Abstract
BACKGROUND AND PURPOSE
Dersalazine sodium (DS) is a new chemical entity formed by combining, through an azo bond, a potent platelet activating factor (PAF) antagonist (UR-12715) with 5-aminosalicylic acid (5-ASA). DS has been demonstrated to have anti-inflammatory effects on trinitrobenzene sulphonic acid (TNBS)-induced colitis in rats and recently in UC patients in phase II PoC. There is Increasing evidence that Th17 cells have an important role in the pathogenesis of inflammatory bowel disease (IBD). The aim of this study was to further characterize the anti-inflammatory effects of DS.
EXPERIMENTAL APPROACH
Effect of DS (10 or 30 mg·kg−1 b.i.d.) on TNBS-induced colitis in rats was studied after 2 and 7 days with special focus on inflammatory mediators. Additionally, its anti-inflammatory properties were analysed in two different models of dextran sodium sulphate (DSS)-induced colitis, BALB/c and C57BL/6 mice, the latter being dependent on IL-17.
KEY RESULTS
DS, when administered for 7 days, showed intestinal anti-inflammatory effects in TNBS-induced colitis; these effects were observed both macroscopically and through the profile of inflammatory mediators (TNF, IL-1β, IL-6 and IL-17). Although the 2 day treatment with DS did not induce intestinal anti-inflammatory effects, it was sufficient to reduce the enhanced IL-17 expression. DS showed beneficial effects on DSS-induced colitis in C57BL/6 mice and reduced colonic pro-inflammatory cytokines IL-1β, IL-6 and IL-17. In contrast, it did not exert intestinal anti-inflammatory effects on DSS-induced colitis in BALB/c mice.
CONCLUSIONS AND IMPLICATIONS
DS exerts intestinal anti-inflammatory activity in different rodent models of colitis through down-regulation of IL-17 expression.
Keywords: dersalazine sodium, experimental colitis, IL-17, PAF antagonist, 5-ASA
Introduction
Ulcerative colitis (UC) is a chronic relapsing inflammatory disease of the large bowel, characterized by chronic diarrhoea, abdominal pain and bleeding due to ulceration of the inner lining of the colon and/or rectum. Acute flares severely impair patient's ability to lead a normal life and may be life-threatening. Treatment goals include remission of symptoms during the acute flare (acute treatment) and control of chronic inflammation to avoid or delay the occurrence of new flares (maintenance treatment). Aminosalicylates are considered to be the first-line therapy for the management of active mild to moderate UC and for maintaining it in remission (Sutherland and MacDonald, 2006a,b; Nielsen and Munck, 2007). Sulphasalazine (SAZ), composed of the sulphapyridine moiety linked to 5-aminosalicylic acid (5-ASA) by an azo bond, was the first drug of this group used in the treatment of human inflammatory bowel diseases (IBDs). The mechanisms of action of this group of drugs have not been fully elucidated, and several mechanisms have been postulated, including antioxidant and/or radical scavenging properties (Nielsen et al., 1998), inhibition of leucocyte chemotaxis (Stenson and Lobos, 1982) and a decreased production of IL-1β, TNF-α, PGs and LTs (Mahida et al., 1991; Kaiser et al., 1999). The high frequency of side effects associated with SAZ, together with the identification of 5-ASA as the active therapeutic moiety, promoted the development of second-generation 5-ASA drugs, like olsalazine, balsalazide and several 5-ASA release systems. It has been reported that these new aminosalicylates show similar efficacy to SAZ with fewer side effects (Loftus et al., 2004), although renal function is mainly affected when high doses are used (Patel et al., 2009).
For resistant or more severe cases of the disease, aminosalicylates presents limited efficacy and then corticosteroids, immunosuppressants or anti-TNF-α agents are used instead; however, adverse effects, an inconvenient dosing schedule and prohibitive price limit their long-term use (Siegel, 2011). For this reason, the development of new therapies that combine efficacy, convenient dosing and lower side effects is an important goal in human IBD therapy.
Dersalazine sodium (DS) is a new chemical entity that constitutes a clear efficacy and safety improvement with respect to 5-ASA releasing systems and provides a new therapeutic alternative to corticosteroids, immunosuppressant and anti-TNF-α treatments. DS combines, through an azo bond, two active molecules for the treatment of UC: 5-ASA and UR-12715, the latter being a compound that displays potent platelet activating factor (PAF) antagonist activity (Gálvez et al., 2000). Thus, and importantly, UR-12715 is an active part of DS when it is released in the colon, rather than acting as an inert carrier of 5-ASA. DS has been found to have a higher efficacy in comparison with the 5-ASA derivative mesalazine in a phase 2a trial as well as a fast onset of action evident after 2 weeks (unpublished observations).
The intestinal anti-inflammatory activity of DS in the trinitrobenzene sulphonic acid (TNBS) experimental model of rat colitis has been previously demonstrated; it was shown to improve the colonic damage induced by the intracolonic administration of the hapten TNBS, as well as to ameliorate the relapse induced by a second administration of this noxious agent, once the initial injury is in process of recovery (Gálvez et al., 2000; 2003). This anti-inflammatory effect was related to the additive effects exerted locally by the two compounds that constitute DS, once they are released in the intestinal lumen after reduction of the azo bond by colonic bacteria. In fact, an inhibitory effect on the production of pro-inflammatory cytokines, like IL-1β and TNF-α, in the inflamed colonic tissue was associated with the beneficial effect of DS exerted in this model of experimental colitis (Gálvez et al., 2003).
Traditionally, Crohn's disease (CD) and UC have been associated with an exaggerated Th1 or Th2 cell response, respectively. However, recent studies have shown that a new subset of T helper cells, Th17 cells, is involved in the development of this inflammatory response, and several cytokines, including IL-21 and IL-23. are involved in regulating their activation and differentiation (Liu et al., 2009; Sarra et al., 2010). The exact mechanism by which Th17 cells contribute to the gut pathology is not completely known; however, there is much evidence indicating that Th17 cytokines can promote the synthesis of other inflammatory cytokines, chemokines and tissue-degrading MMPs by several cell types (Ye et al., 2001; Yen et al., 2006). The effects of DS on the Th17 cytokine network have not been previously reported in either human or animal studies. Hence, in the present study, the intestinal anti-inflammatory properties of DS were further characterized by analysing its effects on the expression of different inflammatory mediators, with special focus on Th17-related cytokines, including IL-17, IL-12A and IL-23, in two different models of experimental colitis: the TNBS model in rats and the dextran sodium sulphate (DSS) model in mice. The results revealed that DS exerts intestinal anti-inflammatory activity in these rodent models of colitis through down-regulation of the expression of these cytokines.
