Abstract
BACKGROUND AND PURPOSE
TNF-related apoptosis-inducing ligand (TRAIL) is currently in clinical trials as a treatment for cancer, but development of resistance is a major drawback. Thus agents that can overcome resistance to TRAIL are urgently needed. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) has been shown to affect cell growth by modulating various cell signalling pathways. Hence, we investigated the effect of cardamonin on the actions of TRAIL.
EXPERIMENTAL APPROACH
The effect of cardamonin on TRAIL was measured by plasma membrane integrity, phosphatidylserine exposure, mitochondrial activity, and activation of caspase-8, caspase-9, and caspase-3 in human colon cancer cells.
KEY RESULTS
Cardamonin potentiated TRAIL-induced apoptosis and this correlated with up-regulation of both the TRAIL death receptor (DR) 4, 5 at mRNA and protein levels. TRAIL-decoy receptor DcR1 was down-regulated by cardamonin. Induction of DRs by cardamonin occurred in a variety of cell types. Gene silencing of the DRs by small interfering RNA (siRNA) abolished the effect of cardamonin on TRAIL-induced apoptosis, suggesting that sensitization was mediated through the DR. Induction of the DR by cardamonin was p53-independent but required CCAAT/enhancer binding protein homologous protein (CHOP); cardamonin induced CHOP, and its silencing by siRNA eliminated the induction of DR5. Cardamonin increased the production of reactive oxygen species (ROS) and quenching ROS abolished its induction of receptors and enhancement of TRAIL-induced apoptosis. Cardamonin also decreased the expression of various cell survival proteins.
CONCLUSIONS AND IMPLICATIONS
Cardamonin potentiates TRAIL-induced apoptosis through ROS-CHOP-mediated up-regulation of DRs, decreased expression of decoy receptor and cell survival proteins. Thus, cardamonin has the potential to make TRAIL more effective as an anticancer therapy.
Keywords: Cardamonin, TRAIL, apoptosis, death receptors, ROS
Introduction
TNF-related apoptosis-inducing ligand (TRAIL), also known as Apo2L and TNFSF10, is a member of the TNF family that induces cell death in various types of cancer cells in vivo and in vitro but has little or no effect on normal cells (Havell et al., 1988). A deficiency of TRAIL in mice has been shown to accelerate malignant tumours, indicating its critical role in cancer (Zerafa et al., 2005). This cytokine has been shown to bind to four membrane-bound death receptors [DR4 (TNFRSF10A), DR5 (TNFRSF10B), DcR1 and DcR2) and a soluble receptor, called osteoprotegrin. Both DR4 and DR5 contain a conserved cytoplasmic region called the death domain that is required for TRAIL-induced apoptosis (Ashkenazi et al., 1999; Bhardwaj and Aggarwal, 2003). In contrast, DcR1 and DcR2 either lack an intracellular domain or have a truncated death domain that negatively controls apoptosis by sequestering the cytokine (Pan et al., 1997; Sheridan et al., 1997). Differential sensitivity of tumour cells to the cytokine may be caused by the higher expression of DR5 in cancer cells than in normal cells (Ichikawa et al., 2001; Koornstra et al., 2003).
Both TRAIL and its receptor agonistic antibodies are currently in clinical trials for treatment of various cancers because of their ability to kill a wide variety of tumour cells specifically, and without activating NF-κB, the transcription factor that controls the expression of cell survival proteins, (Duiker et al., 2006). However, not all human colon cancer cell lines are sensitive to TRAIL due to intrinsic or acquired TRAIL resistance. This is a major obstacle in its presumptive journey to the clinic (Ozoren et al., 2000). The resistance may be due to overexpression of cell survival proteins such as B-cell lymphoma 2 (Bcl-2), B-cell lymphoma extra large (Bcl-xL), X-linked inhibitor of apoptosis protein (XIAP), cellular inhibitor of apoptosis protein (cIAP)-1, cIAP-2 and cellular (FLICE)-like inhibitory protein (cFLIP) or to overexpression of decoy receptors (DcRs) or to limited expression of cell signalling DRs on the cell surface (Nam et al., 2003; Zhang et al., 2005). An agent that can up-regulate the pro-apoptotic mechanisms and down-regulate the anti-apoptotic mechanisms, yet is safe and affordable, is urgently needed if TRAIL is to be an effective therapy.
In the present report, we describe a chalcone, cardamonin (2′,4′-dihydroxy-6′-methoxychalcone), which was first isolated in 1976 from large black cardamom (fruit of Amomum subulatum) (Bheemasankara Rao et al., 1976). Cardamonin has been shown to affect cell growth by modulation of a variety of cell signalling pathways, including mammalian target of rapamycin (Liao et al., 2010), NF-κB (Hatziieremia et al., 2006; Lee et al., 2006; Israf et al., 2007; Kim et al., 2010), cell surface receptors (Ohtsuki et al., 2009), and Wnt/β catenin (Cho et al., 2009). This chalcone has also been shown to bind to lysozyme (He et al., 2006) and human serum albumin (He et al., 2005). Pretreatment with cardamomin has also been shown to protect mice from LPS-induced mortality in conjunction with decreased serum levels of TNF-α, IL-6 and IL-1β secretion (Lee et al., 2006; Kim et al., 2010).
Because resistance to TRAIL has been demonstrated in colorectal cancer (Van Geelen et al., 2004), in the present study we investigated whether cardamonin can affect TRAIL-mediated apoptosis in these tumour cells. The results obtained show that cardamonin can potentiate TRAIL-induced apoptosis through down-regulation of cell survival proteins, down-regulation of DcR, and up-regulation of DRs, which is mediated through the reactive oxygen species (ROS)-mediated CCAAT/enhancer binding protein homologous protein (CHOP) activation.
Experimental procedures
Reagents
A 20 mM solution of cardamonin (from Tocris Bioscience, Ellisville, MO, USA) was prepared in dimethyl sulphoxide, stored at −20°C, and then diluted as needed in cell culture medium. Soluble recombinant human TRAIL was purchased from PeproTech (Rocky Hill, NJ, USA). Penicillin, streptomycin, Roswell Park Memorial Institute medium (RPMI) 1640, FBS and dichlorodihydrofluorescein diacetate (DCF-DA) were purchased from Invitrogen (Carlsbad, CA, USA). Anti-β-actin antibody was obtained from Sigma-Aldrich (St Louis, MO, USA). Antibodies against Bcl-xL, Bcl-2, B-cell lymphoma-2-associated X protein (Bax), cFLIP, PARP, JNK-1, CHOP, phospho-AKT1/2, and annexin V staining kit were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell lines
HCT116 (human colon adenocarcinoma), PC3 and DU145 (human prostate cancer cells), U266 (human multiple myeloma), MIA PaCa-2 (human pancreatic carcinoma cell line) and KBM-5 (human chronic leukemic cells) were obtained from American Type Culture Collection (Manassas, VA, USA). HCT116 and MIA PaCa-2 were cultured in Dulbecco's modified Eagle's medium; PC3, DU145, and U266 were cultured in RPMI1640; and KBM-5 cells were cultured in Iscove's modified Dulbecco's medium (IMDM). All the media were supplemented with 10% FBS, 100 U·mL−1 penicillin, and 100 mg·mL−1 streptomycin except IMDM which contains 15% FBS.
