi: 10.4049/jimmunol.177.3.1975. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang M, O'Connor JP, He M, Tripathi T, Harrison LE. Phosphorylation of PPARγ via active ERK1/2 leads to its physical association with p65 and inhibition of NF-κB. J Cell Biochem. 2003;90:732–744. doi: 10.1002/jcb.10668. [DOI] [PubMed] [Google Scholar]
- Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- Distler JHW, Huber LC, Huber AJ, Reich CF, III, Gay S, Distler O, et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005a;10:731–741. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- Distler JHW, Pisetsky DS, Huber LC, Kalden JR, Gay S, Distler O. Microparticles as regulators of inflammation. Novel players in cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005b;11:3337–3348. doi: 10.1002/art.21350. [DOI] [PubMed] [Google Scholar]
- Esposito K, Ciotola M, Giugliano D. Pioglitazone reduces endothelial microparticles in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1926–1927. doi: 10.1161/01.ATV.0000231512.15115.25. [DOI] [PubMed] [Google Scholar]
- Essayagh S, Xuereb JM, Terrisse AD, Tellier-Cirioni L, Pipy B, Sié P. Microparticles from apoptotic monocytes induce transient platelet recruitment and tissue factor expression by cultured human vascular endothelial cells via a redox-sensitive mechanism. Thromb Haemost. 2007;98:831–837. [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Gasser O, Schifferli JA. Activated polimorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- Gauley J, Pisetsky DS. The release of microparticles by RAW 264.7 macrophage cells stimulated with TLR ligands. J Leukoc Biol. 2010;87:1115–1123. doi: 10.1189/jlb.0709465. [DOI] [PubMed] [Google Scholar]
- Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology. 2004;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Koeppler B, Cohen C, Scloendorff D, Mack M. Differential mechanisms of microparticle transfer to B cells and monocytes: anti-inflammatory properties of microparticles. Eur J Immunol. 2006;36:648–660. doi: 10.1002/eji.200535435. [DOI] [PubMed] [Google Scholar]
- Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocyte/macrophages. Arterioscler Thromb Vasc Biol. 2010;30:1818–1824. doi: 10.1161/ATVBAHA.110.209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- Mayr M, Grainger D, Mayr U, Leroyer AS, Leseche G, Sidibe A, et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–388. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signalling pathway. J Biol Chem. 1999;274:23111–23118. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- Neri T, Armani C, Pegoli A, Cordazzo C, Camazzi Y, Brunelleschi S, et al. Role of NF-κB and PPAR-γ in lung inflammation induced by monocyte-derived microparticles. Eur Respir J. 2011;37:1494–1502. doi: 10.1183/09031936.00023310. [DOI] [PubMed] [Google Scholar]
- Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RG, et al. Cellular origin of procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AE, et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1 dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–343. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- Ray DM, Spinelli SL, Pollock SJ, Murant TI, O'Brien JJ, Blumberg N, et al. Peroxisome proliferator-activated receptor γ and retinoid X receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. doi: 10.1160/TH07-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RC, Keshamouni VG, Jaigirdar SH, Zeng X, Leff T, Thannickal VJ, et al. Deactivation of murine alveolar macrophages by peroxisome proliferator-activated receptor-γ ligands. Am J Physiol Lung Cell Mol Physiol. 2004;286:L613–L619. doi: 10.1152/ajplung.00206.2003. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- Scanu A, Molnarfi N, Brandt KJ, Gruaz L, Dayer JM, Burger D. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J Leukoc Biol. 2008;83:921–927. doi: 10.1189/jlb.0807551. [DOI] [PubMed] [Google Scholar]
- Tesse A, Al-Massarani G, Wangensteen R, Reitenbach S, Martinez MC, Andriantsitohaina R. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, prevents microparticle-induced vascular hyporeactivity through the regulation of proinflammatory proteins. J Pharmacol Exp Ther. 2008;324:539–547. doi: 10.1124/jpet.107.130278. [DOI] [PubMed] [Google Scholar]