Methods
Animals
All animal care and procedures in this study were carried out in accordance with the Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes of the European Union (86/609/EEC). Female Wistar rats (200–210 g), female BALB/c (20–22 g) or female C57BL/6 mice (20–22 g), obtained from Harlan (Barcelona, Spain), were housed in makrolon cages, maintained in air-conditioned animal quarters with a 12 h light–dark cycle and fed standard rodent chow (Panlab A04, Panlab, Barcelona, Spain) and water ad libitum throughout the experiment.
Evaluation of the intestinal anti-inflammatory effect of DS in the TNBS model of rat colitis
Rats were randomly assigned to five groups (n = 10). Three of them received pharmacological treatment with either DS (10 and 30 mg·kg−1) or SAZ (30 mg·kg−1), suspended in 1 mL of carboxymethylcellulose (0.2%, w/v) and Tween 80 (1%, v/v) in distilled water and orally administered by means of an oesophageal catheter every 12 h. The other two groups of rats, non-colitic and colitic control groups, received orally 1 mL of the vehicle used to administer the test compounds. Colonic inflammation was induced in control and treated groups as previously described (Arribas et al., 2009). Briefly, they were anaesthetized with halothane and given 10 mg of TNBS dissolved in 0.25 mL of 50% ethanol (v/v) by means of a Teflon cannula inserted 8 cm through the anus. During and after TNBS administration, the rats were kept in a head-down position until they recovered from the anaesthetic and were then returned to their cages. Rats from the non-colitic group were administered intracolonically 0.25 mL of PBS instead of TNBS. DS or SAZ was administered twice daily from the day of the colitis induction until the day before the rats were killed, which took place 2 or 7 days after the induction of colonic damage. Rats were killed with an overdose of halothane.
In another set of experiments, rats were administered intracolonically either UR-12715 (22.5 mg·kg−1), 5-ASA (7.5 mg·kg−1) or UR-12715 plus 5-ASA (22.5 + 7.5 mg·kg−1), suspended in 1 mL of carboxymethylcellulose (0.2%, w/v) and Tween 80 (1%, v/v) in distilled water. This administration was started the same day as colitis induction and was maintained for the following 7 days, when rats were killed. The doses of UR-12715 and 5-ASA were equivalent to those that, theoretically, could be achieved in the rat colon after complete cleaving of the highest dose of DS when administered orally and adjusted for the molecular weights of the different compounds.
Animal body weights, occurrence of diarrhoea and water and food intake were recorded daily throughout all the experiments. Once the animals were killed, the colon was removed aseptically and placed on an ice-cold plate and opened longitudinally. Afterwards, the colonic segment was cleaned of fat and mesentery, blotted on filter paper, and each specimen was weighed, and its length was measured under a constant load (2 g). Each colon was scored for macroscopically visible damage on a 0–10 scale by two observers unaware of the treatment, according to criteria reported previously (Camuesco et al., 2005), which takes into account the extent and severity of colonic damage. The colon samples were subsequently sectioned in different longitudinal fragments to be used for biochemical determinations or for RNA isolation.
Evaluation of the intestinal anti-inflammatory effect of DS in the DSS model of mouse colitis
Female C57BL/6 mice (7–9 weeks old; approximately 20 g) or female BALB/c mice (7–9 weeks old; approximately 20 g), obtained from Harlan, were randomly assigned to different experimental groups of 10 animals each: treated groups received DS (10 or 30 mg·kg−1day−1, p.o., every 12 h) suspended in 200 µL of carboxymethylcellulose (0.2%, w/v) and Tween 80 (1%, v/v) in distilled water. Also, non-colitic and DSS control groups were included, which received daily, p.o., 200 µL of the vehicle used to administer the test compounds. The colitis was induced in the animals from the control and treated groups by adding DSS (36–50 kDa, MP Biomedicals, Ontario, CA) in the drinking water at different concentrations depending on the mouse strain (Melgar et al., 2005). In C57BL/6 mice, DSS concentration was 3% (w/v) for a period of 5 days, after which DSS was removed, and only tap water was provided for the following week. In BALB/c mice, DSS concentration was 5% (w/v) for the first 5 days, and it was decreased to 1% (w/v) for the following 7 days. Mice from the non-colitic group were administered tap water throughout the experient. DS was administered when colitis was well-established, from the day of the DSS removal (C57BL/6 mice) or when the concentration of DSS was reduced (BALB/c mice) and continued until mice were killed, that is 12 days after the beginning of the experiment.
Animal body weight, the presence of gross blood in the faeces and stool consistency were evaluated daily for each mouse by an observer unaware of the treatment. These parameters were each assigned a score according to the criteria proposed previously and used to calculate a daily average disease activity index (DAI) (Camuesco et al., 2004). Once the animals were killed, the colon was removed aseptically and processed as previously described. Representative whole gut specimens (0.5 cm length) were taken from the distal inflamed region and were fixed in 4% buffered formaldehyde for the histological studies. Equivalent colonic segments were also obtained from the non-colitic group. The remaining colonic tissue was subsequently sectioned in different longitudinal fragments to be used for biochemical determinations or for RNA isolation.
Histological studies
Cross-sections were selected and embedded in paraffin. Full-thickness sections of 5 µm were obtained at different levels and stained with haematoxylin and eosin. The histological damage was evaluated by a pathologist observer who was blinded to the experimental groups, according to the criteria described in Table 1.
Table 1.
Microscopic scoring criteria of full-thickness distal colon sections
| Mucosal epithelium and lamina propia |
| Ulceration: none (0); mild surface (0–25%) (1); moderate (25–50%) (2); severe (50–75%) (3); extensive-full thickness (more 75%) (4). |
| Polymorphonuclear cell infiltrate |
| Mononuclear cell infiltrate and fibrosis |
| Oedema and dilatation of lacteals |
| Crypts |
| Mitotic activity: lower third (0); mild mid third (1); moderate mid third (2); upper third (3) |
| Dilatation |
| Goblet cell depletion |
| Submucosa |
| Polymorphonuclear cell infiltrate |
| Mononuclear cell infiltrate |
| Oedema |
| Vascularity |
| Muscular layer |
| Polymorphonuclear cell infiltrate |
| Mononuclear cell infiltrate |
| Oedema |
| Infiltration in the serosa |
Scoring scale: 0, none; 1 slight; 2, mild; 3, moderate; 4, severe. Maximum score: 59.