Live/dead assay
To measure apoptosis, we used Live/Dead assay kit (Invitrogen). We stained the cells according to the manufacturer's instructions. In this assay, calcein-AM, a nonfluorescent polyanionic dye, is retained by live cells, in which it produces intense green fluorescence through enzymatic (esterase) conversion. In addition, the ethidium homodimer enters cells with damaged membranes and binds to nucleic acids, thereby producing a bright red fluorescence in dead cells. Cells were analysed under a fluorescence microscope (Labophot-2; Nikon, Tokyo, Japan).
Cytotoxicity assay
The effects of cardamonin on TRAIL-induced cytotoxicity were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) uptake method (Yadav et al., 2010a).
Annexin V and propidium iodide
The early indicator of apoptosis was detected by using annexin V/propidium iodide binding kit (Santa Cruz Biotechnology) and then analysed with a flow cytometer (FACS Calibur, BD Biosciences, San Jose, CA, USA) (Yadav et al., 2010a).
Flow cytometry for cell cycle distribution
To determine the effect of cardamonin on the cell cycle, treated and untreated cells were stained with propidium iodide as described previously (Yadav et al., 2010a).
RNA analysis and reverse transcription (RT)-PCR
Total RNA was extracted from the treated cells according to the manufacturer's instructions (Invitrogen), and RT-PCR was performed as described previously (Yadav et al., 2010b).
Analysis of cell surface expression of DR4 and DR5
Treated and untreated cells were stained with phycoerythrin-conjugated mouse monoclonal anti-human DR5 or DR4 (R&D Systems, Minneapolis, MN, USA) for 45 min at 4°C according to the manufacturer's instructions and analysed by flow cytometry with phycoerythrin-conjugated mouse IgG2B as an isotype control (Prasad et al., 2011).
Western blot analysis
To determine the levels of protein expression in the cytoplasm and nucleus, we prepared extracts (Yadav et al., 2010b) and fractionated them by 10% SDS-PAGE. After electrophoresis, the proteins were electro-transferred to nitrocellulose membranes, blotted with each antibody, and detected with a chemiluminescence reagent (GE Healthcare, Piscataway, NJ, USA).
Transfection with siRNA
HCT116 cells were plated in each well of six-well plates and allowed to adhere for 24 h. On the day of transfection, 12 µL Hiperfect transfection reagent (Qiagen, Hilden, Germany) was added to 50 nM siRNA in a final volume of 100 µL culture medium. After 48 h of transfection, cells were treated with cardamonin for 12 h and then exposed to TRAIL for 24 h (Yadav et al., 2010b).
Measurement of ROS
To detect intracellular ROS, cells were pre-incubated with 20 µM DCF-DA for 15 min at 37°C before being treated with 20 µM cardamonin. After 30 min of incubation, the increase in fluorescence resulting from oxidation of DCF-DA to DCF was measured by flow cytometry. The mean fluorescence intensity at 530 nm was calculated. Data were collected from at least 10 000 cells at a flow rate of 250–300 cells·s−1.
Statistical analysis
The data were analysed for mean values and SEM for all treated and vehicle controls. Values were compared using Student's paired t-test; P < 0.05 was considered significant.
Results
The objective of this study was to determine whether cardamonin (see Figure 1A; 2′,4′-dihydroxy-6′-methoxychalcone) potentiates TRAIL-induced apoptosis in human colorectal HCT-116 cells and, if so, to determine the mechanisms by which this chalcone might enhance the effect of this cytokine.
Figure 1.
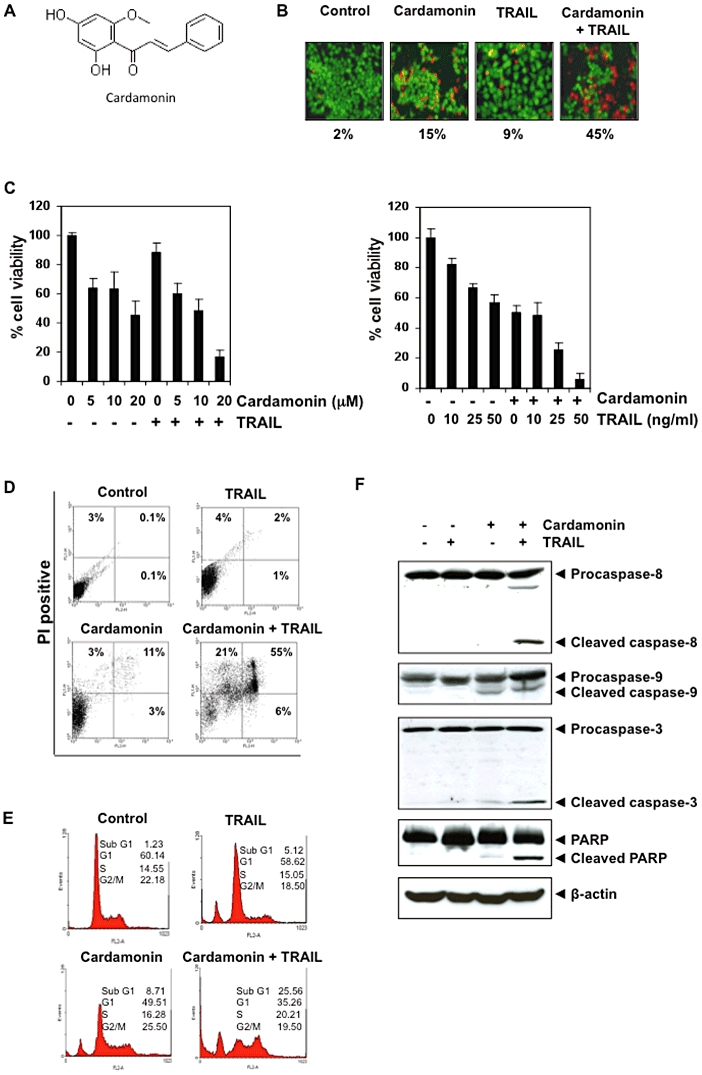
Cardamonin potentiates TRAIL-induced apoptosis of HCT116 cells. (A) Chemical structure of cardamonin. (B) Effect of cardamonin on TRAIL-induced apoptosis by the Live/Dead assay. Cells were pretreated with 20 µM cardamonin for 12 h, the medium was removed, and the cells then exposed to TRAIL for 24 h. Dead cells as a percentage of the number plated is indicated below the photo. (C) Effect of cardamonin on TRAIL-induced apoptosis by the MTT method. Cells were pretreated with indicated concentration of cardamonin for 12 h, the medium was removed, and the cells then exposed to TRAIL for 24 h. Cell viability was then analysed by the MTT method (left panel). Cells were pretreated with 20 µM of cardamonin for 12 h, the medium was removed, and the cells then exposed with indicated concentration of TRAIL for 24 h. Cell viability was then analysed by the MTT method (right panel). (D) Effect of cardamonin on TRAIL-induced apoptosis by the annexin method. Cells were treated with 20 µM cardamonin and TRAIL as described above. Cells were stained with annexin V and analysed by FACS. (E) Effect of cardamonin on TRAIL-induced apoptosis by the propidium iodide method. Cells were treated with 20 µM cardamonin and TRAIL as described above. Cells were stained with propidium iodide and analysed by FACS. (F) Effect of cardamonin on TRAIL-induced apoptosis by the caspase activation method. Whole-cell extracts were prepared and analysed by Western blotting using antibodies against caspase-8, caspase-9, caspase-3 and PARP.