Biochemical determinations in colonic tissue
Myeloperoxidase (MPO) activity was measured according to the technique described by Krawisz et al. (1984); the results are expressed as MPO U g–1 wet tissue; one unit (U) of MPO activity was defined as that degrading 1 µmol hydrogen peroxide min–1 at 25°C. Colonic samples for cytokine (TNF-α, IL-1β, IL-6 and IL-17) determinations and iNOS protein expression were immediately weighed, minced on an ice-cold plate and suspended (1:5 w/v) in a lysis buffer containing 20 mM HEPES (pH 7.5), 10 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid, 40 mM β-glycerophosphate, 2.5 mM magnesium chloride, 1% Igepal®, 1 mM dithiothreitol, 500 µM phenylmethanesulphonyl fluoride, 1 µg·mL−1 aprotinin, 1 µg·mL−1 leupeptin, 1 µg·mL−1 iodoacetamide and 2 mM sodium orthovanadate. The tubes were placed in an orbital rotor (4°C) for 20 min and centrifuged at 9000×g for 10 min at 4°C; the supernatants were frozen at −80°C until assay. The cytokines were quantified by elisa (R&D Systems Europe, Abingdom, UK), and the results are expressed as pg g–1 wet tissue. The iNOS Western blot from colonic tissue was performed as described elsewhere (Comalada et al., 2005). Equal amounts of protein from tissue samples (150 µg) were separated on 7.5% SDS-PAGE. iNOS antibody (Transduction Laboratories, BD Biosciences, Madrid, Spain) was used at a dilution of 1/3000. A primary antibody against β-actin was used as loading control. Peroxidase-conjugated anti-rabbit IgG were used as secondary antibodies.
Analysis of gene expression in colonic samples by RT-PCR
Total RNA from colonic samples was extracted using TRIzol® Reagent (Invitrogen Life Technologies, Madrid, Spain), following the manufacturer's instructions, and was reverse transcribed using oligo (dT) primers (Promega, Southampton, UK). Semiquantitative PCR was performed using specific primers: β-actin (forward: 5′-AATCGTGCGTGACATCAAAG-3′, 5′-ATGCCACAGGATTCCATACC-3′); IL-17 (forward: 5′-TGGACTCTGAGCCGCATTGA-3′, reverse: 5′-GACGCATGGCGGACAATAGA-3′); IL-6 (5′-CTTCCCTACTTCACAAGTC-3′, reverse: 5′-CTCCATTAGGAGAGCATTG-3′); IL12A (forward: 5′-ACGCTACCTCCTCTTCTTG-3′, reverse: 5′-ATGTCGTCCGTGGTCTTC-3′); IL-23 (forward: 5′-GCACACTAGCCTGGAGTGCA-3′, reverse: 5′-TGTCCGAGTCCAGTAGGTGCT-3′). PCR reaction was performed using Go Taq® DNA Polymerase (Promega) in accordance with the manufacturer's recommendations. The PCR mixtures were denatured at 95°C for 3 min, followed by 22 to 35 cycles of denaturation at 94°C for 1 min, annealing at 55–60°C for 45 s and extension at 72°C for 1 min, and a final extension at 72°C for 10 min. PCR products were analysed by electrophoresis in a 1% agarose gel containing ethidium bromide.
Statistics
All results are expressed as the mean ± SEM. Differences between means were tested for statistical significance using a one-way anova and post hoc least significance tests. Differences between proportions were analysed with the chi-squared test. All statistical analyses were carried out with the Statgraphics 5.0 software package (STSC, Bethesda, MD, USA), with statistical significance set at P < 0.05.
Materials
DS ((Z)-2-hydroxy-5-((4-(3-(4-((2-methyl-1H-imidazo(4,5-c)pyridin-1-yl)methyl)-1-piperidinyl)-3-oxo-1-phenyl-1-propenyl)phenyl)azo] benzoic acid sodium salt) was supplied by Palau Pharma S.A. (Barcelona, Spain). All other reagents, unless otherwise stated, were obtained from Sigma (St. Louis, MO).
Results
Effects of DS in TNBS rat colitis after 2 or 7 days of treatment
The administration of DS for 7 days after TNBS instillation, at doses of 10 and 30 mg·kg−1 b.i.d., significantly ameliorated the colonic damage when compared with the colitic control group. This beneficial effect was evidenced macroscopically by a significant reduction, of approximately 2 cm, in the extension of inflamed/necrotic tissue of the colonic segments, thus being assigned a lower macroscopic score than untreated control rats (Table 2). In addition, this anti-inflammatory effect was associated with a significant reduction in the colonic weight/length ratio in comparison with untreated colitic control rats, an index of colonic oedema that is increased significantly as a consequence of the inflammatory process; however, the administration of SAZ, at the dose of 30 mg·kg−1 did not facilitate the macroscopic recovery of the inflamed colonic segment in the same conditions (Table 2). The improvement of the inflammatory process in the presence of DS was also observed biochemically; colonic MPO activity, a marker of neutrophil infiltration that was enhanced in the TNBS control group, was significantly reduced by DS, thus suggesting a lower leucocyte infiltration into the inflamed tissue. SAZ also significantly reduced this enzyme activity in the inflamed colonic tissue (Table 2).
Table 2.
Effects of DS (10 and 30 mg·kg−1) and SAZ (30 mg·kg−1) on colonic macroscopic damage score, weight/length ratio and MPO activity in TNBS experimental colitis in rats following a curative treatment protocol
| Group (n = 10) | Weight/length (mg·cm−1) | Damage score (0–10) | MPO activity (U g–1 wet tissue) |
|---|---|---|---|
| 2 days: | |||
| Non-colitic | 62.8 ± 1.6 | 0 | 32.1 ± 1.2 |
| TNBS control | 118.4 ± 5.1 | 7 (6–8) | 355.8 ± 39.7 |
| DS, 10 mg·kg−1 | 123.4 ± 1.6 | 6 (5–7) | 302.9 ± 19.9 |
| DS, 30 mg·kg−1 | 122.5 ± 5.4 | 6.5 (5–7) | 289.7 ± 11.9 |
| SAZ, 30 mg·kg−1 | 115.7 ± 4.9 | 7 (7–8) | 314.8 ± 23.7 |
| 7 days: | |||
| Non-colitic | 61.2 ± 3.0 | 0 | 24.4 ± 2.4 |
| TNBS control | 169.7 ± 16.0 | 7.5 (6–10) | 82.0 ± 5.4 |
| DS, 10 mg·kg−1 | 108.7 ± 10.6* | 6 (4–7)** | 62.2 ± 6.9* |
| DS, 30 mg·kg−1 | 94.6 ± 8.9** | 5 (4–8)** | 39.9 ± 6.3** |
| SAZ, 30 mg·kg−1 | 165.5 ± 9.5 | 8 (6–9) | 54.8 ± 3.7** |
Colon weight/length ratio and MPO activity data are expressed as mean ± SEM. Damage score for each rat was assigned according to the criteria reported previously (Camuesco et al., 2005), and data are expressed as median (range).
P < 0.05
P < 0.01 versus TNBS control group; all colitic groups statistically differ (P < 0.05) from non-colitic group (not shown).