Cardamonin potentiates TRAIL-mediated cytotoxic effects in colon cancer cells
Whether cardamonin enhances TRAIL-induced cytotoxicity effect was investigated by Live/Dead assay. We found that cardamonin induced up to 15% cytotoxicity while TRAIL alone produced 9% cytotoxicity in HCT116 cells. Interestingly, the combination of cardamonin and TRAIL increased cytotoxicity to 45% (Figure 1B).
Cardamonin potentiates TRAIL-mediated cytotoxicity
To confirm the Live/Dead assay results, we measured the viability and proliferation of the cells by the MTT method. The HCT116 cells were moderately sensitive to either cardamonin or TRAIL. However, pretreatment with cardamonin significantly enhanced TRAIL-induced cytotoxicity, and when TRAIL was added at different doses it potentiated the dose-dependent effect of TRAIL (Figure 1C).
Cardamonin potentiates TRAIL-mediated apoptosis
Next, we examined the effect of cardamonin on TRAIL-induced apoptosis in HCT116 cells by phosphatidylserine externalization using the annexin V/propidium iodide assay. The results shown in Figure 1D (upper panel) indicate that cardamonin enhanced TRAIL-induced apoptosis (including early, late and necrosis) from 7 to 81%. To further determine the effect of cardamonin on TRAIL-induced cytotoxicity, we also investigated the distribution of cells by propidium iodide staining. We found that pretreatment with cardamonin enhanced TRAIL-induced apoptosis from 5 to 26% (Figure 1E). Because activation of caspases is a hallmark of apoptosis, we examined the effect of cardamonin on TRAIL-induced activation of caspase-8, -9, and -3 and on cleavage of PARP. We found that cardamonin enhanced TRAIL-induced activation of all three caspases, thus, leading to enhanced PARP cleavage (Figure 1F). Taken together, the results in Figure 1 suggest that cardamonin enhances TRAIL-induced cytotoxicity and apoptosis in colon cancer cells.
Cardamonin up-regulates the expression of DRs
As TRAIL mediates its activity through the receptors DR4 and DR5, we investigated whether up-regulation of TRAIL-induced apoptosis by cardamonin occurs through modulation of DR5 and DR4 expression. Treatment of HCT116 cells with various concentrations of cardamonin for 24 h increased the expression of TRAIL-R2/DR5 and TRAIL-R1/DR4 in a dose-dependent manner (Figure 2A, left panel).
Figure 2.
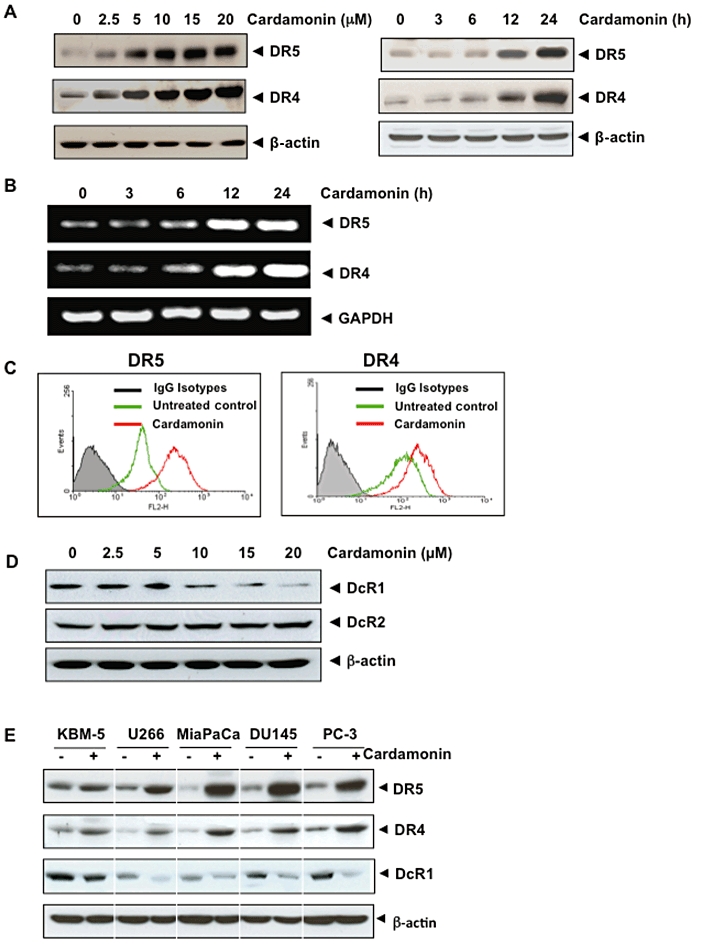
Cardamonin induces the expression of DRs and down-regulates DcRs. (A) Cardamonin induces the expression of DR protein. HCT116 cells (1 × 106 cells per well) were treated with the indicated dose of cardamonin for 24 h (left panel). HCT116 cells (1 × 106 cells per well) were treated at indicated time with 20 µM cardamonin (right panel). Whole-cell extracts were then prepared and analysed for DR expression by Western blotting. (B) Cardamonin induces DR5 gene mRNA expression. Cells (1 × 106 mL−1) were treated with 20 µM cardamonin for indicated times, and total RNA was extracted and examined for expression of DR4 and DR5 by RT-PCR. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control to equalize RNA loading. (C) Cardamonin induces cell surface expression of DRs. HCT116 cells were treated with 20 µM cardamonin for 24 h and analysed for cell surface DR4 and DR5 by immunofluorescent staining and subsequent flow cytometry. Filled greyish peaks: cells stained with a matched control phycoerythrin-conjugated IgG isotype antibody. (D) Cardamonin down-regulates the cell surface expression of DcR1. HCT116 cells were pretreated with indicated dose of cardamonin for 24 h. Whole-cell extracts were prepared and subjected to Western blotting using antibodies specific to DcR1 and DcR2. (E) Cardamonin up-regulates DR5, DR4 and down-regulates DcR1 in various types of cancer cells. Cells (1 × 106 cells) were treated with 20 µM cardamonin for 24 h, after which whole-cell extracts were prepared and analysed by Western blotting. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. Cells were pretreated with 20 µM cardamonin for 24 h and cell viability measured. Cell viability was found to be 91, 93, 90, 88 and 89%, respectively for KBB-5, U266, MiaPaCa, DU145 and PC-3 cell lines.