The colonic inflammation induced by TNBS was characterized by increased levels of colonic TNF-α and IL-1β, determined by elisa, together with a higher colonic iNOS protein expression, determined by Western blotting, in comparison with non-colitic animals (Figure 1). In addition, the expressions of IL-17, IL-12A and IL-6, as determined by RT-PCR, were up-regulated in colitic rats in comparison with healthy rats, whereas IL-23 expression was slightly increased (Figure 2). Treatment of colitic rats with DS resulted in a significant reduction of colonic TNF-α (10 and 30 mg·kg−1) and IL-1β (30 mg·kg−1) (Figure 1). In addition, both doses of DS were able to decrease colonic iNOS protein levels (Figure 1) as well as the expression of IL-17, IL-12A, IL-23 and IL-6, when compared with TNBS control rats (Figure 2). SAZ also reduced the inflammatory markers of colonic damage, although to less extent than DS (Figures 1 and 2); however, it did not modify iNOS expression.
Figure 1.
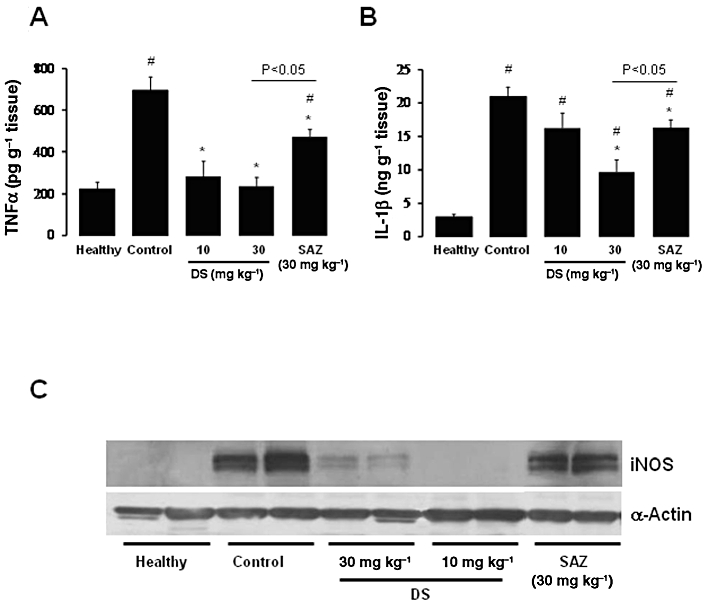
Effects of DS and SAZ treatment on day 7 colonic (A) TNF-α and (B) IL-1β production in TNBS colitis in rats, as quantified by elisa (means ± SEM; *P < 0.05 and **P < 0.01 vs. TNBS control group; # P < 0.01 vs. healthy group). (C) iNOS expression, determined by Western blot analysis.
Figure 2.
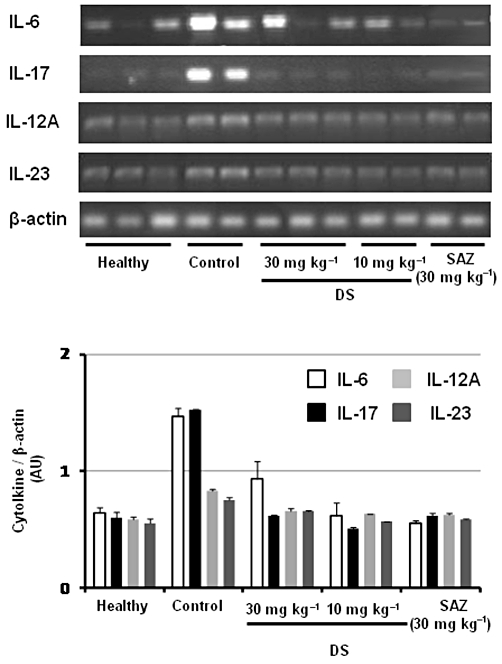
Effects of DS and SAZ treatment on day 7 colonic gene expression of the cytokines IL-6, IL-17, IL-12A and IL-23, analysed by RT-PCR. Representative examples of each treatment group are shown in the upper panel. The lower panel represents averaged data quantified by densitometry.
The intracolonic administration of UR-12715, at 22.5 mg·kg−1 for 7 days after induction of the colonic damage, resulted in an intestinal anti-inflammatory effect (Table 3). This was evidenced both macroscopically, by a reduction in the macroscopic score and in the weight/length ratio, as well as biochemically, since it was able to significantly reduce the colonic MPO activity in comparison with untreated control group. However, when 5-ASA (7.5 mg·kg−1) was administered intracolonically, no beneficial effect was observed macroscopically. The simultaneous administration of UR-12715 and 5-ASA (22.5 mg·kg−1 plus 7.5 mg·kg−1) did not result in any additional beneficial effect in comparison with the effect obtained after the intracolonic administration of the PAF antagonist alone. All the treatments decreased the expressions of IL-6, IL-17, IL-12A and IL-23 (analysed by RT-PCR) in the colonic tissue (Figure 3).
Table 3.
Effects of intracolonic administration of UR-12715 (22.5 mg·kg−1), 5-ASA (7.5 mg·kg−1) and UR-12715 plus 5-ASA (22.5 ± 7.5 mg·kg−1), on colonic macroscopic damage score, weight/length ratio and MPO activity in TNBS experimental colitis in rats following a curative treatment protocol for 7 days
| Group (n = 10) | Weight/length (mg·cm−1) | Damage score (0–10) | MPO activity (units g–1 wet tissue) |
|---|---|---|---|
| Non-colitic | 71.7 ± 5.1 | 0 | 22.3 ± 2.3 |
| TNBS control | 178.1 ± 16.7 | 8 (6–9) | 81.2 ± 6.3 |
| UR-12715, 22.5 mg·kg−1 | 120.8 ± 13.4* | 6 (5–7)* | 47.5 ± 7.3* |
| 5-ASA, 7.5 mg·kg−1 | 188.7 ± 21.6 | 7.5 (7–8) | 71.4 ± 11.0 |
| UR-12715 + 5-ASA 22.5 + 7.5 mg·kg−1 | 128.8 ± 3.9* | 6 (4–7)* | 52.0 ± 3.9* |
Colon weight/length ratio and MPO activity data are expressed as mean ± SEM. Damage score for each rat was assigned according to the criteria reported previously (Camuesco et al., 2005), and data are expressed as median (range).
P < 0.05
**P < 0.01 versus TNBS control group; all colitic groups statistically differ (P < 0.05) from non-colitic group (not shown).
Figure 3.