Next we determined the time at which cardamonin shows optimum induction of DR5 and DR4. Cardamonin induced the expression of DR5 and DR4 in a time-dependent manner (Figure 2A, right panel). The maximum induction was observed 24 h after cardamonin treatment.
To determine whether the induction of TRAIL receptors by cardamonin occurs at the transcriptional level, we measured the expression of mRNA for DR5 and DR4 by RT-PCR after cells had been treated with cardamonin for different periods of time. As shown in Figure 2B, cardamonin increased the transcript for DR5 and DR4 in a time-dependent manner, thus suggesting that cardamonin acts at the transcriptional level.
Whether cardamonin enhances the expression of DRs on the cell surface was also examined. For this, we analysed cell surface expression of DR5 and DR4 in cells exposed to cardamonin. We found that cardamonin increased the cell surface levels of DR5 and DR4 (Figure 2C). The level of DR5 cell surface expression induced by cardamonin was higher than that of DR4. Collectively, these results indicate that cardamonin up-regulates the expression of both DRs on the cell surface.
These data suggest cardamonin also enhances the pro-apoptotic effects of TRAIL in colon cancer cells by up-regulating the expression of DR4 and/or DR5.
Cardamonin down-regulates DcR
Decoy molecules compete with the DRs for ligand binding and thereby inhibit ligand-induced apoptosis (Meng et al., 2000; Merino et al., 2006), so we determined whether cardamonin modulates DcR expression. We found that, indeed, cardamonin decreased the expression of DcR1, but it did not influence the level of DcR2 (Figure 2D) (Prasad et al., 2011). Therefore, cardamonin may also potentiate TRAIL-induced apoptosis by inhibiting DcR1.
Cardamonin-induced up-regulation of DRs is not cell type specific
Whether up-regulation of TRAIL receptors by cardamonin was specific to HCT116 was investigated. We found that cardamonin (20 µM for 24 h) induced the expression of both DR5 and DR4 in all the cell lines examined: leukaemia (KBM-5), myeloma (U266), prostate carcinoma (Mia PaCa-2) and prostate cancer (DU145 and PC3) cells (Figure 2E). Thus the up-regulation of TRAIL receptors by cardamonin was not cell-type specific.
Whether down-regulation of DcRs by cardamonin was specific to HCT116, was also investigated. We found that the down-regulation of DcR1 receptors by cardamonin was also not cell-type specific (Figure 2E).
Cardamonin-induced DR are needed for TRAIL-induced apoptosis
To confirm the roles of DR5 and DR4 in TRAIL-induced apoptosis, we used siRNA specific to DR5 and DR4 to down-regulate the expression of these receptors. Transfection of cells with siRNA for DR5 but not with the control siRNA reduced cardamonin-induced DR5 expression (Figure 3A). Similarly, transfection of cells with siRNA for DR4 reduced the cardamonin-induced DR4 expression (Figure 3A). DR4 siRNA had minimal effect on the cardamonin-induced up-regulation of DR5, and vice versa.
Figure 3.
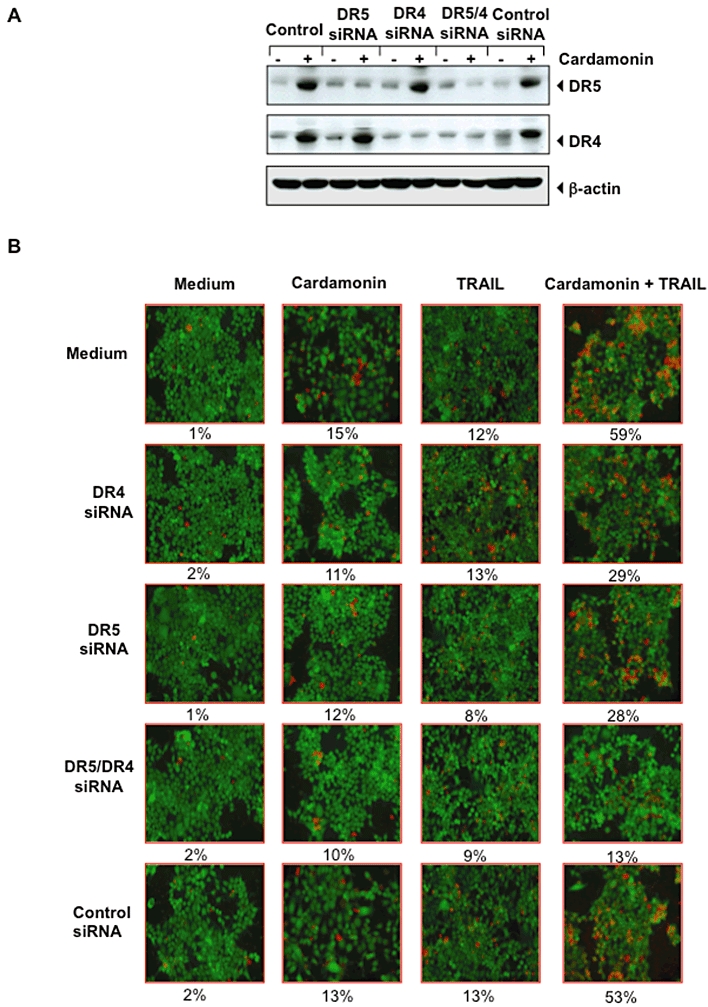
Induction of DRs by cardamonin is needed for sensitization of cells to TRAIL. (A) siRNA down-regulates the expression of TRAIL receptor protein. HCT116 cells were transfected with DR5 siRNA, DR4 siRNA, both siRNAs or control siRNA. After 48 h, cells were treated with 20 µM cardamonin for 24 h, and whole-cell extracts were prepared for Western blotting for DR5 and DR4. (B) Silencing of DRs inhibits the effect of cardamonin on TRAIL. Cells were seeded in a chamber slide and transfected with siRNAs. After 48 h, cells were pretreated with 20 µM cardamonin for 12 h, the medium was removed, and the cells then exposed to TRAIL (25 ng·mL−1) for 24 h. Cell death was determined by the Live/Dead assay.
We next examined whether the suppression of DR5 or DR4 by siRNA could attenuate the sensitizing effects of cardamonin on TRAIL-induced apoptosis using Live/Dead assay. TRAIL-induced apoptosis was effectively abolished in cells transfected with either DR5 or DR4 siRNA (Figure 3B), whereas treatment with control siRNA had no effect. Silencing of DR4 and DR5 reduced the apoptosis from 59 to 29 and 28%, respectively; whereas silencing of both reduced it to 13%.
Cardamonin downregulates the expression of cell survival proteins
Various anti-apoptotic proteins (cIAP-1, cIAP-2, cFLIP, XIAP, Bcl-2, Bcl-xL and survivin), have been shown to induce resistance to TRAIL-induced apoptosis. Therefore, we examined whether cardamonin sensitized the cells to TRAIL through down-regulation of the expression of these cell survival proteins. We found that cardamonin inhibited the expression of cIAP-1, cFLIP, XIAP, Bcl-2 and survivin but had little effect on the expression of Bcl-xL and cIAP-2 (Figure 4). Thus our results suggest that down-regulation of cell survival proteins is one of the mechanisms by which cardamonin potentiates TRAIL-induced apoptosis.
Figure 4.
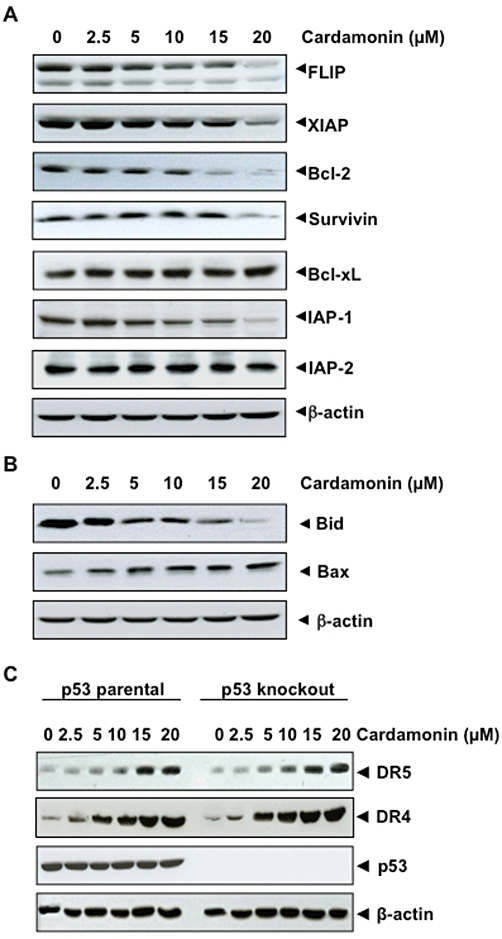
Effects of cardamonin on anti-apoptotic and pro-apoptotic protein expression. (A) Effects of cardamonin on anti-apoptotic protein expression. HCT116 cells were pretreated with indicated dose of cardamonin for 24 h. Whole-cell extracts were prepared and analysed by Western blotting using the antibodies against anti-apoptotic proteins. (B) Effects of cardamonin on pro-apoptotic protein expression. HCT116 cells were pretreated with indicated dose of cardamonin for 24 h. Whole-cell extracts were prepared and analysed by Western blotting using the antibodies against Bid and Bax proteins. (C) Induction of DRs by cardamonin is p53-independent. HCT116 cells that express p53 and those with p53 knockout were pretreated with indicated dose of cardamonin for 24 h. Whole-cell extracts were prepared and analysed by Western blotting using the antibodies against DR5 and p53 proteins. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading.
Cardamonin up-regulates the expression of pro-apoptotic proteins
In our next set of experiments, we found that cardamonin cleaved the pro-apoptotic protein BH3 interacting domain death agonist (Bid) and induced the expression of pro-apoptotic Bax (Figure 4B). These two effects are additional ways cardamonin could enhance the apoptotic effects of TRAIL.
Cardamonin-induced up-regulation of TRAIL receptors is p53-independent
There are reports that suggest that p53 can induce DRs (Wu et al., 1997; Burns et al., 2001), therefore, whether cardamonin induction of TRAIL receptors is mediated through p53 was examined using HCT116 cell lines that lack p53. Cardamonin induced DR5 and DR4 in p53 parental as well as p53 knockout HCT116 cells in a dose-dependent manner (Figure 4C). These results indicate that induction of TRAIL receptors is independent of p53 expression.
Cardamonin induces TRAIL receptors through ROS-dependent mechanism
Induction of DRs by certain agents has been shown to be ROS-dependent (Prasad et al., 2011). Whether cardamonin generates ROS was examined by treating HCT116 cells with cardamonin and measuring ROS by FACS. We found that cardamonin induced ROS in a dose-dependent manner (Figure 5A).
Figure 5.

The effect of cardamonin on TRAIL-induced apoptosis is dependent on ROS. (A) Cardamonin induces ROS production. HCT116 (1 × 106 cells) cells were labelled with DCF-DA, treated with indicated concentration of cardamonin for 1 h, and examined for ROS production by flow cytometer. (B) Cardamonin-induced up-regulation of DR5 and DR4 is mediated by ROS. HCT116 cells (1 × 106 cells) were pretreated with various concentrations of NAC for 1 h and then the cells were treated with 20 µM cardamonin for 24 h. Whole-cell extracts were prepared and analysed by Western blotting for DR5 and DR4. (C) NAC reverses cell death induced by combination of cardamonin and TRAIL. HCT116 cells were pretreated with NAC (10 mM) for 1 h and then treated with 20 µM cardamonin for 12 h. After being washed with PBS, cells were treated with TRAIL (25 ng·mL−1) for 24 h. Cell death was determined by the Live/Dead assay. (D) NAC inhibits caspase activation and PARP cleavage induced by combination of TRAIL and cardamonin. HCT116 cells were treated with NAC, 20 µM cardamonin and TRAIL as indicated above. Whole-cell extracts were prepared and analysed by Western blotting using the relevant antibodies. β-actin was used as a loading control.
Whether cardamonin induction of TRAIL receptors is also regulated by ROS was examined. As shown in the Figure 5B, pretreatment of HCT116 cells with the ROS scavenger N-acetylcysteine (NAC) reduced the cardamonin-induced up-regulation of DR5 and DR4 expression. This suggests ROS plays a critical role in the induction of TRAIL receptors by cardamonin (Figure 5B).
Cardamonin potentiates TRAIL-induced apoptosis through ROS generation
Whether ROS is needed for potentiation of TRAIL-induced apoptosis by cardamonin was examined. As shown in Figure 5C, pretreatment of cells with NAC markedly inhibited the effect of cardamonin on TRAIL-induced cell death from 68 to 18%.
We also found that NAC reversed the effect of cardamonin on TRAIL-induced cleavage of procaspases and PARP (Figure 5D), again suggesting the critical role of ROS in cardamonin's effects on TRAIL.
Cardamonin-induced up-regulation of CHOP and specificity protein 1 (SP1)
It has been shown that the induction of DR by various agents is mediated through activation of CHOP (Yamaguchi and Wang, 2004; Su et al., 2008; Lee et al., 2009; Lim et al., 2009) and SP1 (Moon et al., 2010).
To determine whether cardamonin can induce the expression of CHOP and SP1, we pretreated cells with cardamonin (20 µM) for different times and assayed for CHOP and SP1 expression. We found that cardamonin increased the expression of both CHOP and SP1 (Figure 6A).
Figure 6.