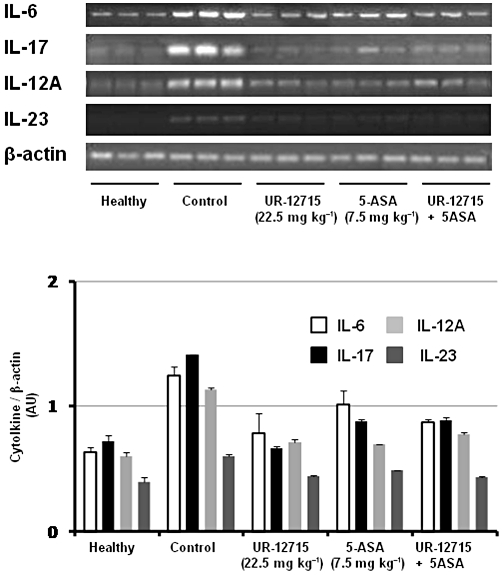
Effects of intracolonic administration of UR-12715 (22.5 mg·kg−1), 5-ASA (7.5 mg·kg−1) and UR-12715 plus 5-ASA (22.5 + 7.5 mg·kg−1) on day 7 colonic gene expression of the cytokines IL-6, IL-17, IL-12A and IL-23, analysed by RT-PCR. Representative examples of each treatment group are shown in the upper panel. The lower panel represents averaged data quantified by densitometry.
However, when the inflammatory response was evaluated macroscopically 2 days after the induction of the colonic damage with TNBS, no significant beneficial effects were observed in terms of colonic score or weight/length ratio with either DS or SAZ (Table 2). Biochemical evaluation of the colonic specimens from colitic rats revealed that none of the treatments significantly ameliorated colonic MPO activity (Table 2) or TNF-α and IL-1β levels or iNOS protein expression (Figure 4). The RT-PCR analysis of the colonic segment revealed that neither DS nor SAZ modified IL-6 expression; in contrast, DS, at both doses assayed, was able to reduce IL-17 expression after 2 days of treatment, whereas SAZ was devoid of any significant effect on the expression of this cytokine (Figure 4). Therefore, IL-17 appears to be a specific, early target gene for DS treatment in TNBS-induced colitis in rats, since its down-regulation precedes the intestinal anti-inflammatory activity.
Figure 4.
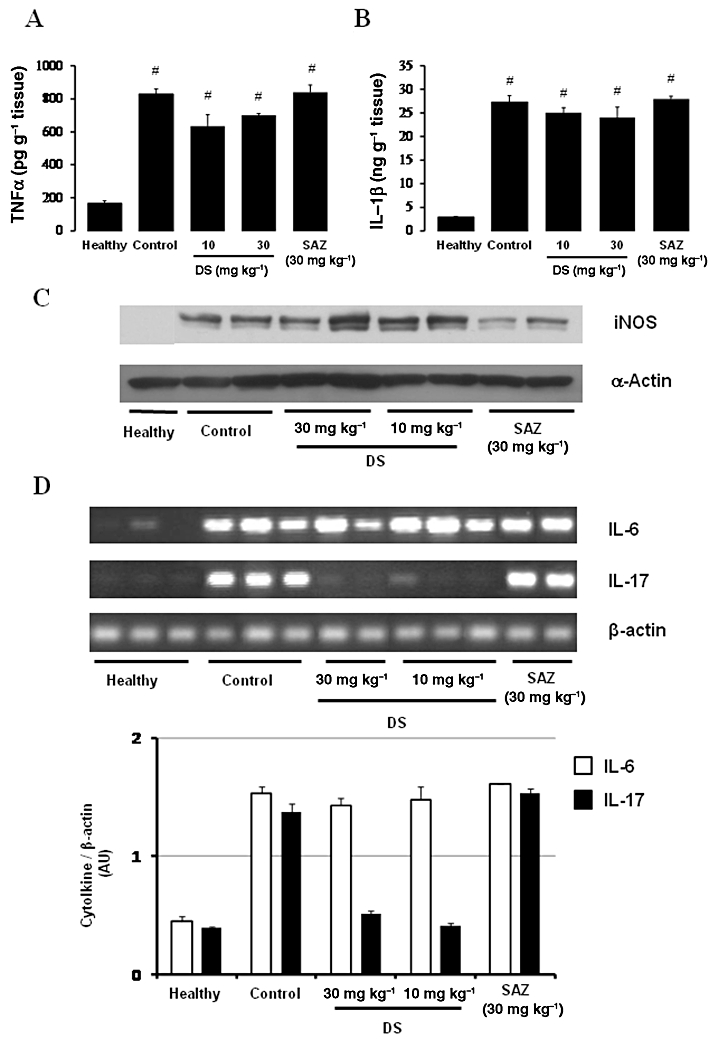
Effects of DS and SAZ treatment on day 2 colonic (A) TNF-α and (B) IL-1β production in TNBS colitis in rats, as quantified by elisa (means ± SEM; #P < 0.01 vs. healthy group). (C) iNOS expression, determined by Western blot analysis. (D) Effects of DS and SAZ on colonic gene expression of the cytokines IL-6 and IL-17, analysed by RT-PCR. Representative examples of each treatment group are shown in the upper panel; the lower panel represents averaged data quantified by densitometry.
Effects of DS in DSS mouse colitis
Once the anti-inflammatory effects of DS in the TNBS model of rat colitic rats were demonstrated, the effects of this compound in the chronic phase of the DSS model of colitis in two strains of mice were evaluated. This model produces inflammation limited to the colonic mucosa that is more closely resembles human UC (Okayasu et al., 1990), as opposed to the TNBS model of rat colitis, which induces a transmural lesion with pathological characteristics similar to CD (Fuss et al., 1996). In order to assure that the possible beneficial effect of the compound was not ascribed to a chemical interaction with DSS, it was administered once the colonic inflammatory process had been established.
The administration of 5% (w/v) DSS dissolved in the drinking water for 5 days to BALB/c mice resulted in a progressive loss in body weight and the excretion of diarrhoeic/blood faeces, thus obtaining a progressive increase in DAI values. When the concentration of DSS was reduced to 1% after this period, constant DAI values were observed with time until the end of the experiment in control colitic mice (Figure 5). The administration of DS (10 or 30 mg·kg−1) did not significantly modify the DAI values in comparison with the untreated control group (Figure 5A). Furthermore, no significant differences among colitic groups were observed when the colonic inflammatory status was evaluated macroscopically (weight/length ratio) or biochemically by determining colonic IL-1β and IL-6 levels (Table 4).
Figure 5.