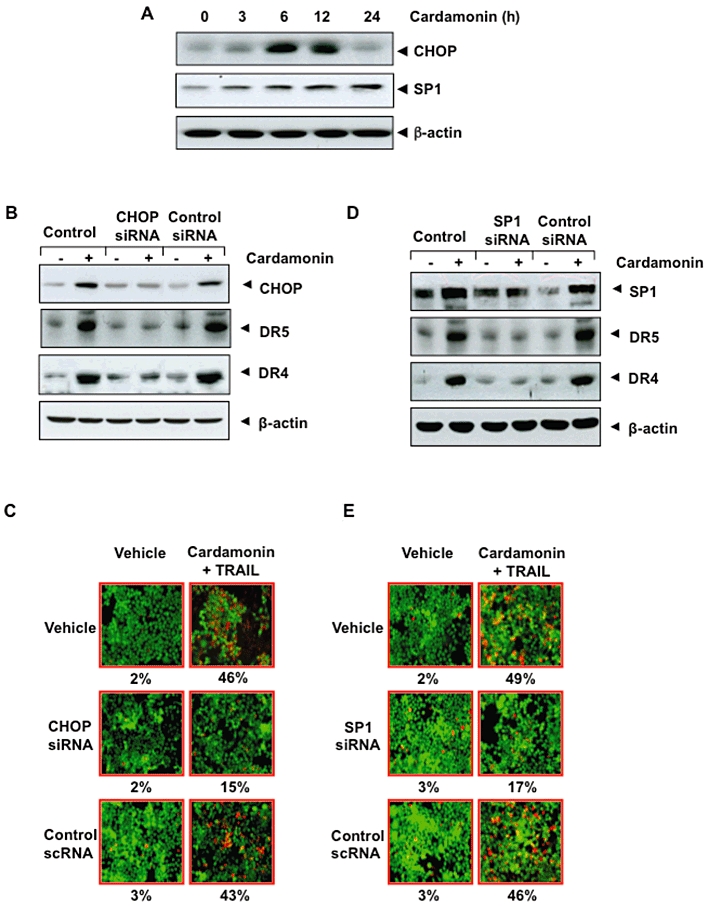
Induction of DRs by cardamonin requires the expression of CHOP and SP1. (A) Time-dependent up-regulation of CHOP and SP1 protein by cardamonin. HCT116 cells (1 × 106 cells per well) were treated with 20 µM cardamonin for the indicated times. Whole-cell extracts were then prepared and analysed by Western blotting for CHOP and SP1. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (B) Silencing of CHOP inhibits the effect of cardamonin on induction of DRs. HCT116 cells were transfected with CHOP siRNA and control siRNA. After 48 h, cells were treated with 20 µM cardamonin for 24 h, and whole-cell extracts were subjected to Western blotting. (C) Silencing of CHOP inhibits the effect of cardamonin on TRAIL. Cells were seeded in a chamber slide and transfected with CHOP siRNAs. After 48 h, cells were pretreated with 20 µM cardamonin for 12 h, the medium was removed, and the cells then exposed to TRAIL (25 ng·mL−1) for 24 h. Cell death was determined by the Live/Dead assay. (D) Silencing of SP1 inhibits the effect of cardamonin on induction of DRs. HCT116 cells were transfected with SP1 siRNA and control siRNA. After 48 h, cells were treated with 20 µM cardamonin for 24 h, and whole-cell extracts were subjected to Western blotting. (E) Silencing of SP1 inhibits the effect of cardamonin on TRAIL. Cells were seeded in a chamber slide and transfected with SP1 siRNAs. After 48 h, cells were pretreated with 20 µM cardamonin for 12 h, the medium was removed, and the cells then exposed to TRAIL (25 ng·mL−1) for 24 h. Cell death was determined by the Live/Dead assay.
Cardamonin-induced up-regulation of TRAIL receptors is mediated through activation of CHOP
We determined whether up-regulation of DR5 and DR4 were dependent on CHOP activation. We used siRNA specific to CHOP to down-regulate its expression. Transfection of cells with siRNA for CHOP down-regulated cardamonin-induced DR5 and DR4 expression (Figure 6B).
We next examined whether the suppression of CHOP by siRNA could attenuate the sensitizing effects of cardamonin on TRAIL-induced apoptosis using Live/Dead assay. TRAIL-induced apoptosis was effectively abolished in cells transfected with CHOP siRNA (Figure 6C), whereas treatment with control siRNA had no effect. Silencing of CHOP reduced the apoptosis from 46 to 15%.
Up-regulation of DRs by cardamonin is mediated through activation of SP1
We also determined whether up-regulation of DR5 and DR4 were dependent on SP1 activation. We used siRNA specific to SP1 to down-regulate its expression. Transfection of cells with siRNA for SP1 down-regulated cardamonin-induced DR5 and DR4 expression (Figure 6D).
We next examined whether the suppression of SP1 by siRNA could inhibit the effects of cardamonin on TRAIL-induced apoptosis using Live/Dead assay. TRAIL-induced apoptosis was effectively suppressed in cells transfected with SP1 siRNA (Figure 6E), whereas treatment with control siRNA had no effect. Silencing of SP1 reduced the apoptosis from 49 to 17%.
Discussion and conclusions
Among 18 different members of the TNF superfamily, TRAIL is the only cytokine from all these apoptosis-inducing cytokines that is being pursued for its anticancer properties in the clinic. However, many human cancer cell types are resistant to apoptosis by this ligand, including chronic lymphocytic leukaemia, astrocytoma, meningioma and medulloblastoma (Ozoren et al., 2000). Thus agents that can sensitize tumour cells to this ligand have a great potential for making cancer therapy more effective. Such agents would exploit the wide variety of mechanisms that have been outlined for the resistance of tumour cells to TRAIL, including overexpression of anti-apoptotic proteins, suboptimal expression of DRs, and overexpression of DcRs.
In the present study, we showed that cardamonin, a component derived from large black cardamom can enhance the apoptotic effects of TRAIL against colon cancer cells. We also demonstrated that cardamonin enhances TRAIL-induced apoptosis through a variety of mechanisms that include down-regulation of survivin, cFLIP, XIAP and Bcl-2, suppression of expression of DcR1, induction of Bax and up-regulation of DRs through regulation of ROS-CHOP-mediated pathway.