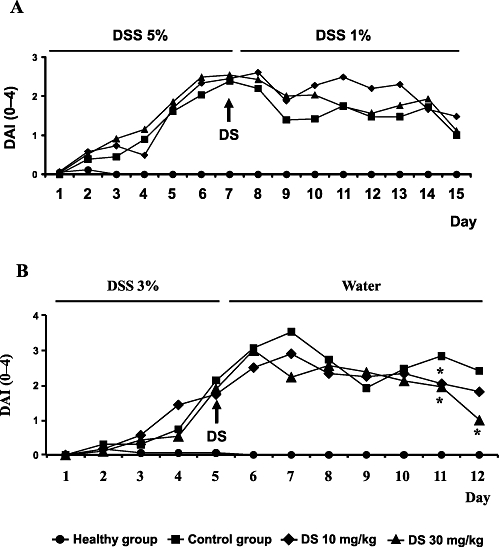
Effects of DS treatment on DAI values in DSS mice colitis based on the criteria proposed previously (Camuesco et al., 2004): (A) BALB/c mice; (B) C57BL/6. Data are expressed as means ± SEM. *P < 0.05 vs. DSS control group.
Table 4.
Effects of DS (10 and 30 mg·kg−1) on colonic weight/length ratio, IL-1β and IL-6 levels in DSS experimental colitis in mice following a curative treatment protocol
| Group (n = 10) | Weight/length (mg·cm−1) | IL-1β (pg·g−1 wet tissue) | IL-6 (pg·g−1 wet tissue) |
|---|---|---|---|
| BALB/c mice | |||
| Non-colitic | 19.2 ± 1.1 | 384.9 ± 23.2 | 62.2 ± 3.6 |
| DSS control | 28.4 ± 0.9 | 1285.0 ± 126.8 | 187.7 ± 14.1 |
| DS, 10 mg·kg−1 | 28.7 ± 0.9 | 1342.0 ± 145.1 | 183.2 ± 15.6 |
| DS, 30 mg·kg−1 | 32.0 ± 1.1 | 1354.9 ± 88.3 | 159.1 ± 24.4 |
| C57BL/6 mice | |||
| Non-colitic | 26.4 ± 1.3 | 581.8 ± 40.8 | 112.2 ± 7.1 |
| DSS control | 36.3 ± 2.4 | 3774.9 ± 439.5 | 1145.3 ± 243.9 |
| DS, 10 mg·kg−1 | 37.7 ± 1.9 | 2559.2 ± 234.8* | 539.0 ± 168.6* |
| DS, 30 mg·kg−1 | 31.5 ± 2.7 | 2544.7 ± 388.4* | 412.2 ± 139.4* |
Data are expressed as mean ± SEM.
P < 0.05 versus DSS control group; all colitic groups statistically differ (P < 0.05) from non-colitic group (not shown).
When DSS (3%) was administered to C57BL/6 mice in the drinking water, a progressive increase in the DAI values was observed with time, which peaked at day 7 in the control group, that is two days after removal of DSS, which were almost constant until the end of the experiment (Figure 5). The treatment of colitic mice with DS, starting when colitis was well established, significantly reduced the DAI values at days 11 and 12 (Figure 5B). Although the macroscopic evaluation of the colonic segments, that is weight/length ratio in the colonic specimens, did not show significant differences among colitic mice (Table 4), microscopic analysis of the colonic samples confirmed the beneficial effects of DS in this model of mouse colitis (Figure 6). Histologically, DSS-induced colitis was characterized by mucosa epithelial ulceration (typically affecting more than 75% of the surface) and crypt hyperplasia accompanied by goblet cell depletion. A chronic inflammatory cell infiltration into the lamina propria was observed, mainly composed of macrophages and lymphocytes, the latter being mainly located close to the muscular layer of the mucosa; neutrophils and eosinophils were scarcely present, even in the ulcerated areas. In the submucosa, there was an intense inflammatory cell infiltrate, mainly lymphocytes. In addition, oedema was present between the mucosa and muscularis layers of the intestine. According to the evaluation criteria (Table 1), a microscopic score of 21.5 (13–35) was assigned to control colitic mice. In contrast, most of the samples from the groups treated with DS showed a noteworthy recovery in the inflammatory process. Mucosal architecture was mostly preserved; only a few animals showed slight/moderate inflammatory infiltrate of mononuclear cells in the lamina propria and a slight oedema in the submucosa. The evaluation of the damage in the both treated groups of colitic mice resulted in reduced microscopic score in comparison with the untreated control group: 8 (7–15) (10 mg·kg−1) and 5 (3–10) (30 mg·kg−1) (P < 0.01 vs. DSS colitic group) (Figure 6). Finally, and in contrast with the data obtained in BALB/c, DS (30 mg·kg−1) treatment of colitic C57BL/6 mice significantly reduced the colonic levels of the pro-inflammatory cytokines IL-1β, IL-6 (Table 4) and IL-17 (Figure 7); the production of these cytokines was significantly increased in colitic mice in comparison with the non-colitic group.
Figure 6.
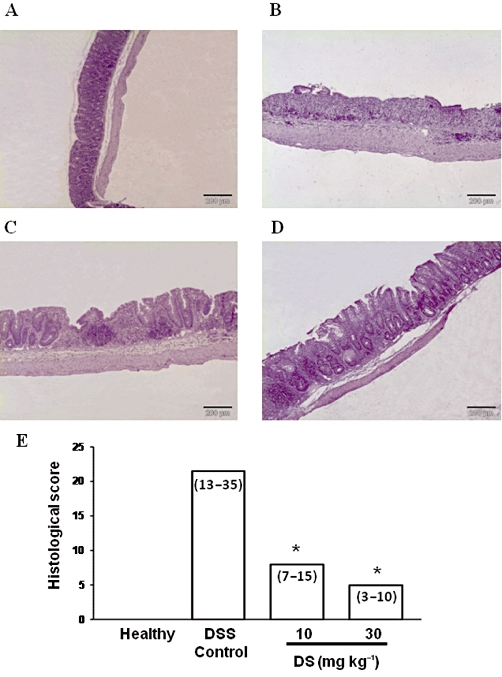
Histological sections of colonic mucosa stained with haematoxylin and eosin, showing the anti-inflammatory effect of DS treatment in DSS mice (C57BL/6) colitis: (A) Healthy; (B) DSS-control; (C) DS 10 mg·kg−1; (D) DS 30 mg·kg−1; and (E) microscopic score assigned according the criteria described in Table 1. Data are expressed as median (range); *P < 0.05 vs. DSS control group.
Figure 7.
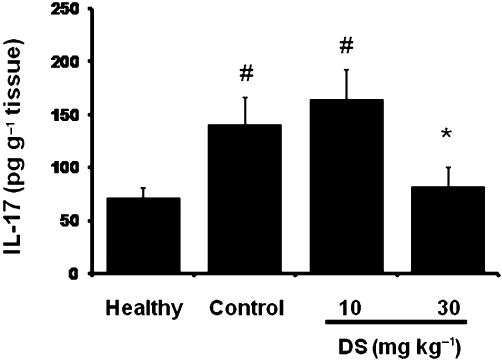
Effects of DS treatment on colonic IL-17 production in DSS mice (C57BL/6) colitis, as quantified by elisa. Data are expressed as means ± SEM; *P < 0.05 vs. DSS control group; #P < 0.05 vs. healthy group.
Discussion
The results obtained in the present study confirm the intestinal anti-inflammatory effects previously reported for DS in the TNBS model of rat colitis (Gálvez et al., 2000; 2003) and support the notion that the PAF antagonist moiety included in its structure plays a key role in its anti-inflammatory properties. Similarly, and in accordance with those previous results, DS showed a higher efficacy than that obtained with SAZ in this experimental model of rat colitis. Although both DS (10 and 30 mg·kg−1) and SAZ (30 mg·kg−1) were able to inhibit the enhanced colonic production of different pro-inflammatory cytokines, like IL-1β and TNF-α, as well as reduce the increased colonic iNOS expression, the inhibition achieved with DS was significantly higher than that obtained with SAZ. Furthermore, only DS was able to significantly reduce the extent of the inflamed/necrosed area in the colonic segment, as well as its weight/length ratio; SAZ was devoid of any significant effect on these macroscopic parameters. Based on their chemical composition, and at the doses assayed in the present study, the amount of 5-ASA that is theoretically provided by DS must be lower than that obtained with SAZ, when these compounds reach the colonic segment and the azo bond is split by colonic bacterial azoreductases (Peppercorn and Goldman, 1972). In the present study, we also showed that the PAF antagonist moiety of DS (UR-12715) was active in experimental colitis when it was administered rectally, thus confirming previous observations (Gálvez et al., 2000). All these facts, together with the current results, suggest that UR-12715 is relevant for the beneficial effect induced by DS in different experimental models of colitis.
Although the aetiology of IBD is not completely known, most studies suggest that this intestinal condition results from the interaction of genetic and environmental factors that ultimately promote an excessive and uncontrolled mucosal immune response that is directed against an unknown component of the normal flora. Classically, this deregulated immune response has been represented by a T helper (Th) 1 or Th2 polarization pattern in CD or UC, respectively. However, the Th17 pathway has recently attracted increasing interest. The Th17 pathway-related cytokines, IL-23 and IL-17, have been shown to be essential for the manifestation of chronic intestinal inflammation (Liu et al., 2009; Sarra et al., 2010). The role of IL-17 in chronic inflammation is mediated through different mechanisms; it contributes to neutrophil migration, expansion and function, and enhances dendritic cell maturation, T-cell priming and cellular (like fibroblasts, endothelial cells, macrophages, epithelial cells) production of inflammatory mediators, including TNF-α, IL-1β, IL-6, IL-8, GM-CSF, iNOS, PGE2, metalloproteases and chemokines (Ye et al., 2001; Yen et al., 2006). Furthermore, IL-17 can synergize with all these pro-inflammatory cytokines to stimulate the release of additional pro-inflammatory cytokines, thus contributing to the maintenance of the inflammatory response in the intestine.
As a consequence, the inhibition of Th17 cytokines may contribute to the suppression in the development of colitis, and they could be considered as interesting targets for treatment strategies of IBD in the future. However, at present, little information is available about the impact of current drugs treatment on Th17 activity in human IBD. In contrast, the neutralization of IL-17 by monoclonal antibodies has been recently reported to show positive results in the treatment of other autoimmune diseases, like rheumatoid arthritis (Genovese et al., 2010). The down-regulation of this cytokine has been demonstrated for some drugs used in IBD therapy in different experimental models of rodent colitis, in which this cytokine has been demonstrated to play a key role (Alex et al., 2009; Zhang et al., 2010). Thus, it has been demonstrated that the intestinal anti-inflammatory effect of the glucocorticoid dexamethasone in the acute phase of TNBS colitis in mice is associated with an inhibition in colonic Th17 effector functions (Daniel et al., 2008). The results obtained in the present study reveal that the intestinal anti-inflammatory effects in the TNBS model of rat colitis of DS are associated with a reduced expression of Th17-related cytokines, including IL-17, IL-23 and IL-12A. However, an interesting question that arises from these in vivo studies is whether this effect constitutes an early event in the anti-inflammatory effect of this compound, or it is secondary to other actions on the complex inflammatory response that take place in intestine. The assays performed in the same experimental model of rat colitis, but evaluating the response at different periods, that is 2 versus 7 days after TNBS instillation, gave some clues about the differences between DS and the conventional 5-ASA releasing products, like SAZ. At the doses assayed, neither of the two compounds significantly reduced the colonic damage after 2 days of treatment, as evidenced either macroscopically (score or weight/length ratio) or biochemically (colonic MPO activity, TNF-α or IL-1β contents, or iNOS expression). However, DS clearly reduced the expression of IL-17 in the inflamed colonic segment, whereas SAZ did not when compared with the untreated colitic control group.
According to these results, an early inhibition in IL-17 production seems to be necessary for the intestinal anti-inflammatory activity of DS, an effect that could be ascribed to its PAF antagonist activity. PAF is a potent pro-inflammatory phospholipid mediator that has been implicated in inducing intestinal inflammation in diseases such as IBD (Sobhani et al., 1992; Thyssen et al., 1996) and necrotizing enterocolitis (Hsueh et al., 2003). It is released early in inflammation by a variety of cell types and acts mainly after binding to its receptor, PAF-R, a GPCR found on a variety of cells, including cells of the immune system (Chao and Olson, 1993). It has been shown that the PAF/PAF-R pathway may influence T-cell responses and promotes the Th17 phenotype (Edwards and Constantinescu, 2009), thus justifying that the PAF-antagonist moiety of DS, once it is released in the colonic lumen, can down-regulate IL-6, IL-17, IL12A and IL-23 production in the inflamed colon of TNBS colitic rats. Similarly, it has been recently reported that PAF blockade exerts a beneficial effect in psoriasis-like skin disease in K5.hTGF-β1 transgenic mice, and this was correlated with a decrease in abnormally elevated mRNA and/or protein levels of T-helper type 17 cell-related cytokines IL-17A, IL-17F, IL-23, IL-12A and IL-6 and its transcription factor signal transducer and activator of transcription 3 (Singh et al., 2011).
The relevant role of IL-17 in the beneficial effect of DS was confirmed when the compound was assayed in the DSS model of mouse colitis in two different strains: C57BL/6 and BALB/c mice, which have been shown to display different susceptibility to this model of experimental colitis due to their genetic background (Melgar et al., 2005). Thus, BALB/c mice have a defective Th1/Th17 response characterized by the development of a Th2 response, while C57BL/6 mice develop a Th1/Th17 response. These differences lead to dissimilar colonic inflammation responses to DSS; in contrast to BALB/c, C57BL/6 mice develop a chronic colonic inflammation associated with a high production of IL-17, IL-12 as well as IL-1, confirming that activated Th17 cells are involved in the chronic development of intestinal inflammation (Melgar et al., 2005). The different efficacy observed with DS in this model can be explained on basis of the involvement of Th17 cytokines in the development of the inflammatory response induced by DSS in each strain. The results obtained in the present study revealed that DS did not exert an intestinal anti-inflammatory effect when colitis was induced in BALB/c mice, but it did in C57BL/6, as evaluated histological and biochemically, an effect associated with a significant decrease in colonic IL-17 production.