Using human colon cancer cells (HCT-116), we demonstrated that cardamonin significantly enhances TRAIL-induced apoptosis and one of the mechanisms involved is down-regulation of anti-apoptotic proteins. Overexpression of cFLIP, an inhibitor of caspase-8, has been linked with resistance to DR-mediated apoptosis (Irmler et al., 1997; Nam et al., 2003). We found that cardamonin down-regulated cFLIP expression. These results are in agreement with the down-regulation of cFLIP by the PPARγ agonist rosiglitazone and sensitization to TRAIL (Kim et al., 2008). We also found that cardamonin down-regulated the expression of survivin. These results are also similar to that noted with TRAIL-sensitization by PPAR-γ agonists (Ma et al., 2009). Resistance to TRAIL-induced apoptosis has been reported to be associated with the overexpression of anti-apoptotic proteins. This chalcone also significantly down-regulated the expression of Bcl-2, which has been linked to the suppression of apoptosis by TRAIL (Fulda et al., 2002). In addition we found that IAP-1 and XIAP were down-regulated by cardamonin, although to a lesser degree. All these proteins have been linked to TRAIL resistance (Nam et al., 2003; Zhang et al., 2005) but how these survival proteins are down-regulated by cardamonin is less clear. One of the potential mechanisms may involve NF-κB (Aggarwal et al., 2006). Israf et al. (2007) showed that cardamonin inhibits NF-κB activation through inhibition of IκBα phosphorylation and this could be the mechanism whereby it down-regulates the expression of cell survival proteins.
Our results also indicate that cardamonin significantly up-regulates the expression of Bax and down-regulates Bid expression; both have been shown to be critical for TRAIL-induced apoptosis (Ravi and Bedi, 2002). Thus, up-regulation of Bax by cardamonin could also contribute to TRAIL-induced apoptosis.
Besides down-regulating cell survival proteins, we found that cardamonin can up-regulate the expression of TRAIL receptors. This effect of cardamonin was not cell-type specific, as induction of receptors was also observed in pancreatic, myeloid leukaemia, multiple myeloma and human prostate adenocarcinoma cell lines. Cardamonin not only induced the expression of protein but also mRNA for the DR. These results are in agreement with those reported with other compounds such as celastrol (Sung et al., 2010) and methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate (Zou et al., 2004). We further found that silencing of DR induction abolished the effect of cardamonin on TRAIL-induced apoptosis, thus suggesting that these receptors play a critical role.
How cardamonin induces these receptors was also investigated in detail. Although several reports suggest that induction of DRs is mediated through expression of p53 (Wu et al., 1997; Burns et al., 2001; Asakuma et al., 2003), we found that these receptors are induced through a p53-independent mechanism, in agreement with those reported previously (Sung et al., 2010). We showed that ROS is the most important upstream signal linked to modulation of TRAIL signalling; firstly, cardamonin induced the expression of ROS in a dose-dependent manner; secondly, quenching of ROS by NAC abolished the cardamonin-induced expression of DRs; and thirdly, quenching of ROS also attenuated the effect of the cardamonin on TRAIL-induced cell death. Thus all these results suggest that ROS has a role in the mechanism of action of cardamonin. These results are also in agreement with those reported previously on induction of DRs by curcumin (Jung et al., 2006), 15-deoxy-Delta12,14-prostaglandin J2 (Su et al., 2008), and celastrol (Sung et al., 2010).
The cell surface DcR proteins DcR1 and DcR2 have been shown to inhibit apoptosis by sequestering TRAIL. Here we demonstrated that cardamonin reduced the expression of DcR1, which allows the availability of ligand for DR4 and DR5 for induction of apoptosis. In contrast, no change in DcR2 occurred. Why DcR1 was differentially down-regulated by cardamonin is unclear. The expression of DcR2 but not DcR1 has shown to be regulated by p53 (Meng et al., 2000; Prasad et al., 2011); cardamonin did not change the expression of p53 and this may explain its inability to down-regulate DcR2.
In addition to this, induction of TRAIL receptors has been shown to be mediated through the induction of CHOP and SP1 (Yoshida et al., 2001; 2005). Firstly, we showed that cardamonin induced the expression of both CHOP and SP1 proteins. Secondly, silencing of the genes for CHOP and SP1 abolished the effect of cardamonin on induction of DRs. Taken together, these results indicate that CHOP and SP1 play an essential role in the action of cardamonin. Like cardamonin, other α, β-unsaturated dienones such as PGJ2 (Su et al., 2008) and curcumin (Jung et al., 2006) have been shown to induce DRs through activation of CHOP.
Overall our studies provide strong evidence that cardamonin potentiates TRAIL-induced apoptosis through the down-modulation of cell survival gene products and up-regulation of DRs. This suggests that it has great potential for making cancer therapy with TRAIL more effective.
Acknowledgments
We thank Walter Pagel, Department of Scientific Publications for carefully editing the manuscript. Dr Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a core grant (CA-16672), and a programme project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center.
Glossary
- CHOP
CCAAT/enhancer binding protein homologous protein
- DcR
decoy receptor
- DR
death receptor
- TRAIL
TNF–related apoptosis-inducing ligand
Conflicts of interest
We have no personal or financial conflict of interest and have not entered into any agreement that could interfere with our access to the data on the research, or upon our ability to analyse the data independently, to prepare manuscripts and to publish them.
References
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Asakuma J, Sumitomo M, Asano T, Asano T, Hayakawa M. Selective Akt inactivation and tumor necrosis actor-related apoptosis-inducing ligand sensitization of renal cancer cells by low concentrations of paclitaxel. Cancer Res. 2003;63:1365–1370. [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A, Aggarwal BB. Receptor-mediated choreography of life and death. J Clin Immunol. 2003;23:317–332. doi: 10.1023/a:1025319031417. [DOI] [PubMed] [Google Scholar]
- Bheemasankara Rao C, Namosiva Rao T, Suryaprakasam S. Cardamonin and alpinetin from the seeds of Amomum subulatum. Planta Med. 1976;29:391–392. doi: 10.1055/s-0028-1097682. [DOI] [PubMed] [Google Scholar]
- Burns TF, Bernhard EJ, El-Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene. 2001;20:4601–4612. doi: 10.1038/sj.onc.1204484. [DOI] [PubMed] [Google Scholar]
- Cho M, Ryu M, Jeong Y, Chung YH, Kim DE, Cho HS, et al. Cardamonin suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2009;390:500–505. doi: 10.1016/j.bbrc.2009.09.124. [DOI] [PubMed] [Google Scholar]
- Duiker EW, Mom CH, de Jong S, Willemse PH, Gietema JA, van der Zee AG, et al. The clinical trail of TRAIL. Eur J Cancer. 2006;42:2233–2240. doi: 10.1016/j.ejca.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- Hatziieremia S, Gray AI, Ferro VA, Paul A, Plevin R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br J Pharmacol. 2006;149:188–198. doi: 10.1038/sj.bjp.0706856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell EA, Fiers W, North RJ. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med. 1988;167:1067–1085. doi: 10.1084/jem.167.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Li Y, Liu J, Hu Z, Chen X. Specific interaction of chalcone-protein: cardamonin binding site II on the human serum albumin molecule. Biopolymers. 2005;79:48–57. doi: 10.1002/bip.20328. [DOI] [PubMed] [Google Scholar]
- He W, Li Y, Tang J, Luan F, Jin J, Hu Z. Comparison of the characterization on binding of alpinetin and cardamonin to lysozyme by spectroscopic methods. Int J Biol Macromol. 2006;39:165–173. doi: 10.1016/j.ijbiomac.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Israf DA, Khaizurin TA, Syahida A, Lajis NH, Khozirah S. Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-kappaB nuclear translocation and Ikappa-B phosphorylation in RAW 264.7 macrophage cells. Mol Immunol. 2007;44:673–679. doi: 10.1016/j.molimm.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Jung EM, Park JW, Choi KS, Park JW, Lee HI, Lee KS, et al. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006;27:2008–2017. doi: 10.1093/carcin/bgl026. [DOI] [PubMed] [Google Scholar]
- Kim YH, Jung EM, Lee TJ, Kim SH, Choi YH, Park JW, et al. Rosiglitazone promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2008;44:1055–1068. doi: 10.1016/j.freeradbiomed.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Ko H, Park JS, Han IH, Amor EC, Lee JW, et al. Dimethyl cardamonin inhibits lipopolysaccharide-induced inflammatory factors through blocking NF-kappaB p65 activation. Int Immunopharmacol. 2010;10:1127–1134. doi: 10.1016/j.intimp.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Koornstra JJ, Kleibeuker JH, van Geelen CM, Rijcken FE, Hollema H, de Vries EG, et al. Expression of TRAIL (TNF-related apoptosis-inducing ligand) and its receptors in normal colonic mucosa, adenomas, and carcinomas. J Pathol. 2003;200:327–335. doi: 10.1002/path.1364. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jung HS, Giang PM, Jin X, Lee S, Son PT, et al. Blockade of nuclear factor-kappaB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J Pharmacol Exp Ther. 2006;316:271–278. doi: 10.1124/jpet.105.092486. [DOI] [PubMed] [Google Scholar]
- Lee TJ, Um HJ, Min S, Park JW, Choi KS, Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009;46:1639–1649. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Liao Q, Shi DH, Zheng W, Xu XJ, Yu YH. Antiproliferation of cardamonin is involved in mTOR on aortic smooth muscle cells in high fructose-induced insulin resistance rats. Eur J Pharmacol. 2010;641:179–186. doi: 10.1016/j.ejphar.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Lim JH, Park JW, Choi KS, Park YB, Kwon TK. Rottlerin induces apoptosis via death receptor 5 (DR5) upregulation through CHOP-dependent and PKC delta-independent mechanism in human malignant tumor cells. Carcinogenesis. 2009;30:729–736. doi: 10.1093/carcin/bgn265. [DOI] [PubMed] [Google Scholar]
- Ma XM, Yu H, Huai N. Peroxisome proliferator-activated receptor-gamma is essential in the pathogenesis of gastric carcinoma. World J Gastroenterol. 2009;15:3874–3883. doi: 10.3748/wjg.15.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng RD, McDonald ER, 3rd, Sheikh MS, Fornace AJ, Jr, El-Deiry WS. The TRAIL decoy receptor TRUNDD (DcR2, TRAIL-R4) is induced by adenovirus-p53 overexpression and can delay TRAIL-, p53-, and KILLER/DR5-dependent colon cancer apoptosis. Mol Ther. 2000;1:130–144. doi: 10.1006/mthe.2000.0025. [DOI] [PubMed] [Google Scholar]
- Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26:7046–7055. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Choi YH, Kim GY. Butein sensitizes human hepatoma cells to TRAIL-induced apoptosis via extracellular signal-regulated kinase/Sp1-dependent DR5 upregulation and NF-kappaB inactivation. Mol Cancer Ther. 2010;9:1583–1595. doi: 10.1158/1535-7163.MCT-09-0942. [DOI] [PubMed] [Google Scholar]
- Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, et al. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003;94:1066–1073. doi: 10.1111/j.1349-7006.2003.tb01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki T, Kikuchi H, Koyano T, Kowithayakorn T, Sakai T, Ishibashi M. Death receptor 5 promoter-enhancing compounds isolated from Catimbium speciosum and their enhancement effect on TRAIL-induced apoptosis. Bioorg Med Chem. 2009;17:6748–6754. doi: 10.1016/j.bmc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Ozoren N, Fisher MJ, Kim K, Liu CX, Genin A, Shifman Y, et al. Homozygous deletion of the death receptor DR4 gene in a nasopharyngeal cancer cell line is associated with TRAIL resistance. Int J Oncol. 2000;16:917–925. doi: 10.3892/ijo.16.5.917. [DOI] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Prasad S, Yadav VR, Ravindran J, Aggarwal BB. ROS and CHOP are critical for dibenzylideneacetone to sensitize tumor cells to TRAIL through induction of death receptors and downregulation of cell survival proteins. Cancer Res. 2011;71:538–549. doi: 10.1158/0008-5472.CAN-10-3121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ravi R, Bedi A. Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res. 2002;62:4180–4185. [PubMed] [Google Scholar]
- Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Su RY, Chi KH, Huang DY, Tai MH, Lin WW. 15-deoxy-Delta12,14-prostaglandin J2 up-regulates death receptor 5 gene expression in HCT116 cells: involvement of reactive oxygen species and C/EBP homologous transcription factor gene transcription. Mol Cancer Ther. 2008;7:3429–3440. doi: 10.1158/1535-7163.MCT-08-0498. [DOI] [PubMed] [Google Scholar]
- Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010;285:11498–11507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Van Geelen CM, de Vries EG, de Jong S. Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist Updat. 2004;7:345–358. doi: 10.1016/j.drup.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, Krantz ID, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- Yadav VR, Prasad S, Kannappan R, Ravindran J, Chaturvedi MM, Vaahtera L, et al. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem Pharmacol. 2010a;80:1021–1032. doi: 10.1016/j.bcp.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yadav VR, Sung B, Prasad S, Kannappan R, Cho SG, Liu M, et al. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med. 2010b;88:1243–1253. doi: 10.1007/s00109-010-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Maeda A, Tani N, Sakai T. Promoter structure and transcription initiation sites of the human death receptor 5/TRAIL-R2 gene. FEBS Lett. 2001;507:381–385. doi: 10.1016/s0014-5793(01)02947-7. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Shiraishi T, Nakata S, Horinaka M, Wakada M, Mizutani Y, et al. Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Res. 2005;65:5662–5667. doi: 10.1158/0008-5472.CAN-05-0693. [DOI] [PubMed] [Google Scholar]
- Zerafa N, Westwood JA, Cretney E, Mitchell S, Waring P, Iezzi M, et al. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005;175:5586–5590. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhu H, Teraishi F, Davis JJ, Guo W, Fan Z, et al. Accelerated degradation of caspase-8 protein correlates with TRAIL resistance in a DLD1 human colon cancer cell line. Neoplasia. 2005;7:594–602. doi: 10.1593/neo.04688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Liu X, Yue P, Zhou Z, Sporn MB, Lotan R, et al. c-Jun NH2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1, 9-dien-28-oate in human lung cancer cells. Cancer Res. 2004;64:7570–7578. doi: 10.1158/0008-5472.CAN-04-1238. [DOI] [PubMed] [Google Scholar]