In conclusion, our data strongly suggest that DS is a novel chemical entity effective in the treatment of UC patients that represents a new therapeutic alternative to corticosteroids, immunosuppressants or anti-TNF-α agents. This compound exerts intestinal anti-inflammatory activity in different rodent models of colitis through down-regulation of IL-17 expression, with the PAF antagonist moiety being the key player for this activity.
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation (SAF2008-02616) with funds from the European Union, and Junta de Andalucia (CTS 164). MC is a recipient of ‘Ramon y Cajal’ Program from Spanish Ministry of Science and Innovation. NG-M and MC-S are predoctoral fellows of the Spanish Ministry of Science and Innovation. DC is a postdoctoral fellow of the Spanish Ministry of Science and Innovation. MER-C is a postdoctoral fellow of CIBEREHD. The CIBEREHD is funded by the Instituto de Salud Carlos III.
Glossary
- 5-ASA
5-aminosalicylic acid
- CD
Crohn's disease
- DAI
disease activity index
- DS
dersalazine sodium
- DSS
dextran sodium sulphate
- IBD
inflammatory bowel disease
- iNOS
inducible NOS
- MPO
myeloperoxidase
- PAF
platelet activating factor
- SAZ
sulphasalazine
- TNBS
trinitrobenzene sulphonic acid
- UC
ulcerative colitis
Conflicts of interest
MM, DB, GJ, JX and JR are employees of Palau Pharma S.A.
References
- Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas B, Rodríguez-Cabezas ME, Camuesco D, Comalada M, Bailón E, Utrilla P, et al. A probiotic strain of Escherichia coli, Nissle 1917, given orally exerts local and systemic anti-inflammatory effects in lipopolysaccharide-induced sepsis in mice. Br J Pharmacol. 2009;157:1024–1033. doi: 10.1111/j.1476-5381.2009.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camuesco D, Comalada M, Rodríguez-Cabezas ME, Nieto A, Lorente MD, Concha A, et al. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol. 2004;143:908–918. doi: 10.1038/sj.bjp.0705941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camuesco D, Peran L, Comalada M, Nieto A, Di Stasi LC, Rodriguez-Cabezas ME, et al. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel Dis. 2005;11:265–271. doi: 10.1097/01.mib.0000160808.30988.d9. [DOI] [PubMed] [Google Scholar]
- Chao W, Olson MS. Platelet-activating factor: receptors and signal transduction. Biochem J. 1993;292:617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Constantinescu CS. Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases. Inflamm Allergy Drug Targets. 2009;8:182–190. doi: 10.2174/187152809788681010. [DOI] [PubMed] [Google Scholar]
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- Gálvez J, Garrido M, Merlos M, Torres MI, Zarzuelo A. Intestinal anti-inflammatory activity of UR-12746, a novel 5-ASA conjugate, on acute and chronic experimental colitis in the rat. Br J Pharmacol. 2000;130:1949–1959. doi: 10.1038/sj.bjp.0703505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez J, Garrido M, Rodríguez-Cabezas ME, Ramis I, de Medina FS, Merlos M, et al. The intestinal anti-inflammatory activity of UR-12746S on reactivated experimental colitis is mediated through downregulation of cytokine production. Inflamm Bowel Dis. 2003;9:363–371. doi: 10.1097/00054725-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology. 1999;116:602–609. doi: 10.1016/s0016-5085(99)70182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15:5784–5788. doi: 10.3748/wjg.15.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EV, Jr, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179–189. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- Mahida YR, Lamming CE, Gallagher A, Hawthorne AB, Hawkey CJ. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut. 1991;32:50–54. doi: 10.1136/gut.32.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;2288:G1328–G1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:160–170. doi: 10.1038/ncpgasthep0696. [DOI] [PubMed] [Google Scholar]
- Nielsen OH, Verspaget HW, Elmgreen J. Inhibition of intestinal macrophage chemotaxis to leukotriene B4 by sulphasalazine, olsalazine, and 5-aminosalicylic acid. Aliment Pharmacol Ther. 1998;2:203–211. doi: 10.1111/j.1365-2036.1988.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Patel H, Barr A, Jeejeebhoy KN. Renal effects of long-term treatment with 5-aminosalicylic acid. Can J Gastroenterol. 2009;23:170–176. doi: 10.1155/2009/501345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972;181:555–562. [PubMed] [Google Scholar]
- Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- Siegel CA. Review article: explaining risks of inflammatory bowel disease therapy to patients. Aliment Pharmacol Ther. 2011;33:23–32. doi: 10.1111/j.1365-2036.2010.04489.x. [DOI] [PubMed] [Google Scholar]
- Singh TP, Huettner B, Koefeler H, Mayer G, Bambach I, Wallbrecht K, et al. Platelet-activating factor blockade inhibits the T-helper type 17 cell pathway and suppresses psoriasis-like skin disease in K5.hTGF-β1 transgenic mice. Am J Pathol. 2011;178:699–708. doi: 10.1016/j.ajpath.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I, Hochlaf S, Denizot Y, Vissuzaine C, Rene E, Benveniste J, et al. Raised concentrations of platelet activating factor in colonic mucosa of Crohn's disease patients. Gut. 1992;33:1220–1225. doi: 10.1136/gut.33.9.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson WF, Lobos E. Sulfasalazine inhibits the synthesis of chemotactic lipids by neutrophils. J Clin Invest. 1982;69:494–497. doi: 10.1172/JCI110474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland LR, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006a;(2):CD000543. doi: 10.1002/14651858.CD000543.pub2. [DOI] [PubMed] [Google Scholar]
- Sutherland LR, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006b;(2):CD000544. doi: 10.1002/14651858.CD000544.pub2. [DOI] [PubMed] [Google Scholar]
- Thyssen E, Turk J, Bohrer A, Stenson WF. Quantification of distinct molecular species of platelet activating factor in ulcerative colitis. Lipids. 1996;31(Suppl.):S255–S259. doi: 10.1007/BF02637086. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hinrichs DJ, Lu H, Chen H, Zhong W, Kolls JK. After interleukin-12p40, are interleukin-23 and interleukin-17 the next therapeutic targets for inflammatory bowel disease? Int Immunopharmacol. 2010;7:409–416. doi: 10.1016/j.intimp.2006.09.024. [DOI] [PubMed] [Google Scholar]


